Abstract
Background and Objectives:
The study aims at the observation of the immunohistochemical expression of CD44s in Oral Squamous Cell Carcinoma (OSCC) and to correlate its expression with prognostic parameters.
Materials and Methods:
A total of 30 cases of OSCC, - 10 cases of each well differentiated (WD SCC), moderately differentiated (MD SCC) and poorly differentiated squamous cell carcinomas (PD SCC) were included in the study. The sections were subjected to immunohistochemical study using CD44s antigen marker. The degree of intensity and distribution of CD44s immunostaining was assessed and correlated with prognostic markers such as tumor stage (tumor size), tumor grade (Broder's histological grading), tumor site, tumor thickness (histological depth of invasion) and nodal status.
Results:
CD44s expression by tumor cells in OSCCs is statistically correlated with tumor grade i.e. Higher mean of CD44s immunoexpression was observed in WD SCC group (10.80 ± 3.97), followed by MD SCC group (5.90 ± 3.38) and PD SCC group showed least CD44s immunoexpression (3.70 ± 4.64). There was no statistical significance observed with respect to the other prognostic markers.
Conclusion:
Based on these observations it can be suggested that the decrease in expression of CD44s in OSCC cells may be due to the reduced cell-to-cell and cell-to-matrix adhesion, resulting in easy detachment from the rigid constitution. Low expression of CD44s in OSCC tissues may be an indicator of tumor invasion and high metastatic potential.
Keywords: CD44s antigen, cell to cell adhesion, cell to matrix adhesion, oral squamous cell carcinoma
INTRODUCTION
Neoplasia or cancer is viewed as a cell cycle disease. Although this concept implies that every tumor is defective in one or more aspects of the cell cycle control, it clearly does not mean that oncogenesis targets only oncogenes and the cell cycle clock. Development of malignancy appears to require aberrations in the cell death machinery, cell-cell and/or cell-matrix interactions that co-operate with cell cycle defects. Many of the processes in which adhesion molecules play central role like anchorage dependent growth, apoptosis, differentiation and migration are those that are characteristically dysregulated in malignancy.[1]
Squamous Cell Carcinoma (SCC) is the most common malignant tumor of the oral cavity, accounting for over 90% of the malignant neoplasms. Nearly 60% of the patients present with an advanced stage of disease and its prognosis is influenced by a variety of factors, the most important being tumor stage (tumor size), tumor site, tumor thickness (depth of invasion), tumor grade (Broder's histological grading) and nodal status.[2,3,4].
CD44 is the major human cell surface receptor for hyaluronate and functions in a diverse range of physiological processes. CD44 may play a role in stimulating in vivo aggressiveness of tumors through hyaluronate-rich stroma.[5] Expression of CD44 has been described to correlate with metastasis in various tumors, although evidence in oral cancers is inconclusive. The purpose of the present study was to examine CD44s expression in OSCC and to investigate its correlation with a number of established prognostic parameters such as tumor stage (tumor size), tumor site, tumor thickness (depth of invasion), tumor grade (Broder's histological grading) and nodal status.
MATERIALS AND METHODS
Thirty cases of OSCC were retrieved from the archives of our college which included 10 cases of well differentiated (WD SCC), 10 cases of moderately differentiated (MD SCC) and 10 cases of poorly differentiated squamous cell carcinomas (PD SCC). Paraffin blocks of all cases were sectioned onto polylysin-coated slides. The avidin-biotin-peroxidase method was performed using the primary monoclonal antibodies against CD44s, standard isoform (Anti-CD44 antigen, clone –BGX- 297). Briefly, the sections were deparaffinized and washed in phosphate-buffered saline (PBS). Endogenous peroxidase activity was blocked using 0.3% solution of hydrogen peroxidase at room temperature for 5 minutes. After microwave treatment for antigen retrieval, primary antibodies were applied for 60 minutes at room temperature and washed in PBS. Linking antibody and HR-peroxidase complex (Biogenex Super Sensitive™ Polymer-HRP detection kit) were added consecutively for 20 minutes at room temperature and washed in PBS. The peroxidase activity was visualized with diaminobenzidine (DAB), applied for 5 minutes.
Evaluation of immunohistochemical staining
The most representative tumor areas were selected for scoring the immunostaining pattern. The degree of positive staining for CD44s antibody was evaluated by a well-established semiquantitative scoring on a scale of 1 to 4 for intensity (I) such as none, mild, moderate and strong; and for Distribution (D) such as none, focal, patchy and diffuse. Tissues with I × D less than or equal to four were considered weakly positive and those with I × D greater than four were designated strongly positive.
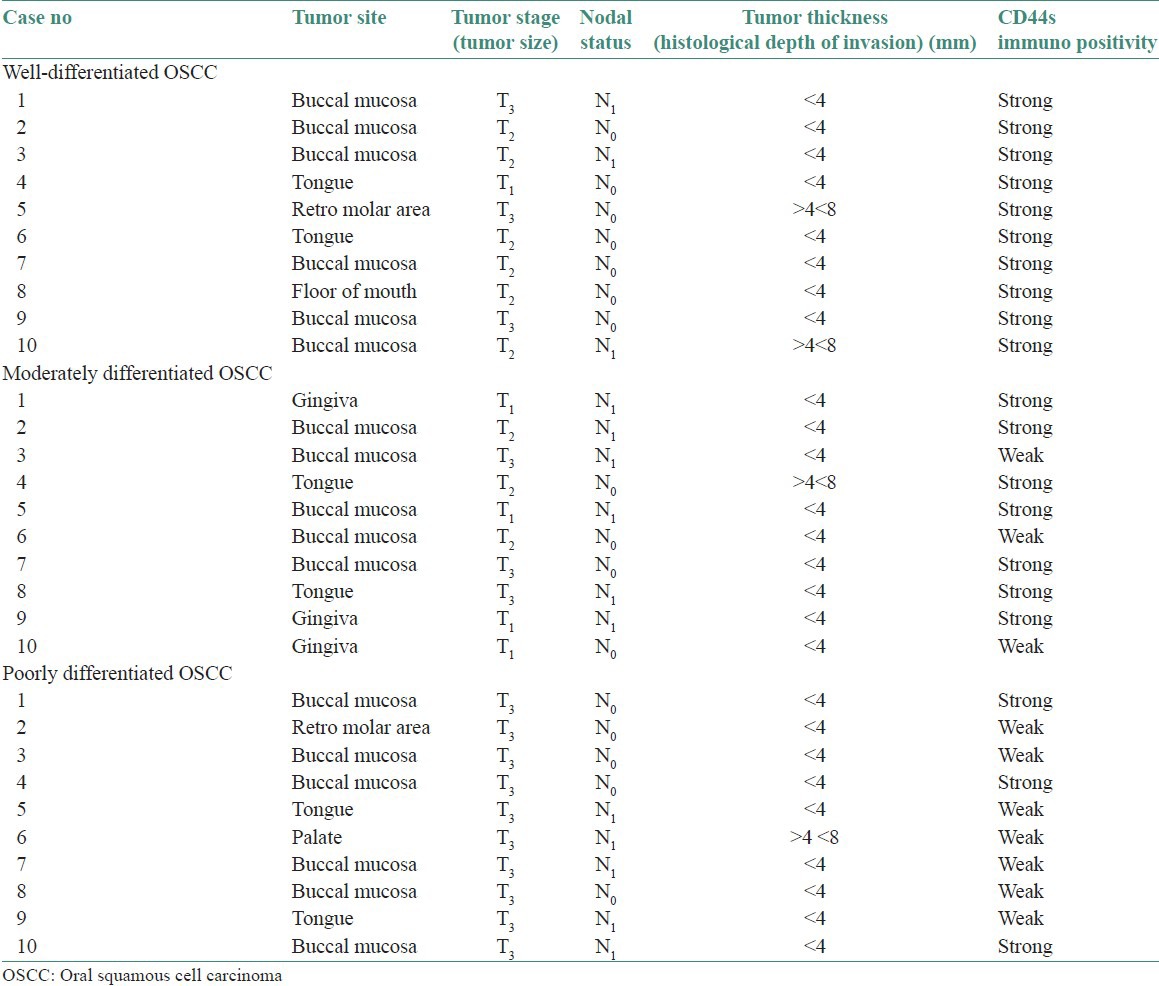
Tumor thickness or histological depth of invasion was assessed objectively using oculometer placed in the microscope. Measurements excluded layers of surface keratin and parakeratin. The cases were separated into three groups: Tumors with depth of invasion equal or less than 4 mm, tumors with depth of invasion greater than 4 mm but less than 8 mm and tumors with depth of invasion greater than 8 mm. The data so obtained for WDSCC, MDSCC0 and PDSCC are listed in Table 1.
Table 1.
CD44s expression in 30 cases of oscc correlating with prognostic markers
RESULTS
Using the above criteria following results were obtained:
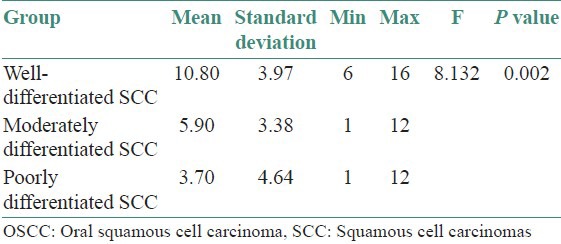
CD44s immunoexpression in the three grades of oral SCC: Higher mean CD44s immunoexpression is observed in WD SCC [Figures 1, 4 and 8] group showed the highest mean 10.80, followed by MD SCC [Figures 2, 7 and 9] group with a mean 5.90 and the PD SCC [Figures 3, 5 and 6] group showed the least CD44s immunoexpression of 3.70. The difference in mean expression between the groups is found to be statistically significant (P value = 0.002) [Table 2, Graph 1]
From the above ANOVA table, we conclude that there is a significant difference between the three groups with respect to the mean CD44s immunoexpression (P < 0.05). In order to find out among which pair of groups there exist a significant difference; multiple comparisons using Bonferroni test were carried out. From the multiple comparisons, we observed that there is a significant difference between WD SCC group and the MD SCC group (P < 0.05). Also, the difference in mean CD44s immunoexpression between WD SCC group and PD SCC group is found to be statistically significant (P < 0.01). But the difference in mean CD44s immunoexpression between MD SCC group and PD SCC group is not statistically significant (P > 0.05) [Table 3]
Association between CD44s immunoexpression and tumor site: The association between site and CD44s immunoexpression is not found to be statistically significant (P > 0.05). Both weakly positive and strongly positive cases were seen in higher numbers in buccal mucosal samples [Table 4, Graph 2]
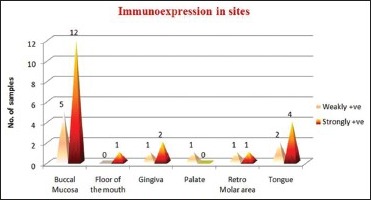
Association between tumor stage and CD44s immunoexpression: The association between tumor stage and CD44s immunoexpression is not found to be statistically significant (P > 0.05). Except in Stage T2, almost all the samples showed strongly +ve CD44s immunoexpression. In Stage T3, the samples are equally present in both weakly +ve and strongly +ve CD44s immunoexpression [Table 5, Graph 3]
Association between nodal status and CD44s immunoexpression: The association between nodal status and CD44s immunoexpression is not statistically significant (P > 0.05). Higher number of N0 samples as well as N1 samples showed strongly +ve CD44s expression category compared to weakly +ve CD44s immunoexpression category [Table 6, Graph 4]
Association between tumor thickness and CD44s immunoexpression: The association between thickness and CD44s immunoexpression category is not statistically significant (P > 0.05). Samples <4 mm tumor thickness showed high CD44s immunoreactivity in strongly positive category when compared with weakly positive CD44s immunoexpression category [Table 7, Graph 5].
Figure 1.
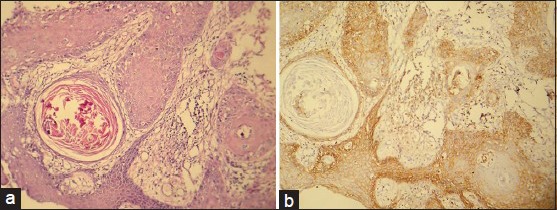
(a) Photomicrograph of well-differentiated sqamous cell carcinoma (H&E stain, ×100). (b) CD44s expression of the same. (IHC stain, ×100)
Figure 4.
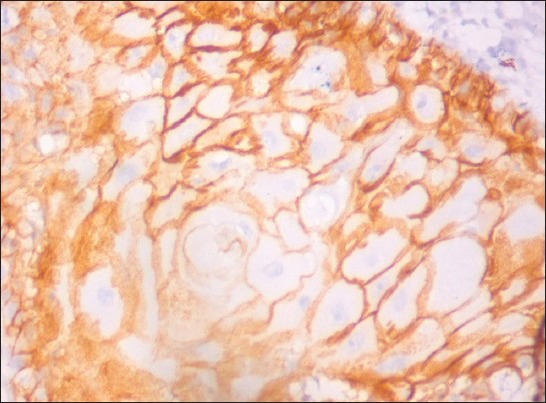
CD44 expression in well differentiated squamous cell carcinoma. (IHC stain, ×400)
Figure 8.
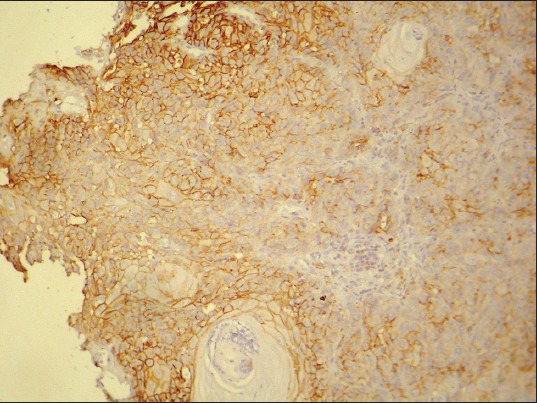
CD44 expression in well differentiated squamous cell carcinoma. (IHC stain, ×100)
Figure 2.
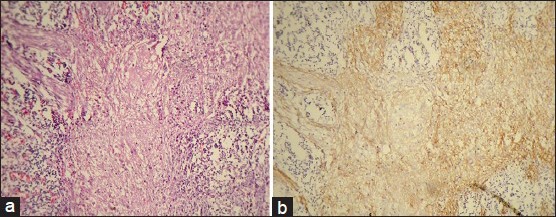
(a) Photomicrograph of moderately differentiated squamous cell carcinoma (H&E stain, ×100). (b) CD44s expression of the same. (IHC stain, ×100)
Figure 7.
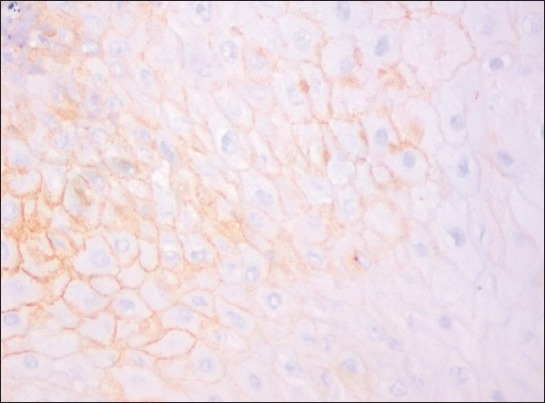
CD44 expression in moderately differentiated squamous cell carcinoma. (IHC stain, ×400)
Figure 9.
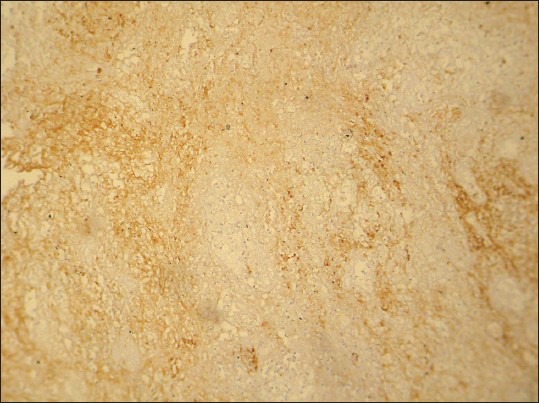
CD44 expression in moderately differentiated squamous cell carcinoma. (IHC stain, ×100)
Figure 3.
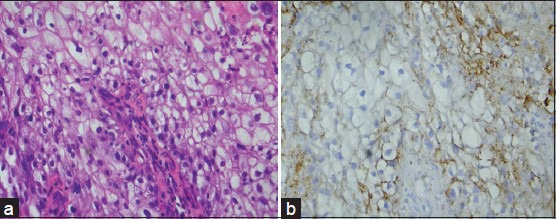
(a) Photomicrograph of poorly differentiated squamous cell carcinoma (H&E stain, ×400). (b) CD44s expression of the same (IHC stain, ×400)
Figure 5.
CD44 expression in poorly differentiated squamous cell carcinoma. (IHC stain, ×400)
Figure 6.
CD44 expression in poorly differentiated squamous cell carcinoma. (IHC stain, ×400)
Table 2.
Analysis of the CD44s immunoexpression in the three grades of OSCC
Graph 1.
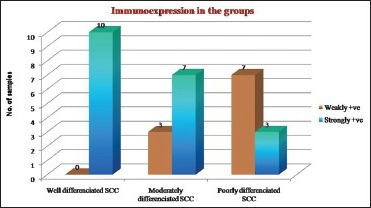
Intensity of CD44s expression in the three grades of OSCC
Table 3.
Multiple comparisons test using Bonferroni method. Dependent variable: Immunoexpression bonferroni
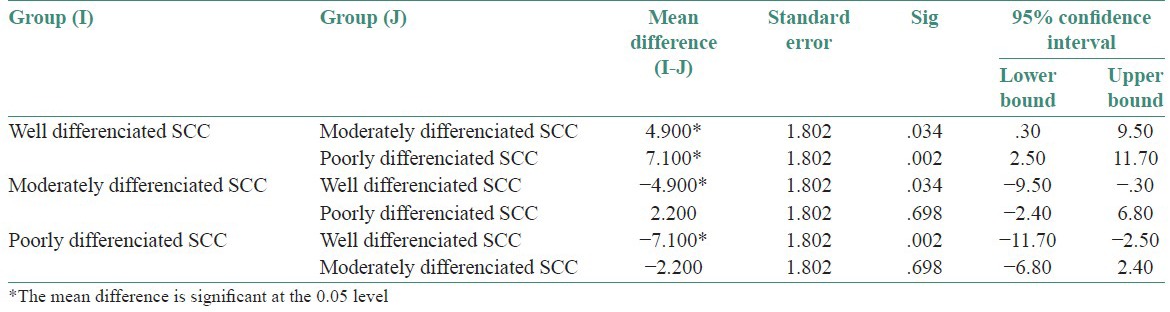
Table 4.
Evaluation of the association between CD44s immunoexpression (weakly +ve and strongly +ve) and tumor site
Graph 2.
CD44s expression in tumor site
Table 5.
Evaluation of the association between CD44s immunoexpression category (weakly +ve and strongly +ve) and tumor stage
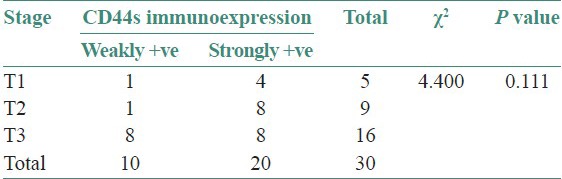
Graph 3.
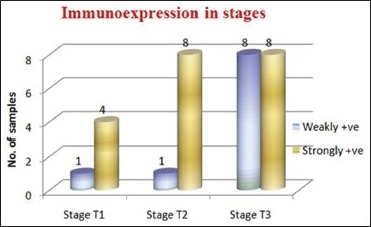
CD44s expression in the tumor stages
Table 6.
Evaluation of the association between CD44s immunoexpression category (weakly +ve and strongly +ve) and nodal status

Graph 4.
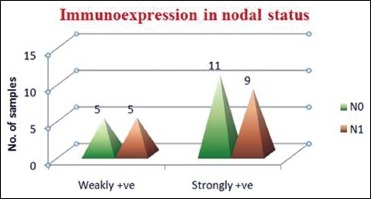
CD44s expression in nodal status
Table 7.
Evaluation of the association between CD44s immunoexpression category (weakly +ve and strongly +ve) and tumor thickness

Graph 5.
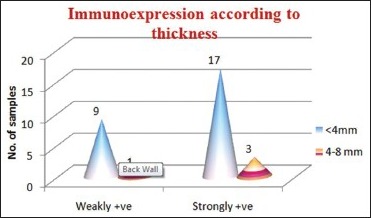
CD44s expression according to tumor thickness
DISCUSSION
CD44 is a multistructural and multifunctional cell surface molecule involved in cell proliferation, cell differentiation, cell migration, angiogenesis, presentation of cytokines, chemokines and growth factors to the corresponding receptors, docking of proteases at the cell membrane, as well as in signaling for cell survival. All these biological properties are essential to the physiological activities of normal cells, but they are also associated with the pathologic activities of cancer cells.[6]
CD44 is known to be a receptor of hyaluronate; functions in lymphocyte homing, cell adhesion and leukocyte activation. The physiological role of the molecule on different tissues remains to be clarified. Recently high molecular weight isoforms of CD44 have been found. These isoforms are characterized by certain insertions in the transmembrane domain. Genomic analysis indicated that the CD44 gene has 20 exons and the region encoding the insertion is composed of 10 exons that are alternatively spliced to produce variable isoforms carrying different membrane proximal inserts. The standard form does not include any of the v1 to v10 exons.[7]
Expression of the variable exons has been correlated with tumor progression and metastasis in a range of cell types. Multiple CD44 isoforms are expressed by normal stratified squamous epithelia, such as the epidermis and the lining of the oral cavity.[8]
Numerous studies on histopathologic features of tumor and host response parameters in OSCC have shown variable prognostic significance. The main prognostic determinate in carcinomas of the oral cavity is stage of the disease. The TNM classification of cancers arising in oral cavity, based upon extent and size of primary tumor, absence/presence and extent of regional lymph node metastasis, is a generally useful and widely applied method for estimating prognosis and planning therapy.[8]
Several studies have indicated that the down regulation of CD44 variants, including CD44v9, v4/5 and v6 is associated with tumor metastasis in OSCC. It has also reported that there is a positive correlation between the reduced immunoexpression of CD44v9, metastasis to lymph nodes and poor survival in OSCC of tongue.[9]
Keeping the above-discussed points in mind, we aimed at studying the relationship of CD44s expression in OSCC with the prognostic parameters.
In the present study, CD44s expression is observed in WD SCC group followed by MD SCC group and PD SCC group. The difference in mean CD44s expression between the groups is found to be statistically significant (P < 0.01). The staining pattern and intensity varied according to the degree of dysplasia and to the degree of differentiation of the SCC. This is in accordance with Herold-Mende et al. (1996),[10] Bahar et al. (1997).,[11] Kunishi et al. (1997).,[7] Kuo et al. (1998).,[12] Stoll et al.,[13] (1999), Kanke et al. (2000).,[14] Fonseca et al. (2001),[15] and Ue et al., (2007).[16]
The decrease in the intensity of the CD44s levels with the increase in the grade of the tumor suggests reduced cell-to-cell adhesion, resulting in easy detachment of the cells from a rigid constitution. Low expression of CD44s in OSCC tissues may be an indicator of high metastatic potential and may be related to lymph node metastasis. So decreased expression of CD44 may correlate with poor prognosis.[17]
In our study no correlation was found between CD44 expression and tumor stage, tumor site, tumor thickness or nodal status. The pattern and the thickness of tumor and presence of lymphatic-vascular invasion are important indicators in prediction of cervical lymph node metastasis.[18,19] The pattern of invasion characterized by infiltrative single cell is associated with a higher frequency of regional lymph node metastasis than the pattern composed of invasive cords of tumor.[8] The majority of tumors included in our study showed nests and sheets pattern. Most of the cases in our study were less than 4-mm tumor thickness and greater than 4 mm but less than 8 mm in thickness cases. The reason being, majority of patients had their tumors diagnosed at an early stage. The most common TNM classifications were T1 N0 M0 and T2 N0 M0. CD44s expression also did not correlate with the nodal status as the tumors were diagnosed early.
CONCLUSION
Based on these findings, we conclude that CD44s immunoexpression might be of prognostic value in assessing the stage of the tumor, invasive capacity and metastatic potential of the tumor. But, further studies on a larger samples are needed to establish the prognostic value of CD44 and its variant isoforms.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Luka Z, Dvorak K. Review article adhesion molecules in biology and oncology. Acta Vet Brno. 2004;73:93–104. [Google Scholar]
- 2.Wenig B. 1st ed. Philadelphia: W B Saunders; 1993. Atlas of head and neck pathology; pp. 715–8. [Google Scholar]
- 3.Tytor M, Olofsson J. Prognostic factors in oral cavity carcinomas. Acta Otolaryngol Suppl. 1992;492:75–8. doi: 10.3109/00016489209136815. [DOI] [PubMed] [Google Scholar]
- 4.Wildt J, Bjerrum P, Elbrand O. Squamous cell carcinoma of the oral cavity: A retrospective analysis of treatment and prognosis. Clin Otolaryngol Allied Sci. 1989;14:107–13. doi: 10.1111/j.1365-2273.1989.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 5.Hudson DL, Speight PM, Watt FM. Altered expression of CD44 isoforms in squamous-cell carcinomas and cell lines derived from them. Int J Cancer. 1996;66:457–63. doi: 10.1002/(SICI)1097-0215(19960516)66:4<457::AID-IJC8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Naor D, Nedvetzki S, Golan I, Melnik L, Faitelson Y. CD44 in Cancer. Crit Rev Clin Lab Sci. 2002;39:527–79. doi: 10.1080/10408360290795574. [DOI] [PubMed] [Google Scholar]
- 7.Kunishi M, Kayada Y, Yoshiga K. Down regulated expression of CD44 variant 6 in oral squamous cell carcinomas and its relationship to regional lymph node metastasis. Int J Oral Maxillofac Surg. 1997;26:280–3. doi: 10.1016/s0901-5027(97)80869-7. [DOI] [PubMed] [Google Scholar]
- 8.Ozer E, Kuyucuo-Lu F. Correlation of CD44 expression with prognostic factors in oral squamous cell carcinoma. East J Med. 1999;4:61–4. [Google Scholar]
- 9.Satoa S, Miyasuchia M, Takekoshia T, Zhaoa M, Kudoa Y, Ogawab I, et al. Reduced expression of CD44 variant 9 is related to lymph node metastasis and poor survival in squamous cell carcinoma of tongue. Oral Oncol. 2000;36:545–9. doi: 10.1016/s1368-8375(00)00049-x. [DOI] [PubMed] [Google Scholar]
- 10.Herold-Mende C, Seiter S, Born AI, Patzelt E, Schupp M, Zöller J, et al. Expression of CD44 splice variants in squamous epithelia and squamous cell carcinomas of the head and neck. J Pathol. 1996;179:66–3. doi: 10.1002/(SICI)1096-9896(199605)179:1<66::AID-PATH544>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Bahar R, Kunishi M, Kayada Y, Yoshiga K. CD44 variant 6 (CD44v6) expressions as a progression marker in benign, premalignant and malignant oral epithelial tissues. Int J Oral Maxillofac Surg. 1997;26:443–6. doi: 10.1016/s0901-5027(97)80010-0. [DOI] [PubMed] [Google Scholar]
- 12.Mark YK, Shih-Jung C, Hsin-Ming C, Sang-Heng K, Liang JH, Chun-Pin C. Expression of CD44s, CD44v5, CD44v6 and CD447-8 in betal quid chewing associated oral premalignant lesions and squamous cell carcinomas in Taiwan. J Oral Pathol Med. 2007;27:428–33. doi: 10.1111/j.1600-0714.1998.tb01980.x. [DOI] [PubMed] [Google Scholar]
- 13.Stoll C, Baretton G, Soost F, Terpe HJ, Domide P, Lohrs U. Prognostic importance of the expression of CD44 splice variants in oral squamous cell carcinomas. Oral Oncol. 1999;35:484–9. doi: 10.1016/s1368-8375(99)00021-4. [DOI] [PubMed] [Google Scholar]
- 14.Kanke M, Fujii M, Kameyama K, Kanzaki J, Tokumaru Y, Imanishi Y, et al. Clinicopathological significance of expression of CD44 variants in head and neck squamous cell carcinoma. Jpn J Cancer Res. 2000;91:410–5. doi: 10.1111/j.1349-7006.2000.tb00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonseca I, Pereira T, Rosa-Santos J, Soares J. Expression of CD44 isoforms in squamous cell carcinoma of the border of the tongue: A correlation with histological grade, pattern of stromal invasion and cell differentiation. Journal Of Surgical Oncology. 2001;76:115–12. doi: 10.1002/1096-9098(200102)76:2<115::aid-jso1021>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Ue T, Yokozaki H, Kagai K, Higashikawa K, Yasui W, Sugiyama M, et al. Reduced expression of CD44 variant exons in oral squamous cell carcinoma and its relationship to metastasis. J Oral Pathol Med. 1998;27:197–01. doi: 10.1111/j.1600-0714.1998.tb01941.x. [DOI] [PubMed] [Google Scholar]
- 17.Carinci F, Stabellini G, Calvitti M, Pelucchi S, Targa L, Farina A, et al. CD44 as prognostic factor in oral and oropharyngeal factor squamous cell carcinoma. J Craniofac Surg. 2002;13:85–9. doi: 10.1097/00001665-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Martínez-Gimeno C, Rodríguez EM, Vila CN, Varela CL. Squamous cell carcinoma of the oral cavity: A clinicopathologic scoring system for evaluating risk of cervical lymph node metastasis. Laryngoscope. 1995;105:728–33. doi: 10.1288/00005537-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Shingaki S, Suzuki I, Nakajima T, Kawasaki T. Evaluation of histopathologic parameters in predicting cervical lymph node metastasis of oral and oropharyngeal carcinomas. Oral Surg Oral Med Oral Pathol. 1988;66:683–8. doi: 10.1016/0030-4220(88)90318-0. [DOI] [PubMed] [Google Scholar]


