ABSTRACT
Pathogenic and nonpathogenic species of bacteria and fungi release membrane vesicles (MV), containing proteins, polysaccharides, and lipids, into the extracellular milieu. Previously, we demonstrated that several mycobacterial species, including bacillus Calmette-Guerin (BCG) and Mycobacterium tuberculosis, release MV containing lipids and proteins that subvert host immune response in a Toll-like receptor 2 (TLR2)-dependent manner (R. Prados-Rosales et al., J. Clin. Invest. 121:1471–1483, 2011, doi:10.1172/JCI44261). In this work, we analyzed the vaccine potential of MV in a mouse model and compared the effects of immunization with MV to those of standard BCG vaccination. Immunization with MV from BCG or M. tuberculosis elicited a mixed humoral and cellular response directed to both membrane and cell wall components, such as lipoproteins. However, only vaccination with M. tuberculosis MV was able to protect as well as live BCG immunization. M. tuberculosis MV boosted BCG vaccine efficacy. In summary, MV are highly immunogenic without adjuvants and elicit immune responses comparable to those achieved with BCG in protection against M. tuberculosis.
IMPORTANCE
This work offers a new vaccine approach against tuberculosis using mycobacterial MV. Mycobacterium MV are a naturally released product combining immunogenic antigens in the context of a lipid structure. The fact that MV do not need adjuvants and elicit protection comparable to that elicited by the BCG vaccine encourages vaccine approaches that combine protein antigens and lipids. Consequently, mycobacterium MV establish a new type of vaccine formulation.
INTRODUCTION
Bacterial pathogens use different secretion systems to release their products into the extracellular environment, tissues, or bloodstream of the host organism (1). Some bacteria and fungi use membrane vesicles (MV) to release a complex group of proteins, polysaccharides, and lipids into the extracellular milieu (1–5). Both pathogenic and nonpathogenic bacteria species secrete MV, providing another means by which they interact with prokaryotic and eukaryotic cells in their environment (1). Typically, MV from pathogenic bacteria contain virulence factors, including toxins, adhesins, and/or immunomodulatory compounds. The packaging of virulence factors in vesicles allows microorganisms to deliver host cell-damaging materials in a concentrated manner. Our group has demonstrated the production of MV in Gram-positive bacteria (6), including Streptococcus pneumoniae (7), Bacillus subtilis (8), and several mycobacterial species, such as the medically important bacillus Calmette-Guerin (BCG) and Mycobacterium tuberculosis (5). Moreover, we showed that mycobacterial MV transport lipids and proteins known to be involved in the subversion of the immune response of the host. Remarkably, MV from pathogenic mycobacteria concentrate lipoproteins, including the well-studied 19-kDa LprG and LprA proteins. These components signal via Toll-like receptor 2 (TLR-2), directly affecting major histocompatibility complex (MHC) class II antigen presentation in macrophages and dendritic cells and modulating the interaction with the host (5).
Tuberculosis (TB) remains a major global health problem. In 2010, there were 8.8 million incident cases of TB, 1.1 million TB-associated deaths among HIV-uninfected people, and an additional 0.35 million among HIV-infected people (9). Efforts to control the disease include the development of point-of-care tests, new TB drugs, the use of the BCG vaccine, and the development of new vaccines. Most of the new TB vaccine candidates that have entered clinical trials fall into one of the following groups: (i) live attenuated vaccines to replace BCG; (ii) subunit vaccines to be given after initial BCG vaccination (10); and (iii) single immunodominant antigens, usually secreted, such as ESAT-6, Cfp10, and Ag85b, along with other adjuvants (10).
Despite the connection between bacterial vesicles and virulence, MV also serve as potential vaccines, since they elicit immune responses that protect against mucosal and systemic bacterial infections, including those caused by Neisseria meningitidis (11), Bordetella pertussis (12), Salmonella enterica serovar Typhimurium (13), Vibrio cholerae (14), and Bacillus anthracis (6). Artificially produced membrane vesicles from the Gram-negative bacterium N. meningitidis constitute the basis of a newly licensed vaccine (VA-MENGOC-BC) (15). Recently, the immune response to artificial MV (liposomes) from Mycobacterium smegmatis showed cross-reactivity against M. tuberculosis antigens in mice (16). These findings suggest the possibility of using MV from M. tuberculosis or other pathogenic mycobacteria as protective vaccines. In this study, we explored whether vaccination with MV induced protective immune responses against experimental murine pulmonary M. tuberculosis infection. The results demonstrated that mycobacterial vesicles elicited protection against an M. tuberculosis aerosol infection in mice and established the potential of MV as components of a new type of vaccine against TB.
RESULTS
Lipoproteins determine the immunogenicity of mycobacterial MV.
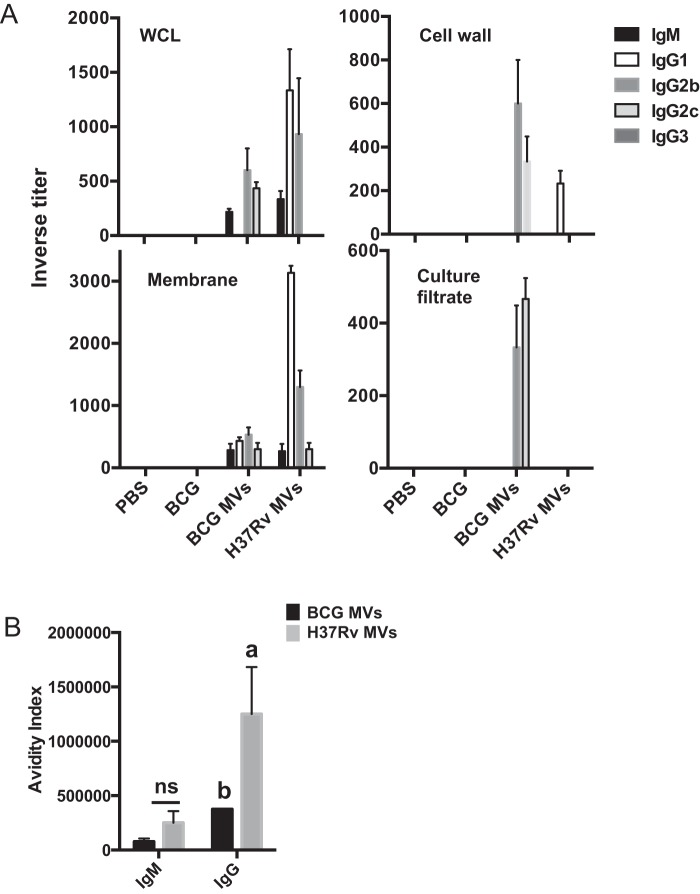
To characterize the immune response to MV in mice, C57BL/6 mice were immunized twice with 2.5 µg of MV from either BCG or H37Rv (based on total weight) subcutaneously (SC) with no adjuvant. The serum antibody response was measured by enzyme-linked immunosorbent assay (ELISA) against a whole-cell M. tuberculosis lysate 42 days after the initial immunization (Fig. 1A). In each experiment, mice that had been injected SC with phosphate-buffered saline (PBS) and mice that had received BCG were used as controls. No detectable M. tuberculosis-specific IgM or IgG antibodies were found in the control groups. The antibody response to the MV differed depending on the source of the immunogen. The response to BCG MV included moderate levels of IgM and IgG2b and lower levels IgG2c. In contrast, H37Rv MV immunization triggered a higher antibody response dominated by IgG1 and IgG2b isotypes (Fig. 1A). Since mycobacterial MV are enriched in lipoproteins and surface-associated proteins (5), we hypothesized that antibodies in sera from BCG or H37Rv MV-immunized mice should preferentially recognize the surface-associated compartments of M. tuberculosis. To test this, we performed ELISA against different mycobacterial subcellular fractions, including membrane, cell wall, cytosol, and culture filtrate proteins (Fig. 1A). BCG MV sera showed an enhanced reactivity against a cell wall fraction, including a mixed IgG2b/c response. When ELISA of a membrane fraction was performed, H37Rv MV immune sera showed the highest antibody levels with a predominant IgG1/2b response. Of note, no antibodies against the cytosolic fraction were detected. The magnitude of the response to culture filtrate proteins was reduced compared to that of the response to cell wall or membrane stimulations, and only BCG MV immune sera contained specific IgG2b/c antibodies reacting against this fraction (Fig. 1A). We also found significant differences in the strength of the antigen-antibody (total IgG) interaction between BCG and H37Rv MV sera against a whole-cell lysate (Fig. 1B). These results show that BCG MV and H37Rv MV induced different antibody responses in terms of isotype, intensity, and avidity and that much of the antibody response was directed to the mycobacterial surface compartments, including the cell membrane and cell wall.
FIG 1 .
Immunogenicity of mycobacterial vesicles. (A) Inverse titers of M. tuberculosis H37Rv-specific antibodies measured by ELISA in serum from C57BL/6 mice (3 per group) immunized with 2.5 µg of BCG or H37Rv MV using a subcutaneous route of injection or 2 × 106 BCG bacilli using the same route after 6 weeks. ELISA was performed on plates coated with 20 µg ml−1 of the indicated mycobacterial subcellular fraction (H37Rv). WCL, whole-cell lysate. (B) Avidity index was determined by titrating ammonium thiocyanate onto plasma total IgG and IgM. Data labeled “a” are statistically significantly different [P < 0.05] from those labeled “b.” Data are means ± standard errors of the means (SEM). The experiment was done three times with similar results. ns, not significant.
To determine the identity of the M. tuberculosis proteins recognized by MV sera, we used nucleic acid programmable protein assays (NAPPA) (Table 1; also, see Fig. S1A and B in the supplemental material). In this technique, mycobacterial proteins are transcribed and translated by a cell-free system and immobilized in situ by means of epitope tags fused to the proteins enabling studies on a proteome scale (17). Using an antibody to the C-terminal glutathione S-transferase (GST) tag, which confirms full-length translation, we detected protein signals for 99% of the printed genes (see Fig. S1A). Further, the arrays were probed with sera from live vaccine BCG-, H37Rv MV-, and PBS-immunized mice and developed with a fluorescence secondary mouse antibody (due to limited amount of arrays, BCG MV antiserum was excluded from the analysis). Arrays were further scanned and normalized by first removing the background signal (first quartile of negative-control spots) and then using median scaling to adjust for array-wide differences in signal intensity. The reactive spots obtained with the BCG or H37Rv MV immunizations were selected when they presented a normalized value higher than 1 and at least a 2-fold change relative to PBS (Table 1). In Fig. S1B we show a representative section of the two arrays, containing some of the most reactive proteins. A total of 36 M. tuberculosis proteins were recognized by BCG and/or H37Rv MV immune sera. Among the proteins identifed, 25 were MV specific, 6 were BCG specific, and 5 were common to sera from both immunizations (Table 1). Of note, the signal intensities were much higher after vesicle immunization than BCG immunization. The four most reactive proteins included the lipoproteins LpqH, Lppx, and PstS1, indicating that lipoproteins elicit the most of the antibody response to MV. Immunoblots on lysates from a BCG mutant with a knockout of lpqH (Δ19 kDa) showed that the 19-kDa reactive band was LpqH, according to its lack of reactivity in sera from MV-vaccinated mice (see Fig. S2 in the supplemental material). The protein VapC6 was also detected with a strong signal, indicating that vesicles also generated antibodies against mycobacterial toxins. Of note, no antibodies to lipoproteins were detected in sera from mice that received the standard vaccination with live BCG. These results indicate that BCG and vesicle immunizations in mice elicit quantitatively and qualitatively different antibody responses. Overall, these results indicate that despite the protein heterogeneity in vesicle composition, only a minor fraction of these proteins determined the immunogenicity of vesicles in terms of antibodies.
TABLE 1 .
Most dominant reactive M. tuberculosis proteins to antisera determined by NAPPAa
| Rv no.b | Gene | Molecular mass (Da) | pI | PBS | BCG | H37Rv MV | Functional category |
|---|---|---|---|---|---|---|---|
| Rv2945c | lppX | 24,140.2 | 4.8103 | 2.5 | 2.3 | 14.2 | Cell wall and cell processes |
| Rv0656c | vapC6 | 13,941.7 | 6.0954 | 0.9 | 0.8 | 11.9 | Virulence, detoxification, adaptation |
| Rv3763 | lpqH | 15,114.8 | 7.1813 | 0.7 | 0.8 | 10.3 | Cell wall and cell processes |
| Rv0934c | pstS1 | 38,211.1 | 5.0223 | 1.3 | 0.3 | 8.5 | Cell wall and cell processes |
| Rv3460c | rpsM | 14,350.7 | 11.5823 | 0.8 | 1.0 | 6.0 | Information pathways |
| Rv1342c | Rv1342c | 13,382 | 9.7927 | 1.4 | 1.3 | 5.4 | Cell wall and cell processes |
| Rv1676 | Rv1676 | 25,859.2 | 9.3164 | 0.7 | 0.8 | 4.8 | Conserved hypotheticals |
| Rv0724A | Rv0724A | 12,187.6 | 4.5522 | 1.1 | 1.0 | 4.4 | Conserved hypotheticals |
| Rv0903c | prrA | 25,253.9 | 4.7448 | 1.3 | 1.3 | 4.1 | Regulatory proteins |
| Rv3100c | smpB | 18,197.7 | 11.3373 | 1.2 | 1.0 | 4.0 | Virulence, detoxification, adaptation |
| Rv3649 | Rv3649 | 81,410.4 | 6.1089 | 0.7 | 0.8 | 3.8 | Information pathways |
| Rv2906c | trmD | 25,134.4 | 5.3006 | 1.4 | 1.2 | 3.5 | Information pathways |
| Rv0510 | hemC | 31,906.3 | 4.585 | 0.8 | 1.3 | 3.1 | Intermediary metabolism and respiration |
| Rv3532 | PPE61 | 41,549.3 | 4.1512 | 0.6 | 0.6 | 3.0 | PE/PPE |
| Rv3850 | Rv3850 | 23,810.9 | 12.1622 | 1.2 | 1.3 | 3.0 | Cell wall and cell processes |
| Rv0776c | Rv0776c | 28,037.1 | 9.5533 | 0.9 | 1.1 | 2.9 | Conserved hypotheticals |
| Rv1009 | rpfB | 38,078.4 | 5.22 | 1.2 | 1.4 | 2.5 | Cell wall and cell processes |
| Rv0825c | Rv0825c | 23,409.8 | 8.8345 | 1.0 | 1.0 | 2.3 | Conserved hypotheticals |
| Rv3497c | mce4C | 38,132.4 | 9.4511 | 1.0 | 2.2 | 2.2 | Virulence, detoxification, adaptation |
| Rv0585c | Rv0585c | 84,041.6 | 9.8652 | 0.9 | 1.1 | 2.1 | Cell wall and cell processes |
| Rv3616c | espA | 39,888.3 | 5.011 | 0.9 | 0.9 | 2.0 | Cell wall and cell processes |
| Rv0029 | Rv0029 | 39,559.2 | 7.9622 | 1.0 | 2.1 | 2.0 | Conserved hypotheticals |
| Rv2607 | pdxH | 25,186.3 | 5.3856 | 0.7 | 1.7 | 1.7 | Intermediary metabolism and respiration |
| Rv3417c | groEL1 | 55,877.5 | 4.7423 | 0.6 | 0.8 | 1.6 | Virulence, detoxification, adaptation |
| Rv3873 | PPE68 | 37,330 | 3.9857 | 0.7 | 0.9 | 1.5 | PE/PPE |
| Rv1701 | Rv1701 | 33,517.3 | 10.7735 | 0.7 | 0.7 | 1.5 | Insertion sequences and phages |
| Rv0139 | Rv0139 | 31,906.3 | 4.585 | 0.6 | 1.0 | 1.3 | Intermediary metabolism and respiration |
| Rv1910c | Rv1910c | 19,849.6 | 6.789 | 0.1 | 1.4 | 1.3 | Cell wall and cell processes |
| Rv3293 | pcd | 51,312.2 | 5.0502 | 0.4 | 1.2 | 1.1 | Intermediary metabolism and respiration |
| Rv0449c | Rv0449c | 47,784.9 | 6.934 | 1.0 | 2.7 | 1.1 | Intermediary metabolism and respiration |
| Rv3622c | PE32 | 9,697.94 | 4.0145 | 0.5 | 0.7 | 1.0 | PE/PPE |
| Rv1484 | inhA | 28,527.8 | 6.0188 | 0.9 | 2.0 | 1.0 | Lipid metabolism |
| Rv3506 | fadD17 | 53,738.8 | 4.9353 | 0.8 | 1.8 | 0.9 | Lipid metabolism |
| Rv1789 | PPE26 | 38,588.7 | 4.1835 | 0.6 | 1.4 | 0.9 | PE/PPE |
| Rv0072 | Rv0072 | 36,410.1 | 10.8351 | 0.5 | 1.0 | 0.7 | Cell wall and cell processes |
| Rv1106c | Rv1106c | 40,741.6 | 7.0104 | 0.4 | 1.4 | 0.3 | Intermediary metabolism and respiration |
Immunoreactive antigens were selected when they showed a signal 2-fold greater that of PBS antiserum and a normalized signal higher than 1.
Proteins in bold are unique toH37Rv MV; proteins in italics are unique to BCG MV; the remaining proteins are common to both BCG and H37Rv MV.
Mycobacterial MV induce a high Th1 response.
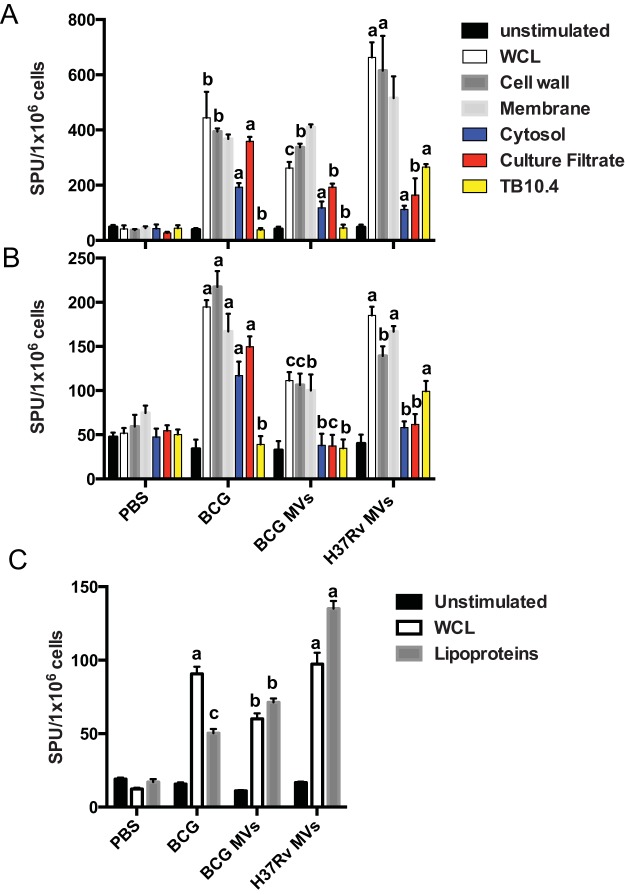
We then studied the cellular response to MV immunization, since a T helper type 1 (Th1) cell response is crucial in protective immunity to many intracellular pathogens, including M. tuberculosis (18). As described above, mice were immunized with two doses of 2.5 µg of BCG or H37Rv MV SC, and the splenic responses of spleens stimulated with M. tuberculosis lysate or the subcellular fraction were studied ex vivo by measuring gamma interferon (IFN-γ)- and interleukin 2 (IL-2)-positive cells by enzyme-linked immunospot (ELISPOT) assays 3 weeks after the last immunization (Fig. 2). Immunization with H37Rv MV triggered the highest IFN-γ splenic responses upon stimulation with M. tuberculosis lysate, with responses being 2-fold higher than those elicited by BCG MV and almost 1.5-fold those observed with BCG immunization (Fig. 2A). Of note, significantly higher responses were observed in MV-immunized mice when subcellular fractions were used for stimulation, including cell wall or membrane, indicating that much of the splenic response was exclusively directed to bacterial cell surface compartments. Supporting this idea was the observation of a reduced response in MV-immunized spleens upon stimulation with culture filtrate proteins compared with live BCG. The specificity of the immunization regimens was tested by restimulation of splenocytes with an M. tuberculosis-specific peptide (TB10.4), producing a recall in the responses of M. tuberculosis MV-immunized spleens only (Fig. 2A). We also measured IL-2 splenic responses upon stimulation with the same preparations (Fig. 2B). In this case, the higher overall responses after stimulation with M. tuberculosis lysate, cell wall, or membrane were observed in live-BCG-immunized spleens, followed by those immunized with H37Rv MV and BCG MV (Fig. 2B). As observed with IFN-γ splenic responses, a markedly higher response was observed in MV-immunized spleens upon stimulation with cell surface-associated fractions. In addition, significantly higher responses were observed in live-BCG-immunized splenocytes upon stimulation with culture filtrate proteins. Immunization with live BCG also induced higher IL-2 splenic responses upon stimulation with cytosol, contrary to what was observed in MV-immunized splenocytes. The specificity of the stimulation with the M. tuberculosis-related TB10.4 peptide was also confirmed (Fig. 2B). The experiment was repeated using isolated T cells from the same set of immunized mice (Fig. 2C). This time isolated T cells were mixed with splenocytes from naive mice and stimulated with either M. tuberculosis lysate or a cell wall fraction enriched in lipoproteins. Both BCG-immunized and H37Rv MV-immunized mice showed the highest number of IFN-γ-producing T cells in whole-cell-lysate (WCL)-stimulated wells. Stimulation with the lipoprotein fraction showed an increase in the response in H37Rv MV-immunized mice, followed by BCG MV- and BCG-immunized mice (Fig. 2C).
FIG 2 .
T cell responses after MV immunization. (A and B) Splenic IFN-γ (A) and IL-2 (B) producers from BCG- or MV-immunized C57BL/6 mice (3 per group) after in vitro stimulation with an M. tuberculosis lysate (WCL) or different subcellular fractions. (C) IFN-γ producing T cells from BCG- or MV-immunized C57BL/6 mice (3 per group) after in vitro stimulation with an M. tuberculosis lysate (WCL) or a lipoprotein-enriched cell wall subcellular fraction. Responses were measured by ELISPOT assay 6 weeks after the initial immunization. Results shown are from one of three independent and similar experiments (values labeled “a” are statistically significantly different from those labeled “b” [P < 0.05] and “c” [P < 0.01]). Data are means ± SEM. SPU, spot-forming units.
These results suggest that immunization with MV elicited a splenic response that was almost exclusively directed to cell surface compartments.
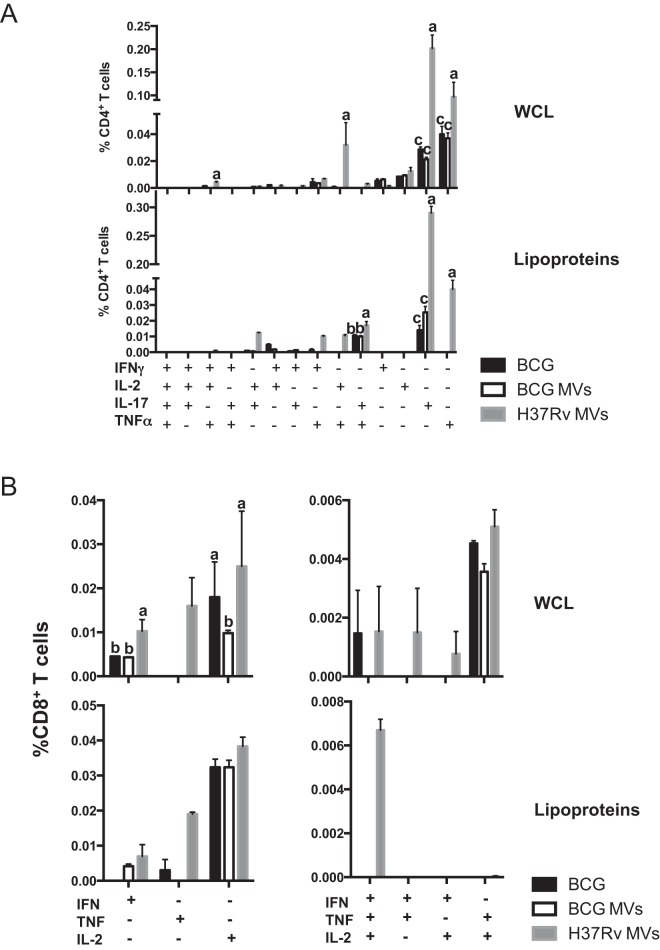
Next, we examined the multifunctionality of the T cell response in MV-immunized mice by intracellular cytokine staining. Mice were immunized as described above, and cytokine production by splenic CD4+ and CD8+ T cells was analyzed at day 42 after restimulation with an M. tuberculosis lysate and a lipoprotein fraction as described above (Fig. 3). Upon restimulation in vitro with an M. tuberculosis lysate, we observed a substantial increase in single-, double-, and triple-cytokine-producing CD4+ T cells in H37Rv MV-immunized mice relative to those immunized with BCG and BCG MV (Fig. 3A). Notably, H37Rv MV-immunized mice showed significantly greater frequencies of IL-17-producing CD4+ T cells, followed by tumor necrosis factor alpha (TNF-α) producers (Fig. 3A). Of note, an almost unique CD4+ T cell population producing TNF and IL-2 was associated with this immunization. The frequency of triple producers was low but still significantly higher in H37Rv MV-immunized mice than in the other groups. When the same splenocytes were stimulated with a lipoprotein fraction, the magnitude and complexity of the response were reduced, and most of the CD4+ T cell subpopulations producing IL-17 and TNF-α were associated with immunizations with H37Rv MV (Fig. 3A). Analysis of CD8+ T cells showed a similar response for BCG and H37Rv MV when splenocytes were stimulated with an M. tuberculosis lysate. Immunization with H37Rv MV showed only a significant increase in the IFN-γ producing CD8+ T cells (Fig. 3B). Stimulation with a lipoprotein fraction did not reduce the magnitude of the response but the complexity. A comparable population of IL-2-positive CD8+ T cells was observed in all the groups. Immunization with H37Rv MV elicited an almost exclusively TNF-α-positive CD8+ T cell population. It is noteworthy that only immunization with BCG and H37Rv MV induced the population producing the three cytokines. As in splenocytes stimulated with M. tuberculosis lysate, the majority of the response was restricted to single-cytokine producers (Fig. 3B).
FIG 3 .
T cell multifunctionality. (A) Frequency of M. tuberculosis specific CD4+ T cells producing IFN-γ, TNF-α, IL-2, or IL-17 measured in splenocytes isolated from mice immunized SC with MV and restimulated with 20 µg ml−1 of H37Rv M. tuberculosis lysate or a lipoprotein-enriched cell wall fraction. (B) Frequency of CD8+ T cells producing IFN-γ, TNF-α, or IL-2 measured as for panel A. Controls included mice that were sham (PBS) immunized and subcutaneously BCG immunized for 6 weeks. The cytokine profile in individual cells was measured by multicolor flow cytometry by gating for CD4+ or CD8+ T cells. All possible combinations of cytokine expression were plotted. The combinations not shown were not detected. The results are representative of two independent and similar experiments (A) and three independent and similar experiments (B) (values labeled “a” are statistically significantly different from those labeled “b” [P < 0.05] and “c” [P < 0.01]). Data are means ± SEM.
These results strongly suggest that immunization of mice with H37Rv MV elicited a Th1 response stronger than and superior to that resulting from BCG MV or BCG immunization, including lipoprotein-specific polyfunctional T cell populations.
Mycobacterium-derived MV protect against M. tuberculosis infection.
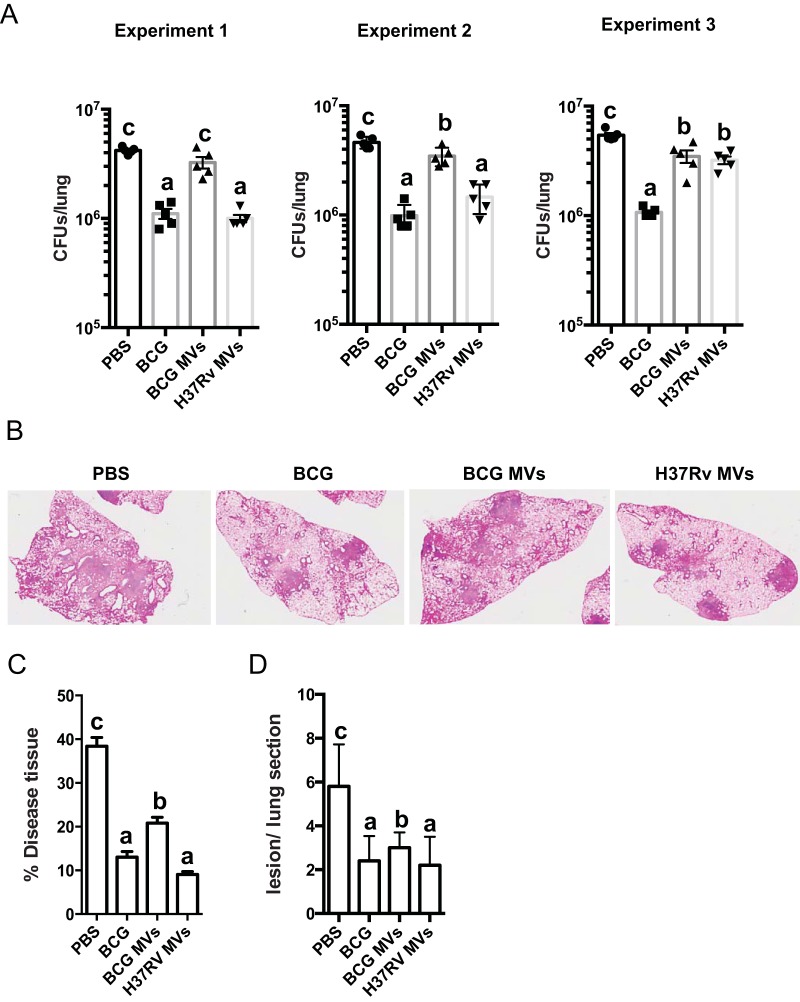
We tested whether immunization with mycobacterial MV was protective in mice against an M. tuberculosis challenge. Immunized mice were infected with approximately with 100 CFU via aerosol, 3 weeks after the last immunization. Eight weeks after infection, lung and spleen bacterial burdens were determined. Mice immunized with PBS or two million bacilli of BCG Pasteur were used as negative and positive controls, respectively (Fig. 4; also, see Fig. S3 in the supplemental material). The experiment was done three times. Two experiments showed a protective effect for H37Rv MV, comparable to that obtained with the control BCG, with a significant reduction of lung CFU relative to levels in PBS-treated mice (Fig. 4A). Of note, immunization with BCG MV was not protective in all experiments. In one experiment, we found almost no reduction in lung CFU in H37Rv MV-immunized mice, comparable to results with unvaccinated mice (PBS) (Fig. 4A). The same trend was observed when CFU were counted in spleens (see Fig. S3). Importantly, in experiments where H37Rv MV resulted in a reduced number of CFU in the spleen, similar to BCG, some mice had numbers of spleen CFU below the limit of detection (see Fig. S3). This result indicates the potential capacity of H37Rv MV to control bacterial replication and dissemination, similar to that of BCG.
FIG 4 .
Immunization with mycobacterial MV protects mice against M. tuberculosis aerosol infection. (A) Bacterial load (CFU) in the lungs of individual C57BL/6 mice, immunized SC with 1 × 106 BCG bacteria or twice SC with 2.5 µg BCG or H37Rv MV was determined at 8 weeks after infection with a low dose of M. tuberculosis H37Rv via aerosol (approximately 100 CFU). The results of three independent experiments are shown. Experimental groups used 5 mice (values labeled “a” are statistically significantly different from those labeled “b” [P < 0.05] or “c” [P < 0.01] using one-way ANOVA). (B) Representative H&E staining images from lungs of C57BL/6 mice SC immunized with BCG or with MV from BCG or H37Rv and infected with M. tuberculosis for 8 weeks. All the images were taken at a magnification of ×2.5. (C) The size of lung lesions was determined from H&E-stained cross sections using computer-assisted image analysis. These data were used to calculate the percentage area of lung tissue occupied by diseased tissue. (D) The number of lesions per lung section was determined by computer-assisted image analysis as for panel C (values labeled “a” are statistically significantly different from those labeled “b” [P < 0.05] or “c” [P < 0.01]). Data are means ± SEM.
Histological analysis of lung sections indicated that the percentage of disease tissue was similar between MV- and BCG-immunized mice relative to PBS. However, both H37Rv MV- and BCG-immunized mice showed a significantly lower percentage of diseased tissue than BCG MV-immunized mice (Fig. 4C). The same held true for the number of foci of infiltrates (Fig. 4D). These results demonstrate that immunization with H37Rv MV without adjuvant protected as efficiently as the current live BCG vaccine.
Boosting BCG vaccine with mycobacterial vesicles enhances humoral and cellular immune responses.
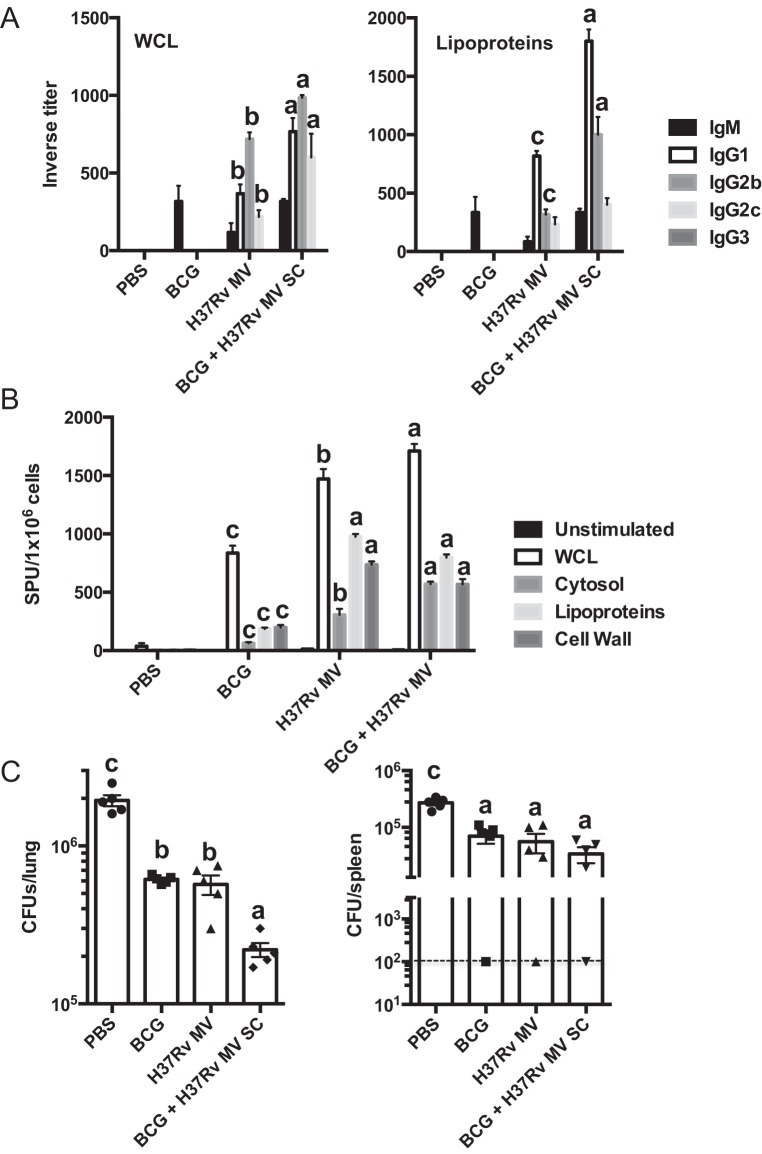
Finally, we tested whether mycobacterial MV had the capacity to improve the performance of the control vaccine BCG. Three weeks after BCG immunization, mice were given boosters of H37Rv MV and M. tuberculosis-specific antibodies, and splenic responses were analyzed 3 weeks after the boosters. When inverse titers were tested against a WCL of M. tuberculosis, a low antibody response, including IgM, was detected in serum of BCG-vaccinated mice (Fig. 5A). When animals were given boosters of H37Rv MV, IgG1, IgG2b, and IgG2c M. tuberculosis-specific antibodies were detected, with IgG2b being the prevalent subclass. Of note, immunization with H37Rv MV elicited a similar antibody profile with lower levels, indicating that the profile observed in the mice given boosters was almost entirely associated with H37Rv MV immunization. Higher levels of antibodies were detected when ELISA was performed against an M. tuberculosis cell wall subcellular fraction enriched in lipoproteins, with major levels for the IgG1 subclass (Fig. 5A).
FIG 5 .
Protection efficacy of MV after administration of boosters to BCG-vaccinated mice. (A) Inverse titers of M. tuberculosis H37Rv-specific antibodies measured by ELISA in serum from C57BL/6 mice (3 per group) immunized subcutaneously with 2.5 µg of H37Rv MV or with 2 × 106 bacilli of BCG and mice that were BCG immunized and given boosters of 2.5 µg of H37Rv MV (BCG + H37Rv MV) after 12 weeks. ELISA was performed in plates coated with 20 µg ml−1 of H37Rv whole-cell lysate (WCL) or a cell wall fraction enriched in lipoproteins. (B) Splenic IFN-γ producers from immunized C57BL/6 mice given MV, BCG, or BCG boosters (3 per group) after in vitro stimulation with M. tuberculosis lysate (WCL) or the indicated subcellular fractions. (C) Bacterial load (CFU) in the lungs of individual C57BL/6 mice (5 per group) immunized subcutaneously with 2.5 µg of H37Rv MV or with 2 × 106 bacilli of BCG and mice immunized with BCG and given a booster of 2.5 µg of H37Rv MV (BCG + H37Rv MV) was determined at 8 weeks after infection with a low dose of M. tuberculosis H37Rv via aerosol (approximately 100 CFU). The results of three independent experiments are shown (values labeled “a” are statistically significantly different from those labeled “b” [P < 0.05] or “c” [P < 0.01] using one-way ANOVA). Data are means ± SEM.
This result showed that boosting BCG vaccination with H37Rv MV elicited a more diverse antibody response than standard BCG immunization.
MV boosting increased 2-fold the number of M. tuberculosis-specific splenic cells producing IFN-γ, after restimulation with a WCL, compared to that in BCG-vaccinated mice (Fig. 5B). The same held true when the same splenocytes were stimulated with M. tuberculosis subcellular fractions, including cell wall, cytosol, and membrane (Fig. 5B). Again, the splenic response observed in H37Rv MV-immunized mice was comparable to that of boosted mice. Remarkably, significantly higher responses were obtained upon M. tuberculosis lipoprotein stimulation in mice receiving boosters than in BCG-vaccinated mice (Fig. 5B). These data strongly suggest that boosting the BCG response with vesicles enhanced the quantity and the quality of the immune response.
Protection efficacy of MV after administration of boosters to BCG-vaccinated mice.
A group of mice from the above-described experiment was infected by the respiratory route using aerosol infection with a low dose of M. tuberculosis. Viable mycobacteria were enumerated in lungs and spleens 8 weeks after challenge. All mice in the BCG-vaccinated groups had significantly fewer bacilli in the lungs than the control mice (Fig. 5C). The level of protection obtained when mice were immunized with H37Rv MV was similar to that seen before. However, mice receiving the H37Rv MV booster had significantly lower bacterial counts than the BCG-vaccinated and MV-vaccinated mice (Fig. 5C). Giving BCG-immunized mice the mycobacterial MV booster did not seem to improve the control of the bacterial dissemination, as all the groups except the PBS-treated mice manifested the capacity to reduce the number of CFU in spleen (Fig. 5C). Histopathological analysis of lung sections showed no difference in the percentage of disease tissue between mice receiving boosters and BCG-immunized mice (data not shown). These results show that giving H37Rv MV boosters to BCG-vaccinated mice can improve humoral and splenic responses and the capacity to reduce the viable bacteria in the lung.
DISCUSSION
Our group recently reported that mycobacterial strains, including M. tuberculosis and BCG, produce MV which are released into the extracellular space (5) and which are involved in iron acquisition (19). Remarkably, only vesicles from the virulent strains are enriched in lipoproteins, which are well known to be TLR2 agonists involved in modulation of host immune response (20). Lipid analysis indicated a prevalence of polar lipids, consistent with an origin from the cellular membrane. Intratracheal administration of MV to mice followed by aerosol challenge resulted in an increase in the bacterial burden in the lungs (5), suggesting a Koch-like phenomenon where an immune response to the MV in the lung worsened the outcome of infection. However, for other bacterial pathogens, such as B. anthracis (6), N. meningitidis (21), Escherichia coli (1), S. pneumoniae (7), and Pseudomonas aeruginosa (1), systemic immunization with MV induces protective immunity (13, 22–27). Presumably, the immune response to bacterial MV-based vaccines neutralizes bacterial toxins and/or is directed to the bacterial surface and membrane antigens, promoting bacterial opsonization and/or complement-mediated killing. In addition, bacterial clearance upon MV immunization could be mediated by Th1 and Th17 cell responses (28). These results, combined with the successful clinical use of an MV-based vaccine against meningococcus (22), suggest that MV have potential for vaccine development.
In this study, we examined the feasibility of using naturally produced MV from two M. tuberculosis strains, BCG and H37Rv, as experimental vaccines against TB in a mouse model. Initially, we observed no effect on the capacity of BCG MV to reduce lung and spleen CFU relative to H37Rv MV- and BCG-immunized mice. However, BCG MV immunization was associated with a reduced percentage of disease lung tissue. Several aspects of the immune response after immunization may explain the differential protection efficacy of BCG MV and H37Rv MV. The first is the specificity and avidity of the antibody response directed to surface M. tuberculosis compartments. Although antibody responses have historically been ignored in studies of TB immunity, there is now strong evidence that some immunoglobulins contribute to host defense (29). Despite the fact that MV are composed of a complex mixture of lipids and 60 to 80 proteins (5), only a few of these proteins elicited strong antibody responses. In agreement with the enrichment of lipoproteins in mycobacterial MV, the antigens found to be immunogenic determinants were in fact three lipoproteins (LpqH, LppX, and PstS1) and one chaperonin, GroEL1. In a recent pilot study, we identified an antibody reactivity pattern to MV-associated proteins in TB patients as a potential biomarker. Patients with disseminated TB lacked antibodies to MV proteins of ~36, 25, and 23 kDa (30), some of which match the mass of the immunogenic lipoproteins identified in this study. These data suggest correspondence between our findings in the mouse model and the antibodies in clinical samples to MV-associated lipoproteins in humans, an association that warrants further investigation. Furthermore, analysis of humoral and cellular responses to subcellular fractions of mycobacteria in mice immunized with MV, including a cell wall fraction enriched in lipoproteins, supports the notion that MV induce immunity mainly against mycobacterial-surface-related compartments. M. tuberculosis may use lipoproteins as a decoy mechanism to escape from macrophage responses by inhibiting antigen processing and presentation, inducing cytokines that modulate inflammation (20, 31, 32). The second potential reason for the different efficacies of the two immunizations is higher levels of splenic multifunctional M. tuberculosis-specific CD4+ T cells. Despite the important role of multifunctional T cells in multiple infectious disease models (33–35), there is not a consensus on the role of these cells during M. tuberculosis infection (36–39). The third is a significantly higher frequency of CD4+ T cells producing IL-17. This result is consistent with previous studies in which mice with higher frequencies of IL-17A-producing CD4+ T cells following vaccination were better protected from an aerogenic challenge with M. tuberculosis (40). Thus, in agreement with protective mechanisms found in other bacterial-MV-based vaccines and the available knowledge of host defenses against mycobacteria, we believe that specific antibodies and T cell responses elicited by MV vaccination may inactivate or neutralize the immunomodulatory properties of lipoproteins to provide a net protective effect. However, the precise mechanism by which mycobacterial MV induce protective immune responses against mycobacterial infection remains elusive, and passive-transfer and T cell-adoptive transfer experiments will be needed to ascertain their contribution to protection.
Mycobacterial-lipoprotein-based vaccines have been shown to produce opposite effects after vaccination. Presumably, the way these lipoproteins are incorporated into these preparations could affect the outcome of the immunization. Of note, vaccination with recombinant vaccinia viruses expressing the 19-kDa and 38-kDa lipoproteins produced a significant reduction in CFU in lung of infected mice at levels comparable to those achieved with BCG (41). Some mycobacterial lipoproteins, including LpqH, were recently shown to activate and modulate CD4+ T cell effector functions in a TLR2-dependent manner, independently of antigen-presenting cells, suggesting that M. tuberculosis lipoproteins can regulate adaptive immunity not only by inducing cytokine secretion and costimulatory molecules but also through direct regulation of T lymphocytes (42). This observation suggests that mycobacterial MV could potentially serve as an adjuvant, since these molecules directly enhance CD4+ T cell memory responses. Recently, some mycobacterial lipids associated with MV, such as PIM2 and PIM6, were used as adjuvants, showing their capacity to modulate the immune response and increase the efficacy of a protective vaccine against M. bovis infection (43). In this context, we also show that subsequent immunization of BCG-vaccinated mice with M. tuberculosis MV improved the level of protection by significantly reducing the number of viable bacteria in lungs compared to those in BCG-vaccinated mice.
We previously proposed that mycobacterial MV may represent the connecting vehicle between phagosomes and the host vesicular system (5). In this context, it is worth noting that mycobacterial MV and exosomes of M. tuberculosis-infected macrophages share 50% of their protein content (44). Moreover, apoptotic vesicles from M. tuberculosis-infected macrophages stimulate CD8+ T cells in vivo and protected mice similarly to mycobacterial MV (45).
Our observations strongly suggest that MV preparations have considerable promise as vaccine components. Naturally obtained mycobacterial MV offer many advantages over conventional vaccines. They can be obtained easily, they deliver many antigens, including all major virulence factors, and unlike live bacteria, they lack the ability to replicate and cause infection or change over time by microevolution of cultures. In this regard, BCG vaccine strains have been shown to diverge significantly from original stocks (46). Furthermore, MV have the significant advantage that they require no adjuvants. Coadministration of MV with live BCG, or boosting of prior BCG vaccination with MV, enhances the protective efficacy against M. tuberculosis. However, we believe that vesicle release is a tightly regulated process, which may be affected by different variables. The fact that only two of three protection studies showed an effect of M. tuberculosis MV indicates that using naturally derived MV from cultures could present reproducibility issues. In this regard, we have observed variability in the antigen recognized by human serum samples of different M. tuberculosis MV batches (our unpublished data). Consequently, producing MV from mycobacteria could involve a major challenge in standardizing preparations and artificial liposomes containing immunogenic antigens could provide a more consistent approach for reliably eliciting protective responses. Indeed, this concept has been already applied to other MV (47). In summary, mycobacterial MV provided protection that was comparable to that obtained with BCG in mice and required no adjuvants, suggesting a new avenue for development of vaccines against TB.
MATERIALS AND METHODS
Mycobacterial culture.
Mycobacteria (Mycobacterium bovis bacillus Calmette-Guerin [BCG, Pasteur strain] and M. tuberculosis strain H37Rv) were grown in a minimal medium (MM) consisting of KH2PO4 (1 g/liter), Na2HPO4 (2.5 g/liter), asparagine (0.5 g/liter), ferric ammonium citrate (50 mg/liter), MgSO4·7H2O (0.5 g/liter), CaCl2 (0.5 mg/liter), and ZnSO4 (0.1 mg/liter), with or without 0.05% (vol/vol) Tyloxapol, containing 0.1% (vol/vol) glycerol, pH 7.0 (As mycobacterial membrane vesicles are isolated from culture supernatant, we use a defined medium [minimal medium] to avoid the presence of undesired contaminants in our preparations.) The M. bovis BCG strain lacking the 19-kDa protein (ΔlpqH) was a gift from D. Young. The ΔlpqH strain was grown in the presence of 50 µg/ml of hygromycin. Cultures were grown for up to 10 days in 850-cm2 roller bottles (Corning) at 37°C. In challenge experiments, Mycobacterium tuberculosis was grown in Middlebrook 7H9 medium (M7H9) supplemented with 10% (vol/vol) OADC (oleic acid-albumin-dextrose-catalase) enrichment (Becton, Dickinson Microbiology Systems, Sparks, MD) and 0.5% (vol/vol) glycerol, with or without 0.05% (vol/vol) Tyloxapol (Sigma).
Subcellular fractionation of Mycobacterium tuberculosis.
M. tuberculosis H37Rv whole-cell lysate and subcellular fractions were obtained as described previously (48). Briefly, 10-day-old cultures of M. tuberculosis H37Rv were harvested by low-speed centrifugation in lysis buffer (0.05 M potassium phosphate, 0.022% [vol/vol] β-mercaptoethanol [pH 6.5], supplemented with protease inhibitors, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 1 mM EDTA), and the cell suspension was treated with a French press. The lysed bacterial suspension was centrifuged at 27,000 × g, yielding a pellet corresponding to the cell wall fraction. The supernatant was treated with RNase for 1 h at 4°C and then ultracentrifuged at 100,000 × g. This produced a pellet (membrane fraction) and a supernatant, corresponding to the cytosolic fraction. To obtain the cell wall fraction enriched in lipoproteins, the mycobacterial cell wall preparation was extracted with Triton X-100 as described previously (20). The presence of lipoproteins in this fraction was assessed by mass spectrometry.
Vesicle isolation and purification.
Vesicles were isolated as described previously (5). Briefly, cells were pelleted (3,450 × g for 15 min at 4°C) from 1,000-ml cultures and the supernatants were filtered through a 0.45-µm-pore-size polyvinylidene difluoride filter (Millipore, Billerica, MA). The supernatant volumes were then concentrated ~20-fold using an Amicon (Millipore) ultrafiltration system with a 100-kDa exclusion filter. The concentrate was then sequentially centrifuged at 4,000 and 15,000 × g (15 min, 4°C) to remove cell debris and aggregates, and the remaining supernatant was then centrifuged at 100,000 × g for 1 h at 4°C to sediment the vesicular fraction into a pellet. The supernatant was then discarded, and the pellet was suspended in 1 ml of 10 mM HEPES, 0.15 M NaCl and mixed with 2 ml of Optiprep solution (Sigma) in 10 mM HEPES and 0.15 M NaCl (yielding a final Optiprep concentration of 35% [wt/vol]). The crude vesicle sample was then overlaid with a series of Optiprep gradient layers ranging from 30 to 5% (wt/vol). The gradients were centrifuged (100,000 × g, 16 h), and 1-ml fractions were removed from the top. The fractions were then dialyzed separately in PBS overnight and again recovered by sedimentation at 100,000 × g for 1 h. Finally, the vesicles were suspended in lipopolysaccharide (LPS)-free PBS. Dry-weight measurements were used to determine the yield of MV preparations.
Immunizations and M. tuberculosis challenge.
Six- to eight-week-old female wild-type (C57BL/6) mice were purchased from the National Cancer Institute (NCI, Frederick, MD). All mice were maintained under specific-pathogen-free conditions and were transferred to biosafety level 3 conditions for infection with M. tuberculosis H37Rv. All procedures involving animals were in compliance with protocols approved by the Albert Einstein College of Medicine Institutional Animal Use and Biosafety Committees. BCG cultures were grown to mid-log phase, washed, suspended in PBS plus 0.05% Tyloxapol (PBS-T), and sonicated before infection. Mice were immunized with BCG subcutaneously (SC) at the scruff of the neck with 2 × 106 CFU in 200 µl for 6 weeks. Mice were also immunized with approximately 2.5 µg (total mass) of MV SC in a final volume of 200 µl of PBS and then boosted 3 weeks later. A group of BCG-immunized mice was boosted SC with a single dose of H37Rv MV 3 weeks later. Three weeks after the last immunization mice were infected via aerosol with M. tuberculosis H37Rv. For aerosol challenge with M. tuberculosis, a low-density freezer stock of H37Rv was grown in 7H9 liquid medium to an A600 of 0.4 to 0.8. A total of 2 × 106 CFU ml−1 of bacteria in PBS-T plus 0.04% (vol/vol) Antifoam Y-30 (Sigma) was placed in a nebulizer attached to an airborne infection system (University of Wisconsin Mechanical Engineering Workshop). Mice were exposed to aerosol for 40 min, during which approximately 100 bacteria were deposited in the lungs of each animal. Tissue bacterial loads in tissues for aerosol infections were determined by plating organ homogenates onto 7H11 agar-OADC plates. Colonies were counted after 21 days of incubation at 37°C.
ELISA.
Blood was obtained 6 weeks after the initial immunization from the retro-orbital plexus. Serum was obtained by centrifugation at 10,000 × g for 20 min and stored at −20°C until use. Serum reactivity was measured by ELISA against a whole-cell lysate preparation of M. tuberculosis H37Rv or cellular fractions from the same strain. ELISA plates (96 wells) were coated with 20 µg ml−1 M. tuberculosis lysate or subcellular fractions in PBS and incubated at 37°C for 1 h and then blocked in PBS plus 3% BSA for 1 h at 37° C or overnight at 4° C. Wells were washed three times in PBS with 0.05% Tween 20 (PBST) and then incubated with serially diluted (2-fold) mouse serum in PBS plus 1% BSA for 1 h at 37°C. After three washes with PBST, wells were incubated with either alkaline phosphatase (AP)-conjugated goat antibody to mouse immunoglobulin G (IgG; heavy plus light chain) or AP-conjugated goat antibody to mouse immunoglobulin M (1:1,000; M chain specific and cross adsorbed with mouse IgG1, IgG2b, IgG2c, IgG3, and IgA) (Southern Biotechnologies) for 1 h at 37°C. The secondary antibody was goat anti-mouse IgG-AP, which reacts with the heavy and light chains of mouse IgG1, IgG2a, IgG2b, and IgG3 and minimally with the light chains of mouse IgM and IgA.
For IgG isotype analysis, IgG1, IgG2c, IgG2b, and IgG3 AP-conjugated secondary antibodies (1:1,000) were used (Southern Biotechnologies). Color was developed with p-nitrophenyl phosphate (Sigma-Aldrich). The antibody titer was defined as the higher dilution of serum resulting in absorbance at 405 nm that was at least 2 times greater than the background, which correspond to PBS (sham immunized mice).
The avidity index was determined by diluting immune sera to a concentration that corresponded to an optical density (OD) of 0.5 in an endpoint ELISA. Antibodies were allowed to react overnight on plates coated with M. tuberculosis total cell lysate. Plates were washed, and various concentrations of ammonium thiocyanate (63 mM to 4 M) were added to the wells. After 20 min of incubation, plates were washed, and the remaining antibody was detected as described above. The avidity index is the concentration of ammonium thiocyanate required to reduce the OD by 50% compared to that in wells with no chaotrope.
Immunoblotting.
To confirm the presence of antibodies specific to the 19-kDa protein in the serum, serum reactivity was measured against a whole-cell lysate preparation of a ΔlpqH (19-kDa lipoprotein) mutant strain of BCG by immunoblotting. Electrophoresis was done with a 12% resolving gel at 100 V. Gels were transferred to nitrocellulose membranes, which were then blocked overnight in a buffer containing 5% milk in PBS with 0.1% Tween 20. Individual channels on a blotting frame were incubated with diluted serum (1:400) in PBS plus 5% milk for 1 h at room temperature or overnight at 4°C. Channels were washed three times with blocking buffer and then incubated with a horseradish peroxidase-conjugated goat antibody to mouse immunoglobulin G (Fc) (Thermo Scientific) for 1 h at room temperature (this secondary antibody reacts with the heavy chains of mouse IgG but not with the light chains of most mouse immunoglobulins and not against mouse IgM or nonimmunoglobulin serum proteins). Membranes were developed using luminol (Pierce).
Serum antibody detection using nucleic acid programmable protein assays (NAPPA).
All 4,217 sequence-verified genes of M. tuberculosis in a mammalian expression vector, pANT7_GST, were obtained from DNASU (http://dnasu.org/DNASU/), including 3,816 clones from strain H37Rv and 401 clones from strain CDC1551. The highly purified plasmid DNAs were prepared and printed onto two amine-coated glass slides in single spots using Genetix QArray2 with 300-µm solid tungsten pins (Molecular Devices, LLC, Sunnyvale, CA). All prepared arrays were stored in a vacuum container at room temperature, as previously reported (49, 50).
The printed DNA was transcribed and translated using a human cell-free expression system (Thermo, Fisher Scientific, Rockford, IL). All proteins were captured in situ by a polyclonal anti-GST antibody, which was confirmed by using mouse anti-GST monoclonal antibody (1:200 dilution) (Sigma-Aldrich, St. Louis, MO), followed by horseradish peroxidase-conjugated sheep anti-mouse antibody (1:500 dilution) (Jackson ImmunoResearch Labs, West Grove, PA), as previously described (49).
All serum screenings were executed automatically in HS 4800 Pro and 400 Pro hybridization stations (Männedorf, Switzerland) with a preset serum screening program. Briefly, the arrays were incubated with a 1:50 dilution of mouse serum in 5% milk in PBST at 4°C overnight, and mouse antibody was detected by using Alexa555 goat-anti-mouse IgG (Jackson ImmunoResearch Labs, West Grove, PA). The slides were scanned using a Tecan (Männedorf, Switzerland) power scanner, and the images were quantified with an Array-Pro analyzer, version 6.3 (Media Cybernetics, Bethesda, MD). Prior to statistical analysis, all fluorescent microarray images were examined to remove false-positive signals caused by the spot shape, dust, and nonspecific binding. Arrays were further scanned and normalized by first removing the background signal (first quartile of negative-control spots) and then using median scaling to adjust for array-wide differences in signal intensity. The reactive spots obtained under BCG-P or H37Rv MV conditions were selected when they presented a normalized value higher than a 2-fold change relative to the value obtained with PBS.
ELISPOT assay.
Splenocytes or isolated T cells were cultured in RPMI in ELISPOT plates (1 × 106/well; Millipore, Danvers, MA) coated with IFN-γ capture antibody (clone R4.6A2; BD Biosciences) or IL-2 capture antibody (clone JES6-1A12: BioLegend). The samples were stimulated with 20 µg ml−1 of M. tuberculosis whole-cell lysate or subcellular fractions or specifically with a lipoprotein-enriched cell wall fraction, and plates were incubated at 37°C for 24 h. After removal of cells, the plates were washed with PBS followed by PBS with 0.05% Tween 20 (PBST). Biotinylated anti-IFN-γ detection antibody (clone 4S.B3; BD Biosciences) and biotinylated anti-IL-2 detection antibody (clone JES6-5H4; BioLegend) were added for 2 h at 37°C, followed by washing with PBST. Streptavidin-alkaline phosphatase (Sigma-Aldrich) was added to the plates for 1 h (37°C), followed by washing and addition of 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium (BCIP-NBT) substrate (Sigma-Aldrich). The reaction was stopped by washing the wells with water, and spots were counted using an ELISPOT plate reader (Autoimmun Diagnostika, Strasberg, Germany).
Intracellular cytokine analysis.
Analysis of cytokine-producing CD4+ and CD8+ T cells was performed 3 weeks after the last immunization. Splenic-cell suspensions were isolated and placed in 96-well plates in RPMI 1640 with 10% fetal calf serum (FCS). Briefly, splenocytes were isolated from finely minced spleens, and cell suspensions were passed through a 70-µm cell strainer, washed, and suspended in fresh medium. The samples were stimulated with 10 µg ml−1 of M. tuberculosis whole-cell lysate (WCL) or cell wall fraction or specifically with a lipoprotein-enriched cell wall fraction. Unstimulated wells served as negative controls in naive mice and surrogates for mycobacterial infections in the immunized and infected mice. Samples were combined with 1 µg ml−1 soluble antibody to mouse CD28 (clone 37.51). After 2 h at 37°C, 10 µg ml−1 of brefeldin A (Sigma) was added to all samples, followed by incubation for 4 h. Cells were stained with blue LIVE/DEAD viability dye (Invitrogen) followed by antibody to FcγRII/III (clone 2.4G2; American Type Culture Collection), with fluorochrome-conjugated monoclonal antibodies for surface staining: antibody to CD3ε (clone 145-2C11; eBioscience), antibody to CD8α (clone 53-6.7; BD Bioscience), antibody to CD4 (clone GK1.5; BD Bioscience), and antibody to CD45R (B220) (clone RA3-6B2; BD Bioscience). Cells were fixed with 2% (vol/vol) paraformaldehyde, washed with permeabilization buffer (PBS with 1 mM Ca2+, 1 mM Mg2+, 1 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 2% [vol/vol] FCS, and 0.1% [wt/vol] saponin) and then blocked in permeabilization buffer plus 5% (vol/vol) normal mouse serum (Jackson ImmunoResearch Laboratories). Intracellular cytokines were detected with fluorochrome-conjugated antibodies to IL-2 (clone JES6-5H4; eBioscience), IFN-γ (clone XMG1.2), TNF-α (MP6-XT22) (both from BD Biosciences), and IL-17 (clone 17B7; eBioscience). Data were acquired on an LSR II flow cytometer (BD Biosciences), and data analysis was performed using FlowJo software v.10 (Tree Star).
Histology.
Lungs were removed and fixed in 10% neutral buffered formalin (Fisher Scientific, Fair Lawn, NJ). Tissues were embedded with paraffin, sectioned at 5-µm thickness, and stained with hematoxylin and eosin stain. Five different lung sections per mouse were analyzed. Images of lung sections were taken using an Axio Observr microscope (Zeiss) at 1.25× or 2.5× or scanned at 2,000 dots per inch (dpi) using an Epson Expression XL scanner. Digitized images were then analyzed using ImageJ software. The number of square pixels was calculated for each distinct lesion. The total disease area represented the area (µm2) occupied by granuloma and the percentage of lung surface affected by pneumonia. The total disease area for the entire lung section was calculated by adding the values for each lesion. The total percentage of diseased tissue was calculated by dividing the total disease area by the entire lung section and multiplying by 100. One random left caudal lobe from five animals was scanned per treatment group.
Statistical analysis.
Standard one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test of the means was used to determine statistical significance of immune responses and protective efficacies of the indicated vaccines. A P value of <0.05 was considered statistically significant.
SUPPLEMENTAL MATERIAL
(A) The 4217 M. tuberculosis genes were spotted into two slides (1 and 2), translated using a cell-free expression system, and detected by an anti-GST antibody. A 97% success rate was attained for protein signal, based on a cutoff of 3 standard deviations above the negative-control value (features containing a nonexpressing plasmid). (B) Sections of NAPPA arrays after probing with sera from PBS-, BCG MV-, and H37Rv MV-immunized mice, developed with an Alexa555-conjugated mouse anti-IgG secondary antibody. Each slide included a mouse IgG spot as a positive control (yellow circles). Some of the proteins showing differential reactivity are highlighted with red circles, including LppX, Rv1676, and PstS1. The complete set of reactive proteins is included in Table 1. Download
Immunoblot analysis of the 19-kDa antigen in MV. An immunoblot of H37Rv MV serum reactivity for WT BCG and the Δlpqh BCG mutant is shown. The asterisk indicates the absence of the 19-kDa antigen. Download
Bacterial load (CFU) in the spleen of individual C57BL/6 mice immunized SC with 1 × 106 BCG bacteria or twice with 2.5 mg BCG or H37Rv MV was determined at 8 weeks after infection with a low dose of M. tuberculosis H37Rv via aerosol (aproximately 100 CFU). The results of three independent experiments are shown. Experimental groups used 5 mice (values labeled “a” are statistically significantly different from those labeled “b” [P < 0.05] or “c” [P < 0.01] using one-way ANOVA). Download
ACKNOWLEDGMENTS
A.C., A.B.-G., J.M.A., and R.P.-R. were supported by a grant from the Bill and Melinda Gates Foundation. S.A.P. acknowledges support from the NIH/NIAID (RO1 AI093649 and PO1 AI063537). Flow cytometry studies were carried out using core facilities supported by the Einstein Cancer Center (NIH/NCI CA013330) and the Einstein Center for AIDS Research (NIH AI-51519). A.B. was supported by “Programa Estrategia de Sostenibilidad 2013-2014, Universidad de Antioquia, Medellín, Colombia.” L.J.C. is a Pew Latin American fellow. D.M.M., J.L., J.M.A., X.Y., and G.W. acknowledge support from the NIAID (AI096213), and J.M.A. also acknowledges support from the NIAID (AI105684).
Footnotes
Citation Prados-Rosales R, Carreño LJ, Batista-Gonzalez A, Baena A, Venkataswamy MM, Xu J, Yu X, Wallstrom G, Magee DM, LaBaer J, Achkar JM, Jacobs WR, Jr., Chan J, Porcelli SA, Casadevall A. 2014. Mycobacterial membrane vesicles induce a protective immune response directed to the surface compartments of Mycobacterium tuberculosis when applied systemically in mice. mBio 5(5):e01921-14. doi:10.1128/mBio.01921-14.
REFERENCES
- 1. Kuehn MJ, Kesty NC. 2005. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 19:2645–2655. 10.1101/gad.1299905 [DOI] [PubMed] [Google Scholar]
- 2. Oliveira DL, Nakayasu ES, Joffe LS, Guimarães AJ, Sobreira TJ, Nosanchuk JD, Cordero RJ, Frases S, Casadevall A, Almeida IC, Nimrichter L, Rodrigues ML. 2010. Biogenesis of extracellular vesicles in yeast: many questions with few answers. Commun. Integr. Biol. 3:533–535. 10.4161/cib.3.6.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oliveira DL, Nakayasu ES, Joffe LS, Guimarães AJ, Sobreira TJ, Nosanchuk JD, Cordero RJ, Frases S, Casadevall A, Almeida IC, Nimrichter L, Rodrigues ML. 2010. Characterization of yeast extracellular vesicles: evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS One 5:e11113. 10.1371/journal.pone.0011113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodrigues ML, Nakayasu ES, Oliveira DL, Nimrichter L, Nosanchuk JD, Almeida IC, Casadevall A. 2008. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryot. Cell 7:58–67. 10.1128/EC.00370-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prados-Rosales R, Baena A, Martinez LR, Luque-Garcia J, Kalscheuer R, Veeraraghavan U, Camara C, Nosanchuk JD, Besra GS, Chen B, Jimenez J, Glatman-Freedman A, Jacobs WR, Jr, Porcelli SA, Casadevall A. 2011. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J. Clin. Invest. 121:1471–1483. 10.1172/JCI44261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rivera J, Cordero RJ, Nakouzi AS, Frases S, Nicola A, Casadevall A. 2010. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc. Natl. Acad. Sci. U. S. A. 107:19002–19007. 10.1073/pnas.1008843107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olaya-Abril A, Prados-Rosales R, McConnell MJ, Martin-Pena R, Gonzalez-Reyes JA, Jimenez-Munguia I, Gomez-Gascon L, Fernandez J, Luque-Garcia JL, Garcia-Lidon C, Estevez H, Pachon J, Obando I, Casadevall A, Pirofski LA, Rodriguez-Ortega MJ. 2014. Characterization of protective extracellular membrane-derived vesicles produced by Streptococcus pneumoniae. J. Proteomics 106C:46–60. 10.1016/j.jprot.2014.04.023 [DOI] [PubMed] [Google Scholar]
- 8. Brown L, Kessler A, Cabezas-Sanchez P, Luque-Garcia JL, Casadevall A. 2014. Extracellular vesicles produced by the Gram-positive bacterium Bacillus subtilis are disrupted by the lipopeptide surfactin. Microbiol. Mol. 93:183–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. WHO 2011. Global tuberculosis control: WHO report. WHO, Geneva, Switzerland [Google Scholar]
- 10. Kaufmann SH. 2010. Future vaccination strategies against tuberculosis: thinking outside the box. Immunity 33:567–577. 10.1016/j.immuni.2010.09.015 [DOI] [PubMed] [Google Scholar]
- 11. Holst J, Martin D, Arnold R, Huergo CC, Oster P, O’Hallahan J, Rosenqvist E. 2009. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 27(Suppl 2):B3–12. 10.1016/j.vaccine.2009.10.014 [DOI] [PubMed] [Google Scholar]
- 12. Fransen F, Stenger RM, Poelen MC, van Dijken HH, Kuipers B, Boog CJ, van Putten JP, van Els CA, van der Ley P. 2010. Differential effect of TLR2 and TLR4 on the immune response after immunization with a vaccine against Neisseria meningitidis or Bordetella pertussis. PLoS One 5:e15692. 10.1371/journal.pone.0015692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alaniz RC, Deatherage BL, Lara JC, Cookson BT. 2007. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 179:7692–7701. 10.4049/jimmunol.179.11.7692 [DOI] [PubMed] [Google Scholar]
- 14. Schild S, Nelson EJ, Camilli A. 2008. Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect. Immun. 76:4554–4563. 10.1128/IAI.00532-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sotolongo F, Campa C, Casanueva V, Fajardo EM, Cuevas IE, González N. 2007. Cuban meningococcal BC vaccine: experiences & contributions from 20 years of application. MEDICC Rev. 9:16–22 [DOI] [PubMed] [Google Scholar]
- 16. Rodriguez L, Tirado Y, Reyes F, Puig A, Kadir R, Borrero R, Fernandez S, Reyes G, Alvarez N, Garcia MA, Sarmiento ME, Norazmi MN, Perez Quinoy JL, Acosta A. 2011. Proteoliposomes from Mycobacterium smegmatis induce immune cross-reactivity against Mycobacterium tuberculosis antigens in mice. Vaccine 29:6236–6241. 10.1016/j.vaccine.2011.06.077 [DOI] [PubMed] [Google Scholar]
- 17. Ramachandran N, Hainsworth E, Bhullar B, Eisenstein S, Rosen B, Lau AY, Walter JC, LaBaer J. 2004. Self-assembling protein microarrays. Science 305:86–90. 10.1126/science.1097639 [DOI] [PubMed] [Google Scholar]
- 18. Flynn JL, Chan J. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93–129. 10.1146/annurev.immunol.19.1.93 [DOI] [PubMed] [Google Scholar]
- 19. Prados-Rosales R, Weinrick BC, Piqué DG, Jacobs WR, Jr, Casadevall A, Rodriguez GM. 2014. Role for Mycobacterium tuberculosis membrane vesicles in iron acquisition. J. Bacteriol. 196:1250–1256. 10.1128/JB.01090-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gehring AJ, Dobos KM, Belisle JT, Harding CV, Boom WH. 2004. Mycobacterium tuberculosis LprG (Rv1411c): a novel TLR-2 ligand that inhibits human macrophage class II MHC antigen processing. J. Immunol. 173:2660–2668. 10.4049/jimmunol.173.4.2660 [DOI] [PubMed] [Google Scholar]
- 21. Fransen F, Hamstra HJ, Boog CJ, van Putten JP, van den Dobbelsteen GP, van der Ley P. 2010. The structure of Neisseria meningitidis lipid A determines outcome in experimental meningococcal disease. Infect. Immun. 78:3177–3186. 10.1128/IAI.01311-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haneberg B, Dalseg R, Wedege E, Høiby EA, Haugen IL, Oftung F, Andersen SR, Naess LM, Aase A, Michaelsen TE, Holst J. 1998. Intranasal administration of a meningococcal outer membrane vesicle vaccine induces persistent local mucosal antibodies and serum antibodies with strong bactericidal activity in humans. Infect. Immun. 66:1334–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keenan J, Day T, Neal S, Cook B, Perez-Perez G, Allardyce R, Bagshaw P. 2000. A role for the bacterial outer membrane in the pathogenesis of Helicobacter pylori infection. FEMS Microbiol. Lett. 182:259–264. 10.1111/j.1574-6968.2000.tb08905.x [DOI] [PubMed] [Google Scholar]
- 24. Kesavalu L, Ebersole JL, Machen RL, Holt SC. 1992. Porphyromonas gingivalis virulence in mice: induction of immunity to bacterial components. Infect. Immun. 60:1455–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, Brunelli B, Pieri A, Santini L, Savino S, Serruto D, Litt D, Kroll S, Welsch JA, Granoff DM, Rappuoli R, Pizza M. 2003. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J. Exp. Med. 197:789–799. 10.1084/jem.20021911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McConnell MJ, Rumbo C, Bou G, Pachón J. 2011. Outer membrane vesicles as an acellular vaccine against Acinetobacter baumannii. Vaccine 29:5705–5710. 10.1016/j.vaccine.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 27. Schild S, Nelson EJ, Bishop AL, Camilli A. 2009. Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera. Infect. Immun. 77:472–484. 10.1128/IAI.01139-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim OY, Hong BS, Park KS, Yoon YJ, Choi SJ, Lee WH, Roh TY, Lötvall J, Kim YK, Gho YS. 2013. Immunization with Escherichia coli Outer Membrane Vesicles Protects Bacteria-Induced Lethality via Th1 and Th17 cell Responses. J. Immunol. 190:4092–4102. 10.4049/jimmunol.1200742 [DOI] [PubMed] [Google Scholar]
- 29. Achkar JM, Casadevall A. 2013. Antibody-mediated immunity against tuberculosis: implications for vaccine development. Cell Host Microbe 13:250–262. 10.1016/j.chom.2013.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ziegenbalg A, Prados-Rosales R, Jenny-Avital ER, Kim RS, Casadevall A, Achkar JM. 2013. Immunogenicity of mycobacterial vesicles in humans: identification of a new tuberculosis antibody biomarker. Tuberculosis (Edinb.) 93:448–455. 10.1016/j.tube.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baena A, Porcelli SA. 2009. Evasion and subversion of antigen presentation by Mycobacterium tuberculosis. Tissue Antigens 74:189–204. 10.1111/j.1399-0039.2009.01301.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harding CV, Boom WH. 2010. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat. Rev. Microbiol. 8:296–307. 10.1038/nrmicro2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13:843–850. 10.1038/nm1592 [DOI] [PubMed] [Google Scholar]
- 34. Wille-Reece U, Flynn BJ, Loré K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA. 2006. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J. Exp. Med. 203:1249–1258. 10.1084/jem.20052433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heeney JL, Plotkin SA. 2006. Immunological correlates of protection from HIV infection and disease. Nat. Immunol. 7:1281–1284. 10.1038/ni1206-1281 [DOI] [PubMed] [Google Scholar]
- 36. Tchilian EZ, Desel C, Forbes EK, Bandermann S, Sander CR, Hill AV, McShane H, Kaufmann SH. 2009. Immunogenicity and protective efficacy of prime-boost regimens with recombinant (delta)ureC hly+ Mycobacterium bovis BCG and modified vaccinia virus Ankara expressing M. tuberculosis antigen 85A against murine tuberculosis. Infect. Immun. 77:622–631. 10.1128/IAI.00685-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, Schoolnik GK, Cassidy JP, Billeskov R, Andersen P. 2011. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat. Med. 17:189–194. 10.1038/nm.2285 [DOI] [PubMed] [Google Scholar]
- 38. Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, Tchilian EZ. 2008. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J. Immunol. 181:4955–4964. 10.4049/jimmunol.181.7.4955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sweeney KA, Dao DN, Goldberg MF, Hsu T, Venkataswamy MM, Henao-Tamayo M, Ordway D, Sellers RS, Jain P, Chen B, Chen M, Kim J, Lukose R, Chan J, Orme IM, Porcelli SA, Jacobs WR., Jr. 2011. A recombinant Mycobacterium smegmatis induces potent bactericidal immunity against Mycobacterium tuberculosis. Nat. Med. 17:1261–1268. 10.1038/nm.2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8:369–377. 10.1038/nrm2146 [DOI] [PubMed] [Google Scholar]
- 41. Zhu X, Venkataprasad N, Ivanyi J, Vordermeier HM. 1997. Vaccination with recombinant vaccinia viruses protects mice against Mycobacterium tuberculosis infection. Immunology 92:6–9. 10.1046/j.1365-2567.1997.00358.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lancioni CL, Li Q, Thomas JJ, Ding X, Thiel B, Drage MG, Pecora ND, Ziady AG, Shank S, Harding CV, Boom WH, Rojas RE. 2011. Mycobacterium tuberculosis lipoproteins directly regulate human memory CD4(+) T cell activation via Toll-like receptors 1 and 2. Infect. Immun. 79:663–673. 10.1128/IAI.00806-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parlane NA, Compton BJ, Hayman CM, Painter GF, Basaraba RJ, Heiser A, Buddle BM. 2012. Phosphatidylinositol di-mannoside and derivates modulate the immune response to and efficacy of a tuberculosis protein vaccine against Mycobacterium bovis infection. Vaccine 30:580–588. 10.1016/j.vaccine.2011.11.055 [DOI] [PubMed] [Google Scholar]
- 44. Giri PK, Kruh NA, Dobos KM, Schorey JS. 2010. Proteomic analysis identifies highly antigenic proteins in exosomes from M. tuberculosis-infected and culture filtrate protein-treated macrophages. Proteomics 10:3190–3202. 10.1002/pmic.200900840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Winau F, Weber S, Sad S, de Diego J, Hoops SL, Breiden B, Sandhoff K, Brinkmann V, Kaufmann SH, Schaible UE. 2006. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity 24:105–117. 10.1016/j.immuni.2005.12.001 [DOI] [PubMed] [Google Scholar]
- 46. Brosch R, Gordon SV, Garnier T, Eiglmeier K, Frigui W, Valenti P, Dos Santos S, Duthoy S, Lacroix C, Garcia-Pelayo C, Inwald JK, Golby P, Garcia JN, Hewinson RG, Behr MA, Quail MA, Churcher C, Barrell BG, Parkhill J, Cole ST. 2007. Genome plasticity of BCG and impact on vaccine efficacy. Proc. Natl. Acad. Sci. U. S. A. 104:5596–5601. 10.1073/pnas.0700869104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen DJ, Osterrieder N, Metzger SM, Buckles E, Doody AM, DeLisa MP, Putnam D. 2010. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proc. Natl. Acad. Sci. U. S. A. 107:3099–3104. 10.1073/pnas.0805532107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rezwan M, Lanéelle MA, Sander P, Daffé M. 2007. Breaking down the wall: fractionation of mycobacteria. J. Microbiol. Methods 68:32–39. 10.1016/j.mimet.2006.05.016 [DOI] [PubMed] [Google Scholar]
- 49. Ramachandran N, Raphael JV, Hainsworth E, Demirkan G, Fuentes MG, Rolfs A, Hu Y, LaBaer J. 2008. Next-generation high-density self-assembling functional protein arrays. Nat. Methods 5:535–538. 10.1038/nmeth.1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Anderson KS, Sibani S, Wallstrom G, Qiu J, Mendoza EA, Raphael J, Hainsworth E, Montor WR, Wong J, Park JG, Lokko N, Logvinenko T, Ramachandran N, Godwin AK, Marks J, Engstrom P, Labaer J. 2011. Protein microarray signature of autoantibody biomarkers for the early detection of breast cancer. J. Proteome Res. 10:85–96. 10.1021/pr100686b [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The 4217 M. tuberculosis genes were spotted into two slides (1 and 2), translated using a cell-free expression system, and detected by an anti-GST antibody. A 97% success rate was attained for protein signal, based on a cutoff of 3 standard deviations above the negative-control value (features containing a nonexpressing plasmid). (B) Sections of NAPPA arrays after probing with sera from PBS-, BCG MV-, and H37Rv MV-immunized mice, developed with an Alexa555-conjugated mouse anti-IgG secondary antibody. Each slide included a mouse IgG spot as a positive control (yellow circles). Some of the proteins showing differential reactivity are highlighted with red circles, including LppX, Rv1676, and PstS1. The complete set of reactive proteins is included in Table 1. Download
Immunoblot analysis of the 19-kDa antigen in MV. An immunoblot of H37Rv MV serum reactivity for WT BCG and the Δlpqh BCG mutant is shown. The asterisk indicates the absence of the 19-kDa antigen. Download
Bacterial load (CFU) in the spleen of individual C57BL/6 mice immunized SC with 1 × 106 BCG bacteria or twice with 2.5 mg BCG or H37Rv MV was determined at 8 weeks after infection with a low dose of M. tuberculosis H37Rv via aerosol (aproximately 100 CFU). The results of three independent experiments are shown. Experimental groups used 5 mice (values labeled “a” are statistically significantly different from those labeled “b” [P < 0.05] or “c” [P < 0.01] using one-way ANOVA). Download