Abstract
Purpose/Background:
Patellofemoral pain (PFP) is a common knee conditions experienced by adolescents and young adults, seen particularly in women. Clinicians and researchers need to understand how proximal, local, or distal factors may influence the development of PFP and affect individuals once they have developed PFP. Proximal factors are the focus of recent studies and the purpose of this systematic review was to determine if females with PFP have hip muscle strength or endurance deficits when compared to their unaffected leg and to comparison groups.
Methods:
A systematic review was conducted to identify relevant studies in the databases PubMed, PEDro, ScienceDirect and EBSCOhost up to June 2013. Data including study design, participants demographic data, and assessments of hip muscle strength or endurance were extracted from individual trials. The mean differences of hip muscles strength or endurance between females with PFP and healthy controls or unaffected side were extracted or calculated from individual trials and, when possible, a meta‐analysis was performed.
Results:
Ten cross‐sectional studies were included in this review. Concerning isometric strength, pooled data reported deficit in hip abduction, extension, external rotation and flexion but no deficit in adduction and internal rotation when compared with healthy controls. When compared with the unaffected side, deficit in hip abduction was reported in two studies and deficit in extension and external rotation in one study. Studies with isokinetic strength evaluation reported deficit in abduction but contradictory results for extensors and rotators in females with PFPS. Finally, one study reported hip endurance deficit in extension and one found no significant differences in hip endurance compared to control subjects.
Conclusion:
The results of this systematic review confirm that females with PFPS have deficit in hip muscle strength compared with healthy controls and the unaffected side but are contradictory concerning endurance.
Level Of Evidence:
2a
Keywords: Endurance, Female, Hip, patellofemoral pain, strength
INTRODUCTION
Patellofemoral pain (PFP) is the most common knee condition experienced by active adolescents and young adults.1 Boling et al2 reported that females are significantly more likely to develop PFP than males while Roush et al3 estimated a prevalence rate of 12‐13% in females 18 to 35 years of age. Symptoms are characterized by anterior, retro or peripatellar pain during activities such as squatting, kneeling, prolonged sitting, ascending or descending stairs, running, hopping, and jumping.4,5 Diagnosis of PFP is clinical in nature, and must exclude pain due to meniscal, cruciate or collateral ligament injuries, patellar tendinopathy, Osgood‐Schlatter or Sinding‐Larsen‐Johansson syndrome, or other pathologic conditions.6
Researchers suggest that PFP is a multifactorial problem.7 Potential impairments that have been associated with PFP include increased quadriceps angle,8 hypermobile patella,8 altered lower limb kinematics,9 muscle dysfunction,10,11,12 and decreased lower limb flexibility.13,14
During the last decade, numerous researchers have investigated the connection between and supported the influence of the hip on PFP.9,10,11,12,15 Powers et al16 reported that impaired muscular control of the hip can increase hip adduction or internal rotation and, therefore, increase the quadriceps angle (Q‐angle) during dynamic movements. Huberti and al17 showed that a 10‐degree increase in the Q‐angle can increase patellofemoral contact pressures by 45% at 20° of flexion of the knee. Repetition of this excessive movement may contribute to development of PFP.18
Based on these studies, authors have hypothesized that deficit of hip muscular strength or endurance may increase femoral movement during functional tasks and contribute to the development of PFP.7,18 Additionally, Powers19 suggested that females are more predisposed to hip neuromuscular deficits than males.19 Prins et al20 performed a systematic review of five studies7,18,21,8,22 assessing hip strength in females with PFP. Because data were insufficiently reported and methodologies varied, the authors chose not to implement a meta‐analysis. The authors reported hip muscle weakness in participants with PFP compared with healthy controls. All included studies tested maximum isometric strength assessed with hand held dynamometry. Additionally, specific research questions for their review were targeted on hip strength and not endurance.
Since the work presented by Prins et al,20 several studies have been performed that evaluated hip muscle function in females with PFP, including strength and endurance evaluation using varied assessment techniques (isometric, isotonic, isokinetic).
Therefore, the purposes of this systematic review were:
To determine if females with PFP have isometric and isokinetic hip muscle strength deficits when compared to their unaffected leg and to comparison groups.
To determine if females with PFP have hip muscle endurance deficits when compared to their unaffected leg and to comparison groups.
A systematic review was conducted and when meta‐analysis was possible, data from individual studies were pooled and analyzed.
METHODS
Search strategy
A systematic search strategy was utilized in order to identify relevant studies in the databases PubMed, PEDro, ScienceDirect and EBSCOhost up to June 2013. The following key words were combined: patellofemoral pain syndrome, anterior knee pain, hip, muscle strength, muscle endurance, female. The search was applied without restrictions on language or year of publication. Study types searched excluded systematic reviews, meta‐analyses, case series, and case reports. Furthermore, this strategy was supplemented by hand searching the references of all articles selected for the review.
Study Selection
Studies were selected using the following criteria: (1) studies had to assess hip muscle strength or endurance in females with PFP; (2) studies that included both males and females had to describe specific results for females; (3) studies had to include a healthy control group. Studies focusing on other knee pathologies were excluded.
The selection of studies was performed independently by two reviewers (JVC and CP) based on the title and the abstract. Articles not excluded by both reviewers were assessed in full‐text and disagreement regarding inclusion was resolved by consensus.
Data Extraction and Synthesis
The first author extracted data including study design (type, author, date), participants (number, age, activity level, PFP definitions) and assessment of hip muscle strength or endurance (nature of contraction, body position, type of fixation and instrument used, number of trials, dynamometer placement, measurement units). When possible, the mean differences (MDs) of hip muscles strength or endurance between females with PFP and healthy controls or unaffected side, were extracted or calculated from individual trials, with matching 95% confidence intervals (CIs). If data were missing, information was requested from the authors. Nature of contraction (isometric, isotonic or isokinetic) and type of instrument used were also determined and recorded for data extraction or calculation.
When meta‐analysis was possible, data from individual studies were pooled with the software package Review Manager 5 (Nordic Cochrane Center, Copenhagen, Denmark) to determine a weighted mean difference (WMD) or a weighted standardized mean difference (WSMD) with a 95% CI.23
Two statistical methods were used to analyze statistical heterogeneity, the chi‐square test for heterogeneity and the I² test. When the chi‐square test is significant, statistical heterogeneity is present.24 The percentage of I² represents the percentage of total variation across studies due to heterogeneity and is interpreted as 25% indicating low heterogeneity, 50% medium heterogeneity, and 75% high heterogeneity.25
The meta‐analysis was conducted using a random‐effects model. If heterogeneity between studies was medium or high or if data were not sufficient for a meta‐analysis, a descriptive analysis was performed.
Methodological Quality Assessment
The authors created a methodological quality assessment list with items from the Newcastle‐Ottawa Scale, the Dutch Cochrane Centre website (http://dcc.cochrane.org/dutch-cochrane-centre), the Cardiff University Systematic Review Network (http:/www.cardiff.ac.uk/insrv/libraries/sure/sysnet), the Scottish Intercollegiate Guidelines Network (http:/www.sign.ac.uk) and work by Higgins et al23 and Lankhorst et al26.
Table 1 lists the resulting 10 questions that were used for assessing the methodological quality of the studies. Two reviewers (JVC and CP) assessed the included studies independently. Disagreements between reviewers were resolved through a consensus procedure. Each item was rated as “positive”, “unclear” or “negative”. Because calculating a summary score is explicitly discouraged by Higgins et al,23 a total score was not calculated.
Table 1.
Methodological Quality Assessment Questions & Scoring
| Methodological Quality Assessment Questions | Scored Positive if: |
|---|---|
| 1. Did the study address an appropriate and clearly focused question? | Research question or hypothesis was that females with PFPS have decreased hip muscle force or endurance compared with healthy controls. |
| 2. Was the study population clearly defined? | The place of recruitment, age and activity level were given. |
| 3. Was patellofemoral pain syndrome clearly defined? | PFPS participants completed a reliable, valid scale responsive in this specific population. The intensity and history of symptoms was clearly documented. |
| 4. Was the method of patellofemoral pain syndrome assessment reported? | The following information was described:
|
| 5. Were the females with PFPS representative of the target population? | The participants were females, actives, between 18 and 35 years of age. |
| 6. How comparable are the females with PFPS and healthy controls with respect to potential confounding factors? | Age and activity level are comparable between both groups. Strength or endurance were normalized to body weight. |
| 7. Were the same exclusion criteria used for both females with PFPS and healthy controls? | The exclusion criteria were described and similar for both groups. |
| 8. Was the method of hip muscle strength or endurance assessment reported? | Body position, type of fixation and instrument used, numbers of trials, order of testing sequences, verbal encouragement, dynamometer placement, measurement units and warm‐up were documented. |
| 9. Was the same method of assessment for both females with PFPS and healthy controls? | The same method of strength or endurance assessment was used for both groups. |
| 10. Were the assessors blinded to the different groups? | Blinded application of strength or endurance assessments was performed. |
Abbreviations: PFPS=patellofemoral pain syndrome.
RESULTS
Flow of Study Selection
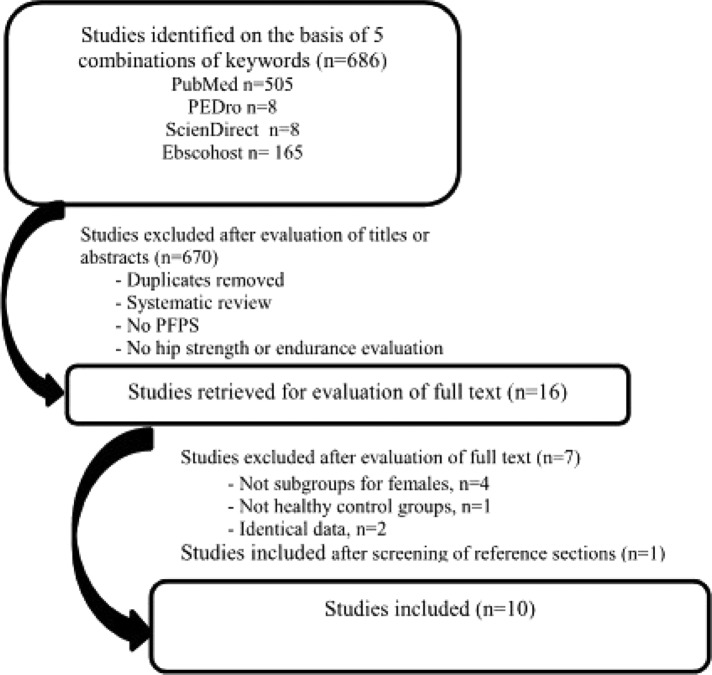
The database search identified 686 potentially relevant articles (Appendix A). After exclusion of 670 studies from titles and abstracts, 16 articles were retrieved for full‐text review. On basis of the full‐text review, the authors' excluded five articles because subgroups for females or healthy control groups were not included. Souza and Powers27,28 and Bolgla et al7,29 each published two articles with some similar data. Data were extracted from both of these articles, but only Souza and Powers27 and Bolgla et al7 were used for citations. One study was added to the review after screening of the reference sections of selected articles.30 Ultimately, 10 studies were included in the systematic review.7,18,21,22,27,30,31,32,33,34 Figure 1 describes the flow chart of the studies selection.
Figure 1.
Flow chart of study selection
Characteristics of the Included Studies
Evaluation of studies
Table 2 reports methodological quality assessment list. Only two studies reported blinded application of evaluation18,32 and five used a functional assessment scale reported as reliable, valid and responsive in population with PFP.22,30,31,32,34 Moreover, studies often insufficiently described the place of recruitment, the activity level of participants, and experience and profession of the clinical investigator.
Table 2:
Summary of Methodological Quality Assessment of Included Papers.
| Quality assessment list | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Baldon et al31 | + | ‐ | + | + | ? | + | + | + | + | ‐ |
| Bolgla et al7 | + | ‐ | ‐ | ‐ | + | + | + | + | + | ‐ |
| Cichanowski et al18 | + | + | ‐ | + | + | + | + | + | + | + |
| Ireland et al21 | + | ‐ | ‐ | + | + | + | ‐ | + | + | ‐ |
| Magalhaes et al32 | + | + | + | + | ‐ | + | + | + | + | + |
| McMoreland et al30 | + | + | + | ‐ | ? | + | + | + | + | ‐ |
| Nakagawa et al33 | + | ‐ | ‐ | + | ? | ‐ | + | + | + | ‐ |
| Robinson and Nee22 | + | ‐ | + | + | ‐ | ? | + | + | + | ‐ |
| Souza and Powers27 | + | + | ‐ | + | ‐ | + | + | + | + | ‐ |
| Willson and Davis34 | + | ‐ | + | + | + | + | + | + | + | ‐ |
Participants
In total, 374 females were included in the studies. The number of participants ranged from 20 22,31 to 100.32 Only one study33 examined both males and females. In the others, all participants were female. The average age of participants ranged from 15.7 21 to 27 years.27Activity level was not specified in five studies.7,22,30,31,33 Table 3 provides the characteristics of participants.
Table 3:
Characteristics of Participants.
| Study | Participants | Activity level | |
|---|---|---|---|
| PFPS | Control | ||
| Baldon et al31 | n= 10 Age (y) : 22.9 (SD : 5.2) | n= 10 Age (y): 23.9 (SD : 2.3) | Not mentioned |
| Bolgla et al7 | n= 18 Age (y): 24.5 (SD : 3.2) | n= 18 Age (y): 23.9 (SD : 2.8) | Not mentioned |
| Cichanowski et al18 | n= 13 Age (y): 19.3 (SD : 1,1) | n= 13 Age (y): 19.5 (SD : 1.3) | Female athletes |
| Ireland et al21 | n= 15 Age (y): 15.7 (SD : 2.7) | n= 15 Age (y): 15.7 (SD : 2.7) | All subjects reported routine participation in either recreational or organized sports |
| Magalhaes et al32 | n= 21 Age (y): 24.1 (SD : 6.3) | n= 50 Age (y): 24.6 (SD : 6.4) | Sedentary (did not perform sports activities any day of the week for at least the previous 6 months) |
| McMoreland et al30 | n= 12 Age (y): 23 (SD : not mentioned ) | n= 12 Age (y): 21 (SD : not mentioned ) | Recreational sports > 30 minutes three times weekly |
| Nakagawa et al33 | n= 20 Age (y): 22.3 (SD : 3.1) | n= 20 Age (y): 21.8 (SD : 2.6) | Not mentioned |
| Robinson and Nee22 | n= 10 Age (y): 21.0 (range 12‐34) | n= 10 Age(y): 26.6 (range 16‐35) | Not mentioned |
| Souza and Powers27 | n= 19 Age (y): 27 (SD : 6) | n= 19 Age (y) : 26 (SD : 4) | Active females |
| Willson and Davis34 | n= 20 Age (y): 23.3 (SD : 3.1) | n= 20 Age (y): 23.7 (SD : 3,6) | Recreational sports that require running or jumping (5/10 on the Tegner activity scale) |
Abbreviations: PFPS=patellofemoral pain syndrome; SD=standard deviation; y=years.
Inclusion criteria for experimental groups
Participants complained of symptoms for a minimum of 4 to 12 weeks.7,21,30,31,32,33,34 Depending on the studies, participants had to report anterior, retro, or peripatellar pain during at least two or three of the following provocative activities: squatting, kneeling, prolonged sitting, ascending or descending stairs, running, hopping, jumping, palpation or compression of medial or lateral patella facet, isometric quadriceps contraction.
Exclusion criteria for both groups
All studies excluded participants if they had previous knee surgery or signs of meniscal, cruciate or collateral ligament injuries, patellar dislocation, Osgood‐Schlatter or Sinding‐Larsen‐Johansson syndrome, or other pathologic conditions. Cichanowski et al18 and Robinson and Nee22 excluded participants with bilateral PFP from their experimental groups,. Additionally, in one study, females over 45 were excluded.27 Table 4 describes the methods of evaluation of strength and endurance for included studies.
Table 4:
Methods of strength and endurance evaluations
| Evaluation | Body position | Stabilisation | Dynamometer position | Range of motion | Contractions | Modalities | |
|---|---|---|---|---|---|---|---|
| Baldon et al2 | Isokinetic muscle strength | Abd /Add: side lying ER/IR:sitting | Strapping | Abd/Add: femoral condyle ER/IR:malleous | Abd/Add: 0° to 30° of abd ER:0° to 30° of ER IR:0° to 30° of IR | Eccentric | Average of 5 repetitions at 30°/s |
| Bolgla et al3 | Isometric muscle strength | Abd: side lying ER:sitting | Strapping | Abd: femoral condyle ER:malleous | N/A | Isometric | Average of 3 trials |
| Cichanowski et al8 | Isometric muscle strength | Abd/Add: side lying ER/IR/Flex:sitting Ext:prone | Manual | Abd/Add: femoral condyle ER/IR:malleouls Ext/Flex:thigh | N/A | Isometric | Best of 2 trials |
| Ireland et al21 | Isometric muscle strength | Abd: side lying ER/IR/Flex: sitting Ext:prone | Strapping | Abd: femoral condyle ER/IR: malleolus Ext/Flex:thigh | N/A | Isometric | Best of 3 trials |
| Magalhaes et al30 | Isometric muscle strength | Abd/Add: side lying ER/IR/Flex:sitting Ext.:prone knee extended | Manual | Abd, IR: lateral malleolus Add, ER:medial malleous Flex/Ext:thigh | N/A | Isometric | Average of 2 trials |
| McMoreland et al31 | Isometric muscle strength | Abd: side lying ER/IR:sitting | Strapping | Abd: femoral condyle ER/IR:malleolus | N/A | Isometric | Average of 3 trials |
| Muscle endurance | Abd: side lying ER/IR:sitting | Strapping | Abd: femoral condyle ER/IR:malleolus | 75% of full active range of motion | Concentric | 30 maximal repetitions at 30°/s | |
| Nakagawa et al33 | Isokinetic muscle strength | Abd: side lying ER:sitting | Strapping | Abd: femoral condyle ER:malleolus | Abd: 0°to 30° of abd ER:5° of IR to 20° of ER | Eccentric | Average of 5 repetitions at 30°/s |
| Robinson and Nee38 | Isometric muscle strength | Abd: side lying ER:sitting Ext:prone knee flexed | Manual | Abd: malleolus ER:malleuols Ext:thigh | N/A | Isometric | Average of 3 trials |
| Souza and Powers40 | Isometric muscle strength | Abd : side lying ER:sitting | Not reported | Abd: femoral condyle ER:malleouls | N/A | Isometric | Average of 3 trials |
| Isokinetic muscle strength | Ext.: prone knee flexed | Not reproted | Ext: thigh | Ext: 30° of flex to 10° of ext | Eccentric and concentric | Average of 10 repetitions at 10°/s | |
| Muscle endurance | Ext.: prone knee flexed | Not reported | Ext : thigh | Ext: 30° of flex to 10° of ext | Eccentric and concentric | 25% of body weight, 2.5 s/repetition | |
| Willson and Davis46 | Isometric muscle strength | Abd: side lying ER:prone | Strapping. | Abd: femoral condyle ER:malleouls | N/A | Isometric | Average of 3 trials |
Abbreviations. Abd=abduction; add=adduction, ER=external rotation; IR=internal rotation; flex=flexion; ext=extension; ant=anterior; post=posterior.
Hip muscles strength and endurance
Isometric muscle strength
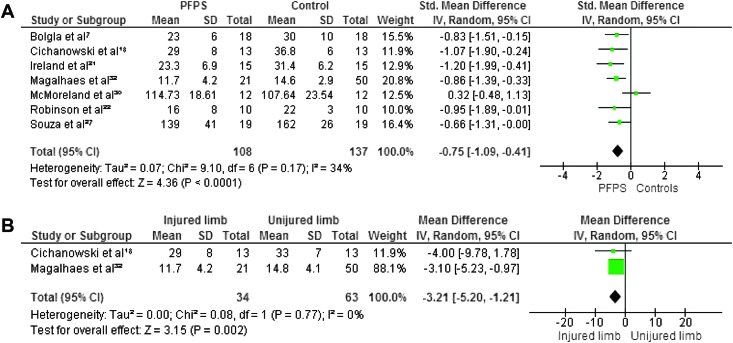
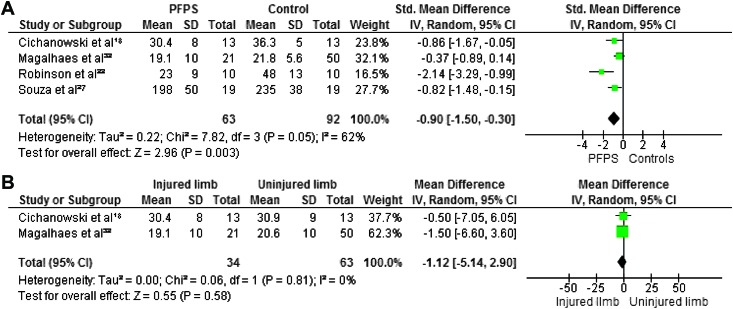
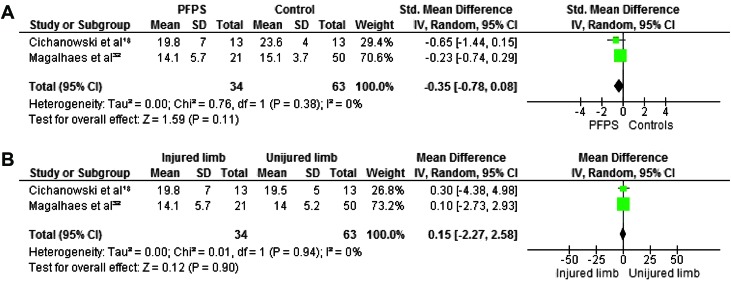
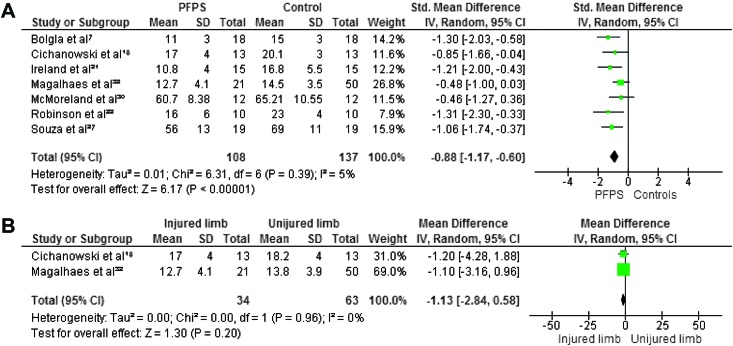
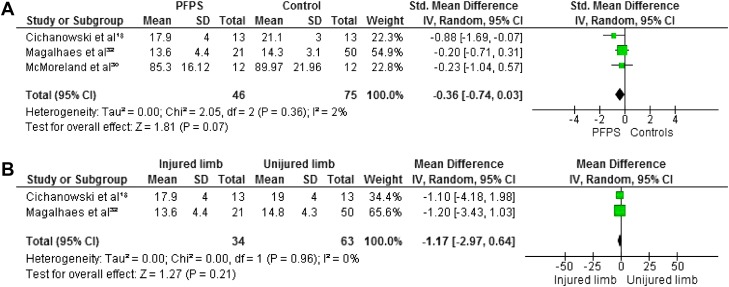
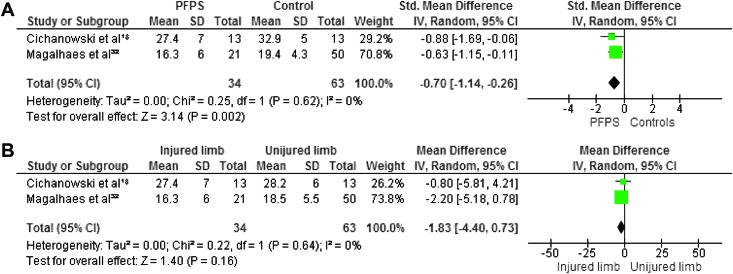
Maximum isometric strength was tested in eight studies. 7,18,21,22,27,30,32,34 In all studies hip abductors and external rotators were evaluated. Four authors investigated hip extensors,8,30,38,40 and three measured internal rotators.8,30,31 Hip flexors and adductors were evaluated in two studies.8,30 The pooled data demonstrated significantly lower strength in females with PFP than in healthy controls for abduction (WSMD, ‐0.75; 95% CI: ‐1.09, ‐0.41),7,18,21,22,27,30,32 external rotation (WSMD, ‐0.88; 95% CI: ‐1.17, ‐0.60),7,18,21,22,27,30,32,3 flexion (WSMD, ‐0.70; 95% CI: ‐1.14, ‐0.26)18,32 and extension (WSMD, ‐0.90; 95% CI: ‐1.50, ‐0.30).18,32,22,27 The pooled data were not significantly lower in females with PFP for strength of adduction (WSMD, ‐0.35; 95% CI: ‐0.78, 0.08) 18,32 and internal rotation (WSMD, ‐0.36; 95% CI: ‐0.74, 0.03).18,30,32 Additionally, Magalhaes et al32 compared the average of both sides in participants with bilateral PFP to healthy controls and reported significant differences in abduction, extension, and external rotation. Willson and Davis34 did not provide sufficient information to calculate MDs with matching 95% CIs, however, they reported a statistically significant decrease in abductor and external rotator strength in females with PFP.
When compared with the unaffected side, the pooled data showed a significant decrease in strength in abduction (WMD, ‐3.21; 95% CI: ‐5.20, ‐1.21) 18,32 and extension (WMD, ‐1.12; 95% CI: ‐5.14, 2.90) 18,32 but no significant differences between both sides for adduction (WMD, 0.15; 95% CI: ‐2.27, 2.58), 18,32 external rotation (WMD, ‐1.13; 95% CI: ‐2.84, 0.58), 18,32 internal rotation ((WMD, ‐1.17; 95% CI: ‐2.97, 0.64) 18,32 and flexion (WMD, ‐1.83; 95% CI: ‐4.40, 0.73). 18,32 Because of missing data and insufficient information to calculate MD with matching 95% CI, the data from Robinson et al22 were not pooled but their study reported a decrease in abduction, external rotation, and extension when compared to the unaffected side. Figures 2‐7 display forest plots of the differences in hip isometric strength between females with PFP and controls (A), and between both sides (B).
Figure 2.
Forest plot of the difference in hip abductors isometric strength between females with PFPS and controls (A) and between both sides (B)..
Figure 7.
Forest plot of the difference in hip extensors isometric strength between females with PFPS and controls (A) and between both sides (B).
Figure 3.
Forest plot of the difference in hip adductors isometric strength between females with PFPS and controls (A) and between both sides (B).
Figure 4.
Forest plot of the difference in hip external rotators isometric strength between females with PFPS and controls (A) and between both sides (B).
Figure 5.
Forest plot of the difference in hip internal rotators isometric strength between females with PFPS and controls (A) and between both sides (B).
Figure 6.
Forest plot of the difference in hip flexors isometric strength between females with PFPS and controls (A) and between both sides (B).
Isokinetic muscle strength
Peak torque during isokinetic evaluation was tested in three studies.27,31,33 Due to high heterogeneity, data from these studies could not be pooled. Baldon et al2 found eccentric peak torques significantly decreased in females with PFP for abduction (MD, ‐34.48; 95% CI: ‐41.81, ‐27.15), adduction (MD, ‐26.48; 95% CI: ‐37.69, ‐15.27), and internal rotation (MD, ‐9.21; 95% CI: ‐16.90, ‐1.52) when compared with healthy controls. Nakagawa et al33 compared females with PFP to a control group and reported significantly decreased eccentric peak torques in females with PFP for abduction (MD, ‐17.00; 95% CI: ‐25.70, ‐8.30) and external rotation (MD, ‐9.00; 95% CI: ‐13.04, ‐4.96). Souza and Powers27 compared eccentric and concentric peak torques of the hip extensors between females with PFP and healthy controls and found concentric peak torques to be significantly lower in females with PFP (MD, ‐16.00; 95% CI: ‐30.28, ‐1.72) but no significant difference between groups for eccentric isokinetic strength measures (MD, ‐5.00; ‐24.52, 14.52).
Muscle endurance
Two studies assessed hip muscle endurance, using isokinetic dynamometer.27,30 Souza and Powers27 evaluated endurance of hip extensors. Subjects were instructed to contract against 25% of body weight and to perform as many repetitions as possible throughout the desired arc of motion. Each repetition was performed in 2.5 seconds. When successful repetition was not achieved (arc of motion or time allotted), the dynamometer power output would drop. A drop to <75% torque output was considered a failed repetition and the test was terminated after two successive failed repetitions. The authors reported significantly fewer repetitions performed in females with PFP than in healthy controls (MD, ‐15.30; 95% CI: ‐20.17, ‐10.43). McMoreland et al30 tested endurance of hip abductors and rotators between females with PFP and healthy controls. Total work (joules) produced during 30 maximal concentric repetitions at 30°/s was used to quantify muscle endurance. Their results showed no significant between‐group difference for total work during tests. Appendix B reports all outcomes and mean differences of hip muscle strength and endurance for the included studies.
DISCUSSION
The aim of this systematic review was to determine if females with PFP have hip muscle strength and endurance deficits when compared to their unaffected leg and to comparison groups (normals). The results confirm that females with PFPS have strength deficits of several hip muscles in comparison with healthy controls and the unaffected side, but are contradictory concerning endurance.
Hip muscle endurance and PFP
Prins et al20 suggested that deficit in hip muscle endurance may contribute to PFP and hypothesized that muscles of patients with PFP have greater deficits in endurance than strength. Only two studies investigated these questions. McMoreland et al30 and Souza and Powers27 evaluated muscle endurance of the hip in females with and without PFP. Souza and Powers27 observed that females with PFP performed 49% less hip extension repetitions when compared with healthy controls. The authors suggested that these results may explain why symptoms in subjects with PFP increase during prolonged activities. These findings may also help to explain observations by Dierks et al35 who described increased hip adduction in subjects with PFPS during prolonged running, especially at the end of the run. McMoreland et al30 reported a moderate correlation between strength and endurance and suggested that clinicians should evaluate both measures separately, however, they found no significant differences in hip endurance compared to control subjects.30 A possible explanation for these contradictory results may be differences in level of pain in the study population as compared to the controls. Indeed, the study of McMoreland et al30 included females with mild patellofemoral pain while other studies included subjects ranging from mild to severe pain. The question concerning whether there is a lack of hip muscle endurance in patients with PFP remains unanswered.
Hip muscle strength and PFP
With regard to isometric strength of the hip, the results of studies indicated that a deficit in hip abduction, extension, external rotation, and flexion but no deficits in adduction and internal rotation when compared with healthy controls. However, differences in the number of studies pooled to evaluate each muscle group were observed. Seven studies were pooled for abduction and external rotation,7,18,21,22,27,30,32 four for extension, 18,22,27,31 three for internal rotation18,30,32 and only two studies for flexion and adduction.18,32 Israel and Richter24 reported that combination of a large number of studies increases the statistical power by reducing the interval confidence and, therefore, by increasing the precision of the estimated population mean difference effect size. The results of studies in which participants were evaluated with isokinetic dynamometry demonstrated strength deficits in abduction but contradictory results were found for peak torques of extensors and rotators. Therefore, more studies are necessary to determine whether concentric, eccentric, or isometric deficits in hip muscles strength are comparable in females with PFP and which deficits is most likely related to function. The isometric strength of the hip in the side with PFP versus the unaffected side was evaluated in three studies. 18,22,32 Data from two studies were pooled and showed a decrease significant in hip abductors compared with the unaffected side.18,32 Data from Robinson and Nee22 also demonstrated a decrease in isometric external rotation and extension strength when examined versus unaffected side.
Cause or consequence of PFP
In a prospective cohort study, Boling et al36 longitudinally followed 1597 healthy participants and included isometric hip muscle strength evaluation in their baseline data collection, using hand held dynamometer. A total of 40 participants developed PFP during the follow‐up period (maximum of 2.5 years of follow up) but initial hip muscle strength deficits were not significantly associated with occurrence of PFP. Finnoff et al37 evaluated baseline isometric hip strength of high school running athletes at the beginning of the running season, using hand held dynamometery. The objective of this prospective study was to determine if pre‐injury hip abductors weakness was associated with the development of PFP when compared to non‐injured groups. Results showed that stronger hip abductors and weaker hip external rotators were risk factors of PFP. In addition, in the injured group, hip abduction and external rotation strengths decreased in post‐injury when compared with their pre‐injury evaluation. To date, it is difficult to determine whether deficits in hip muscle strength are a predisposing factor or consequence of PFP. Furthermore, none of these prospective studies evaluated hip muscle endurance.
Hip muscle strength and motion patterns in PFP
Researchers have shown that abnormal lower leg and thigh motions in the transverse and frontal planes during weight‐bearing activities could affect or increase retropatellar stress when compared to non‐weight bearing activities.16,38 Powers19 determined that subjects with hip weakness demonstrate increased hip adduction, hip internal rotation, and knee valgus when compared with controls. Dierks et al35 compared hip strength and lower extremity kinematics before and after a prolonged run in patients with PFP and controls. The authors reported hip abductor weakness and alteration in the lower extremity kinematics in the PFP group and concluded that weaker hip abductor muscles were associated with increased hip adduction during running. Conversely, Bolgla et al7 reported that females with PFP and hip weakness did not demonstrate altered hip and knee kinematics and other authors have not shown association between hip muscle weakness and lower extremity kinematics during single‐legged 40 cm drop landings in healthy groups.39 Additional investigations are needed to fully understand relationship between hip muscle strength and lower extremity kinematics during functional activities in subjects with PFP.
Effects of interventions
Researchers have recently investigated the effectiveness of hip strengthening for patients with PFP. Four studies, with variable protocols, reported a significant decrease of pain and amelioration of function following an exercise program targeting the hip muscles.40,41,42,43 Dolak et al40 investigated the benefits of a four weeks strengthening program of hip abductors and external rotators compared to quadriceps strengthening. Thirty‐three females with PFP were randomly assigned to a hip strengthening program (hip group) or a quadriceps strengthening program (quadriceps group) for four weeks. After completing the fourth week of rehabilitation, participants from both groups performed similar program of functional weight‐bearing exercises for four additional weeks. The hip group reported less pain than the quadriceps group at four weeks (respectively 43% and 3% on the visual analogic scale). After eight weeks, results were similar for both approaches and showed significant improvement of function and decrease of pain. The authors emphasized the use of isolated hip strengthening exercises in the early rehabilitation stages to in order to reduce pain more efficiently. Moreover, exercises described in the study used simple equipment such as elastic bands and can be easily included in a home training program.
Limitations
Because PFP is a diagnosis is based on a group of symptoms and not a specific test, inclusion criteria often differed between studies. The studies included patients with some variability in terms of localization and history of pain and type of provocative activities. Moreover, only five studies used valid and reliable scales, responsive in this specific population, like the Kujula scale.22,30,31,32,34 The systematic use of the Kujula scale in studies on PFP could allow optimal comparability between participants. PFP is a multifactorial and complex condition and it is evident that the target population is often heterogeneous and could be separated in subgroups. Additionally, participant characteristics were variable or unclear. Females included in the examined studies were sedentary,32athletes,27 reported recreational sports participation,21,34 or their activity level was not specified.7,22,31,32,33 Authors should systematically describe participants demographic data and, similarly, there is a need to provide recommendations for selection of patients with PFP to improve the quality and consistency of research on this syndrome.
Methods for assessing hip strength were variable. Subject and dynamometer position, type of contractions, measurement device and stabilization of the pelvis and upper leg differed among studies. Good to high reliability of hip strength evaluation using hand held or isokinetic dynamometery measures has been reported in the literature,44,45,46 except for measures of the internal rotators, which showed moderate reliability.44 Lower reliability could explain why authors who studied the strength of the internal rotators in female patients with PFP reported no strength deficit. Krause et al45 examined the effect of different testing positions on test–retest reliability and showed that the relative reliability of hip abduction and adduction strength evaluation in the side lying position was higher when using a long lever compared with a short lever. Thorborg et al46 reported less measurement variation in supine than in the side lying position when testing abduction and adduction. These results can explain the lack of agreement between studies and confirm the utility of establishing a standard method for hip strength and endurance evaluations.
Finally, the results of studies need to be interpreted with caution because, in most studies, the primary evaluator was not blinded to subject's condition and bias might have been introduced during evaluation.
CONCLUSION
This systematic review provides strong statistically significant evidence that females with PFP have significant isometric strength deficits in hip abduction, extension, and external rotation when compared with healthy controls. Moderate evidence was obtained concerning isometric strength deficit in flexion because the data of only two studies were pooled. There was no evidence that females with PFP have strength deficits in adduction and internal rotation. Moderate statistical evidence was found for isokinetic deficits in abduction and conflicting evidence for a deficit in rotation and extension. When compared with the uninjured limb, moderate evidence was found for a strength deficit in abduction, conflicting with evidence for deficiencies in extension and external rotation strength and no evidence for internal rotation, adduction and flexion strength deficiencies. Finally, conflicting evidence was found regarding whether a decrease in hip muscular endurance exists in patients with PFP.
Appendix A.
Combinations of keywords for searches in varied Databases.
| Combinations of keywords | |||||
|---|---|---|---|---|---|
| (“Patellofemoral Pain Syndrome” OR “Anterior Knee Pain”) AND (“Muscle Strength” OR “Muscle Endurance”) | 85 | 8 | 8 | 40 | 43 |
| 44 | |||||
| (“Hip” OR “Muscle Strength” OR “Muscle Endurance”) AND (“Patellofemoral Pain Syndrome” OR “Anterior Knee Pain”) | 195 | 0 | 0 | 73 | 45 |
| 46 | |||||
| (“Patellofemoral Pain Syndrome” OR “Anterior Knee Pain”) AND (“Muscle Strength” OR “Muscle Endurance”) AND “Hip” | 40 | 0 | 0 | 22 | 47 |
| 48 | |||||
| 49 | |||||
| (“Hip” OR “Muscle Strength” OR “Muscle Endurance” OR “Muscle Endurance”) AND (“Patellofemoral Pain Syndrome” OR “Anterior Knee Pain”) AND “Female” | 146 | 0 | 0 | 21 | 50 |
| 51 | |||||
| 52 | |||||
| (“Hip” AND “Muscle Strength” OR “Muscle Endurance”) AND (“Patellofemoral Pain Syndrome” OR “Anterior Knee Pain”) AND “Female” | 39 | 0 | 0 | 9 | |
| Total | 505 | 8 | 8 | 165 |
Appendix B.
Outcomes and mean differences of hip muscle strength and endurance
| Study | Modalities | Outcome | MD (95% CI) |
|---|---|---|---|
| Abduction | |||
| Baldon et al31 | Isokinetic strength (eccentric) at 30°/s, (Nm/kg) × 100 | PFPS, 88.89 ±10.27 Controls, 123.37 ± 5.85 P = .008 | ‐34.48 (‐41.81, ‐27.15) |
| Bolgla et al7 | Isometric strength, (Nm/N.m) × 100 | PFPS, 23 ±6 Control, 30±10 P = .0006 | ‐7.00 (‐12.39, ‐1.61) |
| Cichanowski et al18 | Isometric strength, %BW | PFPS, 29 ±8 Control, 36.8±6 P = .010 | ‐7.80 (‐13.24, ‐2.36) |
| Cichanowski et al18 | Isometric strength, %BW | Injured leg, 29±8 Noninjured leg 33±7 P = .003 | ‐4.00 (‐9.78, 1.78) |
| Ireland et al21 | Isometric strength, %BW | PFPS, 23.3 ±6.9 Control, 31.4±6.2 P <.01 | ‐8.10 (‐12.79, ‐3.41) |
| Magalhaes et al32 | Isometric strength, %BW | PFPS, 11.7 ±4.2 Control, 14.6±2.9 P < .002 | ‐2.90 (‐4.34, ‐1.46) |
| Magalhaes et al32 | Isometric strength, %BW | Injured leg, 11.7±4.2 Noninjured leg 14.8 ± 4.1 P <.002 | ‐3.10 [‐5.26, ‐0.94] |
| Magalhaes et al 32 | Isometric strength, %BW | Bilateral PFPS, 9.6 ± 2.8 Control, 14.6 ±2.9 P <.0001 | ‐5.00 (‐6.30, ‐3.70) |
| McMoreland et al30 | Isometric strength, Nm/kg × 100 | PFPS, 114.73 ±18.61 Control, 107.64 ±23.54 P < .0001 | 7.09 (‐9.89, 24.07) |
| McMoreland et al30 | Isokinetic endurance (concentric) at 30°/s, j | PFPS, 493.69 ±174.25 Control, 492.4 ±135.2 P <.001 | 1.29 (‐123.50, 126.08) |
| Nakagawa et al33 | Isokinetic strength (eccentric) at 30°/s, (Nm/kg.m)x100 | PFPS, 56 ±13 Control, 73 ±15 P < .001 | ‐17.00 (‐25.70, ‐8.30) |
| Robinson and Nee22 | Isometric strength, %BW | PFPS, 16 ±8 Control, 22±3 P = .007 | ‐6.00 (‐11.30, ‐0.70) |
| Robinson and Nee22 | Isometric strength, %BW | Injured leg, 16±8 Noninjured leg 20.5±NM | ‐ 4.5 |
| Souza and Powers27 | Isometric strength, Nm/kg × 100 | PFPS, 139 ±41 Control, 162±26 P = .04 | ‐23.00 (‐44.83, ‐1.17) |
| Willson and Davis34 | Isometric strength, %BW | PFPS, 21.1 ±NM Control, 24.9 ±NM P = .05 | ‐3.8 |
| Adduction | |||
| Baldon et al31 | Isokinetic strength (eccentric) at 30°/s, (Nm/kg) × 100 | PFPS, 170.96 ±13,43 Control, 197.44 ±12,11 P =.009 | ‐26.48 (‐37.69, ‐15.27) |
| Cichanowski et al18 | Isometric strength, %BW | PFPS, 19.8 ±7 Control, 23.6 ±4 P = .087 | ‐3.80 (‐8.18, 0.58) |
| Cichanowski et al18 | Isometric strength, %BW | Injured leg, 19.8±7 Noninjured leg, 19.5 ±5 P = .65 | ‐0.30 (‐4.38, 4.98) |
| Magalhaes et al32 | Isometric strength, %BW | PFPS, 14.1 ±5.7 Control, 15.1±3.7 P = NS | ‐1.00 (‐2.88, 0.88) |
| Magalhaes et al32 | Isometric strength, %BW | Injured leg, 14.1±5.7 Noninjured leg 14.0 ±5.2 P > .05 | 0.11 [‐2.72, 2.94] |
| Magalhaes et al32 | Isometric strength, %BW | Bilateral PFPS, 11.4 ±3.3 Control, 15.1 ±3.7 P < .0001 | ‐3.70 (‐5.28, ‐2.12) |
| External rotation | |||
| Baldon et al31 | Isokinetic strength (eccentric) at 30°/s, (Nm/kg) × 100 | PFPS, 51.69 ±2.98 Control, 51.48 ±3.81 P = .96 | 0.21 (‐2.79, 3.21) |
| Bolgla et al7 | Isometric strength, (Nm/N.m) × 100 | PFPS, 11 ±3 Control, 15 ±3 P = .002 | ‐4.00 (‐5.96, ‐2.04) |
| Cichanowski et al18 | Isometric strength, %BW | PFPS, 17 ±4 Control, 20.1 ±3 P = .033 | ‐3.10 (‐5.82, ‐0.38) |
| Cichanowski et al18 | Isometric strength, %BW | Injured leg, 17±4 Noninjured leg, 18.2 ±4 P = .049 | ‐1.20 (‐4.28, 1.88) |
| Ireland et al21 | Isometric strength, %BW | PFPS, 10.8 ±4 Control, 16.8 ±5.5 P < .001 | ‐6.00 (‐9.44, ‐2.56) |
| Magalhaes et al32 | Isometric strength, %BW | PFPS, 12.7 ±4.1 Control, 14.5 ±3.5 P < .01 | ‐1.80 (‐3.29, ‐0.31) |
| Magalhaes et al32 | Isometric strength, %BW | Injured leg, 12.7±4.1 Noninjured leg 13.8 ±3.9 P > .05 | ‐1.10 (‐3.16, 0.96) |
| Magalhaes et al32 | Isometric strength, %BW | Bilateral PFPS, 12.1 ±3.9 Control, 14.5 ±3.5 P < .0001 | ‐2.40 (‐4.12, ‐0.68) |
| McMoreland et al30 | Isometric strength, Nm/kg × 100 | PFPS, 60.7 ±8.38 Control, 65.21 ±10.55 P = .26 | ‐4.51 (‐12.13, 3.11) |
| McMoreland et al30 | Isokinetic endurance (concentric) at 30°/s, j | PFPS, 448.24 ±95.76 Control, 481.03 ±138.38 P = .51 | ‐32.79 (‐125.69, 60.11) |
| Nakagawa et al33 | Isokinetic strength (eccentric) at 30°/s, (Nm/kg.m) × 100 | PFPS, 35 ±0.7 Control, 44 ± 0.6 P < .0001 | ‐ 9.00 (‐13.04, ‐4.96). |
| Robinson and Nee22 | Isometric strength, %BW | PFPS, 16 ±6 Control, 23 ±4 P = .004 | ‐7.00 (‐11.47, ‐2.53) |
| Robinson and Nee22 | Isometric strength, %BW | Injured leg, 16±6 Noninjured leg 20±NM | ‐4 |
| Souza and Powers27 | Isometric strength, Nm/kg × 100 | PFPS, 56 ±13 Control, 69 ±11 P = .002 | ‐13.00 (‐20.66, ‐5.34) |
| Willson and Davis34 | Isometric strength, %BW | PFPS, 9.1 ±NM Control, 10.8 ±NM P = .04 | ‐1.7 |
| Internal rotation | |||
| Baldon et al31 | Isokinetic strength (eccentric) at 30°/s, (Nm/kg) × 100 | PFPS, 113.30 ± 8.33 Control, 122.51 ±9.20 P = .47 | ‐9.21 (‐16.90, ‐1.52) |
| Cichanowski et al18 | Isometric strength, %BW | PFPS, 17.9 ±4 Control, 21.1 ±3 P = .049 | ‐3.20 (‐5.92, ‐0.48) |
| Cichanowski et al18 | Isometric strength, %BW | Injured leg, 17.9±4 Noninjured leg, 19 ±4 P = .11 | ‐1.10 (‐4.18, 1.98) |
| Magalhaes et al32 | Isometric strength, %BW | PFPS, 13.6 ±4.4 Control, 14.3 ±3.1 P < .0001 | ‐0.70 (‐2.19, 0.79) |
| Magalhaes et al32 | Isometric strength, %BW | Injured leg, 13.6±4.4 Noninjured leg 14.8 ±4.3 P > .05 | ‐1.20 (‐3.43, 1.03) |
| Magalhaes et al32 | Isometric strength, %BW | Bilateral PFPS, 12.7 ±3.8 Control, 14.3 ±3.1 P < .0001 | ‐1.60 [‐3.23, 0.03] |
| McMoreland et al30 | Isometric strength, Nm/kg × 100 | PFPS, 85.3 ±16.12 Control, 89.97 ±21.96 P = .56 | ‐4.67 (‐20.08, 10.74) |
| McMoreland et al30 | Isokinetic endurance (concentric) at 30°/s, j | PFPS, 694.42 ±186.15 Control, 656.5 ±191.6 P = .42 | 37.92 (‐113.22, 189.06) |
| Flexion | |||
| Cichanowski et al18 | Isometric strength, %BW | PFPS, 27.4 ±7 Control, 32.9 ±5 P = .033 | ‐5.50 (‐10.18, ‐0.82) |
| Cichanowski et al18 | Isometric strength, %BW | Injured leg, 27.4±7 Noninjured leg, 28.2 ±6 P = .46 | ‐0.80 (‐5.81, 4.21) |
| Magalhaes et al32 | Isometric strength, %BW | PFPS, 16.3 ±6 Control, 19.4 ±4.3 P < .0001 | ‐3.10 (‐5.15, ‐1.05) |
| Magalhaes et al32 | Isometric strength, %BW | Injured leg, 16.3±6 Noninjured leg 18.5 ±5.5 P > .05 | ‐2.20 (‐5.18, 0.78) |
| Magalhaes et al32 | Isometric strength, %BW | Bilateral PFPS, 14.9. ±4.3 Control, 19.4 ±4.3 P < .0001 | ‐4.50 (‐6.47, ‐2.53) |
| Extension | |||
| Cichanowski et al18 | Isometric strength, %BW | PFPS, 30.4 ±8 Control, 36.3 ±5 P = .029 | ‐5.90 (‐11.03, ‐0.77) |
| Cichanowski et al18 | Isometric strength, %BW | Injured leg, 30.4±8 Noninjured leg, 30.9 ±9 P = .56 | ‐0.50 (‐7.05, 6.05) |
| Magalhaes et al32 | Isometric strength, %BW | PFPS, 19.1 ±10 Control, 21.8 ±5.6 P < .0001 | ‐2.70 (‐5.88, 0.48) |
| Magalhaes et al32 | Isometric strength, %BW | Injured leg, 19.1±10 Noninjured leg 20.6 ±10 P > .05 | ‐1.50 (‐6.60, 3.60) |
| Magalhaes et al32 | Isometric strength, %BW | Bilateral PFPS, 15.8 ±9.0 Control, 21.8 ±5.6 P < .0001 | ‐6.42 (‐10.04, ‐2.80) |
| Robinson and Nee22 | Isometric strength, %BW | Injured leg, 23±9 Noninjured leg, 32 ±NM | ‐ 9 |
| Souza and Powers27 | Isometric strength, Nm/kg × 100 | PFPS, 198 ±50 Control, 235 ±38 P = .01 | ‐37.00 (‐65.24, ‐8.76) |
| Souza and Powers27 | Isokinetic strength (eccentric) at 10°/s, Nm/kg × 100 | PFPS, 87 ±34 Control, 92 ±27 P = .59 | ‐5.00 (‐24.52, 14.52) |
| Souza and Powers27 | Isokinetic strength (concentric) at 10°/s, Nm/kg × 100 | PFPS, 78 ±28 Control, 94 ±15 P = .03 | ‐16.00(‐30.28, ‐1.72) |
| Souza and Powers27 | Isotonic endurance, total of repetitions | PFPS, 16.6 ±7.5 Control, 31.9 ±7.8 | ‐ 15.30 (‐20.17, ‐10.43) |
Abbreviations: BMI=body mass index; BW=body weight; CI=confidence interval; M= mean difference; PFPS=patellofemoral pain syndrome
REFERENCES
- 1.Latt. L. Raiszadeh K. Fithian D. Patellofemoral Joint Injuries. In: Kibler W.B. (Ed.) Orthopaedic Knowledge Update: Sport Medicine. Rosemond: The American Academy of Orthopaedic Surgeons; 2009:119‐134 [Google Scholar]
- 2.Boling M Padua D Marshall S, et al. Gender differences in the incidence and prevalence of patellofemoral pain syndrome. Scand J Med Sci Sports. 2010;20:725‐730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roush JR Curtis Bay R Prevalence of anterior knee pain in 18‐35 year‐old females. Int J Sports Phys Ther. 2012;7:396‐401 [PMC free article] [PubMed] [Google Scholar]
- 4.Thomeé R Renström P Karlsson J, et al. Patellofemoral pain syndrome in young women. I. A clinical analysis of alignment, pain parameters, common symptoms and functional activity level. Scand J Med Sci Sports. 1995;5:237‐244 [PubMed] [Google Scholar]
- 5.Thomeé R Augustsson J Karlsson J Patellofemoral pain syndrome: a review of current issues. Sports Med. 1999;28:245‐262 [DOI] [PubMed] [Google Scholar]
- 6.Cook C Hegedus E Hawkins R, et al. Diagnostic accuracy and association to disability of clinical test findings associated with patellofemoral pain syndrome. Physiother Can. 2010;62:17‐24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolgla LA Malone TR Umberger BR, et al. Hip strength and hip and knee kinematics during stair descent in females with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2008;38:12‐18 [DOI] [PubMed] [Google Scholar]
- 8.Piva SR Goodnite EA Childs JD Strength around the hip and flexibility of soft tissues in individuals with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2005;35:793‐801 [DOI] [PubMed] [Google Scholar]
- 9.Aminaka N Pietrosimone BG Armstrong CW, et al. Patellofemoral pain syndrome alters neuromuscular control and kinetics during stair ambulation. J Electromyogr Kinesiol. 2011;21:645‐651 [DOI] [PubMed] [Google Scholar]
- 10.Brindle TJ Mattacola C McCrory J Electromyographic changes in the gluteus medius during stair ascent and descent in subjects with anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 2003;11:244‐251 [DOI] [PubMed] [Google Scholar]
- 11.Cowan SM Crossley KM Bennell KL Altered hip and trunk muscle function in individuals with patellofemoral pain. Br J Sports Med. 2009;43:584‐588 [DOI] [PubMed] [Google Scholar]
- 12.Dierks TA Manal KT Hamill J, et al. Proximal and distal influences on hip and knee kinematics in runners with patellofemoral pain during a prolonged run. J Orthop Sports Phys Ther. 2008;38:448‐456 [DOI] [PubMed] [Google Scholar]
- 13.Utting M. Davies G. Newman J. Is anterior knee pain a predisposing factor to patellofemoral osteoarthrisis? The Knee.2005;12:362‐365 [DOI] [PubMed] [Google Scholar]
- 14.Witvrouw E Lysens R Bellemans J, et al. Intrinsic risk factors for the development of anterior knee pain in an athletic population. A two‐year prospective study. Am J Sports Med. 2000;28:480‐489 [DOI] [PubMed] [Google Scholar]
- 15.Meira EP Brumitt J Influence of the hip on patients with patellofemoral pain syndrome: a systematic review. Sports Health. 2011;3:455‐465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powers CM Ward SR Fredericson M, et al. Patellofemoral kinematics during weight‐bearing and non‐weight‐bearing knee extension in persons with lateral subluxation of the patella: a preliminary study. J Orthop Sports Phys Ther. 2003;33:677‐685 [DOI] [PubMed] [Google Scholar]
- 17.Huberti HH Hayes WC Patellofemoral contact pressures. The influence of q‐angle and tendofemoral contact. J Bone Joint Surg Am. 1984;66:715‐724 [PubMed] [Google Scholar]
- 18.Cichanowski HR Schmitt JS Johnson RJ, et al. Hip strength in collegiate female athletes with patellofemoral pain. Med Sci Sports Exerc. 2007;39:1227‐1232 [DOI] [PubMed] [Google Scholar]
- 19.Powers CM The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40:42‐51 [DOI] [PubMed] [Google Scholar]
- 20.Prins MR van der Wurff P Females with patellofemoral pain syndrome have weak hip muscles: a systematic review. Aust J Physiother. 2009;55:9‐15 [DOI] [PubMed] [Google Scholar]
- 21.Ireland ML Willson JD Ballantyne BT, et al. Hip strength in females with and without patellofemoral pain. J Orthop Sports Phys Ther. 2003;33:671‐676 [DOI] [PubMed] [Google Scholar]
- 22.Robinson RL Nee RJ Analysis of hip strength in females seeking physical therapy treatment for unilateral patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2007;37:232‐238 [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2. Oxford, UK: The Cochrane Collaboration; 2009 [Google Scholar]
- 24.Israel H Richter RR A guide to understanding meta‐analysis. J Orthop Sports Phys Ther. 2011;41:496‐504 [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT Thompson SG Deeks JJ, et al. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557‐560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lankhorst NE Bierma‐Zeinstra SM van Middelkoop M Risk factors for patellofemoral pain syndrome: a systematic review. J Orthop Sports Phys Ther. 2012;42:81‐94 [DOI] [PubMed] [Google Scholar]
- 27.Souza RB Powers CM Predictors of hip internal rotation during running: an evaluation of hip strength and femoral structure in women with and without patellofemoral pain. Am J Sports Med. 2009;37:579‐587 [DOI] [PubMed] [Google Scholar]
- 28.Souza RB Powers CM Differences in hip kinematics muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther. 2009;39:12‐19 [DOI] [PubMed] [Google Scholar]
- 29.Bolgla LA Malone TR Umberger BR Uhl TL Comparison of hip and knee strength and neuromuscular activity in subjects with and without patellofemoral pain syndrome. Int J Sports Phys Ther. 2011;6:285‐296 [PMC free article] [PubMed] [Google Scholar]
- 30.McMoreland A O'Sullivan K Sainsbury D, et al. No deficit in hip isometric strength or concentric endurance in young females with mild patellofemoral pain. Isokinetics and Exercise Science. 2011;19:117‐125 [Google Scholar]
- 31.Baldon Rde M Nakagawa TH Muniz TB, et al. Eccentric hip muscle function in females with and without patellofemoral pain syndrome. J Athl Train. 2009;44:490‐496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magalhães E Fukuda TY Sacramento SN, et al. A comparison of hip strength between sedentary females with and without patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2010;40:641‐647 [DOI] [PubMed] [Google Scholar]
- 33.Nakagawa TH Serrão FV Maciel CD Powers CM Hip and knee kinematics are associated with pain and self‐reported functional status in males and females with patellofemoral pain. Int J Sports Med. 2013;34:997‐1002 [DOI] [PubMed] [Google Scholar]
- 34.Willson JD Davis IS Lower extremity strength and mechanics during jumping in women with patellofemoral pain. J Sport Rehabil. 2009;18:76‐90 [DOI] [PubMed] [Google Scholar]
- 35.Dierks TA Manal KT Hamill J, et al. Lower extremity kinematics in runners with patellofemoral pain during a prolonged run. Med Sci Sports Exerc. 2011;43:693‐700 [DOI] [PubMed] [Google Scholar]
- 36.Boling MC Padua DA Marshall SW, et al. A prospective investigation of biomechanical risk factors for patellofemoral pain syndrome: the Joint Undertaking to Monitor and Prevent ACL Injury (JUMP‐ACL) cohort. Am J Sports Med. 2009;37:2108‐2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finnoff JT Hall MM Kyle K Krause DA, et al. Hip strength and knee pain in high school runners: a prospective study. PM R. 2011;3:792‐801 [DOI] [PubMed] [Google Scholar]
- 38.Li G DeFrate LE Zayontz S, et al. The effect of tibiofemoral joint kinematics on patellofemoral contact pressures under simulated muscle loads. J Orthop Res. 2004;22:801‐806 [DOI] [PubMed] [Google Scholar]
- 39.Lawrence RK 3rd Kernozek TW Miller EJ, et al. Influences of hip external rotation strength on knee mechanics during single‐leg drop landings in females. Clin Biomech (Bristol, Avon).2008;23:806‐13. [DOI] [PubMed] [Google Scholar]
- 40.Dolak KL Silkman C Medina McKeon J, et al. Hip strengthening prior to functional exercises reduces pain sooner than quadriceps strengthening in females with patellofemoral pain syndrome: a randomized clinical trial. J Orthop Sports Phys Ther. 2011;41:560‐570 [DOI] [PubMed] [Google Scholar]
- 41.Earl JE Hoch AZ A proximal strengthening program improves pain, function, and biomechanics in women with patellofemoral pain syndrome. Am J Sports Med. 2011;39:154‐163 [DOI] [PubMed] [Google Scholar]
- 42.Ferber R Kendall KD Farr L Changes in knee biomechanics after a hip‐abductor strengthening protocol for runners with patellofemoral pain syndrome. J Athl Train. 2011;46:142‐149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khayambashi K Mohammadkhani Z Ghaznavi K, et al. The effects of isolated hip abductor and external rotator muscle strengthening on pain health status, and hip strength in females with patellofemoral pain: a randomized controlled trial. J Orthop Sports Phys Ther. 2012;42(1):22‐29 [DOI] [PubMed] [Google Scholar]
- 44.Kollock RO Jr Onate JA Van Lunen B The reliability of portable fixed dynamometry during hip and knee strength assessments. J Athl Train. 2010;45:349‐356 http://dx.doi.org/10.4085/1062-6050-45.4.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krause DA Schlagel SJ Stember BM, et al. Influence of lever arm and stabilization on measures of hip abduction and adduction torque obtained by hand‐held dynamometry. Arch Phys Med Rehabil. 2007;88:37‐42 [DOI] [PubMed] [Google Scholar]
- 46.Thorborg K Petersen J Magnusson SP, et al. Clinical assessment of hip strength using a hand‐held dynamometer is reliable. Scand J Med Sci Sports. 2010;20:493‐501 [DOI] [PubMed] [Google Scholar]