ABSTRACT
The intestines of obese humans and mice are enriched with Erysipelotrichi, a class within the Firmicutes. Clostridium ramosum, a member of the Erysipelotrichi, is associated with symptoms of the metabolic syndrome in humans. To clarify the possible obesogenic potential of this bacterial species and to unravel the underlying mechanism, we investigated the role of C. ramosum in obesity development in gnotobiotic mice. Mice were associated with a simplified human intestinal (SIHUMI) microbiota of eight bacterial species, including C. ramosum, with the SIHUMI microbiota except C. ramosum (SIHUMIw/oCra), or with C. ramosum only (Cra) and fed a high-fat diet (HFD) or a low-fat diet (LFD). Parameters related to the development of obesity and metabolic diseases were compared. After 4 weeks of HFD feeding, the mouse groups did not differ in energy intake, diet digestibility, gut permeability, and parameters of low-grade inflammation. However, SIHUMI and Cra mice fed the HFD gained significantly more body weight and body fat and displayed higher food efficiency than SIHUMIw/oCra mice fed the HFD. Gene expression of glucose transporter 2 (Glut2) in jejunal mucosa and of fatty acid translocase (CD36) in ileal mucosa was significantly increased in the obese SIHUMI and Cra mice compared with the less obese SIHUMIw/oCra mice. The data demonstrate that the presence of C. ramosum in SIHUMI and Cra mice enhanced diet-induced obesity. Upregulation of small intestinal glucose and fat transporters in these animals may contribute to their increased body fat deposition.
IMPORTANCE
Obesity is a growing health problem worldwide. Changes in the proportions of Bacteroidetes and Firmicutes, the two dominant phyla in the human and the murine intestinal tract, link the intestinal microbiota to obesity. Erysipelotrichi, a class within the Firmicutes, increase in response to high-fat feeding in mice. Clostridium ramosum, a member of the Erysipelotrichi, has been linked to symptoms of the metabolic syndrome. We hypothesized that C. ramosum promotes obesity development and related pathologies. Our experiments in gnotobiotic mice show that C. ramosum promoted diet-induced obesity, probably by enhancing nutrient absorption. Identification of obesogenic bacteria and understanding their mode of action enable the development of novel strategies for the treatment of this epidemic disease. Pharmaceuticals that target obesogenic bacteria or their metabolism could help to prevent and treat obesity and related disorders in the future.
INTRODUCTION
The rising prevalence of obesity and its comorbidities, including type 2 diabetes, hypertension, and cardiovascular disease, requires further efforts to better understand the pathogenesis of these conditions. In humans and mice, obesity is accompanied by a reduction of intestinal Bacteroidetes and a concomitant increase of Firmicutes (1, 2). However, this finding was not confirmed in other human studies, which did not show an increased Firmicutes/Bacteroidetes ratio in obese subjects (3, 4). The high proportion of Firmicutes observed in obese mice was in part due to the proliferation of Erysipelotrichi, a bacterial class within this phylum (5). An increased abundance of intestinal Erysipelotrichi was also observed in mice fed a Western diet (6, 7) and in an obese human individual (8). This bloom of Erysipelotrichi suggests a role of these bacteria in obesity development. A recent study in type 2 diabetic women linked the presence of Clostridium ramosum, a member of the Erysipelotrichi, to symptoms of the metabolic syndrome (9). In support of this finding, obesity in human subjects was associated with low bacterial gene content and increased abundance of C. ramosum (10). These studies suggest that C. ramosum may be critically involved in obesity development. However, the underlying mechanisms are obscure.
Several mechanisms have been proposed to explain obesogenic effects of intestinal bacteria. Bacterial glycoside hydrolases cleave nondigestible polysaccharides (dietary fiber) to oligo- and monosaccharides, which subsequently undergo bacterial fermentation mainly to short-chain fatty acids (SCFA). The latter provide additional energy to the host (7, 11, 12). Glucose released during bacterial depolymerization of polysaccharides may promote hepatic lipogenesis (13) and could thereby contribute to obesity development. High-fat diets (HFD) increase intestinal permeability and thereby facilitate the absorption of bacterial lipopolysaccharides (LPS) (14, 15). Increased serum LPS levels were identified as one major cause of obesity-associated low-grade inflammation. Other possible links between obesity and intestinal bacteria include the microbial modulation of appetite (16) or of intestinal lipid absorption (17).
To test the hypothesis that C. ramosum has obesogenic properties, we used gnotobiotic mice harboring a simplified human intestinal microbiota (SIHUMI) composed of eight bacterial species, including C. ramosum, and compared them with mice harboring the same microbial community but without C. ramosum (SIHUMIw/oCra). We hypothesized that SIHUMI mice fed a high-fat diet (HFD) for 4 weeks develop severe obesity, while SIHUMIw/oCra mice will develop no or only moderate obesity. Mice monoassociated with C. ramosum (Cra mice) were investigated to characterize the possible obesogenic effect of this bacterium in the absence of other community members. To elucidate how C. ramosum may contribute to obesity, we tested if this bacterial species (i) increases energy extraction from the diet by delivering SCFA, (ii) facilitates glucose or lipid uptake in the intestine, and/or (iii) reduces gut barrier integrity. The comparison of SIHUMI and SIHUMIw/oCra mice allows a direct link of the observed effects with the presence or the absence of this bacterial species in a defined microbial community.
RESULTS
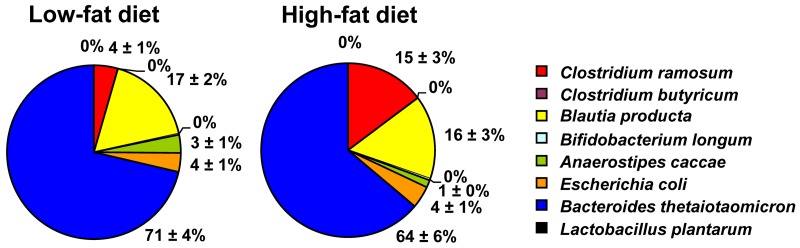
High-fat diet feeding induces obesity and increases the proportion of C. ramosum in SIHUMI mice.
After 4 weeks of dietary intervention, SIHUMI mice fed the low-fat diet (LFD) stayed lean, whereas SIHUMI mice fed the HFD became obese. Accordingly, LFD-fed SIHUMI mice gained less body weight than HFD-fed SIHUMI mice (5.35% ± 3.31% versus 19.94% ± 3.98%, P = 0.014) and had lower body fat percentages than HFD-fed SIHUMI mice (28.78% ± 0.88% versus 32.61% ± 1.10%, P = 0.017). Obesity in HFD-fed SIHUMI mice was accompanied by a higher proportion of C. ramosum in cecal (P = 0.003) (Fig. 1) and colonic (LFD, 5% ± 1%, versus HFD, 16% ± 2%, of total bacteria; P < 0.001) contents. (For absolute cell counts of all bacterial species, see Table S1 in the supplemental material.) Cecal and colonic cell counts of C. ramosum in HFD-fed SIHUMI mice (9.34 ± 0.07 and 9.31 ± 0.06 log10 cells/g dry weight, respectively) were approximately 0.5 log10 cells/g dry weight higher (P < 0.001) than those in LFD-fed SIHUMI mice (8.83 ± 0.04 and 8.77 ± 0.05 log10 cells/g dry weight, respectively). Total bacterial cell counts in cecum (10.24 ± 0.06 versus 10.14 ± 0.04 log10 cells/g dry weight) and colon (10.05 ± 0.02 versus 10.05 ± 0.06 log10 cells/g dry weight) did not differ between LFD-fed and HFD-fed SIHUMI mice. Our finding and the reported associations of C. ramosum with obesity (9, 10, 18) suggest a contribution of this bacterium to obesity development. Therefore, a possible obesogenic effect of C. ramosum was investigated in gnotobiotic mice fed HFD.
FIG 1 .
High-fat diet feeding for 4 weeks increases the cecal proportion of Clostridium ramosum (red) in mice harboring a simplified human intestinal microbiota (SIHUMI) compared with SIHUMI mice fed a low-fat diet for 4 weeks. Bacterial cell numbers were determined by fluorescence in situ hybridization or plating on Rogosa agar (Lactobacillus plantarum). Mean values ± standard errors of the means (SEM) are shown. n = 8 mice per group. For absolute bacterial cell counts, see Table S1 in the supplemental material.
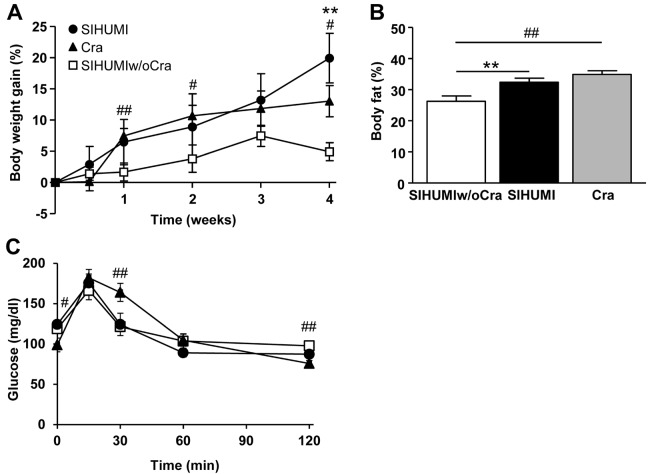
Presence of C. ramosum in gnotobiotic mice fed a semisynthetic HFD promotes obesity.
To test the hypothesis that C. ramosum contributes to diet-induced obesity, we compared SIHUMI, SIHUMIw/oCra, and Cra mice fed HFD. After 4 weeks of HFD feeding, both SIHUMI mice and Cra mice displayed increased body weight gain, body fat percentages (Fig. 2), and adipose tissue weights compared with SIHUMIw/oCra mice (Table 1). On the LFD, the mouse groups did not differ in body weight gain and body fat percentage (see Fig. S1 in the supplemental material), indicating that the obesogenic effect of C. ramosum was restricted to the HFD intervention. HFD-fed SIHUMI and HFD-fed SIHUMIw/oCra mice did not differ in their blood glucose concentrations during the oral glucose tolerance test. However, the blood glucose level in HFD-fed Cra mice decreased more slowly than those in the other two mouse groups (Fig. 2C).
FIG 2 .
Clostridium ramosum increases symptoms of obesity in mice harboring a simplified human intestinal microbiota (SIHUMI) or C. ramosum only (Cra) compared with SIHUMI mice without C. ramosum (SIHUMIw/oCra) after 4 weeks of high-fat diet feeding. (A) Relative body weight gain. (B) Body fat percentage. (C) Blood glucose concentration during oral glucose tolerance test. Mean values ± SEM are shown. n = 8 to 9 mice per group, except for the body fat percentage of Cra mice, for which n = 3, and for the blood glucose concentration of SIHUMI mice, for which n = 4. **, P < 0.01 for SIHUMI versus SIHUMIw/oCra mice; #, P < 0.05, and ##, P < 0.01, for Cra versus SIHUMIw/oCra mice.
TABLE 1 .
Biometric parameters of SIHUMI, SIHUMIw/oCra, or Cra mice, all fed an HFD for 4 weeks
| Parametera | Result for miceb |
||
|---|---|---|---|
| SIHUMIw/oCra | SIHUMI | Cra | |
| Body wt (g) | |||
| Wk 0 | 27.79 ± 0.55 | 27.11 ± 0.62 (0.469) | 28.18 ± 0.49 (0.613) |
| Wk 4 | 29.12 ± 0.41 | 32.43 ± 0.86 (0.003) | 31.83 ± 0.75 (0.005) |
| Wt gain (g [wk 4]) | 1.33 ± 0.40 | 5.31 ± 0.95 (0.001) | 3.65 ± 0.70 (0.009) |
| eWAT (mg/g body wt) | 18.46 ± 2.47 | 28.31 ± 4.23 (0.056) | 32.49 ± 3.74 (0.006) |
| mWAT (mg/g body wt) | 11.81 ± 0.56 | 16.76 ± 1.40 (0.004) | 15.66 ± 1.36 (0.015) |
| sWAT (mg/g body wt) | 9.69 ± 1.21 | 14.15 ± 1.78 (0.048) | 12.87 ± 0.84 (0.052) |
| Energy intake (kJ/day) | 81.20 ± 2.03 | 87.22 ± 6.99 (0.743) | 75.25 ± 2.55 (0.085) |
| Food efficiency (mg/kJ)c | 0.57 ± 0.17 | 2.26 ± 0.40 (0.001) | 1.77 ± 0.37 (0.008) |
| Digestibility of HFD (%) | 92.57 ± 0.43 | 92.27 ± 0.56 (0.681) | 92.60 ± 0.64 (0.963) |
| Digestible energy (kJ/day [wk 3]) | 72.25 ± 6.76 | 70.17 ± 3.97 (0.607) | 68.63 ± 5.37 (1.000) |
eWAT, epididymal white adipose tissue; mWAT, mesenteric white adipose tissue; sWAT, subcutaneous white adipose tissue.
Values are means ± SEM (n = 7 to 9). P values for obese SIHUMI or Cra mice versus less obese SIHUMIw/oCra mice (reference) are shown in parentheses.
Calculated from body weight gain (mg) per consumed energy (kJ) after 4 weeks of HFD feeding.
Leptin gene (Lep) expression was determined in the HFD-fed mice because this adipocyte-derived hormone signals filled energy stores to the brain (19). Lep mRNA levels in epididymal white adipose tissue (eWAT) of obese SIHUMI mice (1.5-fold, P = 0.059) and obese Cra mice (1.8-fold, P = 0.174) were slightly higher than those of less obese SIHUMIw/oCra mice. The improved food efficiency observed for SIHUMI and Cra mice indicates a more efficient conversion of dietary energy into body mass by these mice (Table 1). Since all mice received the same HFD and were housed under the same conditions, it may be concluded that the more pronounced obese phenotype observed for the SIHUMI and Cra mice was mediated by the presence of C. ramosum. The mouse groups did not differ in their energy intakes and the digestibilities of the HFD (Table 1). Therefore, a still unknown bacterial obesogenic mechanism must be responsible for the observed effects. To find indications on how C. ramosum enhanced lipid deposition in SIHUMI and Cra mice in response to HFD feeding, various possible mechanisms were examined.
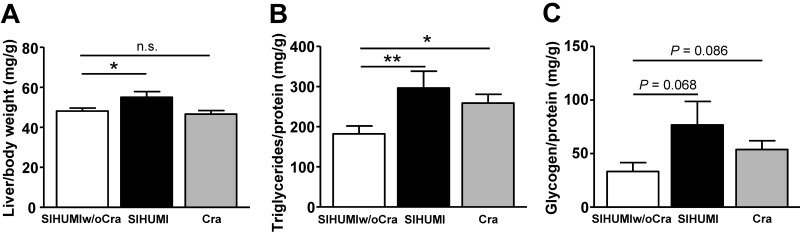
C. ramosum affects liver metabolism in gnotobiotic mice.
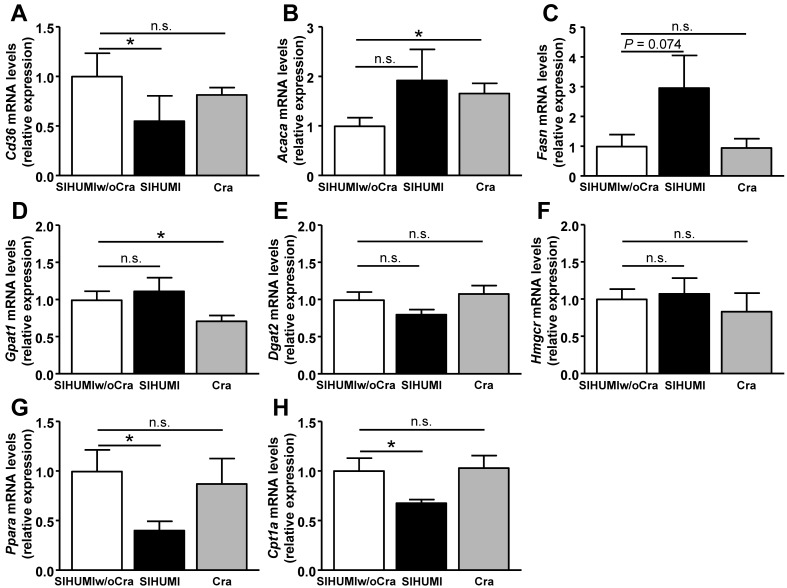
Liver weight in obese SIHUMI mice was higher than that in less obese SIHUMIw/oCra mice or obese Cra mice (Fig. 3A). SIHUMI and Cra mice displayed higher liver triglyceride contents and a tendency for higher liver glycogen contents than SIHUMIw/oCra mice (Fig. 3B and C). To investigate the impact of C. ramosum on liver metabolism in SIHUMI mice in more detail, expression levels of genes involved in lipid transport (fatty acid translocase [Cd36]), lipid synthesis (acetyl coenzyme A [acetyl-CoA] carboxylase 1 [Acaca] and fatty acid synthase [Fasn]), triglyceride synthesis (glycerol-3-phosphate acyltransferase 1 [Gpat1], diacylglycerol acyltransferase 2 [Dgat2]), cholesterol synthesis (HMG-CoA reductase [Hmgcr]), and lipid catabolism (peroxisome proliferator-activated receptor alpha [Ppara] and carnitine palmitoyltransferase 1a [Cpt1a]) were analyzed (Fig. 4). The presence of C. ramosum in SIHUMI mice reduced the mRNA levels of Cd36, Ppara, and Cpt1a significantly and increased those of Fasn (3-fold, P = 0.074) compared with those in SIHUMIw/oCra mice (Fig. 4A, C, G, and H). The data imply reduced lipid uptake and lipid catabolism and elevated fatty acid synthesis in the livers of SIHUMI mice. In contrast to SIHUMI mice, the presence of C. ramosum in monoassociated mice increased the mRNA levels of Acaca and reduced those of Gpat1, indicating increased fatty acid synthesis in conjunction with a reduced triglyceride formation in Cra mice compared with SIHUMIw/oCra mice (Fig. 4B and D). The mRNA levels of Cd36, Gpat1, Ppara, and Cpt1a differed between SIHUMI and Cra mice, suggesting that the effects of C. ramosum on liver metabolism are modulated by the presence of other bacterial species.
FIG 3 .
Liver parameters of mice harboring a simplified human intestinal microbiota (SIHUMI), SIHUMI without C. ramosum (SIHUMIw/oCra), or C. ramosum only (Cra). All mice were fed a high-fat diet for 4 weeks. (A) Relative liver weight. (B) Liver triacylglycerol content. (C) Liver glycogen content. Mean values ± SEM are shown. n = 8 to 9 mice per group, except for SIHUMI liver weight, for which n = 5. *, P < 0.05, and **, P < 0.01, for obese SIHUMI or Cra mice versus less obese SIHUMIw/oCra mice (reference). n.s., not significant.
FIG 4 .
Expression of genes encoding proteins involved in hepatic lipid metabolism in mice harboring a simplified human intestinal microbiota (SIHUMI), SIHUMI without C. ramosum (SIHUMIw/oCra) or C. ramosum only (Cra) after 4 weeks of high-fat diet feeding. (A) Gene coding for fat transport protein fatty acid translocase (Cd36). (B and C) Genes coding for fatty acid synthesis proteins acetyl-CoA carboxylase 1 (Acaca) and fatty acid synthase (Fasn). (D and E) Genes coding for triglyceride synthesis proteins glycerol-3-phosphate acyltransferase 1 (Gpat1) and diacylglycerol acyltransferase 2 (Dgat2). (F) Gene coding for cholesterol synthesis protein HMG-CoA reductase (Hmgcr). (G and H) Genes coding for fatty acid catabolism proteins peroxisome proliferator-activated receptor alpha (Ppara) and carnitine palmitoyltransferase 1a (Cpt1a). Mean values ± SEM are shown. n = 8 to 9 mice per group. *, P < 0.05, and **, P < 0.01, for obese SIHUMI or Cra mice versus less obese SIHUMIw/oCra mice (reference). n.s., not significant.
C. ramosum increases Glut2 transcription in small intestine.
The gut microbiota enhances the extraction of energy from the diet and modulates the expression of genes involved in nutrient absorption and has therefore been proposed to promote obesity development (12, 20). To investigate whether enhanced glucose absorption contributed to the more pronounced obesity of SIHUMI and Cra mice, we analyzed gene expression of two transport proteins involved in small intestinal glucose uptake. The mRNA levels of the gene coding for glucose transporter 2 (Glut2), which facilitates passive glucose absorption, was significantly increased in jejunal mucosa of obese SIHUMI mice (2-fold, P = 0.015) and obese Cra mice (2.6-fold, P = 0.001) compared with less obese SIHUMIw/oCra mice. Moreover, ileal expression of Glut2 was significantly increased in Cra mice (2.3-fold, P = 0.024), whereas the increased ileal Glut2 expression in SIHUMI mice was not statistically significant (1.6-fold, P = 0.250). In contrast to Glut2 expression, mRNA levels of the gene coding for the sodium/glucose cotransporter 1 in jejunal mucosa (Slc5a1) were 1.6-fold lower in SIHUMI mice (P = 0.007) and Cra mice (P = 0.004) than in SIHUMIw/oCra mice. Levels of ileal Slc5a1 gene expression did not differ between the groups. These data indicate that the obesogenic effect of C. ramosum might, at least in part, be mediated by Glut2, which elevates glucose absorption.
C. ramosum increases intestinal SCFA formation in obese SIHUMI mice but not in obese Cra mice.
In humans, SCFA deliver up to 10% of the daily energy needs (21). In addition, SCFA are associated with increased de novo lipogenesis and body fat storage (22, 23). We therefore investigated whether C. ramosum enhanced the formation of SCFA and thereby promoted obesity development in SIHUMI and Cra mice. Acetate concentrations in cecum and colon were higher in SIHUMI mice, but they were lower in Cra mice than in SIHUMIw/oCra mice, all fed HFD (Table 2). To determine the capacity of the microbial communities in SIHUMI and SIHUMIw/oCra mice to form SCFA from HFD, cecal and colonic contents from these mice were incubated under anoxic conditions and SCFA formation was monitored. The in vitro SCFA formation rates by intestinal contents did not differ between SIHUMI and SIHUMIw/oCra mice (see Table S2 in the supplemental material). In accordance with this result, SCFA concentrations in portal vein plasma of SIHUMI and SIHUMIw/oCra mice were also not different (see Table S2), arguing against SCFA as an important factor for obesity development in this mouse model and against increased SCFA absorption in response to the presence of C. ramosum.
TABLE 2 .
Intestinal concentrations of SCFA in SIHUMI, SIHUMIw/oCra, or Cra mice after 4 weeks of high-fat diet feeding
| SCFA | SCFA concn (µmol/g) ina: |
|||||
|---|---|---|---|---|---|---|
| Cecum |
Colon |
|||||
| SIHUMIw/oCra | SIHUMI | Cra | SIHUMIw/oCra | SIHUMI | Cra | |
| Acetic acid | 10.3 ± 0.7 | 15.8 ± 1.9* | 7.6 ± 0.8* | 10.9 ± 0.8 | 16.2 ± 2.5 | 7.1 ± 1.2* |
| Propionic acid | 1.7 ± 0.2 | 2.8 ± 0.5 | 0.1 ± 0.0*** | 1.2 ± 0.2 | 1.5 ± 0.2 | 0.1 ± 0.0*** |
| Butyric acid | 0.4 ± 0.0 | 0.6 ± 0.1 | ND | 0.2 ± 0.1 | 0.2 ± 0.1 | ND |
Values are means ± SEM (n = 8 to 9). ND, not detected. *, P < 0.05, and ***, P < 0.001, for obese SIHUMI or Cra mice versus less obese SIHUMIw/oCra mice (reference).
Gene expression of SCFA-related proteins in colonic mucosa was also determined. Expression of the genes coding for monocarboxylate transporter 1 (Mct1), which facilitates SCFA transport across the brush border membrane (24, 25), free fatty acid receptor 3 (Ffar3), and the downstream-activated gut hormone peptide YY did not differ between SIHUMIw/oCra, SIHUMI, and Cra mice (data not shown). These data suggest that increased formation, uptake, or signaling of SCFA was not causative for obesity development in SIHUMI and Cra mice.
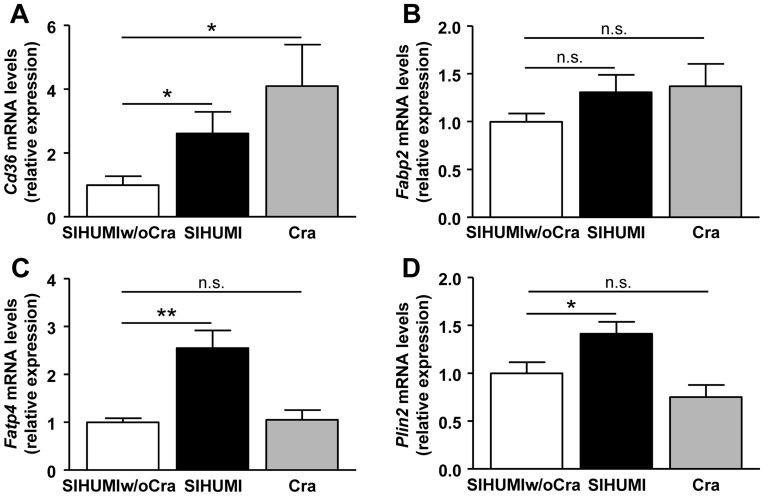
C. ramosum increases gene expression of fat transport proteins in ileum.
We hypothesized that C. ramosum promoted ileal lipid absorption and thereby contributed to the higher body weight gain and fat deposition in the obese SIHUMI and Cra mice compared with the less obese SIHUMIw/oCra mice. To assess the effect of C. ramosum on expression of genes involved in lipid uptake, mRNA levels of the Cd36 gene and the genes coding for intestinal fatty acid binding protein (Fabp2), fatty acid transport protein 4 (Fatp4), and lipid storage protein perilipin 2 (Plin2) were determined in all groups. Ileal gene expression of Cd36 was significantly increased in SIHUMI and Cra mice compared with SIHUMIw/oCra mice (Fig. 5A), while that of Fabp2 only tended to be higher in SIHUMI (P = 0.140) and Cra (P = 0.156) mice than in SIHUMIw/oCra mice (Fig. 5B). Transcription of Fatp4 and Plin2 was increased in ileal mucosa of SIHUMI mice but not in that of Cra and SIHUMIw/oCra mice (Fig. 5C and D). Given that monoassociation of mice with C. ramosum did not increase Fatp4 and Plin2 expression, we propose that upregulation of these genes by C. ramosum was dependent on other members of the SIHUMI community. In conclusion, the data suggest that the obesity-promoting effect of C. ramosum is based on the ability of this bacterium, alone or within a microbial community, to enhance expression of genes involved in ileal lipid absorption.
FIG 5 .
Expression of genes encoding fat transport and storage proteins in ileal mucosa of mice harboring a simplified human intestinal microbiota (SIHUMI), SIHUMI without C. ramosum (SIHUMIw/oCra), or C. ramosum only (Cra). Mice were fed a high-fat diet for 4 weeks. (A) Gene coding for fatty acid translocase (Cd36). (B) Gene coding for intestinal fatty acid binding protein (Fabp2). (C) Gene coding for fatty acid transport protein 4 (Fatp4). (D) Gene coding for fat storage protein perilipin 2 (Plin2). Mean values ± SEM are shown. n = 8 mice per group. *, P < 0.05, and **, P < 0.01, for obese SIHUMI or Cra mice versus less obese SIHUMIw/oCra mice (reference). n.s., not significant.
C. ramosum does not increase gut permeability and does not cause low-grade inflammation.
Obesity is often associated with increased levels of inflammatory markers referred to as low-grade inflammation (26). Diets rich in fat facilitate the uptake of bacterial lipopolysaccharide (LPS), resulting in endotoxemia, an increased inflammatory tone, adiposity, and various symptoms of metabolic disease (27). This has been linked to an HFD-mediated impairment of gut barrier function (14, 15). To investigate whether C. ramosum impairs gut epithelial barrier function, we determined intestinal permeability by measuring the concentration of 4-kDa fluorescein isothiocyanate (FITC)-labeled dextran in plasma following its oral application. High plasma FITC-dextran concentrations indicate increased gut permeability (14). However, SIHUMIw/oCra, SIHUMI, and Cra mice did not differ in their FITC-dextran plasma levels (Table 3). Levels of expression of the genes coding for the tight junction proteins zonula occludens-1 (Zo1) and occludin (Ocln) in small intestinal mucosa were similar in SIHUMIw/oCra, SIHUMI, and Cra mice. SIHUMI and Cra mice even displayed an increased colonic transcription of Zo1 and Ocln (Table 3), arguing also against a disturbed gut barrier in mice harboring C. ramosum. In line with the expression data of epithelial barrier-associated genes, LPS concentrations in all samples were close to or below the detection limit of the method (0.05 IU/ml) (Table 3). Intestinal mRNA levels of the tumor necrosis factor alpha gene (Tnfa) tended to be lower in obese SIHUMI and Cra mice than in less obese SIHUMIw/oCra mice. Tnfa transcription was only increased in liver of SIHUMI mice and in eWAT of Cra mice compared with SIHUMIw/oCra mice (Table 3). In general, Tnfa mRNA levels in all tissues were very low and argue against endotoxemia as a major contributor to obesity in SIHUMI and Cra mice. Taken together, the data demonstrate that the obesogenic effect of C. ramosum in gnotobiotic mice does not involve impaired gut barrier function or endotoxemia.
TABLE 3 .
Parameters of intestinal permeability and low-grade inflammation in SIHUMI, SIHUMIw/oCra, or Cra mice after 4 weeks of high-fat diet feeding
| Parametera | Result for miceb |
||
|---|---|---|---|
| SIHUMIw/oCra | SIHUMI | Cra | |
| Intestinal permeability | |||
| FITC-dextran concn (µg/ml)c | 4.55 ± 0.62 | 4.00 ± 1.20 | 3.29 ± 0.28 |
| Zo1 relative expression in: | |||
| Jejunum | 1.00 ± 0.37 | 1.32 ± 0.42 | 1.71 ± 0.59d |
| Ileum | 1.00 ± 0.08 | 0.87 ± 0.08 | 1.00 ± 0.08 |
| Colon | 1.00 ± 0.12 | 1.99 ± 0.09*** | 1.91 ± 0.24** |
| Ocln relative expression in: | |||
| Jejunum | 1.00 ± 0.08 | 0.80 ± 0.12 | 0.94 ± 0.14 |
| Ileum | 1.00 ± 0.06 | 0.90 ± 0.07 | 0.77 ± 0.05* |
| Colon | 1.00 ± 0.07 | 1.56 ± 0.13** | 1.10 ± 0.07 |
| Low-grade inflammation | |||
| LPS concn (IU/ml)c | 0.06 ± 0.03 | 0.02 ± 0.02 | ND |
| Tnfa relative expression in: | |||
| Jejunum | 1.00 ± 0.14 | 0.58 ± 0.17 | 0.71 ± 0.06 |
| Ileum | 1.00 ± 0.14 | 0.97 ± 0.12 | 0.43 ± 0.06** |
| Colon | 1.00 ± 0.13 | 0.64 ± 0.09* | 1.21 ± 0.19 |
| Liver | 1.00 ± 0.08 | 1.99 ± 0.24** | 1.24 ± 0.15 |
| eWAT | 1.00 ± 0.13 | 1.41 ± 0.15 | 1.78 ± 0.28* |
FITC-dextran, 4 kDa fluorescein isothiocyanate-dextran; LPS, lipopolysaccharides; Ocln, occludin gene; Tnfa, tumor necrosis factor alpha gene; Zo1, zonula occludens-1 gene. Gene expression of Zo1, Ocln, and Tnfa was analyzed in the indicated tissues.
Values are means ± SEM (n = 7 to 9). ND, not detected. *, P < 0.05, **, P < 0.01, and ***, P < 0.001, for obese SIHUMI or Cra mice versus less obese SIHUMIw/oCra mice (reference).
Determined in peripheral blood plasma.
n = 5.
DISCUSSION
HFD feeding changes microbial community composition in favor of C. ramosum.
In SIHUMI mice, HFD feeding and the ensuing obesity were accompanied by an increased abundance of intestinal C. ramosum (Fig. 1). An increase of C. ramosum in response to HFD feeding was also reported for rats harboring the same microbial community (18). This finding is consistent with increased proportions of Erysipelotrichi in obese mice and in a human patient (5–8). However, the reason for the observed increase in C. ramosum in HFD-fed obese SIHUMI mice or SIHUMI rats is unknown. Bile acids produced in response to HFD possibly promoted the growth of C. ramosum, because administration of cholic acid was demonstrated to increase the proportion of intestinal Firmicutes, in particular that of Erysipelotrichi (28). More recent human studies reported a link between the abundance of C. ramosum and parameters of the metabolic syndrome (9, 10). We therefore hypothesized that C. ramosum contributes to obesity development.
C. ramosum promotes accumulation of body fat.
To investigate the hypothesized obesogenic effect of C. ramosum alone or as a member of a defined microbial community, we compared Cra, SIHUMI, and SIHUMIw/oCra mice, all fed HFD. Cra and SIHUMI mice displayed more pronounced symptoms of obesity, such as increased accumulation of body fat compared with SIHUMIw/oCra mice (Fig. 2). Hence, the obesogenic effect of C. ramosum was observed in two different mouse models: mice monoassociated with C. ramosum and mice harboring the SIHUMI community. However, it is not clear whether this effect is restricted to the particular HFD used in our experiments or whether it would also be observed in response to feeding on other energy-rich diets. Moreover, it would be important to find out whether species other than C. ramosum may have obesogenic effects, in particular members of the Erysipelotrichi, including Eubacterium dolichum and Clostridium innocuum, which were increased in the Western diet-associated microbiome (6, 7). Recently, an obesity-promoting effect was demonstrated for the proteobacterium Enterobacter cloacae in monoassociated mice (29). However, the contribution of this endotoxin-producing species to obesity development as a member of a defined microbial community was not investigated in the study by Fei and Zhao (29).
In line with the reported correlation between the fecal abundance of Erysipelotrichi and the hepatic fat content in humans (30), we found high hepatic triglyceride levels in mice harboring C. ramosum (Fig. 3). Unexpectedly, SIHUMI and Cra mice differed in hepatic lipogenesis and oxidation markers, indicating that mechanisms involved in fat storage in adipose tissue differed between these mice. Obesity of SIHUMI and Cra mice was associated with neither a higher energy intake nor impaired gut barrier function or metabolic endotoxemia. Other explanations for differences in the susceptibility to HFD-induced obesity include reduced energy expenditure (5) or reduced thermogenesis as brought about by saturated fatty acids in HFD (31). However, these factors do not explain the differences between the obese SIHUMI or Cra mice and the less obese SIHUMIw/oCra mice, since all mice were fed the same HFD. Therefore, C. ramosum must be responsible for the obesity-promoting effects observed in SIHUMI and Cra mice.
SCFA formation does not explain the obesogenic effect of C. ramosum.
SCFA produced by intestinal bacteria deliver additional energy to the host and could therefore contribute to obesity development (7, 12, 23). However, digestibility of the HFD did not differ between obese and less obese mice. Moreover, three observations argue against the idea that SCFA promoted obesity in SIHUMI and Cra mice: (i) Cra mice became obese despite very low intestinal SCFA concentrations, (ii) SIHUMI and Cra mice stayed lean on LFD even though intestinal SCFA concentrations were as high as those of the respective HFD-fed mice (data not shown), and (iii) SCFA concentrations in portal vein plasma were not increased in obese SIHUMI mice.
Upregulation of Glut2 transcription in small intestine may be involved in the obesogenic effect of C. ramosum.
Gut bacteria have also been proposed to promote obesity by a more efficient absorption of monosaccharides in the intestine (13). Regulation of mucosal gene expression by intestinal bacteria might be critically involved in this effect (20, 32). SIHUMI and Cra mice displayed an improved food efficiency (Table 1), suggesting that C. ramosum alone or as a SIHUMI member promoted the uptake of obesogenic nutrients. The increased small intestinal expression of Glut2 observed in SIHUMI and Cra mice argues in favor of increased glucose absorption as a factor that contributed to the obesogenic effect of C. ramosum. The high glucose uptake may have impaired glucose homeostasis in Cra mice. However, such an effect was not observed in SIHUMI mice, suggesting that the microbial community in SIHUMI mice prevented the prediabetic signs observed in the Cra mice.
Increased transcription of fat transporters in ileum may contribute to the obesogenic effect of C. ramosum.
We hypothesized that elevated lipid absorption could be a mechanism by which C. ramosum promotes obesity. A study comparing conventional and germfree mice revealed that the intestinal microbiota increases intestinal absorption of lipids, resulting in better food efficiency and marked obesity (33). Recently, it has also been demonstrated that gut bacteria induce the absorption of long-chain and medium-chain fatty acids as well as the formation of lipid droplets in intestinal epithelium of zebrafish (17), whereas they do not affect the absorption of triglycerides (34). Proteins such as CD36, FATP4, and FABP2 are involved in intestinal lipid uptake and signaling (35, 36). The protein PLIN2 coats lipid droplets and is involved in intracellular fat storage in enterocytes (37, 38). The increased expression of Cd36 in ileum of Cra and SIHUMI mice suggests that the presence of C. ramosum promoted the transcription of this gene and thereby contributed to obesity development. SIHUMI mice but not SIHUMIw/oCra or Cra mice displayed elevated mRNA levels of Fatp4 and Plin2 in ileum. This indicates that the C. ramosum-mediated increase in transcription of these genes in the SIHUMI mice was dependent on the interaction with other community members.
In summary, this study reveals that the presence of C. ramosum in SIHUMI and Cra mice is accompanied by increased body weight gain and body fat storage compared with SIHUMIw/oCra mice after being fed an HFD for 4 weeks, suggesting that C. ramosum has obesogenic properties under these experimental conditions. Upregulation of Glut2 and Cd36 transcription in small intestinal mucosa in gnotobiotic mice harboring intestinal C. ramosum indicates that this organism promotes body fat accumulation through enhanced intestinal glucose and lipid absorption. Mechanistic research is needed to prove this hypothesis—for instance, by taking advantage of labeled substrates. Which bacterial molecules mediate these obesogenic effects is still unclear and deserves further investigations. We cannot exclude that C. ramosum promoted obesity through additional mechanisms not investigated in this study, including modifications in energy expenditure or in adipose tissue and muscle lipid metabolism. To find out whether the observed ability of C. ramosum to promote obesity is a general feature of this species or restricted to the strain under study, additional C. ramosum strains and other members of the Erysipelotrichi apart from C. ramosum need to be investigated in more complex microbial communities. Unraveling the underlying mechanism may help to develop new strategies in the prevention or treatment of obesity.
MATERIALS AND METHODS
Mice and experimental setup.
Germfree male C3H/HeOuJ mice were obtained from the gnotobiotic animal facility of the German Institute of Human Nutrition, Potsdam-Rehbruecke, Germany. The mice were maintained in positive-pressure isolators (Metall & Plastik, Radolfzell, Germany) under a 12-h light-dark cycle. They were kept individually in polycarbonate cages on irradiated wood chips (25 to 50 kGy) at 22 ± 2°C and 55% ± 5% air humidity. All mice had free access to irradiated standard chow (Altromin fortified type 1310; Altromin, Lage, Germany) and autoclaved water. The animal experiments were approved by the Animal Welfare Committee of the State of Brandenburg (approval no. V3-2347-10-2011). Germfree mice were associated with a simplified bacterial community of human intestinal bacteria (SIHUMI) consisting of Anaerostipes caccae DSM 14662, Bacteroides thetaiotaomicron DSM 2079, Bifidobacterium longum NCC 2705, Blautia producta DSM 2950, Clostridium butyricum DSM 10702, Clostridium ramosum DSM 1402, Escherichia coli K-12 MG1655, and Lactobacillus plantarum DSM 20174 (18). A second group of germfree mice was associated with all SIHUMI members except C. ramosum (SIHUMIw/oCra). Association of germfree mice with bacteria and confirmation of successful colonization were performed as described previously for rats (18). Male offspring of these mice were used for the experiments. A third group of germfree male mice was monoassociated with C. ramosum (Cra) at the age of 6 weeks. Twelve-week-old SIHUMI, SIHUMIw/oCra, and Cra mice (n = 8 to 9) were either fed ad libitum a semisynthetic LFD or an HFD (Table 4) for 4 weeks. Body weight and feed intake were determined weekly. Energy intake and fecal energy loss within 48 h were determined by bomb calorimetry during the third intervention week and used for the calculation of diet digestibility and digestible energy as described before (5). Glucose tolerance was determined using an oral glucose tolerance test 2 days prior to killing. After an overnight fast (16 h), glucose (2 g/kg body weight) was administered orally, and tail vein blood was taken at gavage and 15, 30, 60, and 120 min after gavage. Glucose concentrations were analyzed using Contour glucose sticks (Bayer, Leverkusen, Germany). The total area under the curve (AUC) of blood glucose concentration was calculated.
TABLE 4 .
Composition of the semisynthetic LFD and the HFD and energy measurements
| Parameter | Result for: |
|
|---|---|---|
| HFD | LFD | |
| Ingredients (g/100 g) | ||
| Casein | 27 | 22 |
| Wheat starch | 15 | 38 |
| Maltodextrin | 14 | 14 |
| Sucrose | 10 | 10 |
| Palm kernel fat (Palmin) | 11 | 2 |
| Sunflower oil | 11 | 2 |
| Cellulose | 5 | 5 |
| Mineral mixture | 5 | 5 |
| Vitamin mixture | 2 | 2 |
| Energy (kJ/g)a | 20.6 | 17.3 |
| Energy (%) from: | ||
| Protein | 22.7 | 23.8 |
| Carbohydrate | 32.6 | 65.7 |
| Fat | 44.7 | 10.5 |
Determined by bomb calorimetry.
Intestinal permeability in vivo.
Intestinal permeability was measured using the FITC-dextran assay in the fourth intervention week as previously described (14), with some modifications. Mice were fasted for 6 h and subsequently given 4-kDa FITC-dextran (Sigma-Aldrich, Munich, Germany) by gavage (600 mg/kg body weight [80 mg/ml]). The FITC-dextran solution was prepared with phosphate-buffered saline (PBS: NaCl, 8.0 g/liter; KCl, 0.2 g/liter; Na2HPO4, 1.44 g/liter; KH2PO4, 0.24 g/liter [pH 7.4]) and sterile filtered. One hour after gavage, 90 µl blood was collected from the retrobulbar capillary plexus in heparinized tubes. After centrifugation at 2,000 × g for 5 min, the plasma was diluted 1/5 with PBS. FITC-dextran concentrations were measured spectrophotometrically (Infinite M200 PRO; Tecan, Grödig, Austria) at an excitation wavelength of 485 nm and an emission wavelength of 528 nm. Standards were prepared in PBS, and the generated curve was used to calculate the FITC-dextran concentrations in the collected samples.
Body composition and tissue sampling.
After 4 weeks of dietary intervention, body composition was determined by quantitative magnetic resonance spectroscopy (MQ10; Bruker Minispec, Houston, TX) as previously described (39). Subsequently, the nonfasted mice were anesthetized, and blood was taken from the retrobulbar plexus, followed by cervical dislocation of the mice. Liver, eWAT, mesenteric white adipose tissue (mWAT), and subcutaneous white adipose tissue (sWAT) were weighed. Mucosa was scraped from jejunum, ileum, and colon. All tissues were frozen in liquid nitrogen and stored at −80°C. Gut contents were collected and stored at −20°C for further analyses.
Gut content analyses.
The microbial composition of cecal and colonic contents was analyzed by fluorescence in situ hybridization and plating on Rogosa agar (Oxoid Limited, Hampshire, United Kingdom) (18). SCFA in gut contents were analyzed by gas chromatography as previously described (5), but the sample weight was reduced to approximately 40 mg, and the volume of all solvents was halved. SCFA formation capacity of intestinal contents and SCFA concentrations in portal vein plasma were determined by analyzing three additional SIHUMI and SIHUMIw/oCra mice, respectively (see Text S1 in the supplemental material).
Plasma analyses.
LPS analysis with the Limulus amebocyte lysate (LAL) kinetic chromogenic methodology was performed by Charles River Laboratories (L’Arbresle Cedex, France). Color absorbance was directly proportional to the endotoxin concentration. Prior to the test, peripheral blood plasma was diluted 1/50 with LAL reagent water and heated for 10 min at 70°C.
Liver triacylglycerol and glycogen analyses.
An aliquot of ground liver tissue (50 mg) was used for liver triglyceride and glycogen analysis, respectively. Triglycerides and glycogen were extracted as described previously (5). Triglycerides were enzymatically hydrolyzed by the lipolytic activity of the triglyceride reagent (Sigma-Aldrich) to glycerol and free fatty acids. Free glycerol was measured by coupled enzyme reactions with the free glycerol reagent (Sigma-Aldrich). Liver glycogen was determined with the Starch kit (R-Biopharm, Darmstadt, Germany). Glycogen was hydrolyzed, and the glucose formed was further oxidized, leading to the formation of reduced NADPH. Glycogen and triglyceride contents were related to the protein content of the samples as measured with the detergent-compatible protein assay (Bio-Rad, Hercules, CA), a modified colorimetric Lowry assay. All measurements were performed in triplicates in 96-well plates.
Quantitative real-time PCR.
RNA from intestinal mucosa, liver, and fat tissue was isolated with the peqGOLD TriFast reagent (Peqlab, Erlangen, Germany). Genomic DNA was removed using the Ambion Turbo DNA-free kit (Life Technologies, Darmstadt, Germany). Removal of genomic DNA was considered successful when no amplification of 18S rRNA genes (Rn18s) was detectable. Integrity of RNA was verified by running aliquots on agarose gels stained with ethidium bromide. Complementary DNA was synthesized from 1 µg of total RNA with the RevertAid H Minus first-strand cDNA synthesis kit (Thermo Scientific, Schwerte, Germany). Quantitative PCR was performed with the Applied Biosystems 7900 HT fast real-time PCR system (Life Technologies). The reaction mixture of 5 µl contained 2.5 µl Power SYBR green PCR master mix or TaqMan gene expression master mix (Life Technologies), primers (3 µM each) (for sequences, see Table S3 in the supplemental material), and a cDNA amount corresponding to 5 ng of RNA. An oligonucleotide probe (2 µM) (Table S3) was added to the TaqMan reaction mixture. Gene expression was calculated using the threshold cycle (ΔΔCT) method (40).
Statistical analysis.
Data are presented as means ± standard errors of the means (SEM). The Gaussian distribution of all data was checked with the Kolmogorov-Smirnov test. Normally distributed data sets were tested for statistical significance of difference using Student’s t test, whereas nonnormally distributed data were analyzed with the Mann-Whitney U test using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA). LPS and FITC-dextran outliers were identified with the Grubbs test using a free web calculator (http://graphpad.com/quickcalcs) and removed from the data set.
SUPPLEMENTAL MATERIAL
Supplemental methods. Download
Relative body weight gain during 4 weeks of dietary intervention (A, B, and C) and body fat percentage after 4 weeks of dietary intervention (D) of mice harboring a simplified human intestinal microbiota (SIHUMI), SIHUMI without C. ramosum (SIHUMIw/oCra), or C. ramosum only (Cra). All mice were fed a high-fat diet (HFD) or a low-fat diet (LFD). Values are means ± SEM. n = 8 to 9 mice per group, except for the body fat percentage of Cra mice, for which n = 3. *, P < 0.05, **, P < 0.01, and ***, P < 0.001, for HFD-fed mice versus LFD-fed mice. Download
Bacterial cell counts in the intestine of mice harboring a simplified human intestinal microbiota (SIHUMI) fed low-fat diet (LFD) or high-fat diet (HFD) for 4 weeks.
In vitro short-chain fatty acid (SCFA) formation capacity of SIHUMI and SIHUMIw/oCra communities and portal plasma SCFA concentrations in SIHUMI and SIHUMIw/oCra mice.
Sequences of primers and probes used for quantitative real-time PCR.
ACKNOWLEDGMENTS
We thank Ute Lehmann, Ines Grüner, Sabine Schmidt, Marion Urbich, and Antje Sylvester for excellent technical assistance.
This work was supported by the German Institute of Human Nutrition Potsdam-Rehbruecke, a member of the Leibniz Association.
Footnotes
Citation Woting A, Pfeiffer N, Loh G, Klaus S, Blaut M. 2014. Clostridium ramosum promotes high-fat diet-induced obesity in gnotobiotic mouse models. mBio 5(5):e01530-14. doi:10.1128/mBio.01530-14.
REFERENCES
- 1. Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. 2005. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. U. S. A. 102:11070–11075. 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. 2006. Microbial ecology: human gut microbes associated with obesity. Nature 444:1022–1023. 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 3. Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, Flint HJ. 2008. Human colonic microbiota associated with diet, obesity and weight loss. Int. J. Obes. (Lond.) 32:1720–1724. 10.1038/ijo.2008.155 [DOI] [PubMed] [Google Scholar]
- 4. Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. 2010. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 18:190–195. 10.1038/oby.2009.167 [DOI] [PubMed] [Google Scholar]
- 5. Fleissner CK, Huebel N, Abd El-Bary MM, Loh G, Klaus S, Blaut M. 2010. Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br. J. Nutr. 104:919–929. 10.1017/S0007114510001303 [DOI] [PubMed] [Google Scholar]
- 6. Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. 2009. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 1:6ra14. 10.1126/scitranslmed.3000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. 2008. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3:213–223. 10.1016/j.chom.2008.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferrer M, Ruiz A, Lanza F, Haange SB, Oberbach A, Till H, Bargiela R, Campoy C, Segura MT, Richter M, von Bergen M, Seifert J, Suarez A. 2013. Microbiota from the distal guts of lean and obese adolescents exhibit partial functional redundancy besides clear differences in community structure. Environ. Microbiol. 15:211–226. 10.1111/j.1462-2920.2012.02845.x [DOI] [PubMed] [Google Scholar]
- 9. Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F. 2013. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498:99–103. 10.1038/nature12198 [DOI] [PubMed] [Google Scholar]
- 10. Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T, MetaHIT Consortium. Bork P, Wang J, Ehrlich SD, Pedersen O. 2013. Richness of human gut microbiome correlates with metabolic markers. Nature 500:541–546. 10.1038/nature12506 [DOI] [PubMed] [Google Scholar]
- 11. Hooper LV, Midtvedt T, Gordon JI. 2002. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 22:283–307. 10.1146/annurev.nutr.22.011602.092259 [DOI] [PubMed] [Google Scholar]
- 12. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444:1027–1031. 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 13. Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. 2004. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. U. S. A. 101:15718–15723. 10.1073/pnas.0407076101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. 2008. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57:1470–1481. 10.2337/db07-1403 [DOI] [PubMed] [Google Scholar]
- 15. Erridge C, Attina T, Spickett CM, Webb DJ. 2007. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am. J. Clin. Nutr. 86:1286–1292 [DOI] [PubMed] [Google Scholar]
- 16. Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, De Backer F, Neyrinck AM, Delzenne NM. 2009. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am. J. Clin. Nutr. 90:1236–1243. 10.3945/ajcn.2009.28095 [DOI] [PubMed] [Google Scholar]
- 17. Semova I, Carten JD, Stombaugh J, Mackey LC, Knight R, Farber SA, Rawls JF. 2012. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 12:277–288. 10.1016/j.chom.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Becker N, Kunath J, Loh G, Blaut M. 2011. Human intestinal microbiota: characterization of a simplified and stable gnotobiotic rat model. Gut Microbes 2:25–33. 10.4161/gmic.2.1.14651 [DOI] [PubMed] [Google Scholar]
- 19. Denver RJ, Bonett RM, Boorse GC. 2011. Evolution of leptin structure and function. Neuroendocrinology 94:21–38. 10.1159/000328435 [DOI] [PubMed] [Google Scholar]
- 20. Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. 2001. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291:881–884. 10.1126/science.291.5505.881 [DOI] [PubMed] [Google Scholar]
- 21. McNeil NI. 1984. The contribution of the large intestine to energy supplies in man. Am. J. Clin. Nutr. 39:338–342 [DOI] [PubMed] [Google Scholar]
- 22. Samuel BS, Gordon JI. 2006. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc. Natl. Acad. Sci. U. S. A. 103:10011–10016. 10.1073/pnas.0602187103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Isken F, Klaus S, Osterhoff M, Pfeiffer AF, Weickert MO. 2010. Effects of long-term soluble vs. insoluble dietary fiber intake on high-fat diet-induced obesity in C57BL/6J mice. J. Nutr. Biochem. 21:278–284. 10.1016/j.jnutbio.2008.12.012 [DOI] [PubMed] [Google Scholar]
- 24. Cuff MA, Lambert DW, Shirazi-Beechey SP. 2002. Substrate-induced regulation of the human colonic monocarboxylate transporter, MCT1. J. Physiol. 539:361–371. 10.1113/jphysiol.2001.014241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kirat D, Kato S. 2006. Monocarboxylate transporter 1 (MCT1) mediates transport of short-chain fatty acids in bovine caecum. Exp. Physiol. 91:835–844. 10.1113/expphysiol.2006.033837 [DOI] [PubMed] [Google Scholar]
- 26. Gregor MF, Hotamisligil GS. 2011. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29:415–445. 10.1146/annurev-immunol-031210-101322 [DOI] [PubMed] [Google Scholar]
- 27. Cani PD, Osto M, Geurts L, Everard A. 2012. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 3:279–288. 10.4161/gmic.19625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. 2011. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 141:1773–1781. 10.1053/j.gastro.2011.07.046 [DOI] [PubMed] [Google Scholar]
- 29. Fei N, Zhao L. 2013. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 7:880–884. 10.1038/ismej.2012.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. 2011. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 140:976–986. 10.1053/j.gastro.2010.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takeuchi H, Matsuo T, Tokuyama K, Shimomura Y, Suzuki M. 1995. Diet-induced thermogenesis is lower in rats fed a lard diet than in those fed a high oleic acid safflower oil diet, a safflower oil diet or a linseed oil diet. J. Nutr. 125:920–925 [DOI] [PubMed] [Google Scholar]
- 32. El Aidy S, Merrifield CA, Derrien M, van Baarlen P, Hooiveld G, Levenez F, Doré J, Dekker J, Holmes E, Claus SP, Reijngoud DJ, Kleerebezem M. 2013. The gut microbiota elicits a profound metabolic reorientation in the mouse jejunal mucosa during conventionalisation. Gut 62:1306–1314. 10.1136/gutjnl-2011-301955 [DOI] [PubMed] [Google Scholar]
- 33. Rabot S, Membrez M, Bruneau A, Gérard P, Harach T, Moser M, Raymond F, Mansourian R, Chou CJ. 2010. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 24:4948–4959. 10.1096/fj.10-164921 [DOI] [PubMed] [Google Scholar]
- 34. Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, Felin J, Perkins R, Borén J, Oresic M, Bäckhed F. 2010. The gut microbiota modulates host energy and lipid metabolism in mice. J. Lipid Res. 51:1101–1112. 10.1194/jlr.M002774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Langhans W, Leitner C, Arnold M. 2011. Dietary fat sensing via fatty acid oxidation in enterocytes: possible role in the control of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300:R554–R565. 10.1152/ajpregu.00610.2010 [DOI] [PubMed] [Google Scholar]
- 36. Chevrot M, Martin C, Passilly-Degrace P, Besnard P. 2012. Role of CD36 in oral and postoral sensing of lipids. Handb. Exp. Pharmacol. 209:295–307. 10.1007/978-3-642-24716-3_13 [DOI] [PubMed] [Google Scholar]
- 37. Wolins NE, Brasaemle DL, Bickel PE. 2006. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 580:5484–5491. 10.1016/j.febslet.2006.08.040 [DOI] [PubMed] [Google Scholar]
- 38. Bouchoux J, Beilstein F, Pauquai T, Guerrera IC, Chateau D, Ly N, Alqub M, Klein C, Chambaz J, Rousset M, Lacorte JM, Morel E, Demignot S. 2011. The proteome of cytosolic lipid droplets isolated from differentiated Caco-2/TC7 enterocytes reveals cell-specific characteristics. Biol. Cell 103:499–517. 10.1042/BC20110024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klaus S, Rudolph B, Dohrmann C, Wehr R. 2005. Expression of uncoupling protein 1 in skeletal muscle decreases muscle energy efficiency and affects thermoregulation and substrate oxidation. Physiol. Genomics 21:193–200. 10.1152/physiolgenomics.00299.2004 [DOI] [PubMed] [Google Scholar]
- 40. Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental methods. Download
Relative body weight gain during 4 weeks of dietary intervention (A, B, and C) and body fat percentage after 4 weeks of dietary intervention (D) of mice harboring a simplified human intestinal microbiota (SIHUMI), SIHUMI without C. ramosum (SIHUMIw/oCra), or C. ramosum only (Cra). All mice were fed a high-fat diet (HFD) or a low-fat diet (LFD). Values are means ± SEM. n = 8 to 9 mice per group, except for the body fat percentage of Cra mice, for which n = 3. *, P < 0.05, **, P < 0.01, and ***, P < 0.001, for HFD-fed mice versus LFD-fed mice. Download
Bacterial cell counts in the intestine of mice harboring a simplified human intestinal microbiota (SIHUMI) fed low-fat diet (LFD) or high-fat diet (HFD) for 4 weeks.
In vitro short-chain fatty acid (SCFA) formation capacity of SIHUMI and SIHUMIw/oCra communities and portal plasma SCFA concentrations in SIHUMI and SIHUMIw/oCra mice.
Sequences of primers and probes used for quantitative real-time PCR.