Abstract
Aims and Objective:
To demonstrate the presence, location and pattern of cell proliferation in different histological grades of oral epithelial dysplasia (OED), oral squamous cell carcinoma (OSCC) and normal oral epithelium (NOE) using an antibody directed against the Ki-67 antigen and its intensity of staining evaluated respectively.
Materials and Methods:
A total number of 100 archival paraffin embedded blocks obtained from Department of Oral and Maxillofacial Pathology were studied. The case details were retrieved which consisted of histopathologically diagnosed cases of OSCC (n = 20), low risk OED (n = 30), high risk OED (n = 30) and normal appearing mucosa (n = 20) were taken as standard for comparison. Ki-67 immunostaining was detected. Ki-67 positive cells were counted in the five random high power fields in each case.
Results:
Ki-67 labeling Index (LI) was restricted to the basal and parabasal layers of the normal oral epithelium irrespective of age, sex and site whereas it was seen in the basal, suprabasal and spinous layers in OED. Ki-67 LI is increased in high risk cases than the low risk cases of OED. Ki-67 positive cells in OSCC were located in the periphery of the tumor nests than the center, where frequent mitoses were observed.
Conclusion:
The architectural alteration evaluated by Ki-67 antibody in proliferating cell distribution in the layers of epithelial dysplasias may provide useful information to evaluate the grading of OED. Ki-67 LI increased in high risk cases than low risk cases of OED. This study showed that over expression of Ki-67 antigen between well-differentiated and poorly differentiated OSCC was in accordance with histologic grade of malignancy but not in accordance with moderately differentiated OSCC.
Keywords: Cell proliferation, Ki-67 LI (Labeling Index), Oral epithelial dysplasia, Oral squamous cell carcinoma
INTRODUCTION
The cell is the basic, living, structural and functional unit of the body.[1] Cell proliferation is a biological process of vital importance to all living organisms and is fundamental to both embryonic and post embryonic existence.[2] The control on this important biological process is thought to be lost in cancer[3] and many studies have reported that abnormal cell proliferation appears to be a precursor and may be a predictor of tumorigenesis.[4] The development of cancer is a complex succession of events and multistep process in which the genomes of cancer cells acquire mutant alleles of proto-oncogenes, tumor-suppressor genes and other genes that control, directly or indirectly, cell proliferation.[5] Genetic aberrations are necessary for the affected tumor cell to express malignant phenotype.
Epidemiological studies of oral cancer showed that Southern Asia had the highest incidence of oral cancer, accounting for 18% of all cancers.[6] Oral squamous cell carcinoma (OSCC) being most prevalent type of cancer represents about 91% of the diagnosed cases of malignant tumors of the mouth.[7] The risk factors for oral cancers is closely related to lifestyle, such as tobacco use, alcohol use, poor oral hygiene and betel quid chewing habit.[8] Clinicopathologically, malignant transformation of oral precancerous lesion is observed in up to 17.5% of the cases.[9]
Studies have shown that about 80% of oral cancers were preceded by oral precancerous lesions or conditions.[10] Theories of carcinogenesis suggest that premalignant change may occur in any area of mucous membrane exposed to carcinogens with the risk of developing a second or multiple primary carcinomas.[11,12] The proliferative activity of any tissue or neoplasm can be determined by its growth rate using antibodies directed against specific antigens allowing the simultaneous analysis of cell proliferation and histology.
The two most common immunohistochemical markers used to study cell proliferation are proliferating cell nuclear antigen (PCNA) and Ki-67 antigen.[3]
Of these two markers Ki-67 has been shown to be excellent for the estimation of the growth fraction in both normal and malignant human tissue and this antibody is now used as the usual standard for the assessment of cell proliferation than PCNA as it does not suffer much from the influence of internal and external factors. Its nuclear expression during a defined period of the cell cycle represents an advantage in its use as a biological marker of mitotic activity.[13] Also it has a much shorter half life, thus producing less residual staining after cells have gone through proliferative stage.[14] Its demonstration therefore indicates the proliferative stage of the cell rather than being just residual evidence of the cell that has passed through the stage. Ki-67 is not involved in DNA repair.[15] PCNA is not proliferation specific.[16] Many studies revealed a poor correlation between this antigen and other proliferation markers, in addition to clinical parameters.[17] Consequently, PCNA staining is no longer recommended for use in surgical pathology as it lacks all above mentioned advantages of Ki-67.[18] The fraction of Ki-67 positive cells is often correlated with the clinical course of the disease. Ki-67 marker has been extensively examined in oral epithelial dysplasia (OED) and OSCC.[19] Recently, it was demonstrated that Ki-67 gene suffers “over expression” in epithelial cells of premalignant and malignant oral lesions.[13]
Following the above information, the present study aimed to evaluate the potential association between histologic grades of OED and OSCC by the proliferative marker Ki-67 and compare it with normal appearing mucosa.
MATERIALS AND METHODS
The retrospective study was conducted on sections obtained from the 100 archival paraffin embedded blocks of patients diagnosed histologically as OED and OSCC from the Department of Oral and Maxillofacial Pathology and Microbiology.
Of 100 diagnosed cases 20 were normal mucosa, 60 were OED and 20 were OSCC. According to Kujan et al.,[19] 60
OED were divided 30 each between low risk and high risk OED. OSCCs were subdivided into well-differentiated (WDSCC), moderately differentiated (MDSCC) and poorly differentiated squamous cell carcinoma (PDSCC).
Group-I (n = 20): Normal appearing oral mucosal specimens obtained from the lesional tissue
Group-II (n = 60): Patients diagnosed clinically as potentially malignant disorders and histologically diagnosed as OED
Group-III (n = 20): Patients diagnosed clinically and histologically as WDSCC (n = 7), MDSCC (n = 7) and PDSCC (n = 6).
Immunohistochemical procedure
Immunohistochemical (IHC) detection of Ki-67 was performed using prediluted rabbit monoclonal antibody (6.0 ml), provided by Biocare Medical bearing control number: 901-325-091911, certified by ISO 9001 and 13485 bearing catalog number: PRM 325 AA, which was ready-to-use and has been standardized with Biocare's MACH 2 detection system. This antibody was stored at 2-8ºC.
For IHC procedure two paraffin embedded tissue sections for each case of the above groups were obtained using semiautomatic microtome approximately of 4 μm thickness. Out of two sections, one section was stained by hematoxylin and eosin [Figure 1a–f] while other serial section of the same was stained with Ki-67. For IHC sections were placed on precoated slides.
Figure 1.
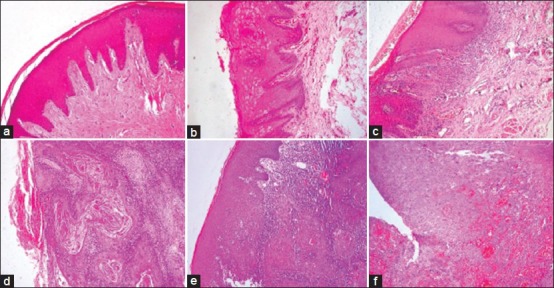
Photomicrograph showing (a) Normal oral mucosal epithelium, (b) Low risk oral epithelial dysplasia(OED), (c) High risk OED, (d) Well differentiated squamous cell carcinoma, (e) Moderately differentiated squamous cell carcinoma and (f) Poorly differentiated squamous cell carcinoma (H&E stain, ×100)
Positive control consisted of paraffin embedded sections of human tonsil tissue with known antigenic reactivity to Ki-67 in the lymphoid follicles and a negative control was performed in all cases by omitting the step of primary antibody during the staining, which resulted in lack of staining in all cases.
All glassware was gently cleaned with running distilled water prior to the usage to avoid background staining and nonspecific deposits on tissue sections. The slides were fixed on a slide warming table at 60ºC for 15 mins. The sections were cleared by passing through two changes of xylene for 10 mins each and rehydrated by passing through two changes of absolute alcohol. Then rinsed thoroughly with distilled water and kept in the distilled water koplin jars until antigen retrieval.
Preparation of antigen retrieval solution: Diva Decloaker solution which was concentrated 10 × was diluted with distilled water in the ratio of 1:10. The slides were placed in above prepared buffer solution in a slide racks and kept in Decloaking chamber. Distilled water of 500 ml was poured in Decloaking chamber before keeping the racks. The chamber was closed with the lid and switched on by pressing the start button on the front panel. The temperature was allowed to rise till 125ºC and then allowed to gradually taper down till 90ºC followed by gradual cooling back to the room temperature. Slides were rinsed thoroughly with distilled water.
IHC staining procedure: All reagents were brought to room temperature prior to immunostaining. Incubations were performed at room temperature in a humidifying chamber and sections were not allowed to dry out during the staining procedure.
The sections were blocked for any endogenous peroxide activity for 5 mins and then washed
Sniper Protein Block was used for 10 mins
primary antibody was applied for 30 mins
MACH 1 polymer wasapplied for 35 mins
Betazoid DAB chromogen was applied for 10 mins
The sections were counterstained with CAT hematoxylin and then rinsed firstly with buffer and later by distilled water. Then slides were mounted in DPX.
The slides were viewed under bright field microscope and compared with their respective H and E sections. Cells were considered to be positive for Ki-67 antigen if there was any staining of nucleoplasm or nucleoli as this is a component of the nuclear matrix and a cell cycle-associated nuclear antigen according to the study by Verheuen et al.,[20]
All sections were evaluated for distribution and intensity of the immunohistochemical reaction product. The staining pattern was observed and made a note of. Diffuse staining of the nucleoplasm and a granular pattern, which stained nucleoli or granules of different size were dispersed throughout the nucleoplasm. Some nuclei showed a mixed pattern; that is strongly stained granules against a diffusely positive background. We classified these nuclei as granular. The staining intensity was classified as strong, moderate or weak.
In this study nuclear distribution was found to be granular, diffuse or a combination of both which is in agreement with previous reports.[18]
Two observers performed the counts twice independent of each other, but from the same areas of the epithelium regardless of staining quality to overcome inter as well as intra observer bias.
Ki-67 LI was determined by the number of positive nuclear profiles/mm2 of epithelial cells. Preferably 5 nonoverlapping areas of high power field (40X = 0.1 mm2 of epithelium) of a IHC slide were captured by Olympus DSLR camera and these areas were viewed for Ki–67 expression. These photographs were analyzed by image analyzer Biowizard Dewinter software 4.1 version with grid system. Positive cells were counted as recommended by Iamaroon et al., (2004).[21] The Ki-67 protein was extensively examined in OED where the number of proliferating cells increased according to the grade of dysplasia and OSCC. The histological examinations mainly focused on the total number of positive cells within the epithelium as an index of malignancy rather than for architectural distribution within the altered epithelium. The nuclear expression of Ki-67 antibody was counted according to epithelial layers/strata as the basal layer, with positive nuclei present just above the basement membrane; parabasal layer, positive nuclei within two layers above the basement membrane and next to the basal layer; and suprabasal layer, with nuclear positivity in layers/strata above the parabasal layer.
Statistics
A descriptive statistical analysis was carried out to tabulate our results. Significance was assessed at 5% level of significance. The values were noted and subjected to Analysis of variance (ANOVA) and Post-hoc Tukey test with P < 0.001 as statistically significant.
RESULTS
The Ki-67 expression was detected in all cases of normal oral epithelium (NOE) and was restricted to the basal and parabasal layers of the epithelium with parabasal layer showing intense staining which is in accordance with a previous report by Takeda et al.[22]
Overall comparison of Ki-67 positivity among study groups showed variable results
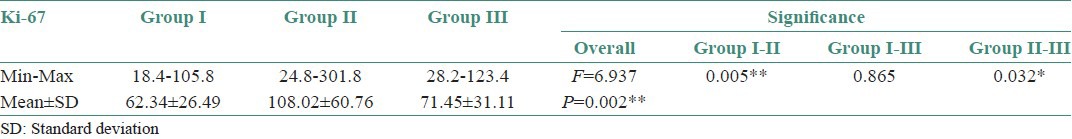
Ki-67 positive cells comparison between Group I [Figures 2a and b] and Group II, showed ‘P’ value of 0.0005 which is strongly significant statistically whereas between Group I and low risk Group II was not statistically significant as the sample size was more for low risk OED than NOE. This was in accordance with the study by Alfredo Maurıcio Batista de Paula et al.,[23] indicating that inflammation induces an increase in the number of epithelial cells in proliferative/cell cycle stage. Comparison between Group I and III was not statistically significant; whereas comparison between Group II and III was moderately significant statistically [Table 1 and Graph 1].
Figure 2.
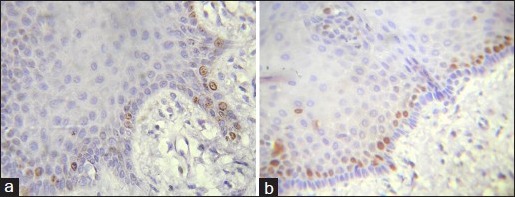
a and b: Photomicrograph showing Ki-67 expression in normal oral mucosa seen in basal and parabasal layer (IHC stain, ×400)
Table 1.
Comparison of Ki-67 in normal oral epithelium, oral epithelial dysplasia and Oral Squamous Cell Carcinoma cases
Graph 1.
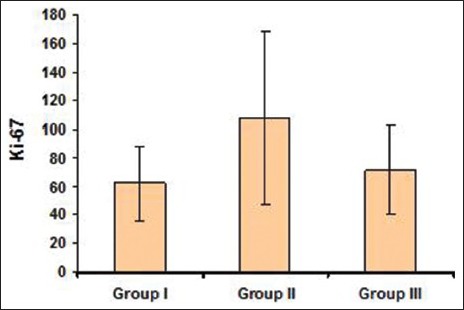
Comparison of Ki-67 in normal oral epithelium, oral epithelial dysplasia and Oral Squamous Cell Carcinoma cases
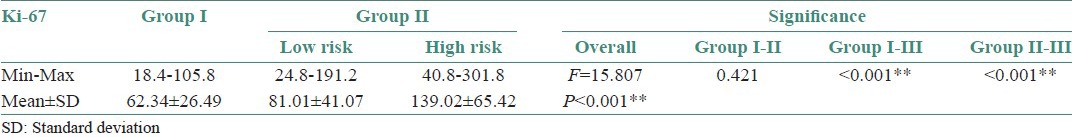
Strong significant differences were observed between Group I and high risk Group II (P < 0.001). Comparison between low risk [Figure 3a and b] and high risk Group II OED [Figure 4a and b] showed significant P value (<0.001) [Table 2 and Graph 2].
Figure 3.
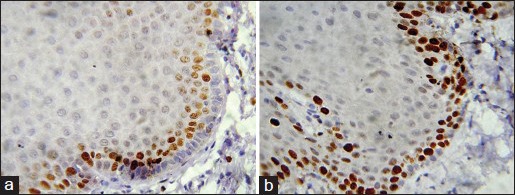
a and b: Photomicrograph showing Ki-67 expression in low risk oral epithelial dysplasia seen in basal, parabasal and spinous layer (IHC stain, ×400)
Figure 4.
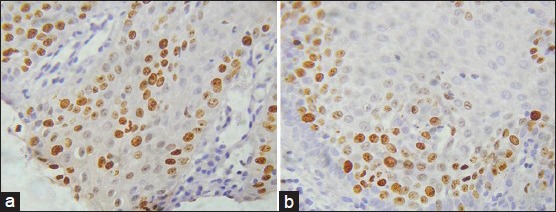
a and b: Photomicrograph showing Ki-67 expression in high risk oral epithelial dysplasia seen in the parabasal as well as superficial spinous layers of the epithelium (IHC stain, ×400)
Table 2.
Comparison of Ki-67 in low risk and high risk Oral Epithelial Dysplasia cases
Graph 2.
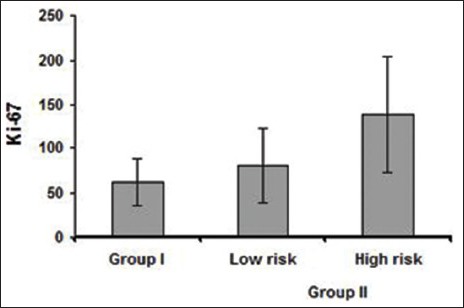
Comparison of Ki-67 in low risk and high risk oral epithelial dysplasia cases
The nuclear Ki-67 positivity was found to be increased and positivity was observed reaching up to the superficial layers of the epithelium, according to the grades of dysplasia.
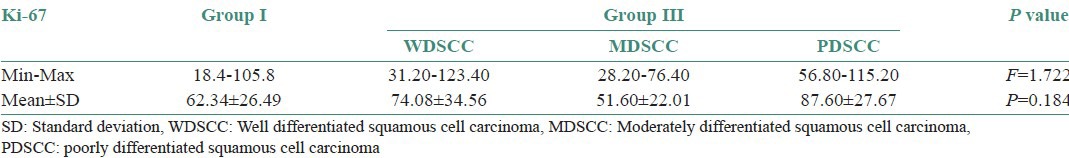
Comparison of Ki-67 positivity among WDSCC, MDSCC and PDSCC
Statistical analysis showed no significant results. The PDSCC showed the highest mean of Ki-67 labeling index LI followed by WDSCC and MDSCC. Ki-67 positivity in OSCC located at the periphery of the tumor nests than the center of WDSCC [Figure 5a and b] which appeared granular but whereas it was diffuse and patchy in most of the PDSCC [Figure 6a and b; Table 3 and Graph 3].
Figure 5.
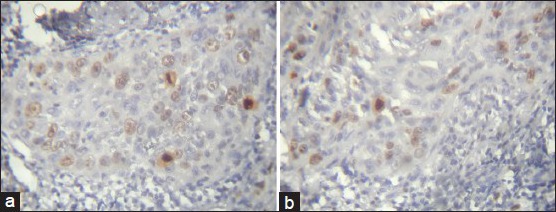
a and b: Photomicrograph showing Ki-67 expression in well-differentiated squamous cell carcinoma seen at peripheral area of tumor islands. Few mitotic figures are also seen (IHC stain, ×400)
Figure 6.
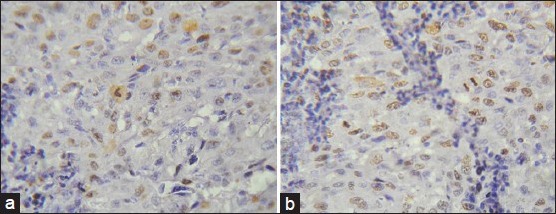
a and b: Photomicrograph showing Ki-67 expression in poorly differentiated squamous cell carcinoma is scattered and diffuse throughout the tumor islands with mitotic figure (IHC stain, ×400)
Table 3.
Comparison of Ki-67 in well-differentiated squamous cell carcinoma, Moderately differentiated squamous cell carcinoma and poorly differentiated squamous cell carcinoma cases
Graph 3.
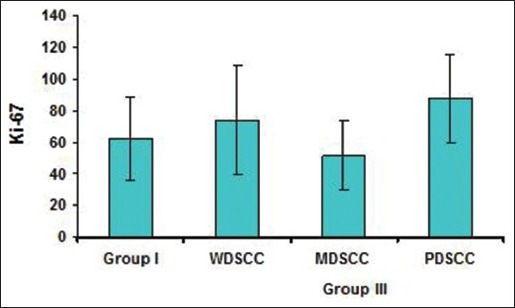
Comparison of Ki-67 in Well differentiated squamous cell carcinoma, moderately differentiated squamous cell carcinoma and poorly differentiated squamous cell carcinoma cases
DISCUSSION
Oral mucosa is made up of stratified squamous epithelium (SSE); the stratification is the result of cell proliferation and sequential differentiation.[24]
Proliferation is a property of stem cells of the basal layers of the SSE and their immediate progeny, the transit-amplifying cells.[25] Differentiation starts when recently divided cells detach from the underlying extracellular matrix.[26] As the differentiating cells mature, they are pushed toward the epithelial surface by the pressure generated in the underlying proliferation compartment.[24]
Proliferation and differentiation are controlled by autocrine and paracrine factors generated by the keratinocytes; the cytokines and growth factors originating in the underlying connective tissue and the circulating systemic factors.[27]
Cell proliferation, a vital biological process, is an important adjunct to histologically based tumor classification and has potential relevance as an indicator of treatment response and relapse. Many studies have reported that abnormal cell proliferation appears to be a precursor and may be a predictor of tumorigenesis.[4]
Various immunohistochemical markers are used to detect cellular proliferation of which Ki-67 is used as a more reliable marker of proliferation in our study.
The monoclonal antibody Ki-67 was first described in 1983 by Johannes Gerdes et al., who suggested that it might be used as a marker for proliferating cells.[28] Immunostaining with antibodies to Ki-67 antigen is well established as a quick and efficient method for evaluating growth fractions of various tumor types because of its distinctive reaction patterns that exclusively involves the proliferating cells.[29] The Ki-67 antibody was first isolated during attempts to raise monoclonal antibodies to antigens specific for Hodgkin and Reed-Sternberg cells.[28] Ki-67 stood out from other antibodies because it only reacted with cells which were proliferating, for example cortical thymocytes and cells in the crypts of the small intestine, whereas it would show no reaction with cells which were known to be in a resting or terminally differentiated state, such as liver cells and neurones.[29] The Ki-67 antigen was named after its place of characterization in Kiel, Germany and because the clone producing the antibody was grown in the 67th well of tissue culture plate.[30] It is a large basic protein found as peptides with molecular weights of 345 kD and 395 kD[31] which have been detected within the nucleus and its gene is located on chromosome 10q25-ter.[32] Ki-67 is not expressed in cells showing an arrest in cell cycle and starts to be expressed in the S-phase, progressively increasing through S and G2 phases which reaches a plateau at mitosis as appropriate stimulation occurs in G1 phase where there is a subsequent increase in the level of Ki-67 protein. If no proper stimulation to proliferate is received then the cell enters Go and production of the Ki-67 protein drops to an undetectable level.[29]
Our aim was to study and interpret the relationship of Ki-67 LI with different histological grades of OED and histologically diagnosed different grades of OSCC. Again these were compared with NOE for the proliferative index. In our study the Ki-67 expression in all the cases of NOE was found to be restricted to the basal and parabasal layers of the epithelium and mainly presented in the parabasal layer where the numbers of proliferating cells were limited when compared to the basal layer of NOE. There were no significant differences of LIs between groups by age, sex and region.
Several lines of evidence, including clinical, experimental and morphological data, support the concept that squamous cell carcinoma of the upper aerodigestive tract arises from noninvasive lesions of the squamous mucosa. These lesions encompass a histological continuum between the normal mucosa at one end and high grade dysplasia/carcinoma in situ, at the other end, establishing a model of neoplastic progression.[33]
Cancer being a genetic disorder, involves multiple alterations of the genome progressively accumulated during a protracted period, the overall effects of which surpass the inherent reparative ability of the cell.[33] Histologically, the majority of oral cancers are OSCC. In the oral cavity, OSCC is thought to develop from precancerous dysplastic lesions by multistep carcinogenesis. In fact, OSCC frequently co-exists with or is surrounded by epithelial dysplasia or leukoplakia. Clinicopathologically, malignant transformation of oral precancerous lesions is observed at a frequency of up to 17.5%,[6] although malignant transformation may rarely also develop directly from normal epithelium.[34] In the course of its progression, visible physical changes take place at the cellular level (atypia) and at the resultant tissue level (dysplasia). These alterations include genetic changes, epigenetic changes, surface alterations and alterations in intercellular interactions. The sum total of these physical and morphological alterations are of diagnostic and prognostic relevance and are designated as precancerous changes.[33] Maerker and Bukradt found a correlation between the development of carcinomas and the grade of dysplasia of the primary lesions. Oral leukoplakia is a precancerous lesion that can exhibit the histopathologic features of OED.[27] The percentage of leukoplakia that progress to invasive OSCC is accepted to be directly related to the severity of dysplastic changes. They range from 5% for leukoplakia with mild to moderate dysplasia to up to 43% for leukoplakia showing with severe dysplasia or carcinoma in situ (CIS). Patient can be presented with multiple lesions at the same site or different site. This phenomenon is usually referred to as field cancerization suggesting that these patients exhibit susceptibility to malignant transformation through the epithelia exposed to exogenous carcinogens (usually tobacco related) that result in higher probability of developing multiple precursor lesions and malignancies at other sites.[35]
OED presents as an alteration of the cellular maturation in the epithelium and as increase in the proliferative activity in suprabasal layers, that is spinous layer, which helps to establish a more objective diagnosis. Studies have revealed that Ki-67 positivity increased according to the proliferative activity and degree of epithelial dysplasia. Thus implicating as a marker of the proliferation and exhibiting the degree of severity of OED.
In the present study, OED group is subdivided as suggested by Kujan et al.,[24] into low risk group and high risk group. In this study proliferation was seen in the basal, parabasal and lower spinous layer in the low risk lesions, whereas it has extended to the superficial part of the spinous layer in high risk lesions. The number of proliferating cells which had stained positive had increased till the superficial layers of the epithelium according to the grade of dysplasia as it is increased in high risk than low risk Group II cases and up to CIS. This increased proliferation in parabasal layers of premalignant oral epithelium is likely related to loss of heterozygosity in 3p, 9p and 17p which behaves as a marker of precancerous fields and increases the risk of developing multiple tumors as stated by Tabor and Brakenhoff et al.[33]
Increase in Ki-67 LI was seen in the basal, parabasal and spinous layers of OED as proliferative activity increased due to cellular alteration. The Ki-67 positivity was constant through every grade in the parabasal layer. The number of Ki-67 positive cells when compared between Group I and low risk Group II, was not significant statistically as the P value obtained was 0.421. In low risk OED maximum expression of Ki-67 was in the basal layer followed by parabasal and then spinous layer which showed the least expression. Whereas, it was highly significant statistically when Group I and high risk Group II were compared (P < 0.001). When Group II low risk OED and high risk OED were compared the P value obtained was < 0.001 was highly statistically significant.
The increased proliferating cell population in both basal and suprabasal layers of OED in this study suggest that proliferating cells might increase not only in a superficial direction but also downward to the basal layer in OED.
Ki-67 positive cells in WDOSCC were located in the periphery of the tumor nests where frequent mitoses were observed than the central areas of squamous maturation which suggest that less differentiated cells are located in peripheral layer and the central cells are highly differentiated with an ability to keratinize, thus no expression of Ki-67 was observed in the central cells of the tumor island.
In MDSCC, Ki-67 expression observed in both peripheral and part of central layer as cells were less differentiated than WDSCC and had shown a lesser proliferation rate when compared to WDSCC which was not in accordance with the previous studies but correlates with the study done by Roland et al., (1994)[36] and Piffko et al., (1996)[37] on OSCC.
PDSCC Ki-67 expression was diffuse and more intense as the cells were less differentiated than WDSCC as well as MDSCC. More number of cells were in proliferative phase and hence showed an increase Ki-67 LI than WDSCC and MDSCC. These findings correlate with previously mentioned studies. The staining of Ki-67 positive cells was patchy in most of the PDSCC whereas it was granular and localized to the nuclei in cases of MDSCC and WDSCC.
In our study when Group I and Group III were compared, no statistical significance was observed, but when Group II and Group III were compared the P value obtained was 0.032 which was moderately significant statistically which signify the fact that dysplastic epithelium holds a high potential for malignant transformation.
In conclusion, we propose that Ki-67 is a reliable proliferative marker which can be used for the diagnosis of OEDs which have tendency to undergo malignant transformation. Information on the growth fraction of the tumors may be used in the assessment of tumor grade and in all tumors which have been studied by Ki-67 staining, a highly significant correlation between Ki-67 staining and the degree of malignancy has been reported. Furthermore, a marked variation in the amount of Ki-67 expression within different tumor grades is observed, indicating that Ki-67 staining may be of use in individual tumor diagnosis and prognosis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.David H Cormach. Textbook of Ham's Histology. In: Barnes D, Winters R, Maxwell ME, editors. 9th ed. Philadelphia: J. B Lippincott Company; 1987. pp. 1–23. [Google Scholar]
- 2.Pardee AB. G1 events and regulation of cell proliferation. Science. 1989;246:603–8. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 3.Tumuluri V, Thomas GA, Fraser IS. Analysis of the Ki-67 antigen at the invasive tumour front of human oral squamous cell carcinoma. J Oral Pathol Med. 2002;31:598–604. doi: 10.1034/j.1600-0714.2002.00042.x. [DOI] [PubMed] [Google Scholar]
- 4.Bacci CE, Gown AM. Detection of cell proliferation in tissue sections. Braz J Med Biol Res. 1993;26:677–87. [PubMed] [Google Scholar]
- 5.Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med. 2002;347:1593–603. doi: 10.1056/NEJMra021902. [DOI] [PubMed] [Google Scholar]
- 6.Saito T, Nakajima T, Mogi K. Immunohistochemical analysis of cell cycle-assosiated proteins P16, Prb, P53, P57 And Ki-67 in oral cancer and precancer with special reference to verrucous carcinomas. J Oral Pathol Med. 1999;28:226–32. doi: 10.1111/j.1600-0714.1999.tb02029.x. [DOI] [PubMed] [Google Scholar]
- 7.Lorz M, Meyer-Breiting E, Bettinger R. Proliferating cell nuclear antigen counts as markers of cell proliferation in head and neck cancer. Eur Arch Otorrhinolaryngol. 1994;251:91–4. doi: 10.1007/BF00179899. [DOI] [PubMed] [Google Scholar]
- 8.Reichart PA. Oral cancer and precancer related to betel and miang chewing in Thailand: A review. J Oral Pathol Med. 1995;24:241–3. doi: 10.1111/j.1600-0714.1995.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 9.Silverman S, Jr, Grosky M, Lozada F. Oral leukoplakia and malignant transformation: A follow-up study of 257 patients. Cancer. 1984;53:563–8. doi: 10.1002/1097-0142(19840201)53:3<563::aid-cncr2820530332>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Reibel J. Prognosis of oral pre-malignant lesions: Significance of clinical, histopathological, and molecular biological characteristics. Crit Rev Oral Biol Med. 2003;14:47–62. doi: 10.1177/154411130301400105. [DOI] [PubMed] [Google Scholar]
- 11.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium clinical implications of multicentric origin. Cancer. 1953;6:963–8. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Gerdes J, Schwab U, Lemke H, Stein H. The production of mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 13.Vieira FL, Vieira BJ, Guimaraes MA, Aarestrup FM. Cellular profile of the peritumoral inflammatory infiltrate in squamous cells carcinoma of oral mucosa: Correlation with the expression of Ki67 and histologic grading. BMC Oral Health. 2008;8:25. doi: 10.1186/1472-6831-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim JJ, Kang S, Lee MR, Pai HK, Yoon HJ, Lee JI, et al. Expression of vascular endothelial growth factor in salivary gland carcinomas and its relation to p53, Ki-67 and prognosis. J Oral Pathol Med. 2003;32:552–61. [PubMed] [Google Scholar]
- 15.Allison RT, Best T. p53, PCNA and Ki-67 expression in oral squamous cell carcinomas: The vagaries of fixation and microwave enhancement of immunocytochemistry. J Oral Pathol Med. 1998;27:434–40. doi: 10.1111/j.1600-0714.1998.tb01981.x. [DOI] [PubMed] [Google Scholar]
- 16.Donzelli M, Negri C, Mandarino A, Rossi L, Prosperi E, Frouin I, et al. Poly (ADP-ribose) synthesis: A useful parameter for identifying apoptotic cells. Histochem J. 1997;29:831–7. doi: 10.1023/a:1026485622824. [DOI] [PubMed] [Google Scholar]
- 17.Bromley M, Rew D, Becciolini A, Balzi M, Chadwick C, Hewitt D, et al. A comparison of proliferation markers (BrdUrd, Ki-67, PCNA) determined at each cell position in the crypts of normal human colonic mucosa. Eur J Histochem. 1996;40:89–100. [PubMed] [Google Scholar]
- 18.Scholzen T, Gerdes J. The Ki -67 protein: From the known and the unknown. J Cell Physiol. 2000;182:311–22. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Kujan O, Oliver RJ, Khattab A, Roberts SA, Thakker N, Solan P. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006;42:987–93. doi: 10.1016/j.oraloncology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Verheijen R, Kuijpers HJ, Schlingemann RO, Boehmer AL, van Driel R, Brakenhoff GJ, et al. Ki-67 detects a nuclear matrix-associated proliferation-related antigen, 1, lntracellular localization during interphase. J Cell Sci. 1989;92:123–30. doi: 10.1242/jcs.92.1.123. [DOI] [PubMed] [Google Scholar]
- 21.Iamaroon A, Khemaleelakul U, Pongsiriwet S, Pintong J. Co-expression of p53 and Ki67 and lack of EBV expression in oral squamous cell carcinoma. J Oral Pathol Med. 2004;33:30–6. doi: 10.1111/j.1600-0714.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 22.Takeda T, Sugihara K, Hirayama Y, Hirano M, Tanuma JI, Semba I. Immunohistological evaluation of Ki-67, p63, CK19 and p53 expression in oral epithelial dysplasias. J Oral Pathol Med. 2006;35:369–75. doi: 10.1111/j.1600-0714.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 23.de Paula AM1, Carvalhais JN, Domingues MG, Barreto DC, Mesquita RA. Cell proliferation markers in the odontogenic keratocyst: Effect of inflammation. J Oral Pathol Med. 2000;29:477–82. doi: 10.1034/j.1600-0714.2000.291001.x. [DOI] [PubMed] [Google Scholar]
- 24.Jones PH. Epithelial stem cells. Bioessays. 1997;19:683–90. doi: 10.1002/bies.950190808. [DOI] [PubMed] [Google Scholar]
- 25.Watt FM. Epidermal stem cells. Markers, patterning and the control of stem cell fate. Philos Trans R Soc Lond B Biol Sci. 1998;353:831–7. doi: 10.1098/rstb.1998.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blumenberg M, Tomic-Canic M, Jiang CK, Yang DR, Magnaldo T, Bernerd F, et al. Regulation of keratin gene expression by harmones, vitamins and growth factors. Pharmacol Skin. 1993;5:75–82. [Google Scholar]
- 27.Tomić-Canić M, Day D, Samuels HH, Freedberg IM, Blumenberg M. Novel regulation of keratin gene expression by thyroid harmone and retinoid receptors. J Biol Chem. 1996;271:1416–23. doi: 10.1074/jbc.271.3.1416. [DOI] [PubMed] [Google Scholar]
- 28.Ross W, Hall PA. Ki67: From antibody to molecule to understanding? Clin Mol Pathol. 1995;48:M113–7. doi: 10.1136/mp.48.3.m113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown DC, Gatter KC. Monoclonal antibody Ki-67: Its use in histopathology. Histopathology. 1990;17:489–503. doi: 10.1111/j.1365-2559.1990.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 30.Gerdes J, Li L, Schlueter C, Duchrow M, Wohlenberg C, Gerlach C, et al. Immunohistochemical and molecular biologic characterization of cell proliferation associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991;138:867–73. [PMC free article] [PubMed] [Google Scholar]
- 31.Fonatsch C, Duchrow M, Reider H, Schluter C, Gerdes J. Assignment of the human Ki-67 gene (MK167) to 10q25-qter. Genomics. 1991;11:476–7. doi: 10.1016/0888-7543(91)90163-9. [DOI] [PubMed] [Google Scholar]
- 32.Karabulut A, Reibel J, Therkildsen MH, Praetorous F, Nielsen HW, Dabelsteen E. Observer variability in the histologic assessment of oral premalignant lesions. J Oral Pathol Med. 1995;24:198–200. doi: 10.1111/j.1600-0714.1995.tb01166.x. [DOI] [PubMed] [Google Scholar]
- 33.Tabor MP, Braakhuis BJ, van der Wal JE, van Diest PJ, Leemans CR, Brakenhoff RH, et al. Comparative molecular and histological grading of epithelial dysplasia of the oral cavity and the oro pharynx. J Pathol. 2003;199:354–60. doi: 10.1002/path.1285. [DOI] [PubMed] [Google Scholar]
- 34.Philias R Garant. A textbook of Oral cells and tissues. In: Arinne Dickson., editor. Illinois: Quintessence publishing co.inc; 2003. pp. 81–4. [Google Scholar]
- 35.MacCallum DE, Hall PA. The biochemical characterisation of the DNA binding activity of the Ki-67 protein. J Pathol. 2000;191:286–98. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH628>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 36.Roland NJ, Caslin AW, Bowie GL, Jones AS. Has the cellular proliferation marker Ki-67 any clinical relevance in squamous cell carcinoma of the head and neck? Clin Otolaryngol Allied Sci. 1994;19:13–8. doi: 10.1111/j.1365-2273.1994.tb01140.x. [DOI] [PubMed] [Google Scholar]
- 37.Piffkó J, Bánkfalvi A, Ofner D, Kusch F, Böcker W, Joos U, et al. In situ assessment of cell proliferation at the invasive front of oral squamous cell carcinoma. Virchows Arch. 1996;429:229–34. doi: 10.1007/BF00198338. [DOI] [PubMed] [Google Scholar]


