Abstract
Backgrounds & Aims
We studied the intestinotrophic hormone glucagon-like peptide-2 (GLP-2) as a possible therapy for non-steroidal anti-inflammatory drug (NSAID)-induced intestinal ulcers. Luminal nutrients release endogenous GLP-2 from enteroendocrine L cells. Since GLP-2 is degraded by dipeptidyl peptidase IV (DPPIV), we hypothesized that DPPIV inhibition combined with luminal administration of nutrients potentiates the effects of endogenous GLP-2 on intestinal injury.
Methods
Intestinal injury was induced by indomethacin (10 mg/kg, sc) in fed rats. The long-acting DPPIV inhibitor K579 was intragastrically (ig) or intraperitoneally (ip) given before or after indomethacin treatment. L-alanine (L-Ala) and 5′-inosine monophosphate (IMP) were co-administered ig after the treatment.
Results
Indomethacin treatment induced intestinal ulcers which gradually healed after treatment. Pretreatment with ig or ip K579 given either at 1 mg/kg reduced total ulcer length, whereas K579 at 3 mg/kg had no effect. Exogenous GLP-2 also reduced intestinal ulcers. The preventive effect of K579 was dose-dependently inhibited by a GLP-2 receptor antagonist. Daily treatment with K579 (1 mg/kg), GLP-2, or L-Ala + IMP after indomethacin treatment reduced total ulcer length. Co-administration (ig) of K579 and L-Ala + IMP further accelerated intestinal ulcer healing.
Conclusion
DPPIV inhibition and exogenous GLP-2 prevented the formation and promoted the healing of indomethacin-induced intestinal ulcers, although high-dose DPPIV inhibition reversed the preventive effect. Umami receptor agonists also enhanced the healing effects of the DPPIV inhibitor. The combination of DPPIV inhibition and luminal nutrient-induced GLP-2 release may be a useful therapeutic tool for the treatment of NSAIDs-induced intestinal ulcers.
Keywords: glucagon-like peptide-2, NSAIDs, taste receptor
INTRODUCTION
Non-steroidal anti-inflammatory drugs (NSAIDs) are often associated with gastroenteropathy and gastrointestinal (GI) bleeding. Advances in enteric mucosal imaging with wireless capsule endoscopy or double-balloon enteroscopy has documented small intestinal mucosal damage accompanying NSAID use. NSAID use is associated with 10% of subjects diagnosed with obscure GI bleeding [1] and a 50% prevalence of small intestinal mucosal injury [2] increasing to 70% in arthritis patients [3]. Nevertheless, the only effective current interventions for the treatment of NSAID-induced intestinal ulcers are discontinuation of NSAID use and conservative measures. Some clinical trials, however, have reported the efficacy of metronidazole, sulfasalazine, and misoprostol for the treatment of NSAID-induced enteropathy, assessed by indirect measures [4–6]. Therefore, preventive as well as accelerative healing therapy is needed to reduce the incidence of NSAID-induced intestinal ulcers and their consequent morbidity.
Glucagon-like peptide-2 (GLP-2), an intestinotrophic hormone released from enteroendocrine L cells [7] has been used to treat short bowel syndrome and inflammatory bowel disease in animal models [8;9] and in humans [10;11]. In mice, GLP-2 reduced indomethacin (IND)-induced mortality, accompanied by decreased intestinal injury [12], suggesting that GLP-2 is a candidate therapy for NSAID-induced enteropathy. The intestinotrophic effect of GLP-2 is mediated by insulin-like growth factor-1 (IGF-1) derived from lamina propria myofibroblasts in the intestinal submucosa [13], which accelerates the proliferation of crypt enterocytes, increasing villus height. Stable GLP-2 analogs are usually used in these studies, since GLP-2 is rapidly degraded by dipeptidyl peptide IV (DPPIV), similar to the related hormone, insulinotropic GLP-1 [14]. DPPIV inhibition enhances the intestinotrophic action of exogenous GLP-2 [15;16]. Therefore, we hypothesized that the DPPIV inhibition prevents ulcer formation and promotes ulcer healing of NSAID-induced enteropathy through the prevention of GLP-2 degradation.
Luminal nutrients evoke hormone release from L cells likely due to the activation of nutrient receptors in their luminal facing projections [17]. Luminal perfusion of the umami taste receptor T1R1/R3 agonists L-glutamate (L-Glu) and 5′-inosine monophosphate (IMP) synergistically increased GLP-2 release into portal vein, stimulating mucosal protective HCO3− secretion in rat duodenum [18;19]. Furthermore, intravenous injection of a DPPIV inhibitor enhanced L-Glu/IMP-induced HCO3− secretion, suggesting that the presence of luminal nutrients combined with DPPIV inhibition augmented the effect of GLP-2 [20]. Thus, we further hypothesized that luminal nutrients combined with the administration of a DPPIV inhibitor further potentiate the therapeutic effect of GLP-2 on NSAID-induced enteropathy.
Here we show that the DPPIV inhibition prevented IND-induced intestinal ulcer formation via activation of the GLP-2 pathway. Furthermore, oral amino acid/IMP and DPPIV inhibitor additively promoted healing of IND-induced intestinal ulcers. The combination of oral nutrients and DPPIV inhibition may potentiate the effect of endogenous GLP-2, offering novel therapeutic options for the treatment of NSAID-induced enteropathy.
Methods
Chemicals and animals
K579 [(2S)-1-[[[4-Methyl-1-(2-pyrimidinyl)-4-piperidinyl]amino]acetyl]-2-pyrrolidinecarbonitrile], NVP DPP 728 dihydrochloride (NVP728; 6-[[2-[[2-(2S)-2-Cyano-1-pyrrolidinyl]-2-oxoethyl]amino]ethyl]amino-3-pyridinecarbono nitrile dihydrochloride) and rat GLP-2 were obtained from Tocris Bioscience (Ellisville, MO). Rat GLP-2(3–33) was synthesized by Bachem Americas, Inc. (Torrance, CA). Indomethacin (IND), L-alanine (L-Ala), inosine 5′-monophosphate (IMP), HEPES and other chemicals were obtained from Sigma Chemical (St. Louis, MO). Indomethacin was freshly dissolved in ethanol. K579 was dissolved in DMSO for stock solution and further diluted in normal saline for the treatment. DMSO (0.1 %) in saline was used as vehicle. GLP-2 and GLP-2(3–33) were dissolved in normal saline containing 0.1 % bovine serum albumin. All studies were performed with approval of the Veterans Affairs Institutional Animal Care and Use Committee (VA IACUC). Male Sprague-Dawley rats weighing 200–250 g (Harlan, San Diego, CA) had free access to food and water.
IND-induced intestinal injury
IND induces gastroenteric injury according to feeding status: gastric mucosal lesions occur in overnight fasted rats, antral ulcers occur in re-fed rats (overnight fasting followed by feeding after IND treatment), whereas small intestinal ulcers are induced in fed rats by a single IND treatment (either intragastric, intraperitoneal or by subcutaneous injection) [21;22]. Therefore, we chose IND treatment in fed rats in order to induce small intestinal ulcers. IND (10 mg/kg) was subcutaneously injected in the morning on Day 0 under brief isoflurane anesthesia (4 %). Portal venous blood was collected under isoflurane anesthesia as described below, followed by euthanasia by terminal exsanguinations at 1 day after (Day 1), or 3 or 7 days after (Day 3 or 7) IND treatment. The gastrointestinal (GI) tract from stomach to terminal ileum was removed and longitudinally opened along anti-mesenteric curvature, since IND-induced intestinal ulcers are mainly induced at mesenteric curvature [23]. After carefully removing the luminal contents and gently rinsing the mucosa in normal saline, the small intestine was cut into about 10 cm segments. The gastric antrum was defined as segment 0, the duodenum as segment 1, the jejunum as segments 2 and 3, and the ileum as segments 4–7. Observed ulcers were typically circular and linearly arranged. The diameter of each ulcer was macroscopically measured with the cumulative ulcer length of all ulcers in each segment calculated. Total ulcer length was defined by the sum of total segment ulcer length.
Experimental protocol
K579 is a long-acting DPPIV inhibitor, due to its lipophilicity and the enterohepatic circulation of its active metabolites [24;25]. To examine the preventive effect of the DPPIV inhibitor on IND-induced intestinal ulcers, K579 (0.3–3 mg/kg) was intragastrically (ig) or intraperitoneally (ip) administered once just before IND treatment with intestinal ulceration assessed 24 hrs after treatment (Day 1). In order to clarify the contribution of GLP-2 relative to that of DPPIV inhibition, the GLP-2 receptor (GLP-2R) antagonist, GLP-2(3–33) (0.01–1 mg/kg, ip) was administered immediately following IND treatment. To confirm the direct effect of GLP-2 on intestinal injury, rat GLP-2 was exogenously administered; 10–100 μg/kg, ip, twice a day (20–200 μg/kg/day) at 0 and 8 hrs after IND treatment.
To examine the healing effect of DPPIV inhibition, K579 (0.3 or 1 mg/kg, ig) was given 24 and 48 hrs after IND treatment (Day 1 and 2), with intestinal ulceration assessed on Day 3. We also tested the effect of exogenous GLP-2 (200 μg/kg/day, ip) or GLP-2(3–33) (1 mg/kg, ip) on ulcer healing.
We further examined the effect of taste receptor activation on intestinal ulcer formation and healing. The animals were treated with L-Glu or L-Ala (400 μmol/kg) + IMP (4 μmol/kg) ig before IND treatment, or with K579 (1 mg/kg, ig), L-Ala + IMP or both at 24 and 48 hrs after IND treatment (Day 1 and 2).
Measurement of GLP-2 in portal venous blood
Plasma concentrations of GLP-2 were measured in portal venous blood samples. amples were collected using a syringe containing 1-μl each of EDTA (0.5 mM) and NVP728 (10 μM). Samples were immediately centrifuged at 5,000 x g for 5 min; plasma was stored at −80°C until use. Plasma was diluted with Tris-HCl buffer (50 mM, pH 7.4) containing Protease Inhibitor Cocktail (1 mg/ml, Sigma) and NVP728 (10 μM). Plasma concentration of total GLP-2 was measured using a GLP-2 (rat) EIA kit (ALPCO Diagnostics, Salem, NH) according to the manufacture’s protocol.
Statistics
All data are expressed as means ± SEM. Data were derived from six rats in each group. Comparisons between groups were made by one-way ANOVA followed by Fischer’s least significant difference test. P values of < 0.05 were taken as significant.
RESULTS
Effect of DPPIV inhibition on IND-induced intestinal ulcer formation
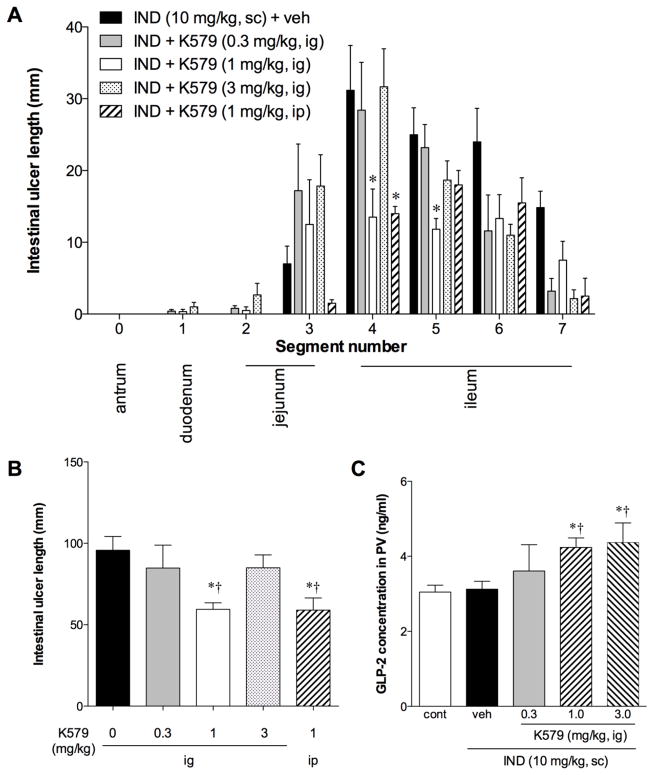
IND treatment reproducibly induced intestinal ulcers (Fig. 1A). The ulcers were primarily observed in the proximal ileum (Fig. 1A and Fig. 2A). Gastric lesions or duodenal ulcers were rarely observed. K579 treatment at 1 mg/kg reduced intestinal ulcer formation (Fig. 1B). K579 predominantly reduced intestinal ulcer formation in the proximal ileum (Fig. 2A). K579 at 0.3 mg/kg had no effect on ulcer formation, whereas 1 mg/kg K579 given either ig or ip inhibited ulcer formation (Fig. 2B). Nevertheless, at high dose (3 mg/kg), the preventive effect of K579 was reversed, consistent with the presence of multiple DPPIV substrates [26].
Fig. 1. Indomethacin (IND)-induced intestinal ulcers in rats.
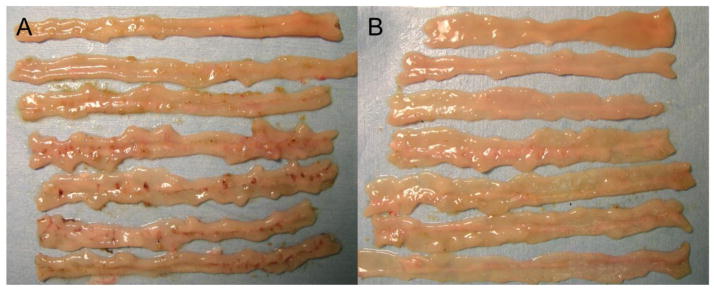
The representative gross appearance of IND-induced intestinal ulcers on Day 1 is shown (A). Pretreatment with K579 (1 mg/kg, ig) reduced intestinal ulcer formation on Day 1 (B).
Fig. 2. Effect of DPPIV inhibition on IND-induced intestinal ulcer formation in rats.
Intestinal ulcers were induced by IND (10 mg/kg, sc). K579 (0.3 – 3 mg/kg) or vehicle was given intragastrically (ig) or intraperitoneally (ip). Intestinal lesions were evaluated at 24 hrs (Day 1) after IND treatment. A: Intestinal ulcer formation in each segment. The small intestine was divided into 7 segments from duodenum (1) to terminal ileum (7); antrum was defined as segment 0. Each data point represents mean ± SEM (n = 6 rats). *p < 0.05 vs. IND + veh group. B: Total intestinal ulcer score. Each data point represents mean ± SEM (n = 6 rats). *p < 0.05 vs. IND + veh group, †p < 0.05 vs. IND + K579 (0.3 mg/kg, ig) group. C: Portal venous (PV) GLP-2 level on Day 1 after IND treatment with or without K579 pretreatment (0.3 – 3 mg/kg, ig). Each data point represents mean ± SEM (n = 6 rats). *p < 0.05 vs. control, †p < 0.05 vs. IND + veh group.
In the ulcer formation study, plasma GLP-2 concentrations in PV blood were compared with those of freely-fed control rats: PV GLP-2 levels were unchanged in IND-treated rats at 24 hrs after the treatment, whereas K579 treatment (1 mg/kg) increased PV GLP-2 concentrations (Fig. 2C), suggesting that DPPIV inhibition increases the local concentrations of GLP-2 by inhibiting its degradation. GLP-2 levels plateaued after treatment with high-dose K579 (3 mg/kg), further suggesting that high-dose K579 prolongs the half-life of bioactive substrates.
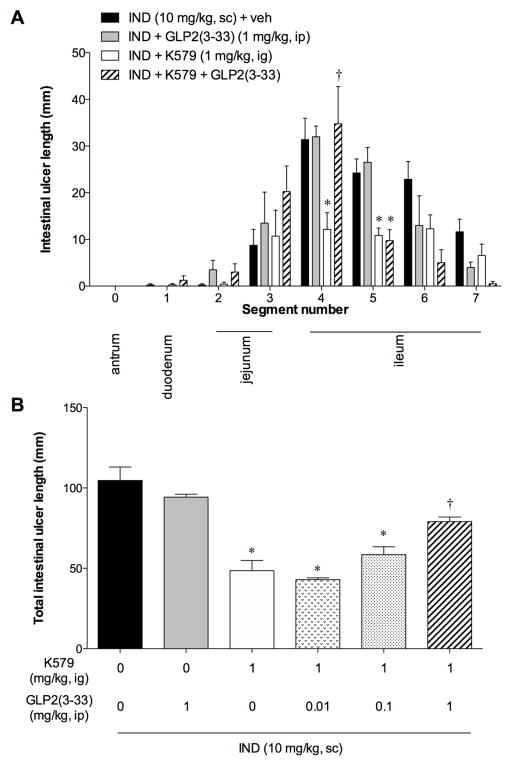
To examine whether the preventive effects of DPPIV inhibitor were mediated by GLP-2, the animals were also treated with a GLP-2R antagonist GLP-2(3–33). GLP-2(3–33) (1 mg/kg, ip) alone had no effect on IND-induced intestinal ulcer formation, whereas GLP-2(3–33) (1 mg/kg) reversed the preventative effects of K579 (1 mg/kg, ig) on ulcer formation (Fig. 3A, B), suggesting that K579 prevents IND-induced intestinal ulcer formation via GLP-2 pathway.
Fig. 3. Effect of a GLP-2 receptor antagonist on the preventive effect of DPPIV inhibition.
GLP-2(3–33) (0.01 – 1 mg/kg, ip) was given after IND treatment with or without K579 pretreatment. Intestinal lesions were evaluated at 24 hrs (Day 1) after IND treatment. A: Intestinal ulcer formation in each segment. Each data point represents mean ± SEM (n = 6 rats). *p < 0.05 vs. IND + veh group. B: Total intestinal ulcer score. Each data point represents mean ± SEM (n = 6 rats). *p < 0.05 vs. IND + veh group, †p < 0.05 vs. IND + K579 (1 mg/kg, ig) group.
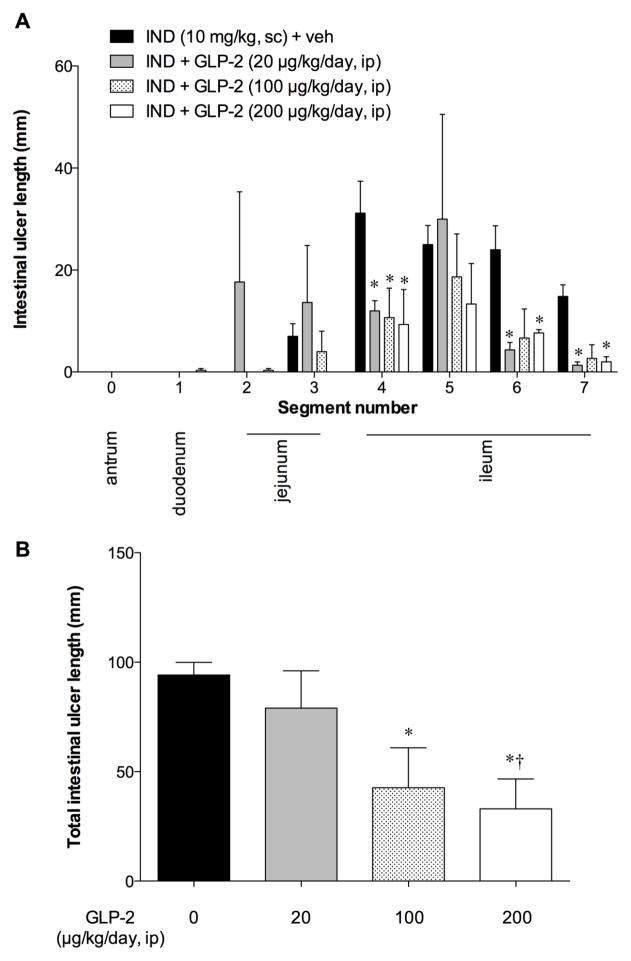
Although exogenous GLP-2 prevents IND-induced enteropathy in mice [12], and is rapidly degraded by DPPIV [14], there has been no report of the effects of exogenous GLP-2 on NSAID-induced enteropathy in rats. Therefore, to confirm the direct effect of GLP-2, we treated the animals with rat GLP-2 (10–100 μg/kg, ip, twice a day, i.e. 20–200 μg/kg/day). Exogenous GLP-2 treatment dose-dependently reduced ulcer length in all segments (Fig. 4A, B), confirming the direct preventative effect of an albeit extremely high-dose GLP-2.
Fig. 4. Effect of exogenous GLP-2 on IND-induced intestinal ulcer formation in rats.
Rat GLP-2 (10 – 100 μg/kg, ip) was given at 0 and 8 hrs after IND treatment (20 – 200 μg/kg/day). Intestinal lesions were evaluated at 24 hrs (Day 1) after IND treatment. Each data point represents mean ± SEM (n = 6 rats). *p < 0.05 vs. IND + veh group. A: Intestinal ulcer formation in each segment. *p < 0.05 vs. IND + veh group. B: Total intestinal ulcer score. *p < 0.05 vs. IND + veh group, †p < 0.05 vs. IND + GLP-2 (20 μg/kg/day, ip) group.
Effect of DPPIV inhibition on healing of IND-induced intestinal ulcers
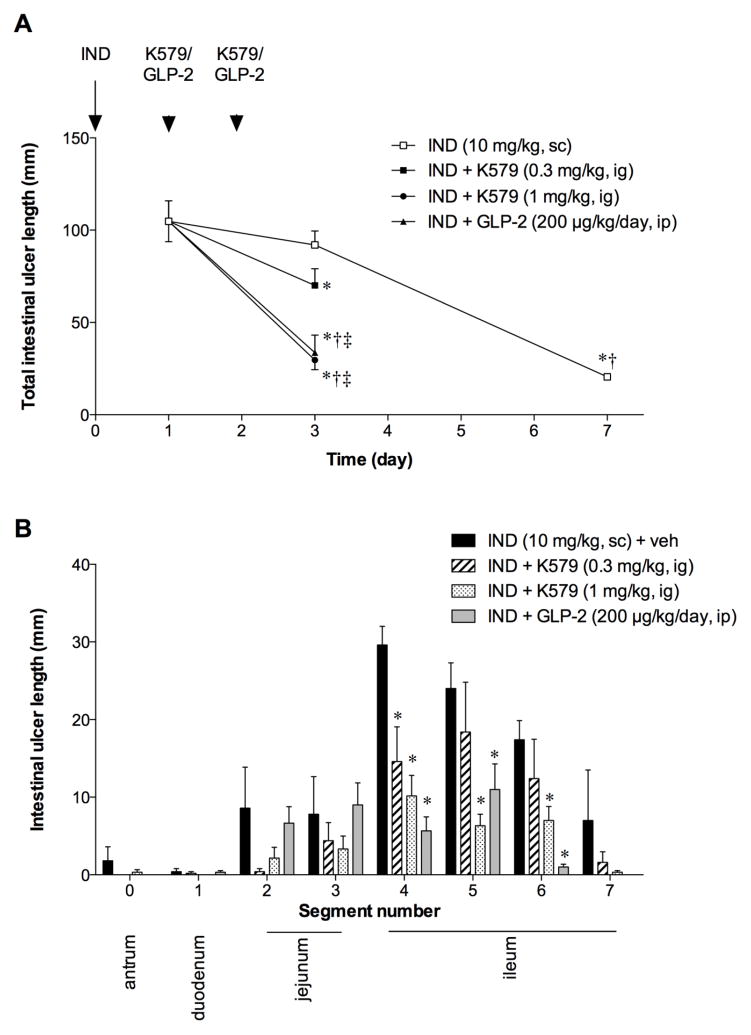
IND-induced intestinal ulcers usually spontaneously heal 7 ~ 10 days after treatment [22]. In our study, intestinal ulcer length was substantially reduced on Day 7, whereas no significant healing was observed on Day 3, compared to Day 1 (Fig. 5A). Therefore, we set Day 3 as the endpoint of the healing study. On Day 3, K579 at 1 mg/kg, but not at 0.3 mg/kg, significantly reduced intestinal ulcer length, compared with the IND group (Fig. 5A). Proximal ileal ulcers were significantly healed by K579 at 0.3 and 1 mg/kg (Fig. 5B). This study suggests that DPPIV inhibition accelerates healing of IND-induced intestinal ulcers. Exogenous GLP-2 (200 μg/kg/day, ip) also promoted ulcer healing, similar to the effect of K-579 (1 mg/kg), whereas the addition of GLP-2(3–33) (1 mg/kg) to K579 treatment reversed the promoting effect of K579 (Fig. 5A, B), further suggesting that DPPIV inhibition-induced healing promotion is mediated by GLP-2 pathway.
Fig. 5. Effect of DPPIV inhibition on ulcer healing of IND-induced intestinal ulcers.
Intestinal ulcers were assessed on Day 3 after IND was given on Day 0. K579 (0.3 or 1 mg/kg, ig, once a day), GLP-2 (100 μg/kg, ip, twice a day), or GLP-2(3–33) (1 mg/kg, ip) with K579 (1 mg/kg, ig) was given on Days 1 and 2. A: Total intestinal ulcer score was assessed on Days 1, 3, and 7 after IND treatment on Day 0. Each data point represents mean ± SEM (n = 6 rats). *p < 0.05 vs. IND + veh group on Day 1, †p < 0.05 vs. IND + veh group on Day 3, ‡p < 0.05 vs. IND + K579 (0.3 mg/kg, ig) group on Day 3. §p < 0.05 vs. IND + K579 (1 mg/kg, ig) group on Day 3. B: Intestinal ulcer score in each segment. Each data point represents mean ± SEM (n = 6 rats). *p < 0.05 vs. IND + veh group.
Effect of L-Ala/IMP on healing of IND-induced intestinal ulcers
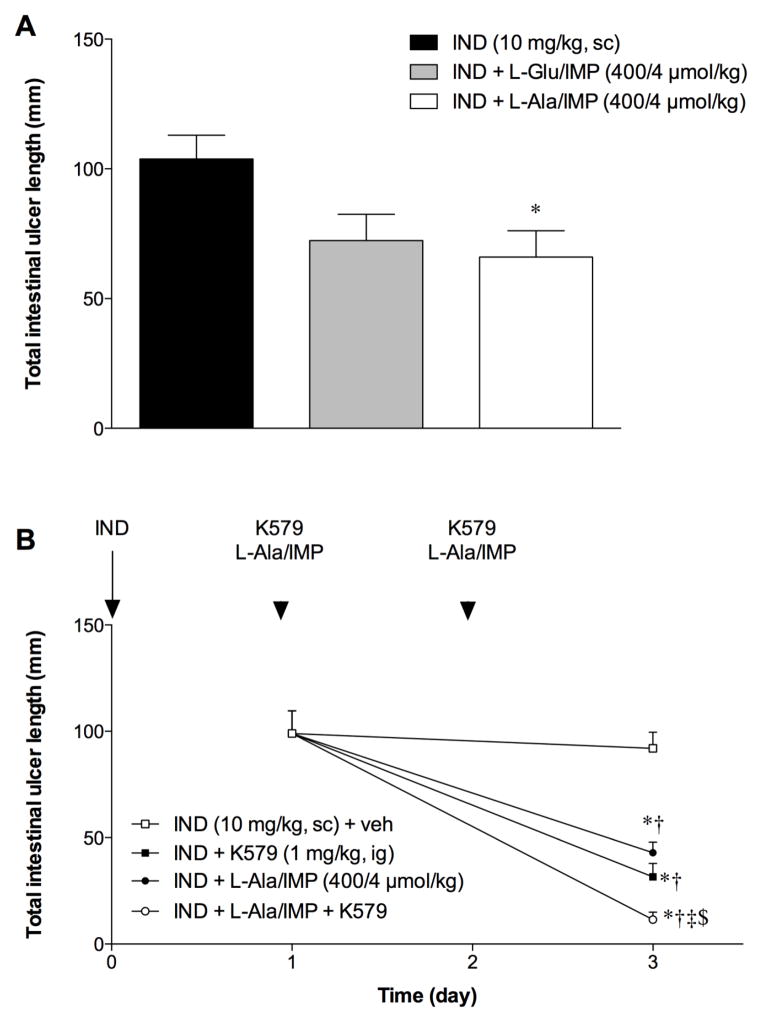
We hypothesized that luminal nutrients, which evoke GLP-2 release, affect the rate of healing of IND-induced ulcers. Since activation of the taste receptor T1R1/R3 with amino acids and IMP increases HCO3− secretion and GLP-2 release in rat duodenum [18;19], we examined the effect of L-Glu/IMP or L-Ala/IMP on intestinal ulcer formation. L-Ala/IMP (400/4 μmol/kg, ig) significantly reduced total intestinal ulcer formation, whereas L-Glu/IMP had no effect (Fig. 6A). Therefore, we next examined the effect of L-Ala/IMP on ulcer healing. L-Ala/IMP (400/4 μmol/kg, ig) significantly reduced total intestinal ulcer length on Day 3, similar to the effect of K579 (1 mg/kg, ig) (Fig. 6B). Co-administration of K579 and L-Ala/IMP further decreased total intestinal ulcer length on Day 3, suggesting that DPPIV inhibition combined with taste receptor activation additively promote healing of IND-induced intestinal ulcers.
Fig. 6. Effect of L-Ala/IMP with DPPIV inhibition on intestinal ulcer healing.
Total intestinal ulcer score was assessed on Days 1 and 3 after IND was given on Day 0. K579 (1 mg/kg, ig) and/or L-Ala/IMP (400/4 μmol/kg, ig) was given once a day on Day 1 and 2. Each data point represents mean ± SEM (n = 6 rats). *p < 0.05 vs. IND + veh group on Day 1, †p < 0.05 vs. IND + veh group on Day 3, ‡p < 0.05 vs. IND + K579 group on Day 3, §p < 0.05 vs. IND + L-Ala/IMP group on Day 3.
PV GLP-2 concentrations during ulcer healing
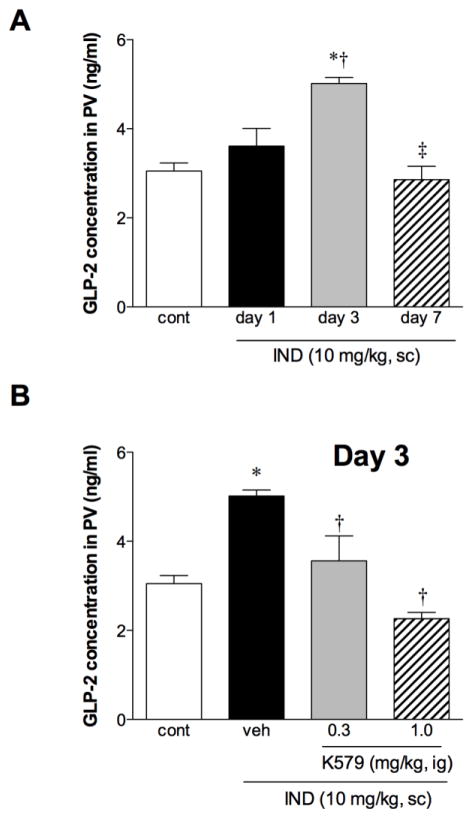
PV GLP-2 concentrations increased on Day 3 and declined on Day 7 during intestinal ulcer healing (Fig. 7A). The elevated GLP-2 concentrations on Day 3 were decreased by K579 post-treatment dose-dependently during ulcer healing (Fig. 7B), corresponding to the accelerated ulcer healing (Fig. 5A). These results suggest that PV GLP-2 concentrations were increased in response to intestinal injury up to Day 3, whereas K579 treatment reverses the increased GLP-2 concentrations by accelerating the healing rate of intestinal ulcers.
Fig. 7. GLP-2 concentration in portal vein (PV) during IND-induced intestinal ulcer healing.
A: PV GLP-2 level on Days 1, 3, and 7 after IND treatment. Each data point represents mean ± SEM (n = 6 rats). *p < 0.05 vs. control, †p < 0.05 vs. IND group on Day 1, ‡p < 0.05 vs. IND group on Day 3. B: PV GLP-2 level on Day 3 after IND treatment with or without K579 daily treatment (0.3 or 1 mg/kg, ig). Each data point represents mean ± SEM (n = 6 rats). *p < 0.05 vs. control, †p < 0.05 vs. IND + veh group on Day 3.
DISCUSSION
We demonstrated that DPPIV inhibition prevented IND-induced intestinal ulcer formation via GLP-2R activation and that DPPIV inhibition promoted healing of IND-induced intestinal ulcers, further enhanced by oral administration of L-Ala/IMP. This is the first study to demonstrate the therapeutic effects of a DPPIV inhibitor on NSAID-induced enteropathy mediated by the GLP-2 pathway. We also confirmed that exogenous GLP-2 prevented injury and promoted ulcer healing in a rat model of NSAID-induced enteropathy. Furthermore, this study suggests that the combination of agonists for L-cell expressing nutrient receptors with DPPIV inhibition accelerates intestinal ulcer healing.
The effects of exogenous GLP-2 or its analog on intestinal mucosal proliferation, healing and growth have been studied in animal models of short bowel syndrome [27]. Exogenous GLP-2 helps heal mucosal injury in inflammatory models of chemical-induced colitis [9], NSAID-induced enteropathy [12], radiation-induced enteritis [28] and post-operative ileus [29], consistent with our results. The effects of endogenous GLP-2 on refeeding-induced intestinal adaptation model were studied using the GLP-2R antagonist GLP-2(3–33) or in GLP-2R knockout (KO) mice [30–32]. DPPIV inhibition enhances the intestinotrophic effect of exogenous GLP-2 in rats and mice [15]. A DPPIV inhibitor in clinical use, sitagliptin, enhances intestinal adaptation in a mouse model of short bowel syndrome [16]. Nevertheless, the preventative or healing effect of DPPIV inhibition on NSAID-induced enteropathy has not been heretofore examined.
The rat NSAID-induced intestinal ulcer model is mechanistically well characterized and reproducible. The proposed mechanisms underlying the formation of NSAID-induced intestinal ulcers include reduced epithelial anion and mucus secretion, hypermotility, reduced blood flow, increased inflammatory cell infiltration, and bacterial translocation [33–35], mostly due to inhibition of prostaglandin synthesis, in addition to direct cytotoxicity of NSAID on epithelial cells [36]. Proven prophylactic or therapeutic treatments for NSAID-induced enteropathy are not currently available, although metronidazole, sulfasalazine and misoprostol have been proposed [4–6]. Proton pump inhibitors (PPIs) and prostaglandin analogs have been used to treat NSAID-induced gastroduodenal lesions [37]. A PPI, lansoprazole or an H2 receptor antagonist lafutidine also inhibit NSAID-induced enteropathy in rats [38;39], although PPIs can exacerbate intestinal injury by inducing microbial dysbiosis [40]. Since DPPIV inhibitors successfully treat type 2 diabetes, and have no effect on blood glucose in normoglycemic individuals [41], our results suggest that clinically-used DPPIV inhibitors could be considered as novel therapies for the prevention and treatment of NSAID-induced enteropathy. Nonetheless, high-dose DPPIV inhibition enhanced NSAID-induced enteropathy, possibly due to half-life prolongation of multiple inflammatory mediators [26], suggesting that use of DPPIV inhibitors for inflammatory conditions may be limited to a narrow range.
Mucosal protection by GLP-2 enhanced by DPPIV inhibition is possibly explained by the secretagogue effects of GLP-2 mediated via VIP and NO release [19]. Since exogenous GLP-2 increases mesenteric blood flow via VIP and NO pathways [42], it may improve the NSAID-induced reduction of blood flow [43]. Since GLP-2 inhibits gastrointestinal motility [44;45], it may also reduce NSAID-induced hypermotility. The GLP-2 analog, GLP-2-linked IgG, reduced inflammation in a post-operative ileus murine model [29], also suggesting the involvement of anti-inflammatory effects. GLP-2R KO mice have increased susceptibility to IND-induced small intestinal injury, attributed to increased bacterial translocation [46]. These studies suggest that exogenous GLP-2 compensates for defense mechanisms impaired by suppressed prostaglandin synthesis and uncontrolled inflammation in the pathogenesis of NSAID-induced enteropathy. Furthermore, exogenous GLP-2 or DPPIV inhibition accelerates crypt cell proliferation [15;47], which may promote healing of NSAID-induced enteropathy.
Patients with active inflammatory bowel disease have increased circulating bioactive GLP-2 as a possible adaptive response to intestinal injury [48]. Our observations that DPPIV inhibition reduced intestinal ulcer formation, and augmented PV GLP-2 concentrations on Day 1, and that GLP-2(3–33) reversed the protective effect of DPPIV inhibition, suggest that DPPIV inhibition enhances the local secretory and trophic effects of GLP-2 released in response to mucosal injury. Although we could not measure bioactive GLP-2, augmented PV GLP-2 concentrations associated with DPPIV inhibition on Day 1 was possibly due to the increased concentrations of GLP-2 in the submucosa, where DPPIV activity degrades locally released GLP-2 [20;49], rather than increasing the concentration of circulating GLP-2.
In contrast, PV GLP-2 concentration increased on Day 3, decreasing to basal levels on Day 7 during spontaneous ulcer healing, suggesting that PV GLP-2 concentration correlates with the amount of mucosal injury. On Day 3, DPPIV inhibition accelerated intestinal ulcer healing and reduced PV GLP-2 level, consistent with our hypothesis that mucosal inflammation correlates with GLP-2 release. This finding also suggests the presence of negative feedback mechanism to regulate GLP-2 release after injury or inflammation, in addition to the increased GLP-2 release in response to mucosal injury. Our finding that GLP-2(3–33) reversed K579-induced acceleration of ulcer healing, further suggested that the effect of K579 was mediated by endogenous GLP-2 during ulcer healing, despite the concomitant decline of PV GLP-2 concentrations. Measurement of bioactive, not total, GLP-2 level at multiple points will clarify the detailed correlation of PV GLP-2 levels with mucosal healing status.
DPPIV inhibition combined with amino acid umami receptor activation enhanced IND-induced intestinal ulcer healing. Since luminal nutrients augment mucosal defense mechanisms via the GLP-2 pathway [19], and DPPIV inhibition potentiates luminal nutrient-induced mucosal defense responses via enhanced effects of endogenous GLP-2 [20], our results support the hypothesis that DPPIV inhibition combined with luminal nutrients enhances intestinal ulcer healing. Administration of the GLP-2R antagonist GLP-2(3–33) or genetic GLP-2R deficiency reverses refeeding-induced intestinal adaptation [30–32]. Furthermore, IGF-1R KO reverses the intestinotrophic effect of exogenous GLP-2 and refeeding-induced intestinal adaptation [13], suggesting that endogenous GLP-2 released in response to luminal nutrients or other stimuli increases crypt cell proliferation via GLP-2R activation, IGF-1 release and IGF-1R activation.
Feeding of L-Glu (1 or 5 %) for 5 days prior to one time NSAID treatment significantly reduced intestinal lesions while daily treatment for 5 days with L-Glu after NSAID treatment accelerated ulcer healing [50], consistent with our finding that luminal amino acids prevent the formation and promote healing of NSAID-induced enteropathy, although one dose of L-Glu/IMP did not significantly inhibit ulcer formation in our study. The combination of metformin, which increases the release of GLP-1 and 2, with a DPPIV inhibitor, prevents 5-fluorouracil-induced mucositis [51]. Supplemental enteral nutrients also enhance the intestinotrophic effect of exogenous GLP-2 [52;53], further supporting our concept in which the combination of GLP-2-releasing nutrients with DPPIV inhibition accelerates intestinal ulcer healing.
In conclusion, a long-acting DPPIV inhibitor prevented formation and promoted healing of IND-induced intestinal ulcers via GLP-2R activation along with locally increased concentration of GLP-2. The combination of a DPPIV inhibitor and luminal GLP-2-releasing nutrients enhanced the rate of intestinal ulcer healing. This study suggests that DPPIV inhibition combined with luminal nutrients is a novel, clinically feasible prophylactic and therapeutic treatment for NSAID-induced enteropathy.
Acknowledgments
We thank Coleen and Bea Palileo for their assistance with manuscript preparation.
Supported by Department of Veterans Affairs Merit Review Award, NIH-NIDDK R01 DK54221 (J. Kaunitz), and the animal core of NIH-NIDDK P30 DK0413 (J. E. Rozengurt).
Abbreviations used
- DPPIV
dipeptidyl peptidase-IV
- IMP
5′-inosine monophosphate
- IND
indomethacin
- L-Ala
L-alanine
- L-Glu
L-glutamate
- GLP
glucagon-like peptide
- GLP-2R
GLP-2 receptor
- PV
portal vein
- T1R
taste receptor type 1
References
- 1.Higuchi K, Umegaki E, Watanabe T, Yoda Y, Morita E, Murano M, Tokioka S, Arakawa T. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol. 2009;44:879–888. doi: 10.1007/s00535-009-0102-2. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto T, Kudo T, Esaki M, Yano T, Yamamoto H, Sakamoto C, Goto H, Nakase H, Tanaka S, Matsui T, Sugano K, Iida M. Prevalence of non-steroidal anti-inflammatory drug-induced enteropathy determined by double-balloon endoscopy: a Japanese multicenter study. Scand J Gastroenterol. 2008;43:490–496. doi: 10.1080/00365520701794121. [DOI] [PubMed] [Google Scholar]
- 3.Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55–59. doi: 10.1016/s1542-3565(04)00603-2. [DOI] [PubMed] [Google Scholar]
- 4.Bjarnason I, Hayllar J, Smethurst P, Price A, Gumpel MJ. Metronidazole reduces intestinal inflammation and blood loss in non-steroidal anti-inflammatory drug induced enteropathy. Gut. 1992;33:1204–1208. doi: 10.1136/gut.33.9.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris AJ, Murray L, Sturrock RD, Madhok R, Capell HA, Mackenzie JF. Short report: the effect of misoprostol on the anaemia of NSAID enteropathy. Aliment Pharmacol Ther. 1994;8:343–346. doi: 10.1111/j.1365-2036.1994.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 6.Hayllar J, Smith T, Macpherson A, Price AB, Gumpel M, Bjarnason I. Nonsteroidal antiinflammatory drug-induced small intestinal inflammation and blood loss. Effects of sulfasalazine and other disease-modifying antirheumatic drugs. Arthritis Rheum. 1994;37:1146–1150. doi: 10.1002/art.1780370806. [DOI] [PubMed] [Google Scholar]
- 7.Drucker DJ. Glucagon-like Peptide 2. The Journal of Clinical Endocrinology and Metabolism. 2010;10:153–156. doi: 10.1016/s1043-2760(98)00136-2. [DOI] [PubMed] [Google Scholar]
- 8.Martin GR, Wallace LE, Sigalet DL. Glucagon-like peptide-2 induces intestinal adaptation in parenterally fed rats with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2004;286:G964–G972. doi: 10.1152/ajpgi.00509.2003. [DOI] [PubMed] [Google Scholar]
- 9.Drucker DJ, Yusta B, Boushey RP, DeForest L, Brubaker PL. Human [Gly2]GLP-2 reduces the severity of colonic injury in a murine model of experimental colitis. Am J Physiol. 1999;276:G79–G91. doi: 10.1152/ajpgi.1999.276.1.G79. [DOI] [PubMed] [Google Scholar]
- 10.Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O’Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011 doi: 10.1136/gut.2010.218271. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchman AL, Katz S, Fang JC, Bernstein CN, Abou-Assi SG. Teduglutide, a novel mucosally active analog of glucagon-like peptide-2 (GLP-2) for the treatment of moderate to severe Crohn’s disease. Inflamm Bowel Dis. 2010;16:962–973. doi: 10.1002/ibd.21117. [DOI] [PubMed] [Google Scholar]
- 12.Boushey RP, Yusta B, Drucker DJ. Glucagon-like peptide 2 decreases mortality and reduces the severity of indomethacin-induced murine enteritis. Am J Physiol. 1999;277:E937–E947. doi: 10.1152/ajpendo.1999.277.5.E937. [DOI] [PubMed] [Google Scholar]
- 13.Rowland KJ, Trivedi S, Lee D, Wan K, Kulkarni RN, Holzenberger M, Brubaker PL. Loss of glucagon-like peptide-2-induced proliferation following intestinal epithelial insulin-like growth factor-1-receptor deletion. Gastroenterology. 2011;141:2166–2175. doi: 10.1053/j.gastro.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Hansen L, Hare KJ, Hartmann B, Deacon CF, Ugleholdt RK, Plamboeck A, Holst JJ. Metabolism of glucagon-like peptide-2 in pigs: role of dipeptidyl peptidase IV. Regul Pept. 2007;138:126–132. doi: 10.1016/j.regpep.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann B, Thulesen J, Kissow H, Thulesen S, Orskov C, Ropke C, Poulsen SS, Holst JJ. Dipeptidyl peptidase IV inhibition enhances the intestinotrophic effect of glucagon-like peptide-2 in rats and mice. Endocrinol. 2000;141:4013–4020. doi: 10.1210/endo.141.11.7752. [DOI] [PubMed] [Google Scholar]
- 16.Okawada M, Holst JJ, Teitelbaum DH. Administration of a dipeptidyl peptidase IV inhibitor enhances the intestinal adaptation in a mouse model of short bowel syndrome. Surgery. 2011;150:217–223. doi: 10.1016/j.surg.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelstoft MS, Egerod KL, Holst B, Schwartz TW. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metab. 2008;8:447–449. doi: 10.1016/j.cmet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Akiba Y, Watanabe C, Mizumori M, Kaunitz JD. Luminal L-glutamate enhances duodenal mucosal defense mechanisms via multiple glutamate receptors in rats. Am J Physiol Gastrointest Liver Physiol. 2009;297:G781–G791. doi: 10.1152/ajpgi.90605.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang JH, Inoue T, Higashiyama M, Guth PH, Engel E, Kaunitz JD, Akiba Y. Umami receptor activation increases duodenal bicarbonate secretion via glucagon-like peptide-2 release in rats. J Pharmacol Exp Ther. 2011;339:464–473. doi: 10.1124/jpet.111.184788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue T, Wang JH, Higashiyama M, Rudenkyy S, Higuchi K, Guth PH, Engel E, Kaunitz JD, Akiba Y. Dipeptidyl peptidase IV inhibition potentiates amino acid- and bile acid-induced bicarbonate secretion in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2012;303:G810–G816. doi: 10.1152/ajpgi.00195.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satoh H, Guth PH, Grossman MI. Role of Food in Gastrointestinal Ulceration Produced by Indomethacin in the Rat. Gastroenterology. 1982;83:210–215. [PubMed] [Google Scholar]
- 22.Hatazawa R, Ohno R, Tanigami M, Tanaka A, Takeuchi K. Roles of endogenous prostaglandins and cyclooxygenase isozymes in healing of indomethacin-induced small intestinal lesions in rats. J Pharmacol Exp Ther. 2006;318:691–699. doi: 10.1124/jpet.106.103994. [DOI] [PubMed] [Google Scholar]
- 23.Iwai T, Ichikawa T, Kida M, Goso Y, Saegusa Y, Okayasu I, Saigenji K, Ishihara K. Vulnerable Sites and Changes in Mucin in the Rat Small Intestine After Non-steroidal Anti-inflammatory Drugs Administration. Dig Dis Sci. 2010;55:3369–3376. doi: 10.1007/s10620-010-1185-6. [DOI] [PubMed] [Google Scholar]
- 24.Takasaki K, Iwase M, Nakajima T, Ueno K, Nomoto Y, Nakanishi S, Higo K. K579, a slow-binding inhibitor of dipeptidyl peptidase IV, is a long-acting hypoglycemic agent. Eur J Pharmacol. 2004;486:335–342. doi: 10.1016/j.ejphar.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 25.Takasaki K, Takada H, Nakajima T, Ueno K, Ushiki J, Higo K. Involvement of the active metabolites in the inhibitory activity of K579 on rat plasma dipeptidyl peptidase IV. Eur J Pharmacol. 2004;505:237–241. doi: 10.1016/j.ejphar.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Lambeir AM, Durinx C, Scharpe S, De MI. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- 27.Scott RB, Kirk D, MacNaughton WK, Meddings JB. GLP-2 augments the adaptive response to massive intestinal resection in rat. Am J Physiol. 1998;275:G911–G921. doi: 10.1152/ajpgi.1998.275.5.G911. [DOI] [PubMed] [Google Scholar]
- 28.Torres S, Thim L, Milliat F, Vozenin-Brotons MC, Olsen UB, hnfelt-Ronne I, Bourhis J, Benderitter M, Francois A. Glucagon-like peptide-2 improves both acute and late experimental radiation enteritis in the rat. Int J Radiat Oncol Biol Phys. 2007;69:1563–1571. doi: 10.1016/j.ijrobp.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 29.Moore BA, Peffer N, Pirone A, Bassiri A, Sague S, Palmer JM, Johnson DL, Nesspor T, Kliwinski C, Hornby PJ. GLP-2 receptor agonism ameliorates inflammation and gastrointestinal stasis in murine postoperative ileus. J Pharmacol Exp Ther. 2010;333:574–583. doi: 10.1124/jpet.109.161497. [DOI] [PubMed] [Google Scholar]
- 30.Shin ED, Estall JL, Izzo A, Drucker DJ, Brubaker PL. Mucosal adaptation to enteral nutrients is dependent on the physiologic actions of glucagon-like peptide-2 in mice. Gastroenterology. 2005;128:1340–1353. doi: 10.1053/j.gastro.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 31.Nelson DW, Murali SG, Liu X, Koopmann MC, Holst JJ, Ney DM. Insulin-like growth factor I and glucagon-like peptide-2 responses to fasting followed by controlled or ad libitum refeeding in rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1175–R1184. doi: 10.1152/ajpregu.00238.2007. [DOI] [PubMed] [Google Scholar]
- 32.Bahrami J, Yusta B, Drucker DJ. ErbB activity links the glucagon-like peptide-2 receptor to refeeding-induced adaptation in the murine small bowel. Gastroenterology. 2010;138:2447–2456. doi: 10.1053/j.gastro.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Takeuchi K, Kita K, Hayashi S, Aihara E. Regulatory mechanism of duodenal bicarbonate secretion: Roles of endogenous prostaglandins and nitric oxide. Pharmacol Ther. 2011;130:59–70. doi: 10.1016/j.pharmthera.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Dynamic regulation of mucus gel thickness in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2000;279:G437–G447. doi: 10.1152/ajpgi.2000.279.2.G437. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi K, Miyazawa T, Tanaka A, Kato S, Kunikata T. Pathogenic importance of intestinal hypermotility in NSAID-induced small intestinal damage in rats. Digestion. 2002;66:30–41. doi: 10.1159/000064419. [DOI] [PubMed] [Google Scholar]
- 36.Omatsu T, Naito Y, Handa O, Mizushima K, Hayashi N, Qin Y, Harusato A, Hirata I, Kishimoto E, Okada H, Uchiyama K, Ishikawa T, Takagi T, Yagi N, Kokura S, Ichikawa H, Yoshikawa T. Reactive oxygen species-quenching and anti-apoptotic effect of polaprezinc on indomethacin-induced small intestinal epithelial cell injury. J Gastroenterol. 2010;45:692–702. doi: 10.1007/s00535-010-0213-9. [DOI] [PubMed] [Google Scholar]
- 37.Hawkey CJ, Karrasch JA, Szczepanski L, Walker DG, Barkun A, Swannell AJ, Yeomans ND. Omeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs. Omeprazole versus Misoprostol for NSAID-induced Ulcer Management (OMNIUM) Study Group. N Engl J Med. 1998;338:727–734. doi: 10.1056/NEJM199803123381105. [DOI] [PubMed] [Google Scholar]
- 38.Yoda Y, Amagase K, Kato S, Tokioka S, Murano M, Kakimoto K, Nishio H, Umegaki E, Takeuchi K, Higuchi K. Prevention by lansoprazole, a proton pump inhibitor, of indomethacin -induced small intestinal ulceration in rats through induction of heme oxygenase-1. J Physiol Pharmacol. 2010;61:287–294. [PubMed] [Google Scholar]
- 39.Kato S, Tanaka A, Kunikata T, Umeda M, Takeuchi K. Protective effect of lafutidine against indomethacin-induced intestinal ulceration in rats: relation to capsaicin-sensitive sensory neurons. Digestion. 2000;61:39–46. doi: 10.1159/000007734. [DOI] [PubMed] [Google Scholar]
- 40.Wallace JL, Syer S, Denou E, de PG, Vong L, McKnight W, Jury J, Bolla M, Bercik P, Collins SM, Verdu E, Ongini E. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314–22. 1322. doi: 10.1053/j.gastro.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 41.Herman GA, Stevens C, Van DK, Bergman A, Yi B, De SM, Snyder K, Hilliard D, Tanen M, Tanaka W, Wang AQ, Zeng W, Musson D, Winchell G, Davies MJ, Ramael S, Gottesdiener KM, Wagner JA. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther. 2005;78:675–688. doi: 10.1016/j.clpt.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, Stoll B, Finegold MJ, Holst JJ, Hadsell D, Nichols BL, Burrin DG. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150–164. doi: 10.1053/j.gastro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Shimizu K, Koga H, Iida M, Haruma K. Microcirculatory changes in experimental mesenteric longitudinal ulcers of the small intestine in rats. Dig Dis Sci. 2007;52:3019–3028. doi: 10.1007/s10620-007-9804-6. [DOI] [PubMed] [Google Scholar]
- 44.Bozkurt A, Naslund E, Holst JJ, Hellstrom PM. GLP-1 and GLP-2 act in concert to inhibit fasted, but not fed, small bowel motility in the rat. Regul Pept. 2002;107:129–135. doi: 10.1016/s0167-0115(02)00095-2. [DOI] [PubMed] [Google Scholar]
- 45.Amato A, Baldassano S, Serio R, Mule F. Glucagon-like peptide-2 relaxes mouse stomach through vasoactive intestinal peptide release. Am J Physiol Gastrointest Liver Physiol. 2009;296:G678–G684. doi: 10.1152/ajpgi.90587.2008. [DOI] [PubMed] [Google Scholar]
- 46.Lee SJ, Lee J, Li KK, Holland D, Maughan H, Guttman DS, Yusta B, Drucker DJ. Disruption of the murine Glp2r impairs Paneth cell function and increases susceptibility to small bowel enteritis. Endocrinol. 2012;153:1141–1151. doi: 10.1210/en.2011-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A. 1996;93:7911–7916. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao Q, Boushey RP, Cino M, Drucker DJ, Brubaker PL. Circulating levels of glucagon-like peptide-2 in human subjects with inflammatory bowel disease. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1057–R1063. doi: 10.1152/ajpregu.2000.278.4.R1057. [DOI] [PubMed] [Google Scholar]
- 49.Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinol. 1999;140:5356–5363. doi: 10.1210/endo.140.11.7143. [DOI] [PubMed] [Google Scholar]
- 50.Amagase K, Ochi A, Kojo A, Mizunoe A, Taue M, Kinoshita N, Nakamura E, Takeuchi K. New therapeutic strategy for amino acid medicine: prophylactic and healing promoting effect of monosodium glutamate against NSAID-induced enteropathy. J Pharmacol Sci. 2012;118:131–137. doi: 10.1254/jphs.11r03fm. [DOI] [PubMed] [Google Scholar]
- 51.Yamazaki K, Yasuda N, Inoue T, Nagakura T, Kira K, Saeki T, Tanaka I. The combination of metformin and a dipeptidyl peptidase IV inhibitor prevents 5-fluorouracil-induced reduction of small intestine weight. Eur J Pharmacol. 2004;488:213–218. doi: 10.1016/j.ejphar.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Nelson DW, Holst JJ, Ney DM. Synergistic effect of supplemental enteral nutrients and exogenous glucagon-like peptide 2 on intestinal adaptation in a rat model of short bowel syndrome. Am J Clin Nutr. 2006;84:1142–1150. doi: 10.1093/ajcn/84.5.1142. [DOI] [PubMed] [Google Scholar]
- 53.Liu X, Murali SG, Holst JJ, Ney DM. Enteral nutrients potentiate the intestinotrophic action of glucagon-like peptide-2 in association with increased insulin-like growth factor-I responses in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1794–R1802. doi: 10.1152/ajpregu.90616.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]