Abstract
Macular degeneration is a progressive, bilateral eye disorder that damages the macula of the human eye. The most common form of macular degeneration is age-related macular degeneration (AMD), which is the leading cause of irreversible blindness in people older than 50 years in developed countries. Autosomal dominant Stargardt disease-3 (STGD3) is an inherited macular dystrophy that has clinical features similar to dry AMD, but occurs at a much earlier age. It is caused by a mutation in the elongation of very-long-chain fatty acids-like 4 (ELOVL4) gene, which is responsible for encoding the elongase enzyme that converts shorter chain fatty acids into C28–C38 very long-chain polyunsaturated fatty acids (VLCPUFAs, total number of carbons ≥24). Diets rich in long-chain polyunsaturated fatty acids (LCPUFAs) have inverse associations with the progression of AMD and STGD3, and a deficiency in retinal LCPUFAs and VLCPUFAs has been detected in AMD retinas and STGD3 animal models. This article systematically summarizes the roles of LCPUFAs and VLCPUFAs in AMD and STGD3, and discusses future research directions.
Keywords: age-related macular degeneration (AMD), autosomal dominant Stargardt disease-3 (STGD3), elongation of very-long-chain fatty acids-like 4 (ELOVL4), long-chain polyunsaturated fatty acid (LCPUFA), very-long-chain polyunsaturated fatty acid (VLCPUFA)
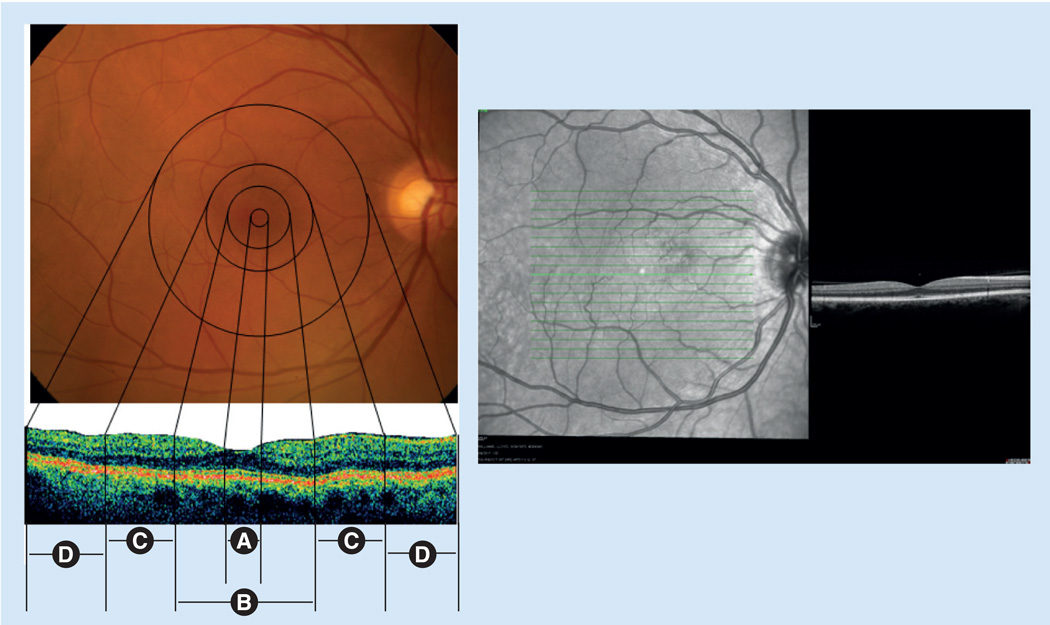
The retina is the inner layer of the eyeball located between the choroid and the vitreous. In the clinical realm, the macula corresponds to an area of the posterior retina 5–6 mm in diameter, centered on the fovea and lying within the major vascular arcades [1]. The macula is responsible for high-resolution visual acuity and color vision, and it is divided anatomically into the foveola, fovea, parafoveal area and perifoveal regions (Figure 1). The fovea has a horizontal diameter of approximately 1.5 mm and contains the highest concentration of cones within the retina. At the margin of the fovea, the retina is relatively thicker (0.55 mm) because of the multiple layers of ganglion cell nuclei present. The foveola is the 0.35 mm region at the bottom of the gradually sloped foveal depression. The annular zone surrounding the fovea can be divided into an inner parafoveal area (0.5 mm), and an outer perifoveal area (1.5 mm) [1]. The retinal pigment epithelium (RPE) is a monolayer of cells, just distal to the photoreceptor layer of the retina that performs functions essential to the health of the retina, including regeneration of visual pigment chromophores, phagocytosis of shed rod and cone outer segments, and maintenance of the blood–ocular barrier.
Figure 1. Clinical and correlated histopathologic views of the human macular region.
The left-hand panels show fundus photographs of the (A) foveola (0.35 mm), (B) fovea (1.5 mm), (C) parafoveal area (0.5 mm) and (D) perifoveal region (1.5 mm). The right-hand panel is a spectral-domain optical coherence tomography image showing the macular region of a normal patient. The clinical and histopathologic views were kindly provided by James Gilman (CRA, Project Administrator) of the John A Moran Eye Center Photography Department, UT, USA.
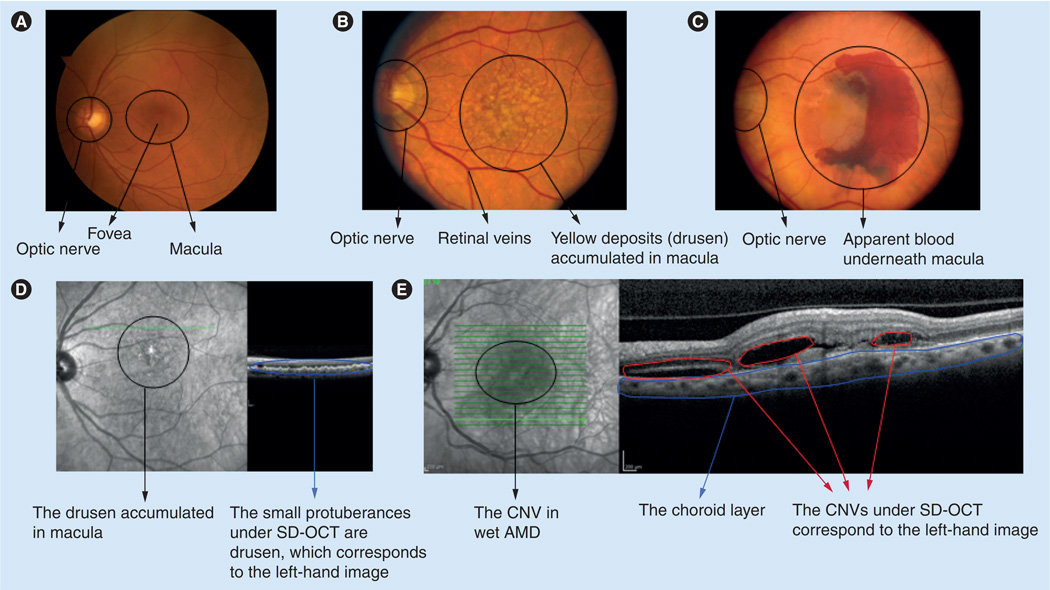
Macular degeneration is a progressive and bilateral eye disorder that damages the macula. Initial symptoms include an increasing need for bright light, a decrease in the intensity or brightness of colors, a gradual increase in the haziness of overall vision and a blurred or blind spot in the center of the field of vision. The most common type of macular degeneration is age-related macular degeneration (AMD), which is the leading cause of irreversible blindness in people older than 50 years in developed countries [2]. There are two forms of AMD known as dry and wet. In its early stage, dry macular degeneration (non-neovascular) is diagnosed when yellowish spots known as drusen consisting of oxidized lipids, proteins and inflammatory debris begin to accumulate in and around the macula (Figure 2). In advanced dry AMD, sharply demarcated areas of retina and RPE become atrophic, causing a condition known as geographic atrophy. With wet macular degeneration (neovascular), new blood vessels grow beneath the retina, which leak blood and fluid (Figure 2) causing permanent damage to light-sensitive retinal cells. These cells eventually die off creating blind spots in central vision. AMD is becoming more prevalent as the population ages, with nearly 30% of Americans over the age of 75 having at least early signs of AMD, and 7% having the late-stage disease [3,4]. The latter number is expected to triple in the next 30–40 years with the increase in the aging population [4].
Figure 2. Clinical views of human macula from different age-related macular degeneration stages.
(A) The clinical view of normal patient macula. (B) The macula of a patient with dry AMD, around which drusen have begun to accumulate. (C) The macula of a patient with wet AMD in which blood vessels underneath the macula have leaked blood and fluid. (D) The SD-OCT image of drusen. (E) The SD-OCT image of CNV.
AMD: Age-related macular degeneration; CNV: Choroidal neovascularization; SD-OCT: Spectral-domain optical coherence tomography. The clinical views were kindly provided by James Gilman (CRA, Project Administrator) of the John A Moran Eye Center Photography Department, UT, USA.
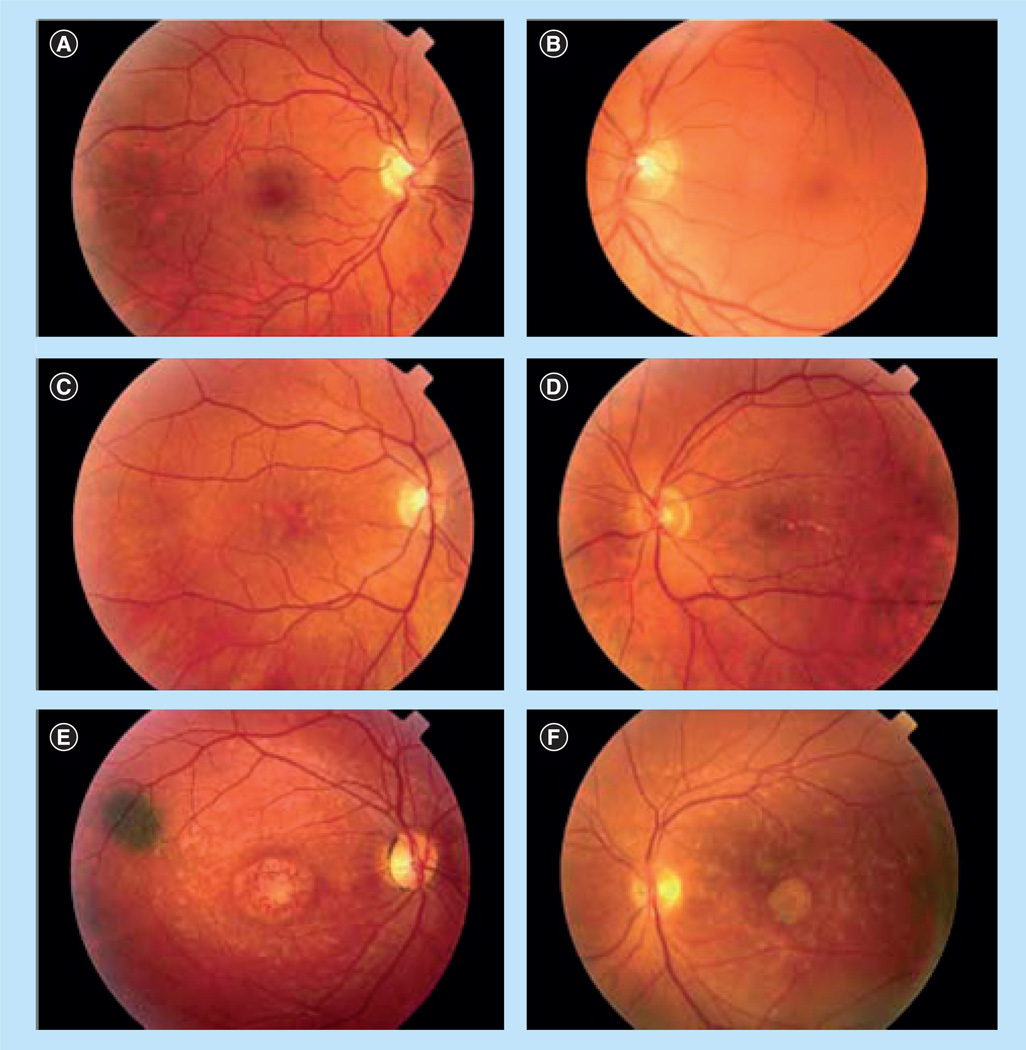
Age-related macular degeneration is a complex multifactorial disease which is attributable to aging, race, pigmentation, iris color, family history, macular degeneration-related susceptibility genes, hypertension, drusen, cardiovascular status, high fat intake, oxidative stress, exposure to sunlight, inflammation and smoking. Treatment options for AMD remain limited because of its complex etiology and pathogenesis. Furthermore, the late onset of AMD makes it challenging to study. This is why considerable research has focused on early-onset inherited macular dystrophies that resemble AMD. Autosomal dominant Stargardt disease-3 (STGD3), which typically starts in the teenage years, is caused by a mutation in the ELOVL4 gene, responsible for elongating long-chain polyunsaturated fatty acids (LCPUFAs) to C28–C38 very-long-chain PUFAs (VLCPUFAs, total number of carbons ≥24) [5]. STGD3 is not as common as AMD, but has similar clinical features that are also characteristic of AMD, including progressive pathology, loss of central vision, atrophy of the RPE, and accumulation of lipofuscin [6]. Thus, investigation of STGD3 has the potential to provide insight into the etiology and pathogenesis of AMD [7]. STGD3 has been divided into 3 phenotypic grades based on the severity of the maculopathy (Table 1), and representative fundus photographs show a varied presentation including macular atrophic flecks, butterfly-pattern dystrophy and bull’s-eye maculopathy (Figure 3) [8].
Table 1.
Definition of autosomal dominant Stargardt macular dystrophy phenotypic grades.
| Grade | Definition |
|---|---|
| 1 | Normal or near-normal macula; mild pigmentary changes and/or a few flecks present; and visual acuity expected to be better than 20/40 |
| 2 | Moderate maculopathy; moderate pigment changes and/or flecks present, but no significant foveal atrophy; and visual acuity expected to be in the 20/40–20/100 range |
| 3 | Advanced maculopathy; foveal atrophy present and may be associated with flecks and pigmentary changes; and visual acuity expected to be 20/200 or worse |
Reproduced with permission from [8] © (2006) American Medical Association. All rights reserved.
Figure 3. Clinical variability of autosomal dominant Stargardt macular dystrophy.
Fundus photographs of selected STGD3 family members with phenotypic grades (Table 1), including (A) grade 1 in the right eye, (B) grade 1 in the left eye, (C) grade 2 in the right eye, (D) grade 2 in the left eye, (E) grade 3 in the right eye and (F) grade 3 in the left eye.
Reproduced with permission from [8]. © (2006) American Medical Association. All rights reserved.
Fatty acids (FAs) are not only a source of energy but are also essential to cell physiological functions which include modulation of cellular metabolism, signal transduction, cell growth and differentiation and membrane lipid composition. The abundance of FAs in the retina, especially in retinal photoreceptor outer segment disc membranes, indicates their importance in retinal function and health [9]. Many clinical and epidemiological studies have demonstrated that the intake of LCPUFAs such as docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) or fish (a major source of both DHA and EPA) has a protective effect against the progression of AMD. Mouse model and human clinical studies indicate that a deficiency of VLCPUFAs in the retina is likely to be a key factor in the macular pathology seen in STGD3 [10,11], but relatively few studies have investigated the role of VLCPUFAs in relation to AMD. In this article, we summarize the roles and possible pathogenic mechanisms of LCPUFAs (DHA, EPA and arachidonicacid [AA]), VLCPUFAs and their related proteins and metabolites in macular degenerations and dystrophies such as AMD and STGD3.
The role of LC- & VLC-PUFAs in macular degeneration
The role of LCPUFAs in macular degeneration
The role of the major ω-3 LCPUFAs (DHA & EPA) in macular degeneration
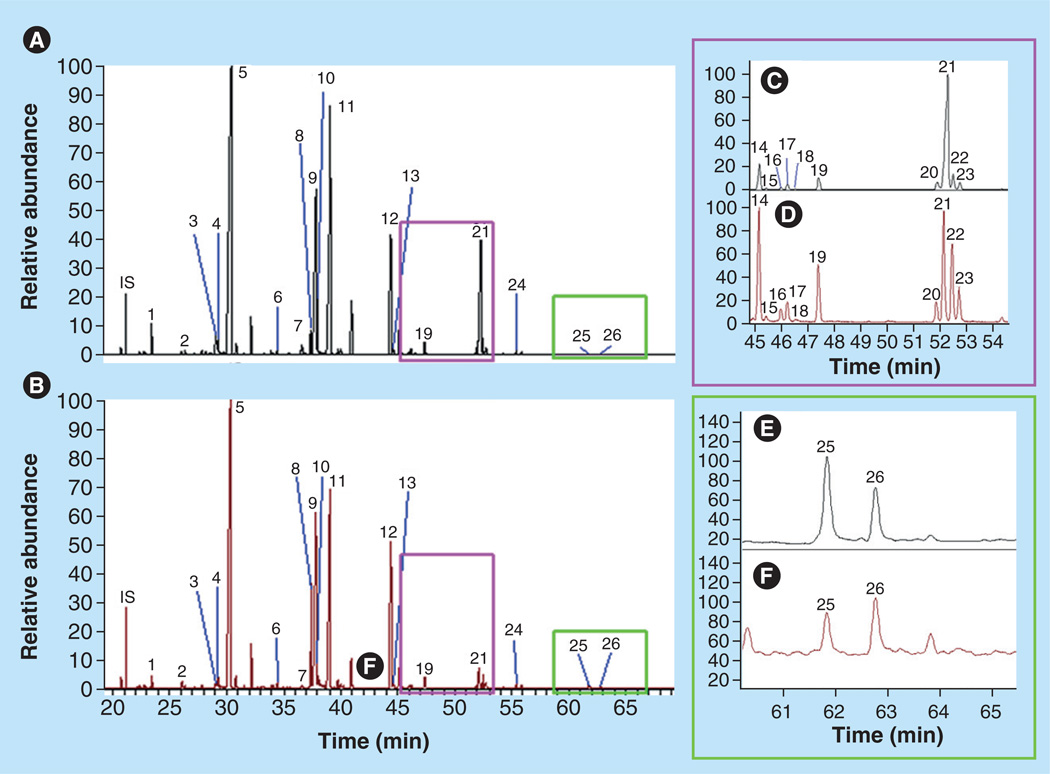
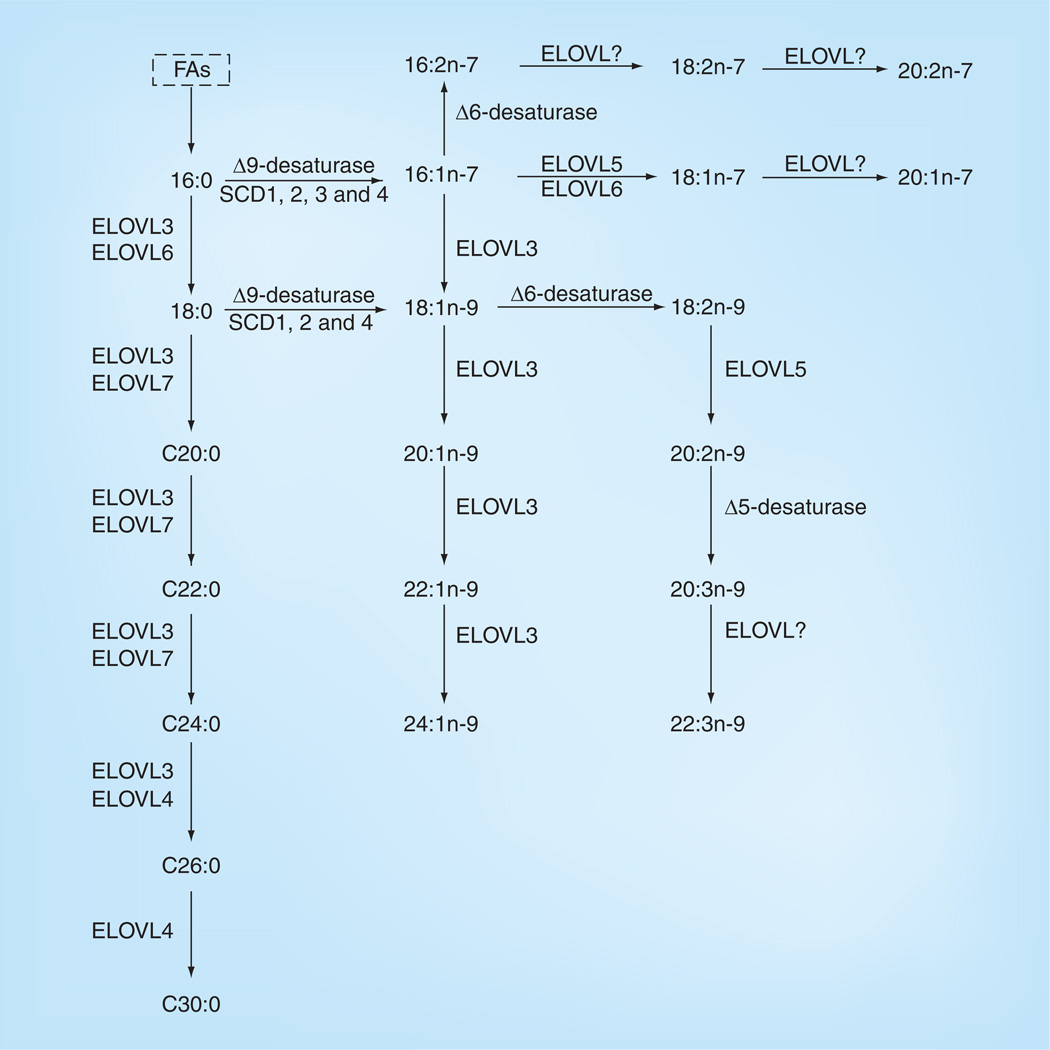
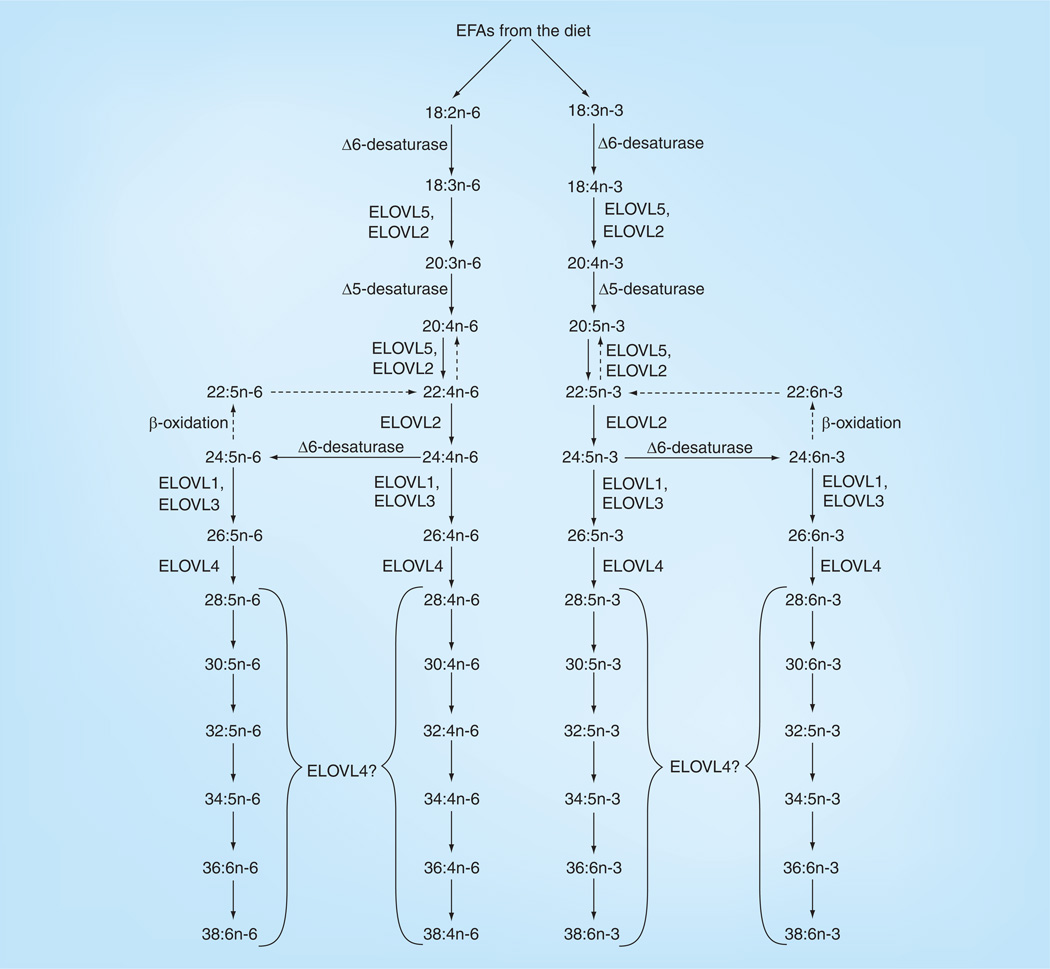
In human retina, 26 long-chain fatty acids (LCFAs) have been identified, including ω-3 and ω-6 saturated, monounsaturated and polyunsaturated FAs (Figure 4 & Table 2). DHA, the most abundant ω-3 LCPUFA in retina, can either be accumulated through diet or converted from linolenic acid (Figures 5 & 6). The concentration of EPA in the retina is relatively low, but it attracts considerable interest, due to the fact that it is one of the major ω-3 LCPUFAs in fish besides DHA, and it is a major precursor for VLCPUFAs.
Figure 4. Gas chromatography–mass spectrometry total ion chromatograms of long-chain fatty acids in retina and retinal pigment epithelium/choroid.
Mass spectrometry was performed under full scan mode (50–650 mau). (A & B) Long-chain fatty acid chromatograms of typical human whole retina (A) and retinal pigment epithelium/choroid (B), respectively.
(C & D) Amplified chromatograms for the purple square in (A & B), respectively. (E & F) Amplified chromatograms for the green square in (A & B), respectively. Peak numbers 1–26 were identified according to standards and the NIST library, and these peaks’ data are shown in Table 3. IS: Internal standard (capric acid).
Reproduced with permission from [10] © The American Society for Biochemistry and Molecular Biology.
Table 2.
Long-chain fatty acid compositions from human retina.
| Peak no.† | Fatty acids‡ | Common name | Classification§ |
|---|---|---|---|
| 1 | 14:0 | Tetradecanoic acid | LCSFA |
| 2 | 15:0 | Pentadecanoic acid | LCSFA |
| 3 | 16:1n-9 | 7-hexadecenoic acid | LCMUFA |
| 4 | 16:1n-7 | 9-hexadecenoic acid | LCMUFA |
| 5 | 16:0 | Palmitic acid | LCSFA |
| 6 | 17:0 | Heptadecanoic acid | LCSFA |
| 7 | 18:3n-3 | α-linolenic acid | LCPUFA |
| 8 | 18:2n-6 | Linoleic acid | LCPUFA |
| 9 | 18:1n-9 | Oleic acid | LCPUFA |
| 10 | 18:1n-7 | 11-octadecenoic acid | LCMUFA |
| 11 | 18:0 | Octadecanoic acid/stearic acid | LCMUFA |
| 12 | 20:4n-6 | Arachidonic acid | LCSFA |
| 13 | 20:5n-3 | 5,8,11,14,17-eicosapentaenoic acid | LCPUFA |
| 14 | 20:3n-6 | 8,11,14-eicosatrienoic acid | LCPUFA |
| 15 | 20:2n-7 | 8,11-eicosatrienoic acid | LCPUFA |
| 16 | 20:2n-9 | 11,14-eicosatrienoic acid | LCPUFA |
| 17 | 20:1n-9 | 9-eicosenoic acid | LCMUFA |
| 18 | 20:1n-7 | 11-eicosenoic acid | LCMUFA |
| 19 | 20:0 | Eicosanoic acid | LCSFA |
| 20 | 22:5n-6 | 4,7,10,13,16-docosapentaenoic acid | LCPUFA |
| 21 | 22:6n-3 | 4,7,10,13,16,19-docosahexaenoic acid | LCPUFA |
| 22 | 22:4n-6 | 7,10,13,16-docosatetraenoic acid (adrenic acid) | LCPUFA |
| 23 | 22:5n-3 | 7,10,13,16,19-docosapentaenoic acid | LCPUFA |
| 24 | 22:0 | Behenic acid | LCSFA |
| 25 | 24:1n-9 | 15-tetracosenoic acid | VLCMUFA |
| 26 | 24:0 | Tetracosanoic acid | VLCSFA |
Peak numbers are shown in chromatograms of Figure 4.
Number of carbon atoms: number of double bonds, the position of first double bond.
Classification of fatty acids.
LCMUFA: Long-chain monounsaturated fatty acid; LCPUFA: Long-chain polyunsaturated fatty acid; LCSFA: Long-chain saturated fatty acid; VLCMUFA: Very-long-chain monounsaturated fatty acid; VLCSFA: Very-long-chain saturated fatty acid.
Figure 5. Biosynthesis of long-chain and very-long-chain saturated fatty acids and some long-chain polyunsaturated fatty acids in mammals.
Pathways are based on published work by various research groups. Only biosynthesis of FAs existing in the retina is shown. FA: Fatty acid.
Figure 6. Biosynthesis of long-chain polyunsaturated fatty acids and very-long-chain polyunsaturated fatty acids in mammals.
Pathways are based on published work by various research groups. The enzymes for the n-3 long-chain polyunsaturated fatty acid pathway are the same for n-6 long-chain polyunsaturated fatty acid synthesis. Enzymatic pathways for n-3 very-long-chain polyunsaturated fatty acid (VLCPUFA) synthesis are better characterized than those for n-6 VLCPUFAs, and we have made the assumption that the same n-3 enzymes would be utilized in n-6 VLCPUFA synthesis.
EFA: Essential fatty acid.
Reproduced with permission from [10] © The American Society for Biochemistry and Molecular Biology.
There are many epidemiology studies examining the relationship between dietary intake of DHA, EPA or fish and prevalence of AMD, and most studies indicate an inverse association between fish/DHA/EPA intake and risk of AMD. Various 5–10 year epidemiological studies have demonstrated that intake of fish (fried, boiled or baked) corresponded with decreased odds of AMD progression including early [12–16], intermediate [12,16] and advanced [13,15,17–20] stages [21,22]. A comparison of fish intake frequency (4 servings/week, 3 servings/week, 2 servings/week, 1 serving/week, 3 servings/month or 1 serving/month) demonstrated that the higher the fish intake frequency, the lower the risk of AMD progression. This association was observed when fish intake frequency was >1 serving/week. An inverse association between intake of DHA/EPA and AMD progression was also observed [19–21,23]. Studies with different intake amounts of DHA and EPA (1, 0.35, 0.06 g/day, ≥64.0 or ≥42.3 mg/day) showed that a higher intake of DHA or EPA correlates with a decreased risk of AMD compared with a lower intake. However, most of the above associations for fish/DHA/EPA did not persist among individuals consuming high dietary levels of linoleic acid (≥5.6 g/day) [12,14,16,21,22]. In fact, a positive association between intake of LA and incidence of AMD was observed [21,22]. Based on the results of these epidemiology studies, the National Eye Institute added 1000 mg of fish oil rich in EPA and DHA in the ongoing Age-Related Eye Disease Study-2 (AREDS2) randomized, placebo-controlled clinical trial [24,201] whose results will be released in 2013.
On the contrary, several other studies have demonstrated different results relative to those cited above. While the intake of total ω-3 LCPUFAs demonstrates a strong relationship with protection against NV-AMD, one study revealed a lack of such a relationship between NV-AMD and dietary pure DHA [20]. No significant association between dietary ω-3 LCPUFAs (including DHA, EPA and fish intake) and the progression of early/late age-related maculopathy (ARM) or AMD was observed, which may be due to a lack in variety of participants or a lower fish intake level than in other studies [25,26]. Furthermore, red blood cell (RBC) levels of AA and DHA were significantly higher in AMD patients than in controls [27].
Besides epidemiological and clinical trials, there are some studies of ω-3 LCPUFA functions in macular degeneration animal models. An EPA-rich diet resulted in significant suppression of choroidal neovascularization (CNV) and CNV-related inflammatory molecules in vivo and in vitro [28], which suggests that frequent consumption of ω-3 LCPUFAs may prevent CNV and lower the risk of blindness caused by AMD. When chemokine receptor mutation (Cx3cr1)−/− mice – a model that develops AMD-like retinal lesions – consume a diet high in ω-3 LCPUFAs, there was a slower progression of retinal lesions when compared with those consuming a low level diet. Some mice given high levels of ω-3 PUFAs even demonstrated lesion reversion [29]. DHA supplementation increased the concentration of DHA in serum and retina and partially counteracted kainic acid-induced retinal damage in rat retina [30].
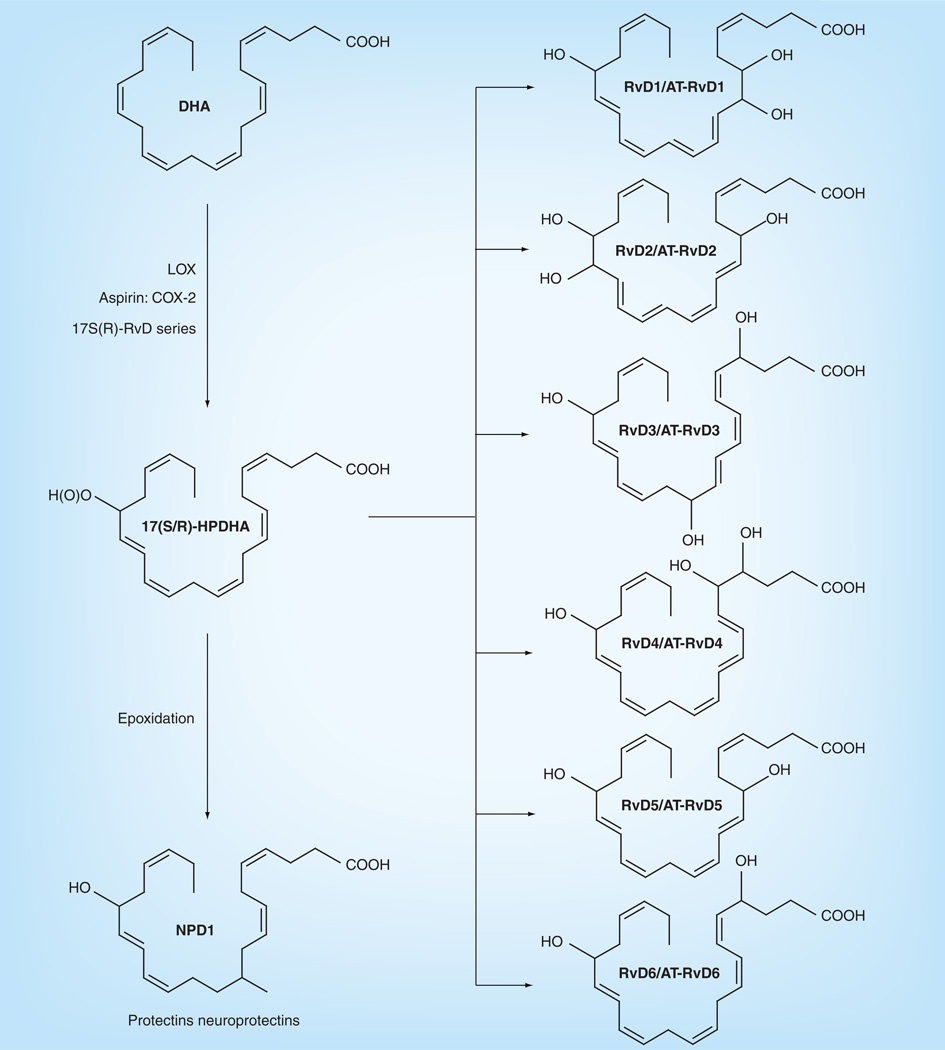
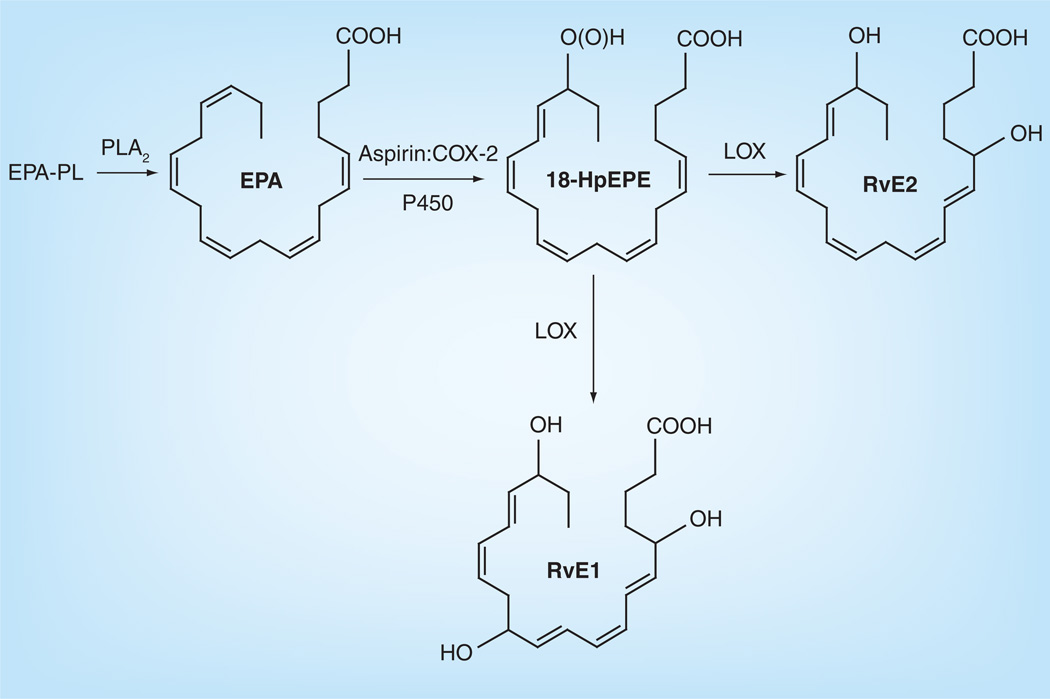
Although most epidemiological and animal studies have concluded that high levels of DHA/fish supplementation can slow the progression of macular degeneration, it is still ambiguous how DHA produces this effect since there are many factors involved, especially in AMD. Among these, oxidative stress in the retina is one of the most important pathogenic factors because it can cause a cytotoxic chain reaction. After oxidative stress takes place, inflammation occurs, which is postulated to be involved in AMD. DHA possesses anti-inflammatory properties, which may act through antiinflammatory pathways in the periphery [31]. Under normal conditions, DHA is retained and protected from peroxidation, but during retinal degeneration, DHA peroxidation takes place along with perturbations of photoreceptor function, damage and cell death [32,33]. During the oxygenation pathways, DHA can be oxidatively synthesized into 10,17S-docosatriene (neuroprotectin D1 [NPD1]) and the resolvin D (RvD) series, including RvD1–6 (Figure 7) [33–35]. NPD1 displays potent anti-inflammatory bioactivity since it turns off several proinflammatory and proapoptotic genes [36–38], downregulates A2E-mediated apoptosis, restores the integrity of RPE and its relationship with the photoreceptor [39,40], counteracts oxidative stress-triggered apoptotic DNA damage in RPE [39,40], protects neural cells from oxidative stress and may be crucial to cell survival [36,38]. RvD1–6, another kind of oxidative metabolite besides NPD1, is derived from DHA (Figure 7) and has inflammatory activity [41,42]. So, not only DHA but also its metabolites, NPD1 and RvD1–6, can protect against the oxidation and inflammation of neural cells. Our laboratory has compared the DHA in AMD and normal patients, and found a decreased level of DHA in AMD patient retinas compared with age-related normal patients [10], which may be attributed to the anti-inflammatory processes as stated above. Consistently, loss of DHA from rat photoreceptors is found in constant light-induced retinal degeneration [43]. Crabb et al. demonstrated that carboxyethyl pyrrole (CEP) protein adducts, the oxidative protein modifications generated uniquely from the docosahexaenoate-containing lipids such as DHA [44], are more abundant in AMD retinal tissue than in the normal retina [45]. The increase of CEP protein adducts in AMD retinas indicates that the increased oxidation of DHA that likely occurs in AMD eyes may lead to a depletion of DHA, which is consistent with our results. The oxidization pathway of EPA is shown in Figure 8 [46,47]. EPA can be metabolized into resolvin E1 (RvE1) which can inhibit inflammation. SanGiovanni et al. recently summarized comprehensive information on the putative actions of ω-3 LCPUFAs on different causes of AMD [48]. Besides the above mentioned enzymic metabolites, there are also nonenzymic products such as 4-hydroxyhexenal (4-HHE), neuroprostanes, neurofurans and neuroketals from DHA and F3 isoprostanes from EPA, which are accurate markers of lipid peroxidation in both animal and human models of oxidative stress [49]. Most of the above products produce oxidative stress and may contribute to cell injury and neurodegeneration. For example, 4-HHE induces cyotoxicity in diverse cell types including rat primary neurons, YPEN-1 prostatic endothelial cells, lens epithelial cells and U937 lymphocytes [50,51]. However, it has also been proved that 4-HHE can prevent tertbutyl hydroperoxide-induced cytotoxicity [52]. Electrophilic cyclopentenone neuroprostanes are potent inhibitors of NF-κB signaling and may contribute to the anti-inflammatory actions of DHA [53].
Figure 7. Enzymatically derived metabolites of docosahexaenoic acid metabolism.
DHA: Docosahexaenoic acid; Rv: Resolvin.
Figure 8. Enzymatically derived metabolites of eicosapentaenoic acid metabolism.
EPA: Eicosapentaenoic acid; PLA: Phospholipase A; Rv: Resolvin.
Dominantly inherited Stargardt’s disease is a juvenile-onset analog of AMD that often leads to legal blindness in teens and young adults. There are three independent mutations causing STGD3 which were identified on exon six of the ELOVL4 gene. The ELOVL4 protein is involved in FA elongation for a series of C28– C38 PUFAs [54], and reduced retinal VLCPUFAs have found in STGD3 mouse retina [11]. Although EPA is thought to be a preferred substrate for VLCPUFA synthesis [55], DHA may also be a precursor for ω-3 VLCPUFAs [56]. Thus, DHA supplementation has been suggested as a potential treatment for STGD3. There is one published clinical case report in which a 15-year-old girl with STGD3 took DHA supplementation at 20 mg/kg body weight per day for 3 months (April-2001 to June-2001) and demonstrated a significant increase in plasma DHA, better vision according to a visual function questionnaire, and improvement of amplitudes from the foveal and parafoveal regions on her multifocal electroretinogram. This suggests that the severity of STGD3 might be alleviated by dietary supplementation of DHA [57,58]. The National Eye Institute and John A. Moran Eye Center have initiated clinical trials of DHA or fish oil supplementation in Stargardt-3 patients as a treatment to slow down disease progression [202,203]. On the other hand, Anderson et al. found no evidence that high levels of DHA in retinal membranes protected photoreceptor cells expressing mutant ELOVL4 [59]. They concluded that DHA is not beneficial for the treatment of retinal degeneration in this animal model of human STGD3 macular dystrophy [59]. The animal model used in Anderson’s paper was produced by a cross of TG2 and Fat-1 mice. Since the Fat-1 protein can convert ω-6 to ω-3 FAs [60], retinal ω-3 LCPUFAs still accumulated in this mouse model despite an ω-3 FA deficient diet. Since EPA is an even better substrate for VLCPUFA synthesis, future studies on STGD3 need to consider fish oils that provide a balanced mixture of DHA and EPA. This is based on results from our laboratory in which the lipids of adipose tissue and RBCs from 18 Utah STGD3 patients with a two base-pair deletion in ELOVL4 were compared with 26 normal family members. We demonstrated that EPA in adipose tissue, a measure of very long-term LCPUFA intake (1–2 years) and EPA/DHA levels in RBC membranes, a measure of medium-term LCPUFA intake (several months) were inversely related to phenotypic severity. Thus, it is evident that dietary factors can influence the severity of an inherited human macular dystrophy [8]. More recently, we also found that DHA concentrations were significantly decreased in TG2 mouse retinas compared with the C57BL/6 control [61]. This may be due to the death of photoreceptor cells, which have the highest concentration of DHA in retina, or because C-terminally truncated ELOVL4 (ELOVL4ΔC) is able to form a dysfunctional elongase complex by interacting strongly with other elongases (ELOVL1–7) similar to wild-type (WT) ELOVL4 [62].
The role of major ω-6 LCPUFAs in macular degeneration
Arachidonic acid (20:4n-6) is the most abundant ω-6 LCPUFA in retina (Figure 4). Similar to DHA, AA can also be either converted from shorter chain ω-6 FAs or accumulated from diet (Figures 5 & 6). Relative to RPE, the amount of AA in rod outer segments (ROS) was 4–16 times lower, and the highest concentrations of AA in human retina are found in phosphatidylcholine (PC) and phosphatidylethanolamine (PE). AA values in RBC lipids were significantly higher in AMD patients than in controls [27], and dietary AA was directly associated with NV-AMD prevalence [19,20]. However, AA levels were significantly lower in the plasma phospholipids of patients with Usher’s syndrome, an inherited autosomal recessive disorder characterized by deafness and visual cell degeneration similar to retinitis pigmentosa [63].
Arachidonic acid serves as both an intra- and intercellular messenger, playing a role in synaptic plasticity and long-term potentiation in the CNS through its ability to diffuse in and out of cells [64]. On the one hand, AA can promote neurite elongation at a low concentration [65], protect against neuronal cell death [66] and depress synaptic transmission in calcium channel currents [67,68]. On the other hand, AA can induce lipid peroxidation, neuronal degeneration [65,69] and excitotoxicity by raising intracellular calcium concentrations [70,71]. Thus, the AA pathway may both promote and protect against neuronal degeneration [72]. A balance between protective and toxic properties of AA pathways may exist; however, the specific conditions that favor protection over toxicity remain poorly understood.
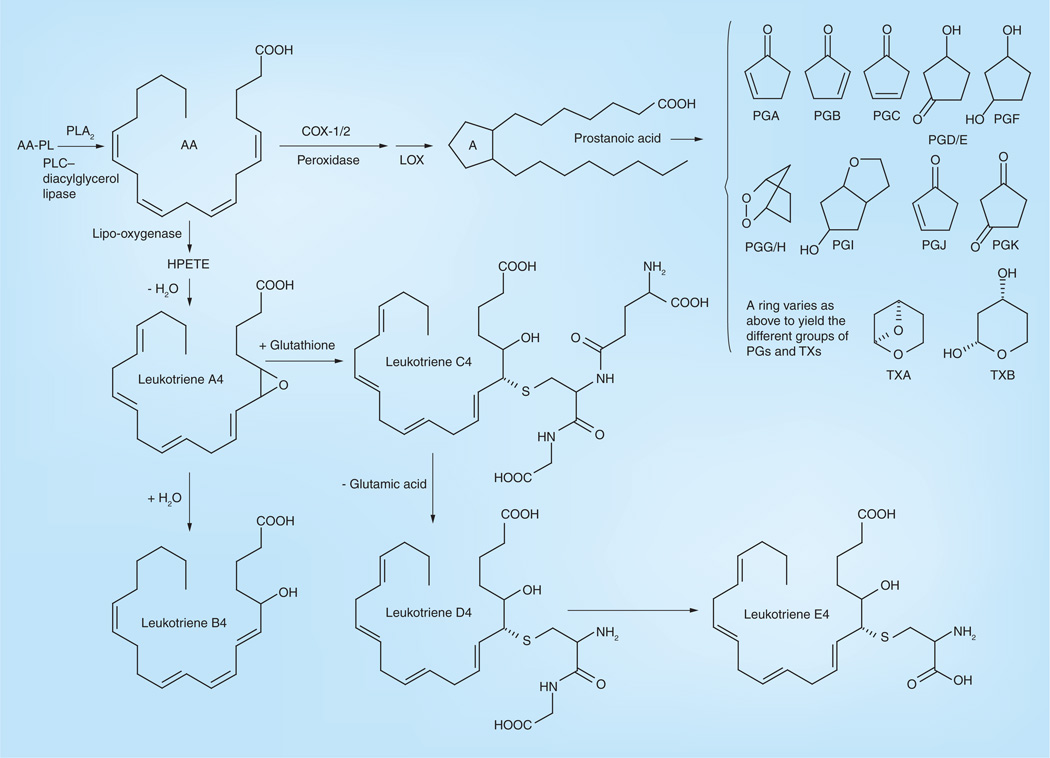
Similar to DHA and EPA, AA can be liberated from membranes by activation of phospholipase A (PLA). Light exposure activates PLAs and induces the release of AA and its metabolites in the retina [73], which have been found to induce apoptosis in retina and other tissues [74,75]. C20 LCPUFAs, including AA and EPA, can be oxidized into eicosanoids. In general, the n-6 eicosanoids are proinflammatory while ω-3 eicosanoids are much less so. AA’s eicosanoids are biologically active lipids that can be divided into three groups: prostaglandins (PG), thromboxanes (TX) and leukotrienes (LT) (Figure 9). Most of these metabolites, such as PGE2, LTB4, PGG/H, LTA4, LTB4, LTC4, LTD4 and LTE4, are believed to act as inflammatory mediators [29,39,76–81], but several of these metabolites such as PGJ2, PGD2 and PGE1 are believed to be anti-inflammatory derivatives [82,83]. Lipoxygenases (LOX) and cyclooxygenases (COX), inducible enzymes that utilize both ω-3 and 6 LCPUFAs, convert LCPUFAs to a number of angiogenic and proinflammatory eicosanoids [48,84]. Similar to DHA and EPA, AA can produce non-enzymic peroxidation including 4-hydroxy-nonenal (4-HNE), acrolein, malondiadehyde, isoprostanes, isoketals and isofurans. These products, especially 4-HNE, also produce oxidative stress and may contribute to cell injury and neurodegeneration [58,85]. In short, AA and most of its metabolites are related with inflammation, nerve cell apoptosis and oxidation, all of which are instigators of macular degeneration. Our laboratory has reported that AMD patient retinas have significantly higher ω-6/ω-3 ratios in LCPUFAs (mainly AA/DHA) compared with normal patient retinas, indicating that an imbalance of the ω-6/ω-3 LCPUFA ratio may be related to AMD disease risk and pathogenesis [10].
Figure 9. Enzymatically derived metabolites of arachidonic acid metabolism.
AA-PL: Arachidonyl-phospholipid; HPETE: Hydroperoxyeicosatetraenoic; PG: Prostaglandin; PLA: Phospholipase A; PLC: Phospholipase C; TX: Thromboxane.
The role of VLCPUFAs in macular degeneration
VLCPUFAs were first discovered in the brain of a patient by Poulos et al. around 25 years ago [86]. One year later, Aveldano et al. detected VLCPUFAs in the whole bovine retina and ROS PC [87] and they demonstrated that, similar to DHA, the concentration of total VLCPUFAs is higher in ROS (13.1 mol % in PC) than in whole retina (2.2 mol % in PC). Until recently, however, VLCPUFAs have generally been overlooked because they are unusually difficult to analyze due to their great length and minor abundance. They exhibit a unique hybrid structure, combining a proximal end with a typical saturated FA character and a distal end more characteristic of common PUFAs, which makes VLCPUFAs of particular interest and importance [54]. VLCPUFAs occur in low amounts in a restricted number of organs such as retina, testes, thymus and brain [5,86–88], and their saturated analogs are essential parts of the skin moisture barrier [89–91].
The role of VLCPUFAs & ELOVL4 in STGD3
ELOVL4 is a fatty acyl elongase that participates in the biosynthesis of C28–C38 VLCFAs, including VLCSFAs and VLCPUFAs, which are relatively abundant in skin (saturated chains), retina, brain and testes (polyunsaturated chains) of mammals, respectively. In cells transfected with mouse Elovl4, Anderson’s laboratory proved that 24:0 was elongated to 28:0 and 30:0, while 20:5n3 and 22:5n3 were elongated to a series of C28–C38 PUFAs [54]. Then, they demonstrated that the ELOVL4 protein is not involved in DHA biosynthesis from the shorter chain FA precursors 18:3n3 and 22:5n3 in the cone photoreceptor derived cell line 661W [55], whereas Monroig et al. proposed that zebrafish Elovl4b may be involved in the biosynthesis of DHA as it has the capacity to elongate 22:5n-3 to 24:5n-3 which can subsequently be desaturated and chain shortened to DHA in peroxisomes [92]. The expression of Elovl4 in mouse retina begins at E15 during embryogenesis and persists in postnatal stages. However, Elovl4 is predominantly expressed in the retinal ganglion cells at P1-P3, followed by predominant expression in the outer nuclear layer at P7, with its final expression enriched in the inner segments of photoreceptors [93].
Zhang and Edwards were the first to identify ELOVL4 as the gene responsible for an autosomal dominant form of Stargardt disease known as STGD3 [94,95]. Genetic studies localized the STGD3 disease locus to a small region on the short arm of human chromosome 6 and application of a positional candidate gene approach identified protein truncating mutations in ELOVL4 in patients with STGD3 [6]. Mutational analysis of the ELOVL4 gene in STGD3 patients revealed that a 5-bp deletion on exon 6 [94–96], two 1-bp deletions (789delT and 794delT) [97], and a 270 stop mutation [98] were identified in large autosomal dominant Stargardt-like macular dystrophy pedigrees from the USA and The Netherlands. Together, all of the above clinical studies confirmed the role of the ELOVL4 gene in STGD3 patients. Nevertheless, it has been suggested that other unknown genes may be responsible for the STGD3-like phenotype in a Chinese pedigree [99].
The effectiveness of a specific gene mutation to cause disease is often evaluated in an animal model. The mouse is one of the more popular animal model choices due to advantages such as having 90% overall similarity to the human genome, relatively short lifespan and inexpensive maintenance [6], but a common criticism of mouse model systems with respect to human macular degeneration is that the mouse retina does not have a macula. However, human macular disease affects the entire retina and not just the macular region, so the incorporation of mutations or known human macular disease genes into mice often results in retinal diseases, making the mouse model system useful [100,101]. Karan et al. produced transgenic mice, including the TG1, TG2 and TG3 lines in which different levels (from low to high) of mutant forms of human ELOVL4 were expressed [102]. These transgenic mice possessed the hallmarks of macular degeneration: accumulation of lipofuscin in the RPE, development of abnormal electrophysiology and atrophy of the RPE and photoreceptors, while there is no evidence that transgenic mice with wild type human ELOVL4 expression exhibit photoreceptor degeneration. TG1, TG2 and TG3 overexpressed human ELOVL4 at levels at around 0.6-, 3.3- and 5.3-fold relative to endogenous mouse ELOVL4. The extent of photoreceptor cell degeneration is greater in the mice with higher expression of mutant ELOVL4 and TG1, TG2 and TG3 lost 50% of photoreceptors at around 18 months, 16 weeks and 6 weeks, respectively. Before significant photoreceptor cell death, the levels of lipofuscin such as A2E and related compounds in the RPE of mutant ELOVL4 transgenic mice retinas were increased. The rate of electroretinogram (ERG) response decrease is faster in mice with higher expression of mutant ELOVL4. TG1 had maximum b-wave amplitudes toward the lower end of the normal range at 22 weeks of age and demonstrated a decline to about one-third the normal amplitude by 84 weeks (1.5 years) of age. TG2 mice had a more rapid decline so that responses were not detectable by 84 weeks. TG3 mice demonstrated greatly reduced responses at 22 weeks and no detectable responses by 35 weeks. Thus, the ELOVL4 transgenic mice have a close phenotype to human STGD3 and AMD and represent an appropriate mouse model for STGD3 and dry AMD [102]. Furthermore, the progressive decline in retinal function of the ELOVL4-transgenic mice makes them an attractive model for tests of therapeutic interventions.
In a heterozygous knock-in mouse model carrying the 5-bp deletion of Elovl4 (E_mut+/−) [103], E_mut+/− mouse retinas showed the presence of both WT and mutant ELOVL4 proteins, ultrastructural abnormalities of cone photoreceptor as early as 2 months of age, accumulation of lipofuscin in RPE, subretinal deposits at later ages and shortening of ROS at approximately 10 months of age. Levels of the FAs 20:5n-3 (P = 0.027), 22:5n-3 (P = 0.040) and 24:6n-3 (P = 0.005) were lower in the retinas of E_mut+/− mice than control mice. Kedzierski’s laboratory generated heterozygous and homozygous knockin mice which carried a human STGD3 mutation with a pathogenic 5-bp deletion and two point mutations in exon 6 [89]. The heterozygous Stgd3 mice expressed equal amounts of both WT and mutant ELOVL4 mRNAs in retina, and they demonstrated no significant changes in retinal morphology. They did, however, accumulate lipofuscin and had reduced visual function, which are early retinal features of the human STGD3 pathology [89]. In this heterozygous Stgd3 knock-in mouse carrying a human pathogenic mutation, two potential mechanisms are involved: truncated protein-induced cellular stress and lipid product deficiency. Further analysis of the mutant retina revealed no detectable cellular stress, but demonstrated selective deficiency of C32–C36 acyl phosphatidylcholines which contain residues of polyunsaturated C28– C36 fatty acids, indicating that selective deficiency of C32–C36 acyl phosphatidyl could lead to the human STGD3 pathology [11]. Since retinal degeneration is the only known phenotype in STGD3, reduced VLCPUFAs in STGD3 retinas may be the cause of photoreceptor cell death [54].
However, their homozygous knock-in Stgd3 mice, which expressed a normal content of mutated Elovl4 mRNA in skin, became dehydrated and died by 6–12 h postpartum. These homozygous STGD3 mice showed a complete absence of acylceramides containing saturated VLCFAs in the epidermis and a significant decrease of VLCPUFAs and protein in the retina, consistent with a role of Elovl4 in the synthesis of the unique very long chain C30–C40 FAs present in skin acylceramides and retina PCs [11,89]. Likewise, we have detected a significant decrease of VLCPUFAs in TG2 and conditional Elovl4-knockout mouse retinas [61]. The studies of Li et al. and Uchida et al. showed that depletion of ceramides with VLCFAs causes defective skin permeability barrier function and neonatal lethality in Elovl4-deficient mice. The absence of Elovl4 results in depletion of ceramides with ω-hydroxy very long chain FAs (Cn ≥C28) in the epidermis and accumulation of ceramides with non ω-hydroxy FAs of C26, implicating C26 FAs as possible substrates of Elovl4 [104,105]. These data demonstrate that ELOVL4 is required for VLCFA synthesis and is essential for the water permeability barrier function of skin.
Similarly, Ayyagari’s laboratory generated a knock-in mouse model with the 5-bp deletion in the Elovl4 gene [91]. The heterozygous (Elovl4+/del) mice exhibit progressive photoreceptor degeneration, while homozygous mice (Elovl4del/del) display scaly and wrinkled skin, have severely compromised epidermal permeability barrier function and die within a few hours after birth. Their results also proved that loss of functional ELOVL4 depletes VLCFAs and the unique ω-O-acylceramides in skin, which leads to neonatal death. Recently, Kedzierski’s laboratory generated transgenic mice (Tg/homozygous Stgd3) with reinstatement of both epidermal Elovl4 expression and VLCFAs synthesis, so that they can survive at least one month. In the meantime, Elovl4 expression and C28–C36 FA synthesis were still lacking in retina. Thus, this Tg/homozygous Stgd3 mouse will facilitate future studies to define the roles of C28–C36 FAs in the Elovl4 expressing tissues such as retina [106].
Raz-Prag et al. generated heterozygous knock-out Elovl4+/− mice and littermate WT control mice of the same age, which demonstrated that although the level of Elovl4 mRNA was reduced in Elovl4+/− retinas, only minimal morphologic abnormalities were found and the retinal function (ERG) was essentially normal in Elovl4+/− retinas compared with the WT control retinas. Systemic FA profiles of Elovl4+/− mice were unremarkable, although the concentrations of several FAs were significantly lower in Elovl4+/− retinas, particularly the monounsaturated FAs [107]. This analysis provides the first in vivo evidence that Elovl4 haploinsufficiency is not the underlying disease mechanism in STGD3. Consistently, Elovl4 haploinsufficiency does not induce early onset retinal degeneration in mice [108], although, disease manifestations worsen as the load of mutant protein increases, consistent with a dominant negative mechanism [102]. These results indicate that a decreased ratio of mutant to normal proteins could be a useful therapeutic strategy. This approach was successfully applied to reduce retinal degeneration in P23H rhodopsin transgenic rats using ribozyme knockdown of mutant RNA [109]. Similar to above homozygous knock-in Elovl4 mouse, the Elovl4 homozygous knockout pups (Elovl4 gene [exon 2, 5-bp] deletion) generated by Raz-Prag et al. were also nonviable [107].
The mechanism underlying the role of ELOVL4 in STGD3 has been studied. Zhang’s laboratory expressed WT and mutant ELOVL4 genes as enhanced green fluorescent protein (EGFP) fusion proteins in transient transfection in NIH-3T3 and HEK293 cells [89,100,101]. The wtELOVL4/EGFP fusion protein localized preferentially to the endoplasmic reticulum (ER) where FAs are synthesized, while the mtELOVL4/EGFP fusion protein does not localize in the ER but rather appears to be mislocalized to other compartments in an aggregated pattern within the cytoplasm. They proved that the ELOVL4 mutation can alter protein localization, enhance aggregate formation and induce cellular stress. They also proposed that ‘ inactivation’ of the WT ELOVL4 protein through sequestration to a non- ER compartment by ELOVL4 mutants may play a role in cellular dysfunction. Ambasudhan et al. also demonstrated that Elovl4 is an ER-resident protein and that the localization of two distinct mutations (a 5-bp deletion and a complex mutation from the same region in exon 6 of this gene) was dramatically changed from an ER to a Golgi distribution [110]. In ER, all identified ELOVL4 mutations produce ELOVL4ΔC that lack a motif for protein retention. Grayson et al. demonstrated that disease-linked ELOVL4ΔC exert a dominant negative effect on wild type ELOVL4, altering its subcellular localization [111]. All of the above observations suggest that the consequences of defective protein trafficking could underlie the molecular mechanism associated with macular degeneration in patients with AMD/STGD3. The apoptosis induced by a mutant ELOVL4 fusion protein may be the mechanism whereby photoreceptor cells degenerate in STGD3.
In summary, the above studies indicate that VLCPUFAs are necessary for normal function of the retina, and defective ELOVL4 protein trafficking and/or altered VLCPUFAs elongation underlies the pathology associated with STGD3. Determination of the role of VLCPUFAs in the retina, discerning the implications of abnormal trafficking of mutant ELOVL4 and evaluation of the depleted VLCPUFA content in the pathology of STGD3, will provide valuable insight in understanding the retinal structure, function and pathology underlying STGD3 and may lead to a better understanding of the process of macular disease in general. Our laboratory has detected deficiency of VLCPUFAs in TG2 mouse retinas [61], which may be due to the ELOVL4 mutation.
The role of VLCPUFAs in AMD
As described above, fish oil and supplementation of DHA/EPA are associated with lower incidence of AMD. Since fish oil components are precursors of VLCPUFAs, it is hypothesized that ELOVL4, the elongase of VLCPUFAs, may be related to AMD. Conley et al. investigated 21 polymorphisms within 15 genes from ARM Caucasian populations, and found a Met299Val variant in the ELOVL4 gene, as well as a Tyr402His variant of exon 9 in the complement factor H (CFH) gene were significantly associated with ARM in case–control allele (p ≤0.001), case– control genotype (p ≤0.001) and case–control family (p < 0.0001) tests [112]. These results support a potential role for multiple pathways in the etiology of ARM, including pathways involved with FA biosynthesis and the complement system. However, Seitsonen et al. investigated the association between AMD and the CFH, ELOVL4, hemicentin-1 (HMCN1) genes in AMD patients from the Finnish population [113]. They found that there is no association between the Met299Val polymorphism in the ELOVL4 gene and Gln5345Arg variant in the HMCN1 gene with AMD, while it confirmed that the Tyr402His polymorphism of the CFH gene is related to AMD. Furthermore, the association of NV-AMD and ELOVL4 was not found by the DeAngelis et al. [114]. Similarly, the association between the M299V variant in the ELOVL4 gene and exudative AMD was also not found in a Chinese population [115].
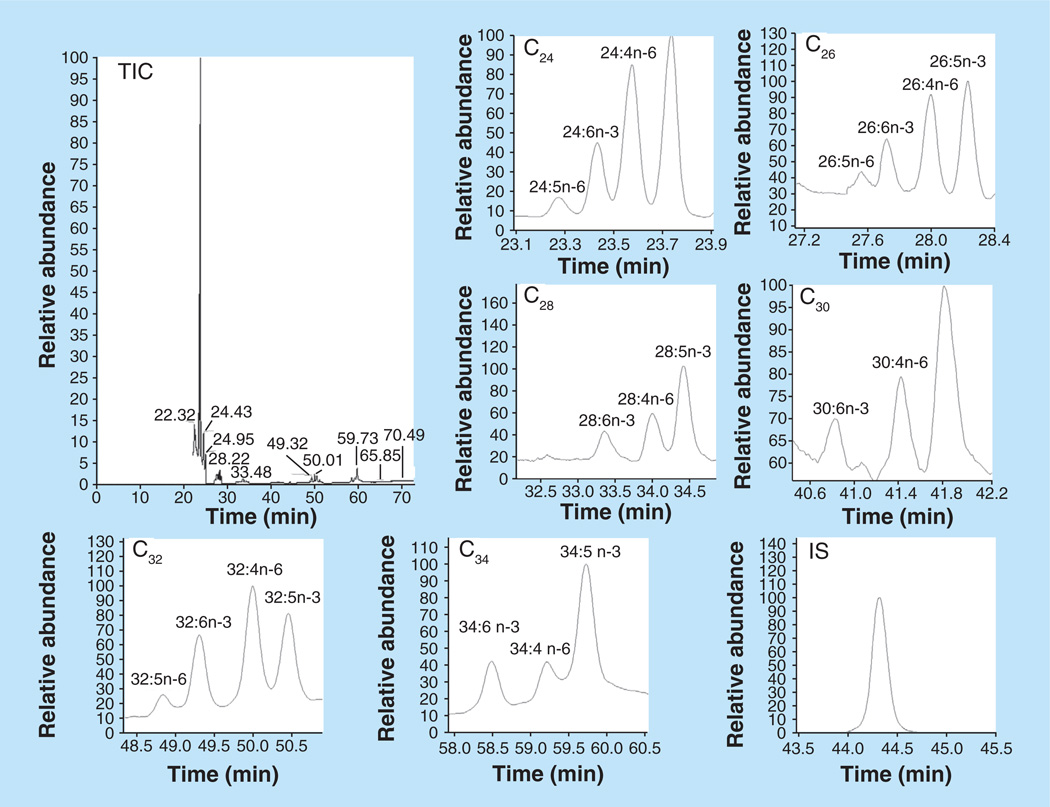
Our laboratory has studied VLCPUFAs in human retina and RPE/choroid (Figure 10 & Table 3) [10]. The latter contained much lower concentrations of VLCPUFAs, which may be due to the fact that RPE cells phagocytize approximately 10% of the photoreceptor outer segment material daily [115]. Unlike LCPUFAs such as DHA, AA, EPA and their precursors, VLC-PUFAs are not present in a normal human diet [116], but must be synthesized from precursors such as 22:4n-6 and 22:5n-3 via a biochemical pathway (Figure 6) featuring the enzymes of the elongation of very LCFAs family along with β-oxidases and desaturases [10,54,55,117,118]. We also have compared the DHA and VLCPUFA concentrations in retinas from AMD and age-matched normal donors and found that both DHA and VLCPUFA levels in AMD retina were significantly lower than in normal donors [10], which is consistent with macular degeneration mouse (TG2) retina [61] and STGD3 mouse retina [11]. This suggests that abnormalities in VLCPUFA levels related to dietary intake of precursors and/or defects in metabolic enzymes may be involved in the pathogenesis of AMD.
Figure 10. Selected ion monitor gas chromatography–mass spectrometry chromatograms of C24–C34 very-long-chain polyunsaturated fatty acids from human retina.
IS: Internal standard (hentriacontanoic acid); TIC: Total ion chromatogram. Reproduced with permission from [10] © The American Society for Biochemistry and Molecular Biology.
Table 3.
Very-long-chain polyunsaturated fatty acid compositions from human retina.
| Peak no. | Fatty acids† | Classification‡ |
|---|---|---|
| 1 | 24:5n-6 | VLCPUFA |
| 2 | 24:6n-3 | VLCPUFA |
| 3 | 24:4n-6 | VLCPUFA |
| 4 | 24:5n-3 | VLCPUFA |
| 5 | 26:5n-6 | VLCPUFA |
| 6 | 26:6n-3 | VLCPUFA |
| 7 | 26:4n-6 | VLCPUFA |
| 8 | 26:5n-3 | VLCPUFA |
| 9 | 28:6n-3 | VLCPUFA |
| 10 | 28:4n-6 | VLCPUFA |
| 11 | 28:5n-3 | VLCPUFA |
| 12 | 30:6n-3 | VLCPUFA |
| 13 | 30:4n-6 | VLCPUFA |
| 14 | 30:5n-3 | VLCPUFA |
| 15 | 32:5n-6 | VLCPUFA |
| 16 | 32:6n-3 | VLCPUFA |
| 17 | 32:4n-6 | VLCPUFA |
| 18 | 32:5n-3 | VLCPUFA |
| 19 | 34:6n-3 | VLCPUFA |
| 20 | 34:4n-6 | VLCPUFA |
| 21 | 34:5n-3 | VLCPUFA |
Number of carbon atoms: number of double bonds: position of double bonds, the position of first double bond. These fatty acids’ peaks are shown in chromatograms of Figure 10.
Classification of fatty acids.
VLCPUFA: Very-long-chain polyunsaturated fatty acid.
Conclusion
In this article, we systematically summarized the roles and possible mechanisms of LCPUFAs and VLCPUFAs in AMD and STGD3. Many epidemiologic studies have demonstrated an inverse association between diets high in ω-3 LCPUFAs such as DHA, EPA and fish (DHA+EPA) and the risk of AMD, which may be explained to some extent by their oxidative metabolisms (such as NPD1, RvD1–6 and RvE1) and their anti-inflammatory activity. While AA, the major ω-6 LCPUFAs in retina, demonstrated protection against neither AMD nor STGD3, it was directly associated with NV-AMD prevalence. This may be attribitable to its potent proinflammatory metabolisms such as PGs, TXs and LTs. Mutations of the ELOVL4 gene which elongates shorter FAs into VLCPUFAs causes STGD3. A deficiency of retinal VLCPUFAs is found in STGD3 and AMD and is thought to play a crucial role in its progression, but performance of definitive clinical and animal studies to cure this condition through direct supplementation of VLCPUFAs remains unfeasible, due to the lack of sufficient quantities of VLCPUFAs. Therefore, the role of dietary alteration of retinal VLCPUFAs in AMD and STGD3 to halt or reverse disease progression remains unclear. Additional basic science and clinical studies should clarify this question in the future.
Future perspective
For the study of the roles of LCPUFAs and VLCPUFAs in macular degenerations and dystrophies, several aspects could be considered in the future.
In interventional studies of LCPUFAs in mouse models of STGD3 and in clinical studies of humans with AMD and STGD3, the source should be fish oil with balanced levels of both EPA and DHA because supplements with DHA only may not be efficient precursors for VLCPUFAs. If sufficient quantities of pure EPA become available, a direct comparison of DHA versus EPA would be of interest.
Since VLCPUFAs are absent in a normal human diet, chemical synthesis of sufficient quantities of relevant VLCPUFAs would be of great value for supplement studies.
Besides oral administration of FAs, intravitreal and subretinal injection into the eye may be a better alternative of administration since it avoids intestinal absorption, the enteroheptic cycle and the blood–ocular barrier, all of which can prevent the accumulation of FAs in retina after oral administration.
Docosahexaenoic acid cannot be distinctly increased in mouse feeding studies, while rats consuming a DHA rich diet can accumulate more retinal DHA relative to control rats [30]. Thus, the rat model with macular degeneration could be more proper for feeding studies.
A decreased ratio of mutant to normal ELOVL4 protein may be a useful therapeutic strategy for treating STGD3 disease. Increasing normal ELOVL4 protein by delivering normal ELOVL4 cDNA packaged as a proper vector through subretinal injection, or decreasing mutant ELOVL4 protein by ribozyme knockdown of mutant RNA, may be feasible and attractive therapeutic strategies for the treatment of STGD3.
A deficiency of VLCPUFAs has been detected in mouse models of macular degeneration, but it is unclear whether the deficiency or the degeneration happens first. Study of LCPUFAs and VLCPUFAs in transgenic mice before retina degeneration begins, may unravel this mystery and generate new insights to help improve the dietary treatment of macular degenerations and dystrophies.
Executive summary.
Background
-
▪
Macula is anatomically divided into foveola, fovea, parafoveal area and perifoveal area.
-
▪
Age-related macular degeneration (AMD), the most common type of macular degeneration, can be further subdivided into dry and wet classifications. Stargardt disease (STGD3), with similar clinical symptoms to AMD, is an early onset, autosomal dominant macular degeneration.
The role of major ω-3 long-chain polyunsaturated fatty acid (docosahexaenoic acid & eicosapentaenoic acid) in macular degeneration
-
▪
Most epidemiological and animal studies showed a inverse relationship between dietary intake of docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) or fish, and prevalence of AMD. Both an increase in dietary intake and frequency of dietary intake resulted in a lowered risk of AMD progression. DHA is detected at a lower concentration in AMD retina and retinal pigment epithelium (RPE) compared with normal retina and RPE.
-
▪
The metabolites of DHA and EPA can explain the beneficial effects of ω-3 long-chain polyunsaturated fatty acid (LCPUFAs) to some extent.
-
▪
After one study demonstrated that the severity of STGD3 can be alleviated by dietary supplementation of DHA, the National Eye Institute and John A. Moran Eye Center have initiated clinical trials of DHA or fish oil supplementation in STGD3 patients.
-
▪
DHA was lower in AMD retina and RPE compared with normal patient. Consistently, TG2 mouse retinas contained lower DHA than control mouse retinas.
The role of major ω-6 LCPUFAs in macular degeneration
-
▪
Arachidonic acid (AA) and most of its metabolites are closely related with inflammation, nerve cell apoptosis and oxidation, all of which are instigators of macular degeneration.
The role of very-long-chain polyunsaturated fatty acids & ELOVL4 in STGD3
-
▪
ELOVL4 is a fatty acyl elongase that participates in the biosynthesis of C28–C38 VLCFAs, both saturated and unsaturated. ELOVL4 exists in restricted organs such as retina, brain, testes and skin.
-
▪
STGD3 is caused by the mutation in the ELOVL4 gene including 5-bp deletion on exon 6, two 1-bp deletions (789delT and 794delT), and a 270 stop mutation.
-
▪
Many STGD3 mouse models with ELOVL4 gene mutations, such as TG1, TG2, TG3 and heterozygous/homozygous knock-in/knockout mice, were produced. Very-long-chain polyunsaturated fatty acids (VLCPUFAs) are deficient in STGD3 transgenic mouse retina, which indicates an important role of VLCPUFAs in retinal function.
-
▪
The ELOVL4 mutation can cause the ELOVL4 protein to be mislocalized from the endoplasmic reticulum where fatty acids are synthesized to other compartments in an aggregated pattern with the cytoplasm, which may be the mechanism of action of the ELOVL4 mutation in STGD3.
The role of VLCPUFAs in AMD
-
▪
The correlation between the Met299Val variant in the ELOVL4 gene and AMD needs further study.
-
▪
VLCPUFAs are detected to be lower in concentration in the retina and RPE of AMD patients compared with age-matched normal patients.
Future perspective
-
▪
Compared with pure DHA, fish oil or even pure EPA are better interventional study alternatives for use in clinical studies of human and mouse models with macular degeneration. The chemical synthesis of sufficient VLCPUFAs would be of great value to supplement studies. Intravitreal and subretinal injection can be better alternatives to oral administration.
-
▪
Developing a decreased ratio of mutant to normal ELOVL4 protein may be a useful therapeutic strategy for the treatment of STGD3 disease.
Acknowledgments
This work was supported by grants from the Steinbach Foundation, the Macula Vision Research Foundation, the Foundation Fighting Blindness, and a departmental grant from Research to Prevent Blindness.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1. Bernstein PS. Macular biology. In: Berger JWF, SL, Maguire MG, editors. Age-related Macular Degeneration. Vol. 1. Mosby, Inc., MO, USA; 1999. ▪▪ Comprehensively introduces age-related macular degeneration (AMD).
- 2.Hampton GR, Nelsen PT. Principles and Practice. Age Related Macular Degeneration. Raven Press, NY, USA; 1992. [Google Scholar]
- 3.Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J. Clin. Nutr. 1995;62(Suppl. 6):S1448–S1461. doi: 10.1093/ajcn/62.6.1448S. [DOI] [PubMed] [Google Scholar]
- 4.Beatty S, Boulton M, Henson D, Koh HH, Murray IJ. Macular pigment and age related macular degeneration. Br. J. Ophthalmol. 1999;83(7):867–877. doi: 10.1136/bjo.83.7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poulos A, Sharp P, Johnson D, White I, Fellenberg A. The occurrence of polyenoic fatty acids with greater than 22 carbon atoms in mammalian spermatozoa. Biochem. J. 1986;240(3):891–895. doi: 10.1042/bj2400891. ▪▪ First paper to define very-long-chain polyunsaturated fatty acids (VLCPUFAs).
- 6.Vasireddy V, Wong P, Ayyagari R. Genetics and molecular pathology of Stargardt-like macular degeneration. Prog. Retin. Eye Res. 2010;29(3):191–207. doi: 10.1016/j.preteyeres.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zack DJ, Dean M, Molday RS, et al. What can we learn about age-related macular degeneration from other retinal diseases? Mol. Vis. 1999;5:30. [PubMed] [Google Scholar]
- 8. Hubbard AF, Askew EW, Singh N, Leppert M, Bernstein PS. Association of adipose and red blood cell lipids with severity of dominant Stargardt macular dystrophy (STGD3) secondary to an ELOVL4 mutation. Arch. Ophthalmol. 2006;124(2):257–263. doi: 10.1001/archopht.124.2.257. ▪▪ Introduces in detail the association of adipose and red blood cell lipids with severity of autosomal dominant Stargardt disease-3 (STGD3).
- 9.Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 1983;22(2):79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 10. Liu A, Chang J, Lin Y, Shen Z, Bernstein PS. Long-chain and very long-chain polyunsaturated fatty acids in ocular aging and age-related macular degeneration. J. Lipid Res. 2010;51(11):3217–3229. doi: 10.1194/jlr.M007518. ▪▪ Shows that long-chain polyunsaturated fatty acids (LCPUFAs) and VLCPUFAs were lower in AMD retina and retinal pigment epithelium than normal patients.
- 11. McMahon A, Jackson SN, Woods AS, Kedzierski W. A Stargardt disease-3 mutation in the mouse ELOVL4 gene causes retinal deficiency of C32–C36 acyl phosphatidylcholines. FEBS Lett. 2007;581(28):5459–5463. doi: 10.1016/j.febslet.2007.10.050. ▪▪ Shows that C32–C36 acyl phosphatidylcholines were deficient in STGD3 mice retinas.
- 12.Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch. Ophthalmol. 2003;121(12):1728–1737. doi: 10.1001/archopht.121.12.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chua B, Flood V, Rochtchina E, Wang JJ, Smith W, Mitchell P. Dietary fatty acids and the 5-year incidence of age-related maculopathy. Arch. Ophthalmol. 2006;124(7):981–986. doi: 10.1001/archopht.124.7.981. [DOI] [PubMed] [Google Scholar]
- 14.Tan JS, Wang JJ, Flood V, Mitchell P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch. Ophthalmol. 2009;127(5):656–665. doi: 10.1001/archophthalmol.2009.76. [DOI] [PubMed] [Google Scholar]
- 15.Augood C, Chakravarthy U, Young I, et al. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am. J. Clin. Nutr. 2008;88(2):398–406. doi: 10.1093/ajcn/88.2.398. [DOI] [PubMed] [Google Scholar]
- 16.Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, ω-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch. Ophthalmol. 2006;124(7):995–1001. doi: 10.1001/archopht.124.7.995. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology. 1995;102(10):1450–1460. doi: 10.1016/s0161-6420(95)30846-9. [DOI] [PubMed] [Google Scholar]
- 18.Smith W, Mitchell P, Leeder SR. Dietary fat and fish intake and age-related maculopathy. Arch. Ophthalmol. 2000;118(3):401–404. doi: 10.1001/archopht.118.3.401. [DOI] [PubMed] [Google Scholar]
- 19.SanGiovanni JP, Chew EY, Clemons TE, et al. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch. Ophthalmol. 2007;125(5):671–679. doi: 10.1001/archopht.125.5.671. [DOI] [PubMed] [Google Scholar]
- 20.SanGiovanni JP, Chew EY, Agron E, et al. The relationship of dietary ω-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS Report No. 23. Arch. Ophthalmol. 2008;126(9):1274–1279. doi: 10.1001/archopht.126.9.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho E, Hung S, Willett WC, et al. Prospective study of dietary fat and the risk of age-related macular degeneration. Am. J. Clin. Nutr. 2001;73(2):209–218. doi: 10.1093/ajcn/73.2.209. [DOI] [PubMed] [Google Scholar]
- 22.Seddon JM, Rosner B, Sperduto RD, et al. Dietary fat and risk for advanced age-related macular degeneration. Arch. Ophthalmol. 2001;119(8):1191–1199. doi: 10.1001/archopht.119.8.1191. [DOI] [PubMed] [Google Scholar]
- 23. Sangiovanni JP, Agron E, Meleth AD, et al. ω-3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS Report No 30, a prospective cohort study from the Age-Related Eye Disease Study. Am. J. Clin. Nutr. 2009;90(6):1601–1607. doi: 10.3945/ajcn.2009.27594. ▪ A 12-year epidemiology study showing that ω-3 LCPUFAs can protect against AMD.
- 24.SanGiovanni JP, Chew EY, Clemons TE, et al. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case–control study: AREDS Report No. 22. Arch. Ophthalmol. 2007;125(9):1225–1232. doi: 10.1001/archopht.125.9.1225. [DOI] [PubMed] [Google Scholar]
- 25.Mares-Perlman JA, Brady WE, Klein R, Vanden Langenberg GM, Klein BE, Palta M. Dietary fat and age-related maculopathy. Arch. Ophthalmol. 1995;113(6):743–748. doi: 10.1001/archopht.1995.01100060069034. [DOI] [PubMed] [Google Scholar]
- 26.Sanders TA, Haines AP, Wormald R, Wright LA, Obeid O. Essential fatty acids, plasma cholesterol, and fat-soluble vitamins in subjects with age-related maculopathy and matched control subjects. Am. J. Clin. Nutr. 1993;57(3):428–433. doi: 10.1093/ajcn/57.3.428. [DOI] [PubMed] [Google Scholar]
- 27.Ouchi M, Ikeda T, Nakamura K, Harino S, Kinoshita S. A novel relation of fatty acid with age-related macular degeneration. Ophthalmologica. 2002;216(5):363–367. doi: 10.1159/000066178. [DOI] [PubMed] [Google Scholar]
- 28.Koto T, Nagai N, Mochimaru H, et al. Eicosapentaenoic acid is anti-inflammatory in preventing choroidal neovascularization in mice. Invest. Ophthalmol. Vis. Sci. 2007;48(9):4328–4334. doi: 10.1167/iovs.06-1148. [DOI] [PubMed] [Google Scholar]
- 29.Tuo J, Ross RJ, Herzlich AA, et al. A high ω-3 fatty acid diet reduces retinal lesions in a murine model of macular degeneration. Am. J. Pathol. 2009;175(2):799–807. doi: 10.2353/ajpath.2009.090089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizota A, Sato E, Taniai M, Adachi-Usami E, Nishikawa M. Protective effects of dietary docosahexaenoic acid against kainate-induced retinal degeneration in rats. Invest. Ophthalmol. Vis. Sci. 2001;42(1):216–221. [PubMed] [Google Scholar]
- 31.Orr SK, Bazinet RP. The emerging role of docosahexaenoic acid in neuroinflammation. Curr. Opin. Invest. Drugs. 2008;9(7):735–743. [PubMed] [Google Scholar]
- 32.Organisciak DT, Darrow RM, Jiang YL, Blanks JC. Retinal light damage in rats with altered levels of rod outer segment docosahexaenoate. Invest. Ophthalmol. Vis. Sci. 1996;37(11):2243–2257. [PubMed] [Google Scholar]
- 33. Bazan NG. Homeostatic regulation of photoreceptor cell integrity: significance of the potent mediator neuroprotectin D1 biosynthesized from docosahexaenoic acid: the Proctor Lecture. Invest. Ophthalmol. Vis. Sci. 2007;48(11):4866–4881. doi: 10.1167/iovs.07-0918. Biography 4–5. ▪ Along with [35], these papers demonstrated the oxidization pathway of docosahexaenoic acid (DHA).
- 34.Bazan NG, Marcheselli VL, Cole-Edwards K. Brain response to injury and neurodegeneration: endogenous neuroprotective signaling. Ann. NY Acad. Sci. 2005;1053:137–147. doi: 10.1196/annals.1344.011. [DOI] [PubMed] [Google Scholar]
- 35. Serhan CN. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes and neuroprotectins. Curr. Opin. Clin. Nutr. Metab. Care. 2005;8(2):115–121. doi: 10.1097/00075197-200503000-00003. ▪ Along with [33], these papers demonstrated the oxidization pathway of DHA.
- 36.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl Acad. Sci. USA. 2004;101(22):8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bazan NG. ω-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10(2):136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- 38.Lukiw WJ, Cui JG, Marcheselli VL, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J. Clin. Invest. 2005;115(10):2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bazan NG. Lipid signaling in neural plasticity, brain repair, and neuroprotection. Mol. Neurobiol. 2005;32(1):89–103. doi: 10.1385/MN:32:1:089. [DOI] [PubMed] [Google Scholar]
- 40.Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc. Natl Acad. Sci. USA. 2007;104(32):13152–13157. doi: 10.1073/pnas.0705949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serhan CN, Arita M, Hong S, Gotlinger K. Resolvins, docosatrienes, and neuroprotectins, novel ω-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids. 2004;39(11):1125–1132. doi: 10.1007/s11745-004-1339-7. [DOI] [PubMed] [Google Scholar]
- 42.Tian H, Lu Y, Sherwood AM, Hongqian D, Hong S. Resolvins E1 and D1 in choroid-retinal endothelial cells and leukocytes: biosynthesis and mechanisms of anti-inflammatory actions. Invest. Ophthalmol. Vis. Sci. 2009;50(8):3613–3620. doi: 10.1167/iovs.08-3146. [DOI] [PubMed] [Google Scholar]
- 43. Li F, Cao W, Anderson RE. Protection of photoreceptor cells in adult rats from light-induced degeneration by adaptation to bright cyclic light. Exp. Eye Res. 2001;73(4):569–577. doi: 10.1006/exer.2001.1068. ▪ DHA loss was found in adult rats from light-induced degeneration.
- 44.Crabb JW, Miyagi M, Gu X, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc. Natl Acad. Sci. USA. 2002;99(23):14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gu X, Meer SG, Miyagi M, et al. Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J. Biol. Chem. 2003;278(43):42027–42035. doi: 10.1074/jbc.M305460200. ▪ Carboxyethyl pyrrole can be used as a biomarker for AMD.
- 46.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of ω-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002;196(8):1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the ω-3 lipid mediator resolvin E1. J. Exp. Med. 2005;201(5):713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. SanGiovanni JP, Chew EY. The role of ω-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog. Retin. Eye Res. 2005;24(1):87–138. doi: 10.1016/j.preteyeres.2004.06.002. ▪ Comprehensively summarizes information on the putative actions of ω-3 LCPUFAs in different causes of AMD.
- 49.Song WL, Lawson JA, Reilly D, et al. Neurofurans, novel indices of oxidant stress derived from docosahexaenoic acid. J. Biol. Chem. 2008;283(1):6–16. doi: 10.1074/jbc.M706124200. [DOI] [PubMed] [Google Scholar]
- 50.Long EK, Murphy TC, Leiphon LJ, et al. Trans-4-hydroxy-2-hexenal is a neurotoxic product of docosahexaenoic (22:6; n-3) acid oxidation. J. Neurochem. 2008;105(3):714–724. doi: 10.1111/j.1471-4159.2007.05175.x. [DOI] [PubMed] [Google Scholar]
- 51.Choudhary S, Xiao T, Srivastava S, et al. Toxicity and detoxification of lipid-derived aldehydes in cultured retinal pigmented epithelial cells. Toxicol. Appl. Pharmacol. 2005;204(2):122–134. doi: 10.1016/j.taap.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 52.Ishikado A, Nishio Y, Morino K, et al. Low concentration of 4-hydroxy hexenal increases heme oxygenase-1 expression through activation of Nrf2 and antioxidative activity in vascular endothelial cells. Biochem. Biophys. Res. Comm. 2010;402(1):99–104. doi: 10.1016/j.bbrc.2010.09.124. [DOI] [PubMed] [Google Scholar]
- 53.Musiek ES, Brooks JD, Joo M, et al. Electrophilic cyclopentenone neuroprostanes are anti-inflammatory mediators formed from the peroxidation of the ω-3 polyunsaturated fatty acid docosahexaenoic acid. J. Biol. Chem. 2008;283(29):19927–19935. doi: 10.1074/jbc.M803625200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Agbaga MP, Brush RS, Mandal MN, Henry K, Elliott MH, Anderson RE. Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids. Proc. Natl Acad. Sci. USA. 2008;105(35):12843–12848. doi: 10.1073/pnas.0802607105. ▪▪ Proved that ELOVL4 protein is involved in fatty acid elongation for a series of C28–C38 fatty acids.
- 55.Agbaga MP, Brush RS, Mandal MN, Elliott MH, Al-Ubaidi MR, Anderson RE. Role of ELOVL4 protein in the biosynthesis of docosahexaenoic acid. Adv. Exp. Med. Biol. 2010;664:233–242. doi: 10.1007/978-1-4419-1399-9_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roqueta-Rivera M, Stroud CK, Haschek WM, et al. Docosahexaenoic acid supplementation fully restores fertility and spermatogenesis in male Δ-6 desaturase-null mice. J. Lipid Res. 2010;51(2):360–367. doi: 10.1194/jlr.M001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. MacDonald IM, Hebert M, Yau RJ, et al. Effect of docosahexaenoic acid supplementation on retinal function in a patient with autosomal dominant Stargardt-like retinal dystrophy. Br. J. Ophthalmol. 2004;88(2):305–306. doi: 10.1136/bjo.2003.024299. ▪ DHA supplementation can alleviate the the severity of STDG3.
- 58.Nanda BL, Nataraju A, Rajesh R, Rangappa KS, Shekar MA, Vishwanath BS. PLA2 mediated arachidonate free radicals: PLA2 inhibition and neutralization of free radicals by anti-oxidants–a new role as anti-inflammatory molecule. Curr. Top. Med. Chem. 2007;7(8):765–777. doi: 10.2174/156802607780487623. [DOI] [PubMed] [Google Scholar]
- 59.Li F, Marchette LD, Brush RS, et al. DHA does not protect ELOVL4 transgenic mice from retinal degeneration. Mol. Vis. 2009;15:1185–1193. [PMC free article] [PubMed] [Google Scholar]
- 60.Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427(6974):504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- 61. Barabas P, Liu A, Xing W, et al. Conditional ablation of retinal ELOVL4 reveals a key role in synthesis of VLC-PUFAs and photoreceptor light responses. Presented at: The Association for Research in Vision and Ophthalmology 2011; 1–15 May 2011; Fort Lauderdale, FL, USA. ▪▪ DHA and VLCPUFAs were lower in macular degeneration mice models.
- 62.Okuda A, Naganuma T, Ohno Y, et al. Hetero-oligomeric interactions of an ELOVL4 mutant protein: implications in the molecular mechanism of Stargardt-3 macular dystrophy. Mol. Vis. 2010;16:2438–2445. [PMC free article] [PubMed] [Google Scholar]
- 63.Bazan NG, Scott BL, Reddy TS, Pelias MZ. Decreased content of docosahexaenoate and arachidonate in plasma phospholipids in Usher’s syndrome. Biochem. Biophys. Res. Comm. 1986;141(2):600–604. doi: 10.1016/s0006-291x(86)80215-7. [DOI] [PubMed] [Google Scholar]
- 64.Williams JH, Errington ML, Lynch MA, Bliss TV. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature. 1989;341(6244):739–742. doi: 10.1038/341739a0. [DOI] [PubMed] [Google Scholar]
- 65.Okuda S, Saito H, Katsuki H. Arachidonic acid: toxic and trophic effects on cultured hippocampal neurons. Neuroscience. 1994;63(3):691–699. doi: 10.1016/0306-4522(94)90515-0. [DOI] [PubMed] [Google Scholar]
- 66.Bazan NG., Jr Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochim. Biophys. Acta. 1970;218(1):1–10. doi: 10.1016/0005-2760(70)90086-x. [DOI] [PubMed] [Google Scholar]
- 67.Keyser DO, Alger BE. Arachidonic acid modulates hippocampal calcium current via protein kinase C and oxygen radicals. Neuron. 1990;5(4):545–553. doi: 10.1016/0896-6273(90)90092-t. [DOI] [PubMed] [Google Scholar]
- 68.Fraser DD, Hoehn K, Weiss S, MacVicar BA. Arachidonic acid inhibits sodium currents and synaptic transmission in cultured striatal neurons. Neuron. 1993;11(4):633–644. doi: 10.1016/0896-6273(93)90075-3. [DOI] [PubMed] [Google Scholar]
- 69.Chan PH, Fishman RA. Transient formation of superoxide radicals in polyunsaturated fatty acid-induced brain swelling. J. Neurochem. 1980;35(4):1004–1007. doi: 10.1111/j.1471-4159.1980.tb07100.x. [DOI] [PubMed] [Google Scholar]
- 70.Volterra A, Trotti D, Cassutti P, et al. High sensitivity of glutamate uptake to extracellular free arachidonic acid levels in rat cortical synaptosomes and astrocytes. J. Neurochem. 1992;59(2):600–606. doi: 10.1111/j.1471-4159.1992.tb09411.x. [DOI] [PubMed] [Google Scholar]
- 71.Miller B, Sarantis M, Traynelis SF, Attwell D. Potentiation of NMDA receptor currents by arachidonic acid. Nature. 1992;355(6362):722–725. doi: 10.1038/355722a0. [DOI] [PubMed] [Google Scholar]
- 72.Simonian NA, Coyle JT. Oxidative stress in neurodegenerative diseases. Ann. Rev. Pharmacol. Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- 73.Reme CE, Grimm C, Hafezi F, Marti A, Wenzel A. Apoptotic cell death in retinal degenerations. Prog. Retin. Eye Res. 1998;17(4):443–464. doi: 10.1016/s1350-9462(98)00009-3. [DOI] [PubMed] [Google Scholar]
- 74.Tang DG, Chen YQ, Honn KV. Arachidonate lipoxygenases as essential regulators of cell survival and apoptosis. Proc. Natl Acad. Sci. USA. 1996;93(11):5241–5246. doi: 10.1073/pnas.93.11.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hafezi F, Steinbach JP, Marti A, et al. The absence of c-fos prevents light-induced apoptotic cell death of photoreceptors in retinal degeneration in vivo. Nat. Med. 1997;3(3):346–349. doi: 10.1038/nm0397-346. [DOI] [PubMed] [Google Scholar]
- 76.Scholl S, Kirchhof J, Augustin AJ. Pathophysiology of macular edema. Ophthalmologica. 2010;224(Suppl. 1):8–15. doi: 10.1159/000315155. [DOI] [PubMed] [Google Scholar]
- 77.Reichenbach A, Wurm A, Pannicke T, Iandiev I, Wiedemann P, Bringmann A. Muller cells as players in retinal degeneration and edema. Graefes. Arch. Clin. Exp. Ophthalmol. 2007;245(5):627–636. doi: 10.1007/s00417-006-0516-y. [DOI] [PubMed] [Google Scholar]
- 78.Grimm C, Wenzel A, Hafezi F, Yu S, Redmond TM, Reme CE. Protection of Rpe65-deficient mice identifies rhodopsin as a mediator of light-induced retinal degeneration. Nat. Genet. 2000;25(1):63–66. doi: 10.1038/75614. [DOI] [PubMed] [Google Scholar]
- 79.Hanna N, Peri KG, Abran D, et al. Light induces peroxidation in retina by activating prostaglandin G/H synthase. Free Radic. Biol. Med. 1997;23(6):885–897. doi: 10.1016/s0891-5849(97)00083-x. [DOI] [PubMed] [Google Scholar]
- 80.Topol EJ, Smith J, Plow EF, Wang QK. Genetic susceptibility to myocardial infarction and coronary artery disease. Hum. Mol. Genet. 2006;15(Spec. No 2):R117–R123. doi: 10.1093/hmg/ddl183. [DOI] [PubMed] [Google Scholar]
- 81.Blom HM, Van Rijswijk JB, Garrelds IM, Mulder PG, Timmermans T, Gerth van Wijk R. Intranasal capsaicin is efficacious in non-allergic, non-infectious perennial rhinitis. A placebo-controlled study. Clin. Exp. Allergy. 1997;27(7):796–801. doi: 10.1046/j.1365-2222.1997.670842.x. [DOI] [PubMed] [Google Scholar]
- 82.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 1999;5(6):698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 83.Ladewig MS, Ladewig K, Guner M, Heidrich H. Prostaglandin E1 infusion therapy in dry age-related macular degeneration. Prostaglandins Leukot. Essent. Fatty Acids. 2005;72(4):251–256. doi: 10.1016/j.plefa.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 84.Manev H, Uz T, Qu T. Early upregulation of hippocampal 5-lipoxygenase following systemic administration of kainate to rats. Restor. Neurol. Neurosci. 1998;12(2–3):81–85. [PubMed] [Google Scholar]
- 85.Miyata T, Inagi R, Asahi K, et al. Generation of protein carbonyls by glycoxidation and lipoxidation reactions with autoxidation products of ascorbic acid and polyunsaturated fatty acids. FEBS Lett. 1998;437(1–2):24–28. doi: 10.1016/s0014-5793(98)01079-5. [DOI] [PubMed] [Google Scholar]
- 86.Poulos A, Sharp P, Singh H, Johnson D, Fellenberg A, Pollard A. Detection of a homologous series of C26–C38 polyenoic fatty acids in the brain of patients without peroxisomes (Zellweger’s syndrome) Biochem. J. 1986;235(2):607–610. doi: 10.1042/bj2350607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Aveldano MI. A novel group of very long chain polyenoic fatty acids in dipolyunsaturated phosphatidylcholines from vertebrate retina. J. Biol. Chem. 1987;262(3):1172–1179. ▪ First paper to detect VLCPUFAs in the retina.
- 88.Aveldano MI. Long and very long polyunsaturated fatty acids of retina and spermatozoa: the whole complement of polyenoic fatty acid series. Adv. Exp. Med. Biol. 1992;318:231–242. doi: 10.1007/978-1-4615-3426-6_19. [DOI] [PubMed] [Google Scholar]
- 89.McMahon A, Butovich IA, Mata NL, et al. Retinal pathology and skin barrier defect in mice carrying a Stargardt disease-3 mutation in elongase of very long chain fatty acids-4. Mol. Vis. 2007;13:258–272. [PMC free article] [PubMed] [Google Scholar]
- 90.Cameron DJ, Tong Z, Yang Z, et al. Essential role of ELOVL4 in very long chain fatty acid synthesis, skin permeability barrier function, and neonatal survival. Int. J. Biol. Sci. 2007;3(2):111–119. doi: 10.7150/ijbs.3.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vasireddy V, Uchida Y, Salem N, Jr, et al. Loss of functional ELOVL4 depletes very long-chain fatty acids (>or = C28) and the unique ω-O-acylceramides in skin leading to neonatal death. Hum. Mol. Genet. 2007;16(5):471–482. doi: 10.1093/hmg/ddl480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Monroig O, Rotllant J, Cerda-Reverter JM, Dick JR, Figueras A, Tocher DR. Expression and role of ELOVL4 elongases in biosynthesis of very long-chain fatty acids during zebrafish Danio rerio early embryonic development. Biochim. Biophys. Acta. 2010;1801(10):1145–1154. doi: 10.1016/j.bbalip.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 93.Zhang XM, Yang Z, Karan G, et al. ELOVL4 mRNA distribution in the developing mouse retina and phylogenetic conservation of ELOVL4 genes. Mol. Vis. 2003;9:301–307. [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang K, Kniazeva M, Han M, et al. A 5-bp deletion in ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. Nat. Genet. 2001;27(1):89–93. doi: 10.1038/83817. ▪▪ Demonstration that mutation of ELOVL4 is related to STGD3.
- 95.Edwards AO, Donoso LA, Ritter R., 3rd A novel gene for autosomal dominant Stargardt-like macular dystrophy with homology to the SUR4 protein family. Invest. Ophthalmol. Vis. Sci. 2001;42(11):2652–2663. [PubMed] [Google Scholar]
- 96.Vrabec TR, Tantri A, Edwards A, Frost A, Donoso LA. Autosomal dominant Stargardt-like macular dystrophy: identification of a new family with a mutation in the ELOVL4 gene. Am. J. Ophthalmol. 2003;136(3):542–545. doi: 10.1016/s0002-9394(03)00227-7. [DOI] [PubMed] [Google Scholar]
- 97. Bernstein PS, Tammur J, Singh N, et al. Diverse macular dystrophy phenotype caused by a novel complex mutation in the ELOVL4 gene. Invest. Ophthalmol. Vis. Sci. 2001;42(13):3331–3336. ▪ Demonstrates diverse macular dystrophy phenotypes are caused by a novel complex mutation in the ELOVL4 gene.
- 98.Maugeri A, Meire F, Hoyng CB, et al. A novel mutation in the ELOVL4 gene causes autosomal dominant Stargardt-like macular dystrophy. Invest. Ophthalmol. Vis. Sci. 2004;45(12):4263–4267. doi: 10.1167/iovs.04-0078. [DOI] [PubMed] [Google Scholar]
- 99.Lai Z, Zhang XN, Zhou W, Yu R, Le YP. Evaluation of the ELOVL4 gene in a Chinese family with autosomal dominant STGD3-like macular dystrophy. J. Cell. Mol. Med. 2005;9(4):961–965. doi: 10.1111/j.1582-4934.2005.tb00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ambati J, Anand A, Fernandez S, et al. An animal model of age-related macular degeneration in senescent Ccl-2-or Ccr-2-deficient mice. Nat. Med. 2003;9(11):1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 101.Marmorstein AD, Marmorstein LY. The challenge of modeling macular degeneration in mice. Trends Genet. 2007;23(5):225–231. doi: 10.1016/j.tig.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 102. Karan G, Lillo C, Yang Z, et al. Lipofuscin accumulation, abnormal electrophysiology, and photoreceptor degeneration in mutant ELOVL4 transgenic mice: a model for macular degeneration. Proc. Natl Acad. Sci. USA. 2005;102(11):4164–4169. doi: 10.1073/pnas.0407698102. ▪ The mutant ELOVL4-trangenic mouse is a successful macular degeneration model.
- 103.Vasireddy V, Jablonski MM, Mandal MN, et al. ELOVL4 5-bp-deletion knock-in mice develop progressive photoreceptor degeneration. Invest. Ophthalmol. Vis. Sci. 2006;47(10):4558–4568. doi: 10.1167/iovs.06-0353. [DOI] [PubMed] [Google Scholar]
- 104.Li W, Sandhoff R, Kono M, et al. Depletion of ceramides with very long chain fatty acids causes defective skin permeability barrier function, and neonatal lethality in ELOVL4 deficient mice. Int. J. Biol. Sci. 2007;3(2):120–128. doi: 10.7150/ijbs.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Uchida Y, Holleran WM. ω-O-acylceramide, a lipid essential for mammalian survival. J. Dermatol. Sci. 2008;51(2):77–87. doi: 10.1016/j.jdermsci.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 106.McMahon A, Butovich IA, Kedzierski W. Epidermal expression of an ELOVL4 transgene rescues neonatal lethality of homozygous Stargardt disease-3 mice. J. Lipid Res. 2011;52(6):1128–1138. doi: 10.1194/jlr.M014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Raz-Prag D, Ayyagari R, Fariss RN, et al. Haploinsufficiency is not the key mechanism of pathogenesis in a heterozygous ELOVL4 knockout mouse model of STGD3 disease. Invest. Ophthalmol. Vis. Sci. 2006;47(8):3603–3611. doi: 10.1167/iovs.05-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li W, Chen Y, Cameron DJ, et al. ELOVL4 haploinsufficiency does not induce early onset retinal degeneration in mice. Vision Res. 2007;47(5):714–722. doi: 10.1016/j.visres.2006.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lewin AS, Drenser KA, Hauswirth WW, et al. Ribozyme rescue of photoreceptor cells in a transgenic rat model of autosomal dominant retinitis pigmentosa. Nat. Med. 1998;4(8):967–971. doi: 10.1038/nm0898-967. [DOI] [PubMed] [Google Scholar]
- 110.Ambasudhan R, Wang X, Jablonski MM, et al. Atrophic macular degeneration mutations in ELOVL4 result in the intracellular misrouting of the protein. Genomics. 2004;83(4):615–625. doi: 10.1016/j.ygeno.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 111.Grayson C, Molday RS. Dominant negative mechanism underlies autosomal dominant Stargardt-like macular dystrophy linked to mutations in ELOVL4. J. Biol. Chem. 2005;280(37):32521–32530. doi: 10.1074/jbc.M503411200. [DOI] [PubMed] [Google Scholar]
- 112.Conley YP, Thalamuthu A, Jakobsdottir J, et al. Candidate gene analysis suggests a role for fatty acid biosynthesis and regulation of the complement system in the etiology of age-related maculopathy. Hum. Mol. Genet. 2005;14(14):1991–2002. doi: 10.1093/hmg/ddi204. [DOI] [PubMed] [Google Scholar]
- 113.Seitsonen S, Lemmela S, Holopainen J, et al. Analysis of variants in the complement factor H, the elongation of very long chain fatty acids-like 4 and the hemicentin 1 genes of age-related macular degeneration in the Finnish population. Mol. Vis. 2006;12:796–801. [PubMed] [Google Scholar]
- 114.DeAngelis MM, Ji F, Kim IK, et al. Cigarette smoking CFH APOE ELOVL4, and risk of neovascular age-related macular degeneration. Arch. Ophthalmol. 2007;125(1):49–54. doi: 10.1001/archopht.125.1.49. [DOI] [PubMed] [Google Scholar]
- 115.Gu H, Cui L, Liu NP. (Association of M299V variant in ELOVL4 gene with exudative age-related macular degeneration in a Chinese population) Zhonghua Yan Ke Za Zhi. 2010;46(2):125–128. [PubMed] [Google Scholar]
- 116.Elner VM. Retinal pigment epithelial acid lipase activity and lipoprotein receptors: effects of dietary ω-3 fatty acids. Trans. Am Ophthalmol. Soc. 2002;100:301–338. [PMC free article] [PubMed] [Google Scholar]
- 117.McMahon A, Kedzierski W. Polyunsaturated very-long-chain C28–C36 fatty acids and retinal physiology. Br. J. Ophthalmol. 2010;94(9):1127–1132. doi: 10.1136/bjo.2008.149286. [DOI] [PubMed] [Google Scholar]
- 118. Suh M, Clandinin MT. 20 5n-3 but not 22:6n 3 is a preferred substrate for synthesis of n 3 very-long- chain fatty acids (C24–C36) in retina. Curr. Eye Res. 2005;30(11):959–968. doi: 10.1080/02713680500246957. ▪ 20 5 n-3 but not 22:6 n 3 is a preferred substrate for the synthesis of n 3 VLCPFAs.
- 119. Denic V, Weissman JS. A molecular caliper mechanism for determining very long-chain fatty acid length. Cell. 2007;130(4):663–677. doi: 10.1016/j.cell.2007.06.031. ▪ Introduces the molecular caliper mechanism for determining very-long-chain fatty acid length.
Websites
- 201. Age-Related Eye Disease Study 2. [Accessed 14 June 2006]; (AREDS2) http://clinicaltrials.gov/ct2/show/NCT00345176?term=AREDS2&rank=1. Record of the clinical trial information of Age-Related Eye Disease Study 2 (AREDS2).
- 202.NIH. Effect of DHA Supplements on Macular Function in Patients With Stargardt Macular Dystrophy and Stargardt-Like Macular Dystrophy. [cited 9 May 2003];2007 Dec 11; http://clinicaltrials.gov/ct2/results?term=03-EI0179.
- 203.University of Utah. DHA Supplementation in Patients With STGD3. www.clinicaltrial.gov/ct2/show/NCT00420602?term=STGD3&rank=1. [Google Scholar]