Abstract
Introduction:
Dental follicle (DF) is an ectomesenchymal tissue that surrounds the developing tooth germ and contains precursor cells for cementoblasts, periodontal ligaments and osteoblasts. Radiographically, the DFs are seen as semicircular radiolucencies around unerupted teeth. However, if the DFs are larger than 2.5 mm, they are considered to be a pathological change.
Aims and Objectives:
The purpose of this study is to assess the cell proliferation activity of DF surrounding an asymptomatic impacted third molar teeth using the Ki 67 proliferation marker and to evaluate the variation of cell proliferation depending on the age factor.
Materials and Methods:
Forty-four specimens of DFs associated with impacted mandibular third molars fully covered by mucosa or bone were surgically removed from 44 patients. The patients were divided into 2 age groups. Twenty of forty-four DFs were between 18 and 29 years (Group 1) and 24 were 30 years and above (Group 2). Ki-67 immunostaining was evaluated in epithelial component of the DFs.
Results:
Ki 67 expression was found to be 60% in Group 1 and 75% in Group 2. Statistically significant differences were found among the two groups in both the basal layer and the supra-basal layer.
Conclusion:
This study shows that DFs have more proliferative potential in older people as compared to the young and squamous metaplasia may be an early sign of developing lesions of odontogenic origin. Therefore, clinicians should be aware that histopathological changes could be found in DFs without clinical and radiographic alterations.
Keywords: Cell proliferation, Dental follicle, Ki-67
INTRODUCTION
Impacted third molar operations are one of the most commonly performed surgical operations in oral surgery. Although an indication for symptomatic third molar extraction is well known, there is no general consensus on the need for surgical removal of asymptomatic impacted third molars.[1,2,3,4] Dental follicle (DF) is an ectomesenchymal tissue that surrounds the developing tooth germ and contains precursor cells for cementoblasts, periodontal ligaments and osteoblasts.[5] Radiographically, the DFs are seen as slight semicircular radiolucencies around unerupted teeth. However, if the DFs are larger than 2.5 mm, they are considered to be a pathological change.[6] Differences in the proliferation rates of the oral epithelial cells or odontogenic epithelial components of DF may play an important role in the pathogenesis of epithelial tumors and odontogenic cysts.[7]
Ki-67, a nuclear antigen is expressed throughout the cell cycle but not in the G0 phase and has been used mainly as a cell proliferation marker.[8] The purpose of this study is to assess the cell proliferation activity of DF surrounding the asymptomatic impacted third molar teeth using the Ki-67 proliferation marker and to evaluate the variation of cell proliferation depending on the age factor.
MATERIALS AND METHODS
Forty-four specimens of DFs associated with impacted mandibular third molars fully covered by mucosa or bone were surgically removed from 44 patients. The age of the patients’ ranged from 18 to 62 years (mean 32, standard deviation 5). The contours of the tooth and of the pericoronal space were traced on tracing paper using the X-ray viewer. The widest point of the follicular space was measured using a graduated scale. Subjects who had follicular space >2.5 mm were excluded from the study.
All operations were carried out under local anesthesia through conventional third molar surgeries. Dental follicles were carefully removed and the specimens were fixed in 10% buffered formalin for 1 to several days and embedded in paraffin wax. They were sliced into serial 3-μm-thick sections and processed for routine histological and subsequent immunohistochemical examinations.
Immunohistochemical staining was performed using the streptavidin-biotin method. Briefly, the 3 mm sections were deparaffinized in xylene and dehydrated through a series of baths containing decreased concentrations of ethanol. The sections were then heated in citrate buffer (10 mM, pH 6.0) at 120ºC for 15 minutes (pressure cooker) for antigen retrieval, rinsed 3 times in de-ionized distilled water and endogenous peroxidase activity was blocked using 3% hydrogen peroxide in methanol for 30 minutes. The sections were then incubated with the primary antibody to Ki-67 (Neomarkers, 7 mlt, ready to use) for 3 hours at room temperature and then stained according to the streptavidin-biotin method. The immunoreaction was visualized with 3-Amino-9-Ethylcarbazole (AEC) as a chromogen. The slides were counterstained with Mayer's hematoxylin solution and mounted in Entellan (Merck KGaA, Darmstadt, Germany). For negative control, the addition of primary antibody was omitted and the sections of tonsilwas used as a positive control. Clear brown nuclei, regardless of staining intensity, were considered Ki-67-positive. Each slide was examined under a multi-head light microscope and scored by a pathologist.
Evaluating Ki-67 immunostaining
Ki-67 immunostaining was evaluated in epithelial component of DF. Epithelial components of DF were classified as basal layer of squamous epithelium and supra-basal layer. Inflammatory cells, rests of the odontogenic epithelium and calcified follicles in mesenchymal components were also noted.
The positivity of Ki-67 was evaluated by counting the number of positive cells per 1000 cells in both basal and upper part of the epithelium of DF. The percentage of Ki-67 positive cells was calculated for each case.
x2 test was used to compare data between groups.
RESULTS
The patients were divided into 2 age groups. Twenty cases out of 44 DF were between the age group of 18 and 29 (Group 1) and 24 were of 30 years and above (Group 2).
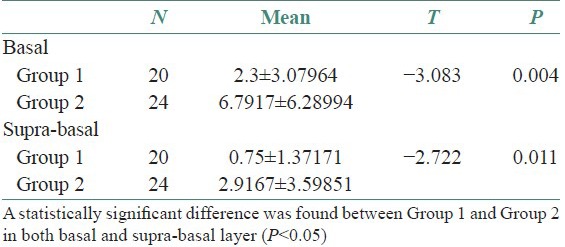
Histologically, all of the DFs were lined with squamous epithelium. Fibrous connective tissues were seen under the epithelium [Figure 1]. The histologic examination of DF specimens showed 50% and 75% squamous proliferation, 90% and 96% inflammatory cells, 5% and 42% calcification, 35% and 16% reduced enamel in Group 1 and Group 2 cases respectively [Table 1]. However, one specimen was not stained appropriately with Ki-67 in Group 1 and six specimens were not stained properly in Group 2. Ki 67 expression was found to be 60% in Group 1 [Figure 2] and 75% in Group 2 [Figure 3]. In Group 1, the distribution of Ki-67 positive cells was mainly confined to the basal location. The positive cells were rarely observed in the supra-basal layer. In Group 2, positive nuclei were confined to both basal and supra-basal portions of the epithelium and more than those in control group. Statistically significant differences were found among the two groups in both the basal layer and the supra-basal layer [Table 2].
Figure 1.
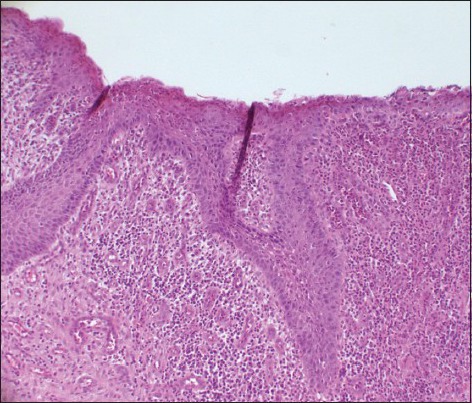
The photomicrograph shows DF lined with squamous epithelium. Squamous proliferation is obvious in dental follicle (DF). The connective tissue is fibrous with inflammatory cells (H&E stain, ×100)
Table 1.
Histologic data of some components
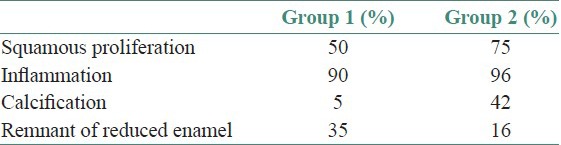
Figure 2.
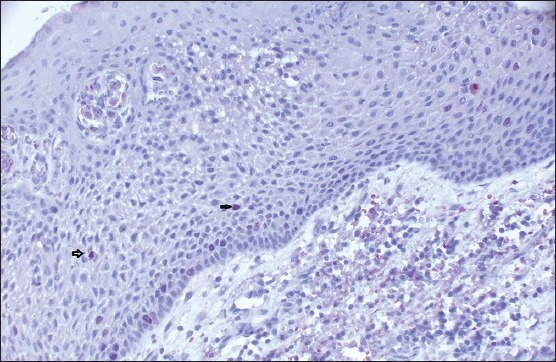
The photomicrograph shows the distribution of Ki-67-positive cells in Group 1. Ki-67-positive cells were mainly confined to the basal location. The positive cells were rarely observed on supra-basal layer (IHC stain, ×100)
Figure 3.
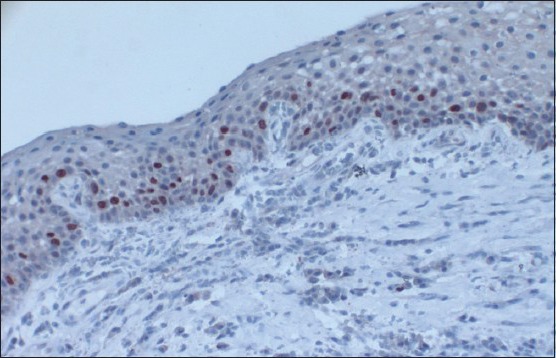
The photomicrograph shows the distribution of Ki-67-positive cells in Group 2. Ki-67-positive nuclei were confined to both basal and supra-basal portions of the epithelium and were more than those in control group (IHC stain, ×100)
Table 2.
Mean Ki-67 expression in Group 1 and Group 2
DISCUSSION
An indication for extraction of the symptomatic wisdom teeth is evident. However, there is no consensus about the extraction of the asymptomatic wisdom teeth. Recently, a small number of immunohistochemical studies gave us an idea about the extraction of the asymptomatic wisdom teeth.[2,3,7,9,10] The common thought of these studies is that it may help to determine any histopathological changes that can be seen in DFs of wisdom teeth which seem to be asymptomatic and radiographically normal.
Cell proliferation can be followed up by the expression of some cell proteins such as proliferating cell nuclear antigen (PCNA) and Ki-67. Ki-67 is widely used and believed to be a reliable marker which indicates the status of cell proliferation. It is a nuclear antigen expressed during G1, S, M and G2 periods of cell cycle. During the interphase, the antigen can be exclusively detected within the nucleus, whereas in mitosis most of the protein is relocated onto the surface of the chromosomes. Briefly, the Ki-67 protein is present during all active phases of the cell cycle (G1, S, G2 and mitosis), but it does not exist in resting cells (G0), which makes it an excellent marker in determining the so-called growth fraction of a given cell population.[8,11] In many studies, Ki-67 protein has been shown to be a biomarker in the evaluation of the proliferative activity and progression from normal to dysplastic and neoplastic changes. Therefore, we preferred Ki-67 proliferation marker in this study.
Dental follicles, present in unerupted teeth, are histologically characterized by fibrous connective tissue with variable amounts of reduced enamel epithelium and epithelial remnants of dental lamina.[2,4] Although the importance of proliferative potential of odontogenic epithelium in DFs has not been completely elucidated yet, the origins of dentigerous cysts and keratocystic odontogenic tumors have been attributed to odontogenic epithelium.[7,9,12,13] Several studies have evaluated the expression of cell proliferation markers in the epithelial lining of dental pathologies such as odontogenic cystic lesions. However, only a few studies with a limited number of specimens, exist on the proliferative potential of DFs of asymptomatic unerupted teeth. On the basis of these points, evaluation of proliferation potentials of DFs and its association with age and inflammation may be important for deciding the removal of asymptomatic unerupted teeth.
In literature, some authors noted that squamous metaplasia is one of the important histopathological changes in DF. In this study, we determined the squamous metaplasia in all of the follicles. Aldelsperger et al.,[2] pointed out that cellular proliferation might be active in DFs showing squamous metaplasia, which is not observed in healthy DFs. Oliviera et al.,[14] reported that a higher frequency of PCNA-positive cases was found in DF with squamous metaplasia. They also emphasized that follicles with squamous metaplasia could represent signs of development of odontogenic lesions. In our study, squamous epithelium was classified as basal and supra-basal layer and Ki 67 expression was assessed in both portions. Although Ki-67 expression was mainly observed in basal layer of Group 1, it was observed both in basal and supra-basal layers of Group 2 cases. These findings indicate that there is correlation between Ki-67 proliferation and age. We believe that this correlation may suggest a possible effect of old age on proliferation of epithelial cells. In addition, previous studies also showed that the number of Ki-67 positive cells was found to be increased in the presence of marked inflammatory cell infiltrat.[4,15,16,17]
Chronic irritations such as chronic inflammation may stimulate proliferation of oral epithelial cells.[9,18] Yadav et al.,[18] found that a significant relationship exists between increase in patient's age and inflammation of the DF. Authors believed that longer the follicular tissues remain in the bone, more is the possibility of an inflammatory reaction within the connective tissue. They also noted a significant relationship between inflammation and the presence of squamous epithelial hyperplasia. Generally, it is accepted that factors such as chronic inflammation or age-induced effects may lead to squamous metaplasia in DF. In the present study, there were no significant differences between the two groups in relation to the inflammation although we found a correlation with proliferation index and age. This result is in agreement with the previous studies that noted that the chronic inflammation may cause chronic irritation and may stimulate proliferation of oral epithelial cells.[2,19,20]
In contrary to these studies, some authors showed that pathological changes were more in younger age. In the Venta et al.'s[21] study, impacted third molars had little changes at age 38 years and these changes have been reported more in age range of 20-32 years than 32-38 years old. Also according to Rakprasitkul et al's[19] study, more pathological changes were seen in younger people, but no correlation between increasing expression of apoptotic and proliferation markers and age in this study was noted.
Despite the existence of these data, it has been showed that the growth factors and cytokines released by the inflammatory infiltrates might be responsible for the greater proliferative activity.
In literature, Ki-67 expression pattern in DF was similar to that of us. Most of the studies showed that Ki-67 positive cells are located in the basal layer of squamous epithelium in benign pathologies. Our results is in agreement with Edamatsu et al.,[9] and Saraçoglu et al.[4] They reported that expression of Ki-67 was seen in some epithelial cells neighboring the basal membrane in DFs. However, it is known that Ki-67 immunoreactivitiy was predominantly within supra-basal layer in odontogenic cystic lesions and tumors. In this regard, it can be assumed that there might be a trend for increase in Ki-67-positive cells from normal epithelium to odontogenic cystic lesions and tumors. The expression of Ki-67 extending to the basal and upper layers of the epithelium and its close relation with hyper-proliferative state are also supported with the other markers such as EGFR, survivin and syndecan-1 in the recent studies.[14,22]
Additionally, the findings of this study suggest that the absence of radiographic disease is not necessarily reflective of the absence of a disease. Results of our immunohistochemical study also showed that there is a strong correlation between the expression of Ki-67 in DF and age. Glosser and Campbell[3] reported that they diagnosed cyst formation in 31 follicles of 96, which radiographically had normal follicular space and they also indicated that this formation was even more in elderly patients. Adelsperger et al.,[2] examined 100 impacted third molars that showed no radiographic evidence of pathology. The fact that 34% of them showed squamous metaplasia suggests that the cystic change is equivalent to that found in dentigerous cyst. Soft-tissue pathosis was significantly higher in individuals over the age of 21 years in the same study. When specimens with evidence of pathosis were compared with specimens without pathosis, there was a significant difference in PCNA in the diseased specimens. Their findings suggest that lack of radiographic appearance of the disease is not a reliable indicator of the absence of a disease and that the prevalence of soft-tissue pathosis is higher than generally assumed from radiographic evaluation alone.
In conclusion, this study shows that DFs have more proliferative potential in older people as compared to the young and squamous metaplasia may be an early sign of developing lesions of odontogenic origin. Therefore, clinicians should be aware that histopathological changes could be found in DFs without clinical and radiographic alterations. Although there were conflicting data about the risk of the development of cysts and tumors around unerupted teeth, the biological behavior of the DF can be determined with further studies.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.NIH consensus development conference for removal of third molars. J Oral Surg. 1980;38:235–6. [PubMed] [Google Scholar]
- 2.Adelsperger J, Campbell JH, Coates DB, Summerlin DJ, Tomich CE. Early soft tissue pathosis associated with impacted third molars without pericoronal radiolucency. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:402–6. doi: 10.1016/s1079-2104(00)70119-3. [DOI] [PubMed] [Google Scholar]
- 3.Glosser JW, Campbell JH. Pathologic change in soft tissue associated with radiographically ‘normal’ third molar impactions. Br J Oral Maxillofac Surg. 1999;37:259–60. doi: 10.1054/bjom.1999.0061. [DOI] [PubMed] [Google Scholar]
- 4.Saraçoðlu U, Kurt B, Günhan Ö, Güven O. MIB-1 expression in odontogenic epithelial rests, epithelium of healthy oral mucosa and epithelium of selected odontogenic cysts. An immunohistochemical study. Int J Oral Maxillofac Surg. 2005;34:432–5. doi: 10.1016/j.ijom.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Stanley HR, Krogh H, Pannkuk E. Age changes in the epithelial components of follicles (dental sacs) associated with impacted third molars. Oral Surg Oral Med Oral Pathol. 1965;19:128–39. doi: 10.1016/0030-4220(65)90226-4. [DOI] [PubMed] [Google Scholar]
- 6.Eliasson S, Heimdahl A, Nordenram A. Pathological changes related to long-term impaction of third molars. A radiographic study. Int J Oral Maxillofac Surg. 1989;18:210–2. doi: 10.1016/s0901-5027(89)80055-4. [DOI] [PubMed] [Google Scholar]
- 7.Curran AE, Damm DD, Drummond JF. Pathologically significant pericoronal lesions in adults: Histopathologic evaluation. J Oral Maxillofac Surg. 2002;60:613–7. doi: 10.1053/joms.2002.33103. [DOI] [PubMed] [Google Scholar]
- 8.Tumluri V, Thomas GA, Fraser IS. Analysis of Ki-67 antigen at the invasive tumor front of human oral squamous cell carcinoma. J Oral Pathol Med. 2003;31:598–604. doi: 10.1034/j.1600-0714.2002.00042.x. [DOI] [PubMed] [Google Scholar]
- 9.Edamatsu M, Kumamoto H, Ooya K, Echigo S. Apoptosis-related factors in the epithelial components of dental follicles and dentigerous cysts associated with impacted third molars of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:17–23. doi: 10.1016/j.tripleo.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Cabbar F, Guler N, Comunoglu N, Sencift K, Cologlu S. Determination of potential cellular proliferation in the odontogenic epithelia of the dental follicle of the asymptomatic impacted third molars. J Oral Maxillofac Surg. 2008;66:2004–11. doi: 10.1016/j.joms.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Macluskey M, Ogden GR, Gren M, Chisholm DM, Schor SL, Schor AM. The association between epithelial proliferation and disease progression in the oral mucosa. Oral Oncol. 1999;35:409–14. doi: 10.1016/s1368-8375(99)00014-7. [DOI] [PubMed] [Google Scholar]
- 12.Kramer IRH, Pindborg JJ, Shear M. 2nd ed. Berlin: Springer; 1992. Histological typing of odontogenic tumours. [DOI] [PubMed] [Google Scholar]
- 13.Philipsen HP. Keratocyst odontogenic tumour. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. World Health Organization classification of tumours. LyonFrance: IARC Press; 2005. [Google Scholar]
- 14.Oliveira MG, Lauxen Ida S, Chaves AC, Rados PV, Sant’Ana Filho M. Odontogenic epithelium: Immunolabeling of Ki-67, EGFR and survivin in pericoronal follicles, dentigerous cysts and keratocystic odontogenic tumors. Head Neck Pathol. 2011;5:1–7. doi: 10.1007/s12105-010-0216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paula AM, Carvalhais JN, Domingues MG, Barreto DC, Mesquita RA. Cell proliferation markers in the odontogenic keratocysts: Effect of inflammation. J Oral Pathol Med. 2000;29:477–82. doi: 10.1034/j.1600-0714.2000.291001.x. [DOI] [PubMed] [Google Scholar]
- 16.Li TJ, Browne RM, Matthews JB. Expression of epidermal growth factor receptors by odontogenic jaw cysts. Virchows Arch A Pathol Anat Histopathol. 1993;423:137–44. doi: 10.1007/BF01606588. [DOI] [PubMed] [Google Scholar]
- 17.Rodu B, Tate AL, Martinez Jr., MG The implications of inflammation in odontogenic keratocysts. J Oral Pathol Med. 1987;16:518–21. doi: 10.1111/j.1600-0714.1987.tb00684.x. [DOI] [PubMed] [Google Scholar]
- 18.Yadav M, Meghana SM, Deshmukh A, Godge P. The wisdom behind third molar extraction: A clinicopathologic study. Int J Oral Maxillofac Pathol. 2011;2:7–12. [Google Scholar]
- 19.Rakprasitkul S. Pathologic changes in the pericoronal tissues of unerupted third molars. Quintessence Int. 2001;32:633–8. [PubMed] [Google Scholar]
- 20.Baykul T, Saglam AA, Aydin U, Başak K. Incidence of cystic changes in radiographically normal impacted lower third molar follicles. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:542–5. doi: 10.1016/j.tripleo.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Venta I, Ylipaavalniemi P, Turtola L. Clinical outcome of third molars in adults followed during 18 years. J Oral Maxillofac Surg. 2004;62:182–5. doi: 10.1016/j.joms.2003.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Nadalin MR, Fregnani ER, Silva-Sousa YT, Perez DE. Syndecan-1 (CD138) and Ki-67 expression in odontogenic cystic lesions. Braz Dent J. 2011;22:223–9. doi: 10.1590/s0103-64402011000300008. [DOI] [PubMed] [Google Scholar]


