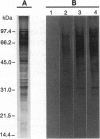
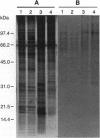
Abstract
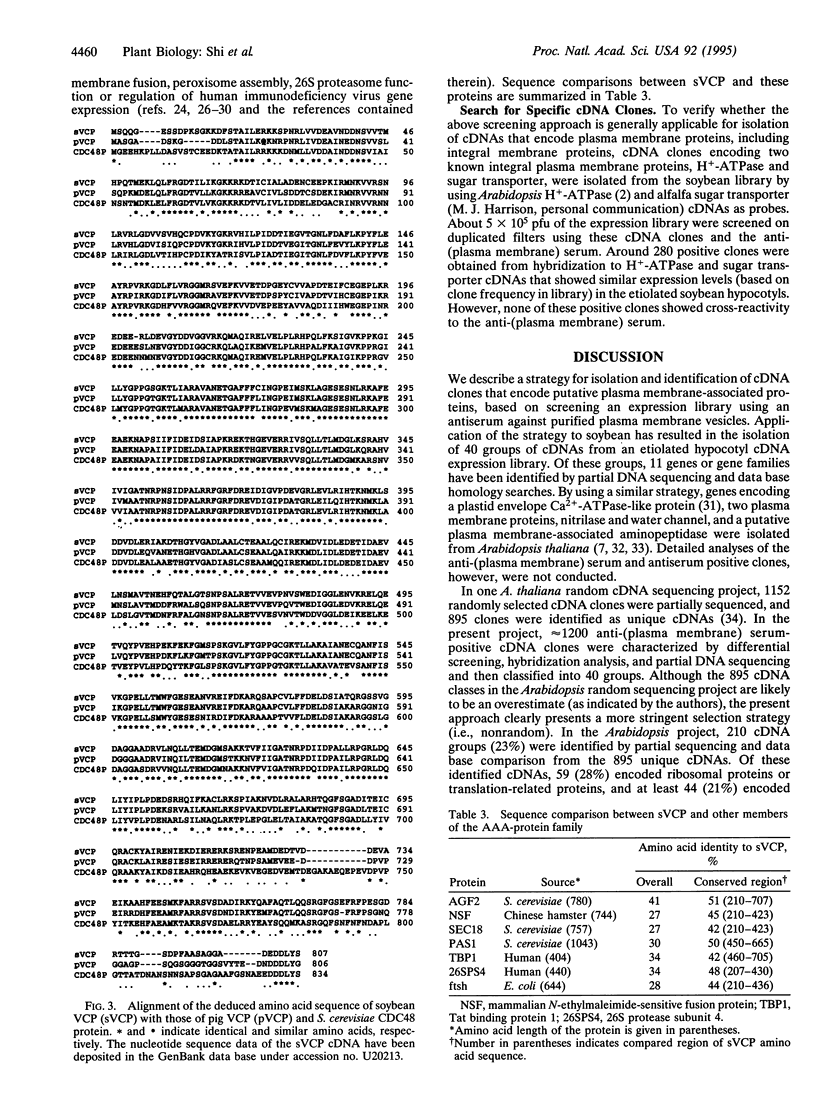
An approach was developed for the isolation and characterization of soybean plasma membrane-associated proteins by immunoscreening of a cDNA expression library. An antiserum was raised against purified plasma membrane vesicles. In a differential screening of approximately 500,000 plaque-forming units with the anti-(plasma membrane) serum and DNA probes derived from highly abundant clones isolated in a preliminary screening, 261 clones were selected from approximately 1,200 antiserum-positive plaques. These clones were classified into 40 groups by hybridization analysis and 5'- and 3'-terminal sequencing. By searching nucleic acid and protein sequence data bases, 11 groups of cDNAs were identified, among which valosin-containing protein (VCP), clathrin heavy chain, phospholipase C, and S-adenosylmethionine:delta 24-sterol-C-methyltransferase have not to date been cloned from plants. The remaining 29 groups did not match any current data base entries and may, therefore, represent additional or yet uncharacterized genes. A full-length cDNA encoding the soybean VCP was sequenced. The high level of amino acid identity with vertebrate VCP and yeast CDC48 protein indicates that the soybean protein is a plant homolog of vertebrate VCP and yeast CDC48 protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. D., Kelley J. M., Gocayne J. D., Dubnick M., Polymeropoulos M. H., Xiao H., Merril C. R., Wu A., Olde B., Moreno R. F. Complementary DNA sequencing: expressed sequence tags and human genome project. Science. 1991 Jun 21;252(5013):1651–1656. doi: 10.1126/science.2047873. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bartling D., Seedorf M., Mithöfer A., Weiler E. W. Cloning and expression of an Arabidopsis nitrilase which can convert indole-3-acetonitrile to the plant hormone, indole-3-acetic acid. Eur J Biochem. 1992 Apr 1;205(1):417–424. doi: 10.1111/j.1432-1033.1992.tb16795.x. [DOI] [PubMed] [Google Scholar]
- Bartling D., Weiler E. W. Leucine aminopeptidase from Arabidopsis thaliana. Molecular evidence for a phylogenetically conserved enzyme of protein turnover in higher plants. Eur J Biochem. 1992 Apr 1;205(1):425–431. doi: 10.1111/j.1432-1033.1992.tb16796.x. [DOI] [PubMed] [Google Scholar]
- Blackbourn H. D., Barker P. J., Huskisson N. S., Battey N. H. Properties and partial protein sequence of plant annexins. Plant Physiol. 1992 Jul;99(3):864–871. doi: 10.1104/pp.99.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. Y., Erickson H. P. Cell surface annexin II is a high affinity receptor for the alternatively spliced segment of tenascin-C. J Cell Biol. 1994 Jul;126(2):539–548. doi: 10.1083/jcb.126.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G. B., Dauwalder M., Roux S. J. Purification and immunolocalization of an annexin-like protein in pea seedlings. Planta. 1992;187:1–9. [PubMed] [Google Scholar]
- Dean M. F., Martin H., Sansom P. A. Characterization of a thioredoxin-related surface protein. Biochem J. 1994 Dec 15;304(Pt 3):861–867. doi: 10.1042/bj3040861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droillard M. J., Rouet-Mayer M. A., Bureau J. M., Lauriere C. Membrane-Associated and Soluble Lipoxygenase Isoforms in Tomato Pericarp (Characterization and Involvement in Membrane Alterations). Plant Physiol. 1993 Dec;103(4):1211–1219. doi: 10.1104/pp.103.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakle K. A., Bernstein M., Emr S. D. Characterization of a component of the yeast secretion machinery: identification of the SEC18 gene product. Mol Cell Biol. 1988 Oct;8(10):4098–4109. doi: 10.1128/mcb.8.10.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciello I., Chinali G. A modified alkaline lysis method for the preparation of highly purified plasmid DNA from Escherichia coli. Anal Biochem. 1993 Aug 1;212(2):394–401. doi: 10.1006/abio.1993.1346. [DOI] [PubMed] [Google Scholar]
- Fröhlich K. U., Fries H. W., Rüdiger M., Erdmann R., Botstein D., Mecke D. Yeast cell cycle protein CDC48p shows full-length homology to the mammalian protein VCP and is a member of a protein family involved in secretion, peroxisome formation, and gene expression. J Cell Biol. 1991 Aug;114(3):443–453. doi: 10.1083/jcb.114.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaber R. F., Copple D. M., Kennedy B. K., Vidal M., Bard M. The yeast gene ERG6 is required for normal membrane function but is not essential for biosynthesis of the cell-cycle-sparking sterol. Mol Cell Biol. 1989 Aug;9(8):3447–3456. doi: 10.1128/mcb.9.8.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Grimes H. D., Breidenbach R. W. Plant plasma membrane proteins : immunological characterization of a major 75 kilodalton protein group. Plant Physiol. 1987 Dec;85(4):1048–1054. doi: 10.1104/pp.85.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. F., Surowy T. K., Sussman M. R. Molecular cloning and sequence of cDNA encoding the plasma membrane proton pump (H+-ATPase) of Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1234–1238. doi: 10.1073/pnas.86.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A., Luthman M. Tissue distrubution and subcellular localization of bovine thioredoxin determined by radioimmunoassay. Biochemistry. 1978 Sep 19;17(19):4071–4077. doi: 10.1021/bi00612a031. [DOI] [PubMed] [Google Scholar]
- Huang L., Berkelman T., Franklin A. E., Hoffman N. E. Characterization of a gene encoding a Ca(2+)-ATPase-like protein in the plastid envelope. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10066–10070. doi: 10.1073/pnas.90.21.10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte H., Desprez T., Amselem J., Chiapello H., Rouzé P., Caboche M., Moisan A., Jourjon M. F., Charpenteau J. L., Berthomieu P. An inventory of 1152 expressed sequence tags obtained by partial sequencing of cDNAs from Arabidopsis thaliana. Plant J. 1993 Dec;4(6):1051–1061. doi: 10.1046/j.1365-313x.1993.04061051.x. [DOI] [PubMed] [Google Scholar]
- Kammerloher W., Fischer U., Piechottka G. P., Schäffner A. R. Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant J. 1994 Aug;6(2):187–199. doi: 10.1046/j.1365-313x.1994.6020187.x. [DOI] [PubMed] [Google Scholar]
- Kirsch T., Beevers L. Uncoating of clathrin-coated vesicles by uncoating ATPase from developing peas. Plant Physiol. 1993 Sep;103(1):205–212. doi: 10.1104/pp.103.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller K. J., Brownstein M. J. Use of a cDNA clone to identify a supposed precursor protein containing valosin. Nature. 1987 Feb 5;325(6104):542–545. doi: 10.1038/325542a0. [DOI] [PubMed] [Google Scholar]
- Kunau W. H., Beyer A., Franken T., Götte K., Marzioch M., Saidowsky J., Skaletz-Rorowski A., Wiebel F. F. Two complementary approaches to study peroxisome biogenesis in Saccharomyces cerevisiae: forward and reversed genetics. Biochimie. 1993;75(3-4):209–224. doi: 10.1016/0300-9084(93)90079-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loferer H., Bott M., Hennecke H. Bradyrhizobium japonicum TlpA, a novel membrane-anchored thioredoxin-like protein involved in the biogenesis of cytochrome aa3 and development of symbiosis. EMBO J. 1993 Sep;12(9):3373–3383. doi: 10.1002/j.1460-2075.1993.tb06011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus F., Chamberlain S. H., Chu C., Masiarz F. R., Shin S., Yee B. C., Buchanan B. B. Plant thioredoxin h: an animal-like thioredoxin occurring in multiple cell compartments. Arch Biochem Biophys. 1991 May 15;287(1):195–198. doi: 10.1016/0003-9861(91)90406-9. [DOI] [PubMed] [Google Scholar]
- Melin P. M., Pical C., Jergil B., Sommarin M. Polyphosphoinositide phospholipase C in wheat root plasma membranes. Partial purification and characterization. Biochim Biophys Acta. 1992 Jan 24;1123(2):163–169. doi: 10.1016/0005-2760(92)90107-7. [DOI] [PubMed] [Google Scholar]
- Peters J. M., Harris J. R., Lustig A., Müller S., Engel A., Volker S., Franke W. W. Ubiquitous soluble Mg(2+)-ATPase complex. A structural study. J Mol Biol. 1992 Jan 20;223(2):557–571. doi: 10.1016/0022-2836(92)90670-f. [DOI] [PubMed] [Google Scholar]
- Pleasure I. T., Black M. M., Keen J. H. Valosin-containing protein, VCP, is a ubiquitous clathrin-binding protein. Nature. 1993 Sep 30;365(6445):459–462. doi: 10.1038/365459a0. [DOI] [PubMed] [Google Scholar]
- Sauer N., Friedländer K., Gräml-Wicke U. Primary structure, genomic organization and heterologous expression of a glucose transporter from Arabidopsis thaliana. EMBO J. 1990 Oct;9(10):3045–3050. doi: 10.1002/j.1460-2075.1990.tb07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller G. E., Harmon A. C., Sussman M. R. Characterization of a calcium- and lipid-dependent protein kinase associated with the plasma membrane of oat. Biochemistry. 1992 Feb 18;31(6):1721–1727. doi: 10.1021/bi00121a020. [DOI] [PubMed] [Google Scholar]
- Sentenac H., Bonneaud N., Minet M., Lacroute F., Salmon J. M., Gaymard F., Grignon C. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992 May 1;256(5057):663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993 Mar 25;362(6418):318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Tsay Y. F., Schroeder J. I., Feldmann K. A., Crawford N. M. The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell. 1993 Mar 12;72(5):705–713. doi: 10.1016/0092-8674(93)90399-b. [DOI] [PubMed] [Google Scholar]
- Verhey S. D., Gaiser J. C., Lomax T. L. Protein Kinases in Zucchini (Characterization of Calcium-Requiring Plasma Membrane Kinases). Plant Physiol. 1993 Oct;103(2):413–419. doi: 10.1104/pp.103.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. W., Wilcox C. A., Flynn G. C., Chen E., Kuang W. J., Henzel W. J., Block M. R., Ullrich A., Rothman J. E. A fusion protein required for vesicle-mediated transport in both mammalian cells and yeast. Nature. 1989 Jun 1;339(6223):355–359. doi: 10.1038/339355a0. [DOI] [PubMed] [Google Scholar]
- Zinser E., Paltauf F., Daum G. Sterol composition of yeast organelle membranes and subcellular distribution of enzymes involved in sterol metabolism. J Bacteriol. 1993 May;175(10):2853–2858. doi: 10.1128/jb.175.10.2853-2858.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]