Abstract
Chondroblastic osteosarcoma (COS), a subgroup of intramedullary osteosarcoma (OS), is the most common osteosarcoma that occurs in adolescents and early adulthood. The COS has similar clinical and radiological features to those of conventional OS. We present a case of 20-year-old male patient with the chief complaint of pain and swelling in the left zygomatic region. The computed tomography (CT) and three-dimensional (3D) CT face showed erosion, calcific foci, sunray type of spicules suggestive of OS. On fine-needle aspiration cytology (FNAC) examination, initial diagnosis was malignant chondroid lesion, with differential diagnosis of mesenchymal chrondrosarcoma, COS on incisional biopsy and finally COS on excisional biopsy. The patient underwent radical resection of left zygomatic arch, followed by chemotherapy. Although clinically unsuspected in this unusual site, histopathology along with immunohistochemistry (IHC) results confirmed the COS. Because zygomatic location of COS is very rare, this report aimed to discuss clinical, radiographic, histopathologic, IHC findings and diagnostic pitfalls of COS in light of the literature.
Keywords: Bone neoplasm, chondroblastic osteosarcoma, zygomatic bone
INTRODUCTION
Osteosarcoma (OS) is derived from primitive bone-forming mesenchymal cells with the tendency for bone formation. The OS, the second most frequent primary malignant bone tumor, is usually found in long bones.[1] Although it is the most common malignancy of long bones after multiple myeloma, it is a relatively rarer entity in the craniofacial region consisting of about 6.5-7% of all OS.[2] In craniofacial region, mandibular tumors arise more frequently in posterior body and ramus. Maxillary lesions are discovered more commonly in the inferior portion (alveolar ridge, sinus floor, palate) than the superior aspect (zygoma, orbital rim).[3] Despite sharing common histopathological features, craniofacial OS and OS of long bones are distinct biological entities. Chondroblastic osteosarcoma (COS) accounts for about 25% of all cases of OS. An extensive research has revealed only few well-documented cases of OS of zygomatic bone and no case of COS of zygomatic bone. This article is presented to share our experience with a case of very rare COS of left zygomatic bone in a 20-year-male patient and to review the relevant literature.
CASE REPORT
A 20-year-old male patient presented with the chief complaint of pain and swelling in the left zygomatic region since 2 months. He had history of trauma in left malar region, which caused a swelling. The swelling was initially small, gradually grew larger and reached to a present size of 7 × 6 cm in 2 months time, along with history of pain since one month. He had no significant medical history. On general and systemic examinations the patient was apparently healthy. No cervical lymphadenopathy was evident. The mouth opening was adequate. No significant findings were observed on intra-oral examination. Extra-oral examination showed a solitary, diffuse, swelling in the left zygomatic region, extending superioinferiorly from infra-orbital ridge to angle of mandible and anteroposteriorly 2 cm from ala of nose to pre-auricular region. [Figure 1] On palpation, the swelling was tender, non mobile, hard in consistency and fixed to the underlying structures, with no localized rise in temperature of the overlying skin. There was no evidence of nasal obstruction or ophthalmologic signs of extension of the lesions into these anatomical regions. There were also no signs of neurosensory deficit associated with the infraorbital nerve. The computed tomography (CT) scan and three- dimensional (3D) CT face showed erosion of medial aspect of left zygomatic arch [Figures 2 and 3]. Peripheral arc like multiple conglomerated calcific density foci with associated soft tissue swelling was noticed extending inferiorly into left infratemporal fossa. Sunray types of spicules were also noted perpendicular to cortex of zygomatic arch. These imaging features were suggestive of a malignant lesion. Fine needle aspiration cytology (FNAC) examination from the lesion was carried out, which revealed chondroid matrix, with oval chondrocytes, vacuolated granular cytoplasm, raising a suspicion of malignant chondroid lesion.
Figure 1.

Extraoral photograph showing swelling and extension over left zygomatic region
Figure 2.
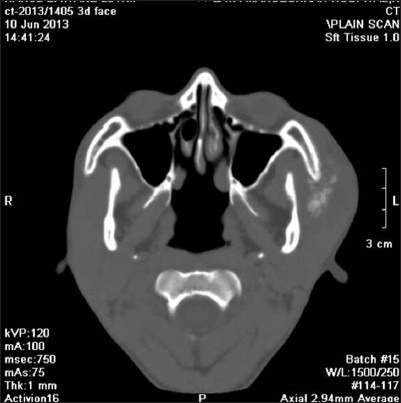
Computed tomography axial section showing erosion, calcific foci, sunray type of spicules perpendicular to cortex of left zygomatic arch
Figure 3.

Three-dimensional CT face showing tumor mass associated with left zygomatic arch
An incisional biopsy was done under local anesthesia from the left zygomatic region. As extensively, cellular chondroid areas were seen, it was difficult to exclude the possibility of a chondrosarcoma (CS) and incisional biopsy was suggestive of differential diagnosis of mesenchymal CS and COS. Patient underwent radical excision of tumor along with the left zygomatic arch under general anesthesia. Lateral tarsorrhaphy was carried out. Excisional biopsy specimen revealed well-circumscribed solid, single soft tissue specimen, reddish-brown, with localized hemorrhagic areas, 5.5 × 4.5 cm in size, round to oval in shape, firm in consistency with rough surface texture [Figure 4]. Hematoxylin and eosin (H and E) stained sections of the excisional biopsy specimen showed cellular tumour composed of spindle- shaped cells arranged in diffuse fashion in osteoid and chondroid matrices. Most of the tumor was composed of highly cellular connective tissue stroma with formation of large amounts of chondroid matrix [Figure 5] and tumor osteoid areas [Figure 6]. The malignant spindled cells were set within lobules of malignant cartilage with only focal neoplastic bone formation. [Figure 7] The chondroid areas were with few atypical chondrocytes, [Figure 8] with binucleation and neoplastic mesenchymal cells [Figure 9] along with malignant cells with osteoid. [Figure 10] Higher-power view of an area of bone differentiation shows that atypical neoplastic osteoblasts are associated with a partially mineralized osteoid stroma. [Figure 11] An immunohistochemistry (IHC) study was done. The IHC profile for a panel of antibodies showed the tumor cells were diffusely positive for vimentin [Figure 12a and b] and focally positive for S100 protein, [Figure 12c] and proliferating marker Ki-67(MIB1) showed 60% staining. The majority of neoplastic cells were diffusely positive for vimentin, characterizing them as of mesenchymal origin. The S-100 protein was focally positive for chondroblastic component. The expression of Ki-67(MIB1) was statistically elevated in higher-grade tumors and younger patients. There appears to be a relationship between proliferative tumor activity and tumor grade, location and metastasis. The histopathology and IHC confirmed the diagnosis of COS. Possibility of metastasis to the lungs and brain was ruled out after a CT thorax and brain were done, respectively. Although the patient was treated surgically with wide margins of resection and is doing well presently but knowing the notorious nature of COS in the head and neck region, the patient is kept under a close long-term follow-up.
Figure 4.
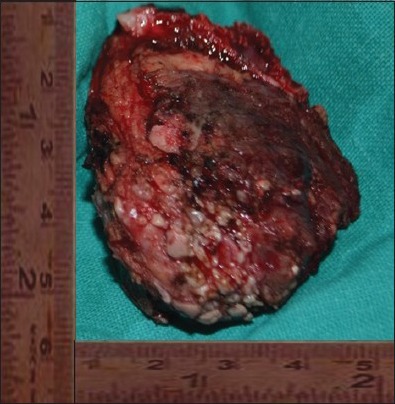
Excisional biopsy of the resected tumor mass
Figure 5.
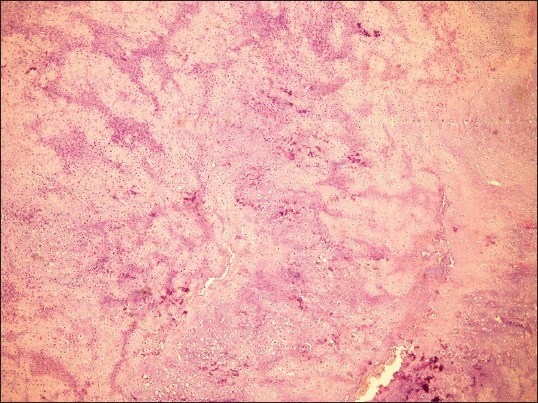
Scanner view showing highly cellular connective tissue stroma with formation of large amounts of chondroid matrix. (H&E stain, ×40)
Figure 6.
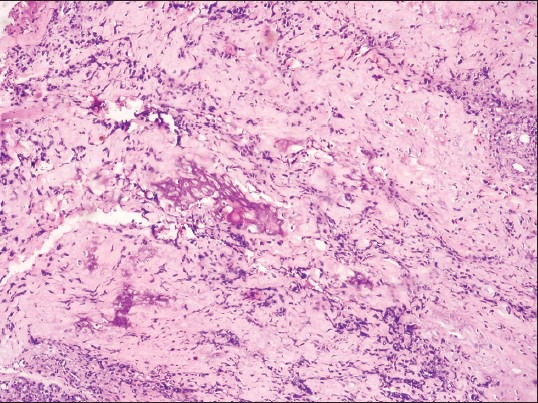
Photomicrograph showing tumor osteoid areas. (H&E stain, ×100)
Figure 7.

Photomicrograph showing malignant spindled cells set within lobules of malignant cartilage with only focal neoplastic bone formation. (H&E stain, ×200)
Figure 8.
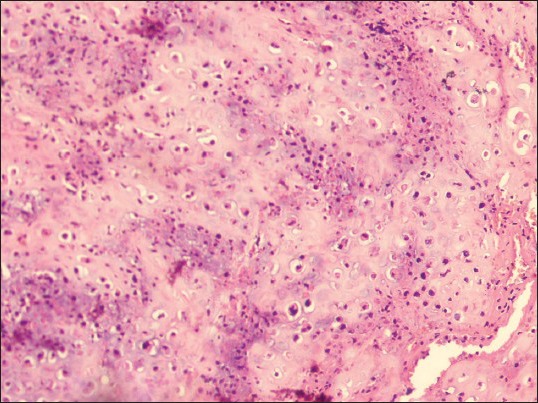
Photomicrograph showing chondroid areas with few atypical chondrocytes. (H&E stain, ×200)
Figure 9.

Photomicrograph showing chondroid areas with few atypical chondrocytes, with binucleateation and neoplastic mesenchymal cells. (H&E stain, ×200)
Figure 10.
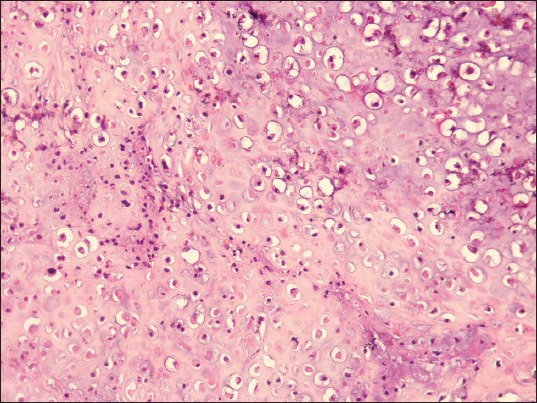
Photomicrograph showing malignant cells with osteoid. (H&E stain, ×200)
Figure 11.

Higher-power view of an area of bone differentiation shows that atypical neoplastic osteoblasts are associated with a partially mineralized osteoid stroma. (H&E stain, ×400)
Figure 12.

(a) Immunohistochemical stain positive for Vimentin (IHC stain, ×100), (b) Immunohistochemical stain positive for Vimentin, (IHC stain, ×200) (c) Immunohistochemical stain positive for S-100 protein (IHC stain, ×400)
DISCUSSION
Osteosarcoma is the most common primary bone malignancy in childhood and adolescence. The etiology of human OS and the various interactions that occur between the host and tumor cells are still unknown. OS has a bimodal age distribution, having the first peak during adolescence, coinciding with the pubertal growth spurt and the second peak in older adulthood is more likely to represent a second malignancy, frequently related to Paget's disease. This suggests a close relationship between the adolescent growth spurt and OS. The incidence is higher in males than in females, with a higher incidence in blacks and hispanics than in whites. OS commonly occurs in the long bones of the extremities and the other possible locations are the skull or jaw (8%) and the pelvis (8%).[1] OS makes up to about 20% of all the sarcomas of the skeleton and relatively rarer entity in the craniofacial region only about 6.5-7% of all OS.[2] In craniofacial region, frequency of involvement of maxilla and mandible is almost same. Mandibular tumors arise more frequently in posterior body and ramus. Maxillary lesions are discovered more commonly in the inferior portion (alveolar ridge, sinus floor, palate) than the superior aspect (zygoma, orbital rim). The main clinical manifestations of OS of jaws are pain of variable intensity, swelling of bone and adjacent soft tissues.[3] The radiographic findings can be variable. Conventional OS are lytic, blastic or mixed. As in OS of the long bones, craniofacial OS often exhibit cortical destruction and extension into the soft tissue. However, unlike long bone OS; they often do not exhibit periosteal reactive bone.[4] The CT and magnetic resonance imaging (MRI) also aid in ascertaining the full extent of the lesion (intramedullary and soft tissue extension) and for identifying skip lesions or drop metastases.[5] The 2002, World Health Organization (WHO) classification scheme divides OS into primary (with 7 subtypes) and secondary types. The primary types include conventional, parosteal, telangiectatic, small cell, low-grade central, periosteal and high-grade surface. The secondary type encompasses syndrome and radiation-associated OS.[6] Smith et al. evaluated 496 cases of the head and neck OS and found that the subtypes of OS arising in gnathic and skull-based OS were similar. However, high-grade tumors were more commonly encountered in the skull and other craniofacial bones (67% high-grade histology in extragnathic sites compared with 53% in gnathic sites).[7] All types of OS have malignant osteoid, although the quantity may be scant. Osteoid is dense, pink, curvilinear amorphous collagen often arranged in a lace-like arrangement. It has a heterogeneous morphology and the cells can be spindled, clear, epithelioid, plasmacytoid or anaplastic giant cells. A mixture is often seen within the same tumor. Conventional OS is divided into three subcategories depending on the predominant matrix formation: chondroblastic, fibroblastic and osteoblastic. There is controversial evidence as to whether there is prognostic differentiation among these sub-classifications. From a diagnostic perspective, recognition of these subtypes is important for appropriate diagnosis, particularly on small biopsy samples, to avoid misclassifying a tumor as a CS or fibrosarcoma. In the craniofacial bones, COS is the most common type followed by osteoblastic. It is important to distinguish craniofacial CS from a COS. The latter will contain osteoid. De-differentiated CS by definition consists of a high-grade sarcoma juxtaposed to a well-differentiated CS.[6] Most authorities currently believe that even though a lesion is composed chiefly of malignant cartilage, it should be diagnosed as OS if significant malignant osteoblasts and tumor osteoid or bone can be identified since course of the lesion will probably be that of an OS rather than of a CS.[8] Distinguishing CS from COS can be difficult and highly subjective, especially on a small biopsy specimen. This distinction is critical in determining the most accurate prognosis and appropriate treatment modality, as adjuvant chemotherapy with surgery is standard treatment for OS, whereas CS is generally treated by surgical excision alone. Cartilaginous neoplasms have recently been shown to frequently (56%) harbour gene mutations in the metabolic enzymes isocitrate dehydrogenase 1 (IDH1) and IDH2 (IDH1 > IDH2), whereas other mesenchymal tumors lack these genetic aberrations.[9] Ezrin is a useful IHC marker for differential diagnosis between COS and conventional CS with a specificity of 100%.[10] New investigation results, indicate that galectin-1 (GAL-1) expression is a powerful marker to distinguish COS and conventional CS and is usually negative in conventional CS and the final diagnosis needs to incorporate histopathology results.[11] Takahama Junior et al. (2003), in a study with 25 cases of OS, observed that, according to the histological type of the tumor, patients with the chondroblastic type had a higher survival rate when compared to patients with the osteoblastic type.[11,12] Death rates for OS have been declining by about 1.3% per year. The overall 5-year survival rate for OS is 68%, without significant gender difference. The age of the patient is correlated with the survival, with the poorest survival among older patients. Complete surgical excision is important to ensure an optimum outcome. Tumor staging, presence of metastases, local recurrence, chemotherapy regimen, anatomic location, size of the tumor and percentage of tumor cells destroyed after neo-adjuvant chemotherapy have effects on the outcome.[1]
CONCLUSION
Craniofacial OS remain enigmatic in many ways and a number of difficulties related to their diagnosis and treatment are yet to be resolved. The diagnosis has a severe and invasive therapeutic consequence for the patient and therefore, it is very important to confirm such a diagnosis by IHC. COS is a histopathological diagnosis. It is important to differentiate the COS from CS and other types, as prognosis depends on the type of OS. Only when the essential tumor aspect of neoplastic bone differentiation is biopsied, can the pathologists make the correct diagnosis of COS; otherwise, a misdiagnosis of CS might occur. IHC in our case proved to be useful in the confirmation of the diagnosis. Although the histopathological picture of OS of long bones and craniofacial bones exhibit similarity, their management modalities may not necessarily be the same. Perhaps the time has come for histopathologists to re-examine diagnostic criteria for craniofacial OS.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 2.Zarbo RJ. Malignant non odontogenic neoplasms of the jaws. In: Regezi JA, Sciubba J, editors. Oral pathology: Clinical- pathologic correlations. 2nd ed. Philadelphia: WB Saunders; 1993. pp. 436–2. [Google Scholar]
- 3.Neville BW, Damm DD, Allen CM, Bouquet JE. 2nd edition. Elsevier: Saunders; 2005. Oral and Maxillofacial Pathology; pp. 544–7. [Google Scholar]
- 4.Lee YY, Van Tassel P, Nauert C, Raymond AK, Edeiken J. Craniofacial osteosarcomas: Plain film, CT, and MR findings in 46 cases. AJR Am J Roentgenol. 1988;150:1397–2. doi: 10.2214/ajr.150.6.1397. [DOI] [PubMed] [Google Scholar]
- 5.Gillespy T, 3rd, Manfrini M, Ruggieri P, Spanier SS, Pettersson H, Springfield DS. Staging of intraosseous extent of osteosarcoma: Correlation of preoperative CT and MR imaging with pathologic macroslides. Radiology. 1988;167:765–7. doi: 10.1148/radiology.167.3.3163153. [DOI] [PubMed] [Google Scholar]
- 6.Barnes L. 2nd Ed. New York: Marcel Dekker Inc; 2001. Surgical pathology of the head and neck; pp. 1058–1. [Google Scholar]
- 7.Smith RB, Apostolakis LW, Karnell LH, Koch BB, Robinson RA, Zhen W, et al. National Cancer Data Base report on osteosarcoma of the head and neck. Cancer. 2003;98:1670–80. doi: 10.1002/cncr.11716. [DOI] [PubMed] [Google Scholar]
- 8.Rajendran R. Benign and malignant tumors of oral cavity. In: Rajendran R, Sivapathasundaram B, editors. Shafer's Text Book of Oral Pathology. 5th ed. New Delhi: Elsevier; 2006. pp. 187–8. [Google Scholar]
- 9.Kerr DA, Lopez HU, Deshpande V, Hornicek FJ, Duan Z, Zhang Y, et al. Molecular distinction of chondrosarcoma from chondroblastic osteosarcoma through IDH1/2 mutations. Am J Surg Pathol. 2013;37:787–5. doi: 10.1097/PAS.0b013e31827ab703. [DOI] [PubMed] [Google Scholar]
- 10.Salas S, de Pinieux G, Gomez-Brouchet A, Larrousserie F, Leroy X, Aubert S, et al. Ezrin immunohistochemical expression in cartilaginous tumours: A useful tool for differential diagnosis between chondroblastic osteosarcoma and chondrosarcoma. Virchows Archiv. 2009;454:81–7. doi: 10.1007/s00428-008-0692-8. [DOI] [PubMed] [Google Scholar]
- 11.Machado I, López Guerrero JA, Navarro S, Mayordomo E, Scotlandi K, Picci P, et al. Galectin-1 (GAL-1) expression is a useful tool to differentiate between small cell osteosarcoma and Ewing sarcoma. Virchows Arch. 2013;462:665–1. doi: 10.1007/s00428-013-1423-3. [DOI] [PubMed] [Google Scholar]
- 12.Junior AT, de Abreu Alves F, Pinto CA, Carvalho AL, Kowalski LP, Lopes MA. Clinicopathological and immunohistochemical analysis of twenty-five head and neck osteosarcomas. Oral Oncol. 2003;39:521–30. doi: 10.1016/s1368-8375(03)00017-4. [DOI] [PubMed] [Google Scholar]


