Abstract
Study Design:
Case Series.
Background and Purpose:
Myofascial trigger points (MTrPs) are a common occurrence in many musculoskeletal issues and have been shown to be prevalent in both subjects with nonspecific low back pain and whiplash associated disorder. Trigger point dry needling (DN) has been shown to reduce pain and improve function in areas such as the cervical and lumbar spine, shoulder, hip, and knee, but has not been investigated in the thoracic spine. The purpose of this case series was to document the use of DN with intramuscular electrical stimulation (IES) in subjects with nonspecific thoracic spine pain.
Case Description:
The subjects were both active duty military males aged 31 and 27 years who self‐referred to physical therapy for thoracic spinal pain. Physical examination demonstrated thoracic motor control dysfunction, tissue hypertonicity, and tenderness to palpation of bilateral thoracic paraspinal musculature in both subjects. This indicated the presence of possible MrTPs. Objective findings in the first subject included painful thoracic flexion and bilateral rotation in each of these planes of movement. Pain reduction was observed when postural demands of the spine and trunk musculature were reduced through positional changes. Patient 1 demonstrated pain with posterior to anterior (P/A) pressure at T9 to T12. The second subject had bilaterally limited and painful thoracic rotation actively with normal passive rotation and demonstrated pain with P/A pressure at T4 to T7.
Intervention:
The subjects were treated with DN and IES for a total of two visits each. DN was performed to paraspinal and multifidus musculature at the levels of elicited pain with P/A testing and IES set at a frequency level of 4 (1.5Hz) for 20 minutes.
Outcomes:
Subject 1 reported reduced pain with standing flexion from a 62mm VAS score on initial evaluation to 26mm at his second visit. Subject 2 reported being “quite a bit better” in symptoms on the GROC following his second treatment. His VAS score reported following weightlifting activities changed from 43mm on initial evaluation to 20mm at his second visit. Both subjects also demonstrated a 10 degree improvement in active thoracic spinal rotation (on the right for Subject 1 and bilateral for Subject 2) following their second treatment.
Discussion:
Both subjects demonstrated motor control dysfunctions and pain with P/A pressure in the thoracic spine. With the use of DN and IES, immediate reduction was seen in subject perceived symptoms, and pain free ROM was improved. Extended treatment and follow up was not plausible due to the high pace tempo and demands of their operational training schedule. With research indicating the influence of MTrPs on a multitude of musculoskeletal issues and the prevalence of thoracic spine pain, further research is indicated for examining the effects of DN and IES for motor control and painful conditions occurring in the thoracic spine.
Level of Evidence:
Level 4
Keywords: Dry needling, intramuscular electrical stimulation, myofasical trigger points, thoracic spine pain
BACKGROUND AND PURPOSE
The prevalence of thoracic spine pain (TSP) has been reported to range from 3 to 22% in the general population.1 Rates of thoracic spine pain in varying professions have shown a lifetime prevalence of 77% in healthcare professionals and a one year prevalence's of 55% and 54.8% in performing artists and manual laborers respectively.2 TSP can be a debilitating condition with many possible causes, including osteoporosis, hyperkyphosis, ankylosing spondylitis, and degenerative changes, as examples.3 While TSP may have a variety of causes, the results of a study performed by Wood et al4 indicates that it may also be nonspecific. Wood et al4 examined thoracic MRIs of 99 asymptomatic individuals and found that 73% were positive for anatomic pathology in one or more thoracic levels. These results indicate that anatomic pathology in many cases may not be a pain generator and that TSP may be more nonspecific much like how low back pain has been reported in the literature.
Myofascial Pain Syndrome (MPS) may play a role in nonspecific spine pain, and is a well‐recognized and common cause of pain.5,6 Prevalence of MPS ranges from 21‐85% in subjects with regional pain complaints7 and is defined as sensory, motor, and autonomic symptoms, arising from myofascial trigger points (MTrPs).8 A MTrP is a tender spot in a muscle often with a palpable taut band of tissue that elicits pain referral when pressure is applied.6,9 Active MTrPs refer pain that is “recognized” by the subject, while a latent trigger point has increased muscle tension and shortening but does not elicit spontaneous pain.10 MTrPs can be found in many muscles, which can contribute to various musculoskeletal problems, such as joint dysfunction, tendonitis, craniomandibular dysfunction, tension headaches, radiculopathies, and disk pathologies.5 Specifically related to the spine, it has been found that individuals with nonspecific chronic low back pain have greater number of trigger points, associated with higher pain levels and worse quality sleep than the general population.11 Compared to the general population, a greater number of trigger points were also found in a population of subjects with whiplash associated disorders (WAD) and the number of trigger points was related to pain intensity.12
It is theorized that MTrPs may be caused by sustained muscle contractions at low levels,13 muscle overload,10 unaccustomed concentric and eccentric contractions,14 low load repetitive tasks, and sustained postures.13,15,16 Increased end plate noise and excessive release of acetylcholine is found in active MTrPs which causes sustained muscle contractions leading to an ischemic and hypoxic environment.13,14,17‐19 This ischemic and hypoxic environment leads to decreased pH which is capable of exciting nociceptors.13,20‐23 Along with decreased pH, an increases in Substance P, calcitonin‐gene‐related peptide (CGRP), bradykinin (BK), serotonin (5‐HT), norepinephrine, tumor necrosing factor‐alpha, and interleukin‐1 beta have been seen in active MTrPs which also contribute to sensitization and stimulation of nociceptors located within the muscle.13,21‐23 This stimulation of nociceptors from active trigger points may cause peripheral and central sensitization through continued nociceptive signals to the dorsal horn and therefore these trigger points are important to address in patients with chronic pain conditions.13,24‐29
Dry needling (DN) is a common technique used to treat MTrPs. This technique is performed by inserting a small monofilament needle into an active or latent trigger point in an effort to elicit a local twitch response and eliminate the MTrP. DN to active trigger points has been shown to decrease Substance P and CGRP, as well as to reduce endplate noise when a local twitch response is elicited.13,30,31 DN can reduce pain, normalize the chemical environment of a MTrP, and restore range of motion and muscle activation patterns.13,32‐36 DN of latent trigger points has been shown to elicit a short term segmental anti‐nociceptive effect and normalize muscle activation patterns in the rotator cuff muscles.34,36
Research literature on the impact of DN in a variety of musculoskeletal conditions is expanding in both quality and quantity. In a Cochrane review on acupuncture and DN for low back pain, the authors concluded that DN (when used cautiously) is a useful adjunct to other therapies for chronic LBP.37 In a recent meta‐analysis, Kietrys et al38 recommended the use of DN over sham or placebo treatments for upper quarter conditions with MTrPs in order to reduce pain immediately and at 4 weeks.
A moderate number of randomized controlled trials (RCT) have been performed looking at the effects of DN in a variety of populations and conditions. Mayoral et al39 showed that DN of active MTrPs pre‐operatively in subjects awaiting a total knee arthroplasty (TKA) demonstrated less pain and analgesic use at one month as compared to a sham group. Tekin et al7 examined subjects with MPS in the cervical, thoracic, and scapula musculature through a RCT and found that their Visual Analog Scale (VAS) scores were decreased and quality of life scores were improved following the use of DN. Casaneuva et al40 showed decreased VAS scores immediately and at 6 weeks using DN in a population with fibromyalgia. The authors also saw improvements in the VAS fatigue scale, short form SF‐36, and pressure pain threshold.40 Pain‐pressure sensitivity was reduced in a RCT performed by Mejuto‐Vasquez et al41 looking at DN in subjects with acute mechanical neck pain. Subjects receiving DN showed decreased pain 10 minutes and one week after treatment, as well as significant range of motion increases and wide spread decreases in pressure pain sensitivity.41
Other lower levels of evidence have been published in the form of case studies documenting the use of DN in subjects with low back pain, posterior hip pain, posterior knee pain, and shoulder adhesive capsulitis.42‐44 Rainey42 showed that DN with intramuscular electrical stimulation (IES) decreased pain and increased ROM and function within two treatment sessions in a subject with chronic low back and posterior hip pain. No literature was found examining the use of DN in a population with TSP.
The purpose of this case series was to document the use of DN with IES in the thoracic spine and the immediate outcomes. Due to the high pace tempo and demands of the subjects' operational training schedule, the ability to perform multiple treatment sessions and long term follow up was a challenge. Therefore, this case series examined the subjects' immediate outcomes after only two DN sessions.
The subjects featured in this case series gave informed consent to participate in the study and were informed that the data of the case reports would be submitted for publication. This case series was not required to be reviewed or approved by a US Navy Institutional Review Board.
CASE DESCRIPTION
The first subject in this case series was a 31‐year‐old active duty military male who self‐referred to physical therapy for TSP that had persisted for 8 weeks. He reported a “spasm type” pain with thoracic flexion and bilateral rotation in each respected movement plane. The subject did not report a known cause of initial pain onset and reported pain was not getting better or worse at the time of initial evaluation. No radiographs were ordered, due to the sub‐acute nature of his symptoms and absence of trauma.
Pain was made worse with forward bending, rotating, and activities that loaded the thoracic spine, such as lifting his child and wearing military body armor. No pain was reported with taking a deep breath, decreasing the likelihood of a rib dysfunction being present. Reduced pain was accomplished for a few minutes after self‐soft tissue mobilization to the thoracic paraspinal musculature utilizing a lacrosse ball. He was cleared for red flags and denied any risk factors for cancer, including weight loss, night pain, fatigue, or a previous history of cancer. No neurological symptoms were reported or found and the subject was overall in good health. Subject's goals were to eliminate pain with weightlifting and forward bending and to be able to return to pain free military training. He was scheduled to leave for further military training the day after evaluation, so accurate assessment and effective treatment was important.
The second subject was a 27‐year‐old active duty military male with complaints of TSP since 2006, which started with a weight‐lifting injury when he felt a back spasm during deadlifting. He had previous thoracic spine radiographs ordered and evaluated by his primary care physician, due to the chronicity of his symptoms. These radiographs were negative for bony abnormality. Pain was elicited following heavy weight‐lifting workouts the day following his lifting activities. He reported a sensation of stiffness and occasional pain with a deep breath. Pain was relieved with a few days of rest, but always returned following a lifting workout. He was cleared for red flags, had no signs or symptoms of neurologic involvement, and was overall in good health. Subject's goals were to be able to return to pain free weight‐lifting without the need for multiple rest days following a workout.
Outcome tools utilized in this case series included the Visual Analog Scale (VAS), Numeric pain rating scale (NPRS), and the Global Rating of Change scale (GROC). The VAS was used to evaluate the subjects' primary complaint via pain rating upon initial evaluation and post treatment. Upon initial evaluation, Subject 1 marked his VAS pain level at 61 mm during standing forward flexion. Subject 2 marked his pain at 43mm, but reported as high as 68mm in the last two weeks. The VAS has been found to be a reliable measure of pain with an ICC of .97 (95% CI .96‐.98).45 This tool consists of a 100mm line with two descriptors to describe pain levels. The far left indicates no pain, with the far right edge indicating extreme pain.46 Jensen et al46 investigated how to interpret VAS scores and levels of change. Groups were found based on levels of pain as follows: No pain 0 to 1.4mm, mild pain 27 to 28mm, moderate pain 56 to 58mm and severe pain 83 to 87mm. These groupings align well with the groups described by Kelly47, which indicate “mild pain” as less than 30mm, “moderate pain” as 31 to 69 and “severe pain” as greater than 70mm. Both subjects fell into the “moderate pain” group upon initial evaluation. Change scores of 13.3 on the VAS indicate “a little pain relief”, 20mm changes indicate “some relief”, 43.7mm indicate “a lot of relief” and finally 61.6mm changes indicate “complete relief”.46
During objective movement testing, a quick verbal NPRS ranging from 0 (no pain) to10 (worst pain imaginable) was used. The NPRS has been shown to be reliable and valid with a clinically meaningful change of 2 points.48,49
The GROC was completed by both subjects following treatment. The GROC consists of a 15 point scale ranging from ‐7 (a very great deal worse) to 0 (about the same) to +7 (a very great deal better). The minimally clinically important difference (MCID) has been reported to be +3 with +3 to +4 indicating small changes, +4 to +5 indicating moderate changes and +6 to +7 indicating large changes.50 The construct validity of the GROC has been shown with a Pearson correlation between the Modified Oswestry and the GROC of .78.51 A Modified Oswestry was not utilized in this case series, because its questions were designed for lower level activities of daily living (ADLs). The subjects in this case series were high level military operators, their level of function would result in not reaching the MCID on a tool such as the Modified Oswestry throughout the treatment period and likely cause a “floor effect”.
Initial Clinical Impression
For Subject 1, the subjective evaluation indicated a differential diagnosis to include joint impairments in the thoracic facet joints and or costovertebral joints, muscle strain, MPS, and motor control dysfunction. With no red flags, neurologic signs, or mechanism of injury, imaging was not considered necessary. The subjective evaluation of Subject 2 led to the same differential diagnosis as Subject 1.
EVALUATION
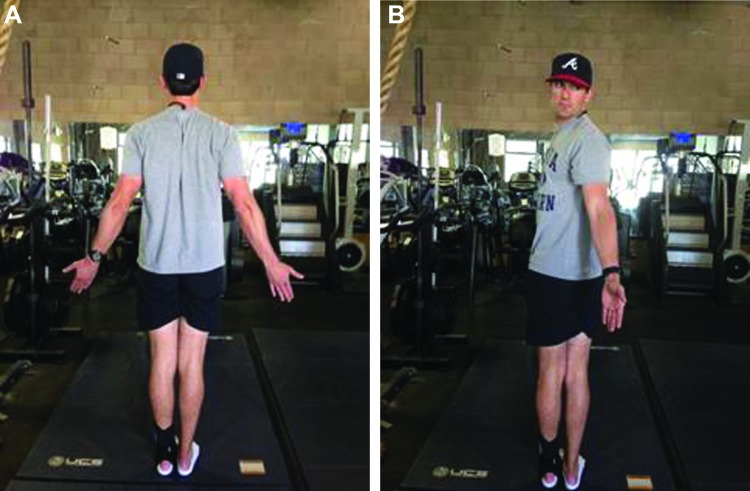
Specific movement pattern “bench marks” were utilized to deem a movement pattern within functional limits (WFL). These bench marks were taken from the Selective Functional Movement Assessment (SFMA), which is a comprehensive movement based assessment system used to classify fundamental movement patterns, identify musculoskeletal dysfunction and direct manual therapy and therapeutic exercise interventions.52 Standing forward bending (multi‐segmental flexion; Figures 1A‐B) was WFL if the subject was able to achieve a posterior weight shift of the hips and pelvis relative to the base of support and touch their toes without bending the knees, while maintaining a uniform spinal curve and a sacral angle of >70 degrees. The sacral angle (Figure 1C) is measured by assessing the angle of the sacrum relative to the vertical axis as the subject bends forward. In functional forward bending patterns, as a subject flexes forward, an appreciable amount (>70 degrees) of sacral anterior tilt occurs. Multi‐segmental flexion has an intra‐reliability of .46 to .85 across raters of various experience levels.53
Figure 1.
A. Multi‐Segmental Flexion (start), B. Multi‐Segmental Flexion (finish), C. Sacral Angle.
Standing backward bending (multi‐segmental extension) was WFL if the subject was able to anterior weight shift (ASIS past the toes) and raise and maintain the arms at >170 degree flexion angle, while performing general spinal extension (scapula spines past the heels) and maintaining a uniform spinal curve. Multi‐segmental extension has an intra‐reliability of .25 to .87 across raters of various experience levels.53
Standing rotation (multi‐segmental rotation; Figure 2A‐B) was WFL if the subject was able to rotate the trunk 50 degrees and pelvis 50 degrees (summative rotation of 100 degrees), while preventing any spinal or pelvic deviation or excessive knee flexion. In order to look more specifically at thoracic spine mobility versus motor control dysfunction, a lumbar locked position was utilized to examine thoracic rotation ROM. Multi‐segmental rotation has an intra‐reliability of .52 to .89 (right) and .52 to .77 (left) across raters of various experience levels.53
Figure 2.
A. Multi‐Segmental Rotation (start), B. Multi‐Segmental Rotation (start).
Lumbar locked thoracic rotation (Figure 3A‐B) has been shown to be reliable with in session inter‐rater reliability of .85 to .95 and a Minimally Detectable Change (MDC) of 2.8 to 6.3 degrees.53 The in session intra‐rater reliability is .86 to .95 with an MDC of 2.1 to 5.9 degrees.54 The lumbar locked position consists of the subject being in a kneeling position sitting on their heels with the forearm of one arm resting on the treatment table (elbow flexed at 90 degrees) providing upper extremity support and in line with the midline of the body; the other arm is placed behind the low back. The position of the subject's arm behind the low back was intended to limit movement of the shoulder girdle and capture isolated thoracic spine rotation, preventing a false‐positive limitation secondary to an anterior shoulder girdle tissue extensibility limitation (e.g. pectoral musculature hypertonicity). This arm position can also help to limit a false negative test by reducing the likelihood of compensation with scapular and/or glenohumeral hypermobility and making it appear that increased thoracic motion exists when it doesn't. While keeping the hips and forearm in position, the subject rotates the thoracic spine to its limit. This kneeling position in hip and lumbar flexion is utilized to minimize hip and lumbar spine motion during thoracic rotation, due to the flexed position in which both are placed. Thoracic rotation ROM can be assessed visually and/or with a bubble inclinometer positioned between the scapular spines at the T1 to T2 level. This motion is performed both actively and passively (with assistance from the administrator). Lumbar locked thoracic rotation has been reported in the literature53; however, most have utilized the “arm behind the head” position versus the “behind the low back” position. The authors of this case series believe the “arm behind the low back” position is superior secondary to the previously mentioned reasons.
Figure 3.
A. Lumbar Locked Thoracic Rotation (start), B. Lumbar Locked Thoracic Rotation (finish).
It is the thought process presented by the creators of the SFMA and the authors opinion that testing movement patterns both actively and passively, as well as changing the position of testing (e.g. spinal weight bearing versus non‐weight bearing), is important in order to alter motor control demands on the body.52 Throughout this process, the impairments may be distinguished as either a mobility dysfunction (tissue extensibility and/or joint restriction) or motor control dysfunction. It has been theorized by the creators of the SFMA that when a movement elicits the same degree of limitations and/or pain in multiple testing positions (e.g. spinal weight bearing and non‐weight bearing) or when both active and passive ROM are limited, a mobility dysfunction is likely present and further biomechanical assessment would be needed to determine structural dysfunction (i.e. tissue extensibility and/or joint restriction).52 A motor control dysfunction is likely present when movement dysfunction and/or pain are not consistent and improve with less challenging postural positions (e.g. spinal non‐weight bearing) or when active ROM is limited, but passive ROM is normal.52
Subject 1: Postural examination was assessed visually for structural changes and deformity and revealed increased thoracic kyphosis and bilateral paraspinal hypertonicity in the mid‐thoracic spine. Movement pattern assessment via active range of motion (AROM) testing was performed and assessed visually for both quality and quantity of movement. Upon AROM examination, standing forward flexion elicited a VAS pain score of 61mm in the lower thoracic spine, but ROM was WFL. Pain started as his fingers reached mid‐thigh, but he was able to reach his toes. Standing spinal extension was WFL with no pain reported. Standing rotation was WFL with reported pain (3/10 on NRPS) at end range bilaterally.
Upon movement testing, Subject 1 had 55 degrees of left rotation in the lumbar locked position with no pain and no change in ROM when passive ROM was applied. There was 45 degrees of right rotation with a 10 degree increase in ROM when passive ROM was applied; slight pain (2/10) was reported with both active and passive ROM to the right. All lumbar locked thoracic rotation movements were objectively assessed using a bubble inclinometer. To further evaluate thoracic spine flexion, a quadruped position was used to reduce the motor control demand of the spine and trunk musculature (non‐weight bearing) compared to the previously evaluated standing position. The subject was asked to arch his back up as high as he could and reported pain (4/10 on NPRS) with ROM WFL. Palpation examination revealed tissue hypertonicity and tenderness along bilateral thoracic paraspinals. Joint accessory examination revealed pain with posterior to anterior (P/A) pressure through the spinous process of T9 to T12 with no hypo‐ or hypermobility noticed. No painful symptoms were found with costovertebral P/A testing.
Subject 2: Postural examination showed the shoulders were slightly anterior to the ear with no significant changes in thoracic spinal curvature. Standing forward and backward bending, as described above, did not elicit pain and was WFL. Standing rotation elicited a “sense of tightness” with a VAS score of 43mm and was restricted 25% in both directions. The lumbar locked position was once again utilized to investigate thoracic spine rotation. Forty degrees of rotation was achieved actively in both directions and 50 degrees was achieved with passive overpressure. He also felt a “sense of tightness” with this testing position, but no increase in pain. Palpation examination also revealed tissue hypertonicity and tenderness along bilateral thoracic paraspinals. Joint accessory examination revealed pain (4/10 on NPRS) with P/A testing to the T4 to T7 spinal levels with no hypo‐ or hypermobility noticed. No pain was reported with costovertebral P/A testing. He reported his pain intensity ranging from 5 to 7/10 on the NPRS the day following his weightlifting activities.
Second Clinical Impression
Subject 1: From the results of examination, this subject demonstrated thoracic spine paraspinal hypertonicity and tenderness in the lower half of the thoracic spine and a spinal flexion motor control dysfunction. The motor control dysfunction was indicated by the reduction in pain when postural demands of the spine and trunk musculature were reduced through positional changes. No joint restrictions were indicated through P/A testing, and demonstrated by his ability to go through full ROM.
Subject 2: A motor control dysfunction was also seen in this subject, but in a rotational direction. This was indicated by increased rotation ROM actively versus passively. He also demonstrated thoracic spine paraspinal hypertonicity and tenderness upon paraspinal tissue palpation assessment located in the upper half of the thoracic spine.
INTERVENTION
DN with the use of IES was chosen to be used in both subjects as it is an effective way to reduce muscle hypertonicity and MTrPs, allowing the administrator to directly influence the multifidus musculature, which could be difficult with other soft tissue techniques due to the overlying paraspinals. A 60mm length by .30mm diameter monofilament needle was used to treat both the paraspinal and multifidus musculature at the levels of elicited pain with P/A testing. Risks were discussed with the subject to include pneumothorax and infection along with the common side effect of short term muscle soreness. Neither subject had any contraindications for DN, such as a local infection, bleeding disorders, immune suppression, or significant fear of needles.
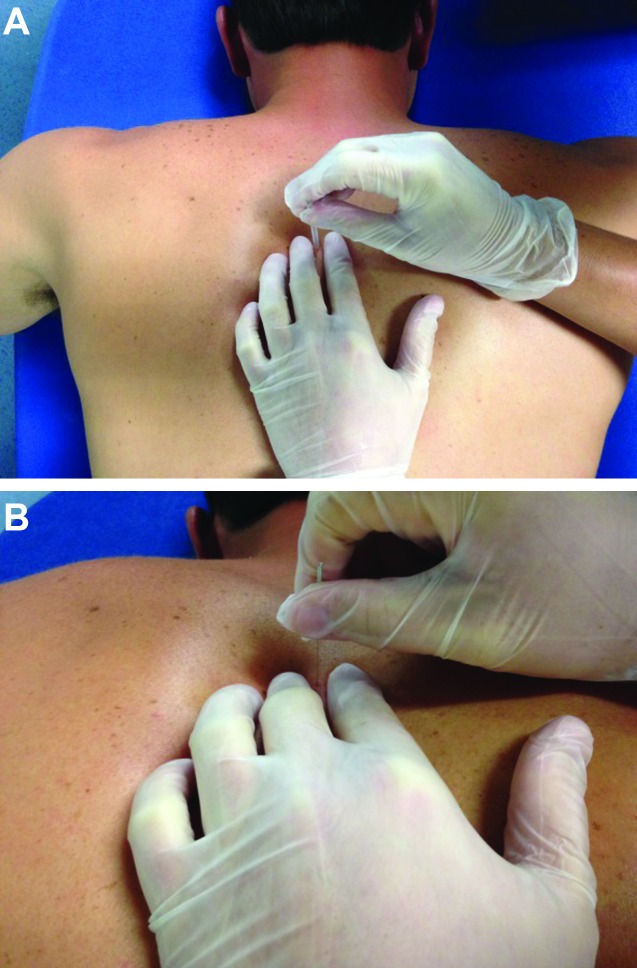
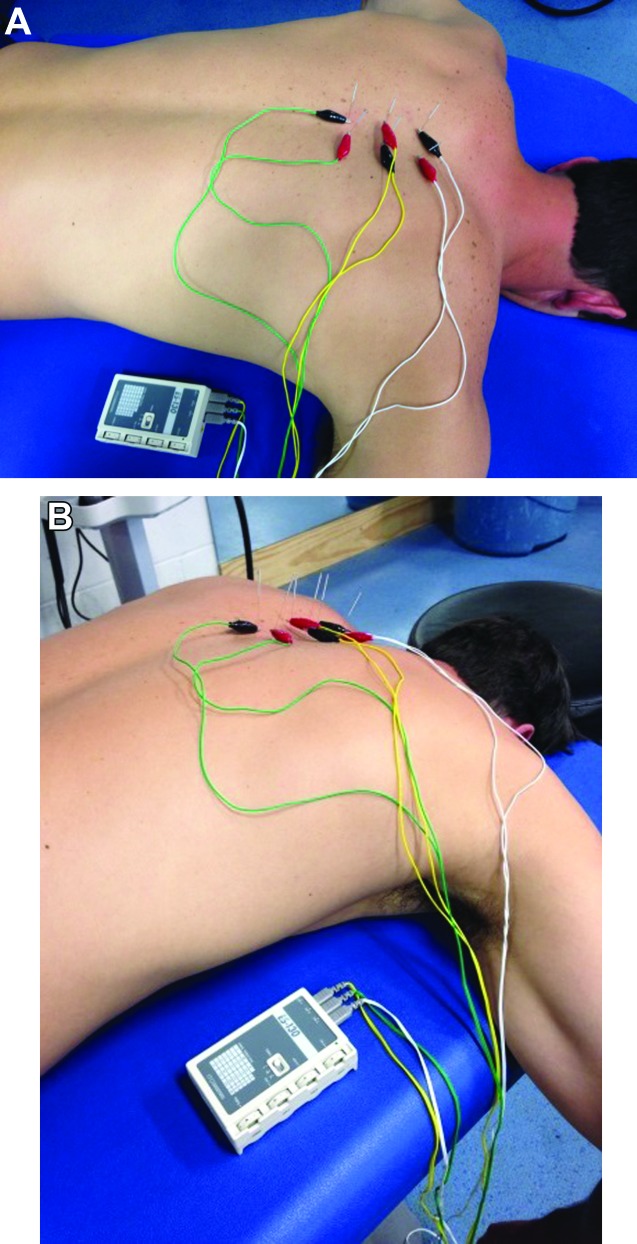
The DN technique included standard protocol of disinfection of the skin with 70% isopropyl alcohol followed by insertion of the needle within one finger breadth (subjects' finger) of the spinous process until lamina was contacted by the tip of the needle to ensure that the multifidus was being treated. This needle placement is the standard distance used for DN in the multifidus. The purpose of using the finger breadth of the subject is to allow for variance of distance from the spinous process based on the subject's body size. Both sides of the spinal levels were treated at T9 to T12 for Subject 1 and T4 to T7 for Subject 2 (Figure 4A‐B). The needles were left in situ and the IES unit (Figure 5A‐B) was attached. The use of IES was indicated, due to the chronicity of symptoms in both cases and clinician experience using IES in the multifidus to reduce both pain and hypertonicity. The IES unit (Figure 6) utilized was an ES‐130 by ITO® (Japan), which is a three channel unit (6 leads) that produces a pulsed asymmetric biphasic square waveform. The 6 leads had alligator clips attached, which allowed for easy attachment onto the needle shafts. Upon attachment to the needles, the IES unit was set with a frequency level of 4 (1.5Hz) and the intensity increased to subject comfort, which ranged from level 4 to 5/10 and was at an intensity that elicited repeated muscular contraction. A frequency of 1.5Hz allowed for 1 to 2 muscle contractions per second at this setting. The IES unit was allowed to run for 20 minutes in all treatment sessions.
Figure 4.
A. Needle Placement, B. Needle Placement (close‐up).
Figure 5.
A. Dry Needling set‐up with IES (view 1), B. Dry Needling set‐up with IES (view 2).
Figure 6.

IES Unit (ES‐130 by ITO®).
DISCUSSION
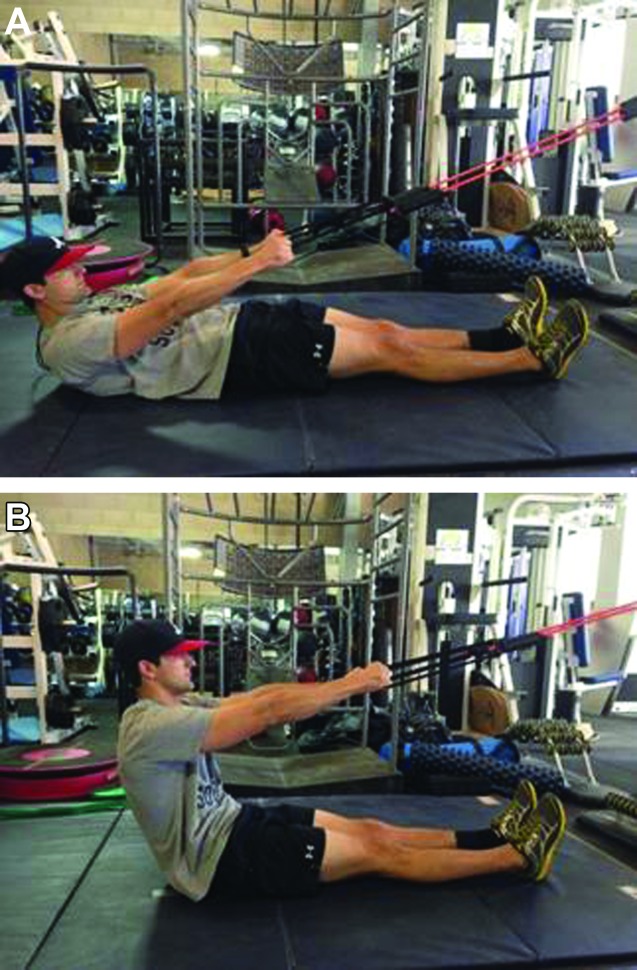
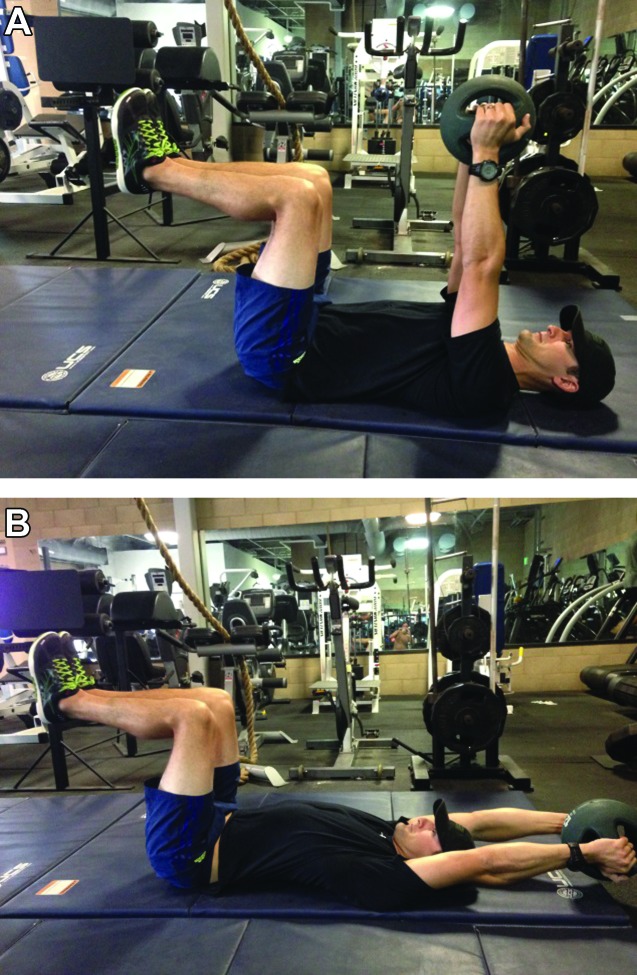
Subject 1: Due to the short treatment window for Subject 1, he was only treated once before having to return to operational military training. The spinal levels treated with the DN technique described above were T9 to T12. He was given a Home Exercise Program (HEP) that included supine assisted sit‐up exercises (Figure 7A‐B). This exercise was selected to address his motor control dysfunction by creating a situation in which his body could go through pain free flexion ROM. Progression was accomplished by reducing assistance as a way to increase motor control demands. Following DN, one set of 20 supine assisted sit‐up exercises was performed. Directly following treatment, the subject was asked to complete the GROC and standing forward bending was re‐evaluated. The subject rated himself as a +5 (quite a bit better) on the GROC and pain was reduced on the VAS to 23mm with forward bending with continued ability to touch his toes.
Figure 7.
A. Assisted Sit‐ups (start), B. Assisted Sit‐ups (finish).
Subject 1 returned for treatment 19 days later following 2 ½ weeks of military training. He reported that the two days following treatment he felt “a little better” and pain was now intermittent instead of constant. The day of his second treatment he rated himself on the GROC as 0 (about the same). Upon evaluation, he had a VAS score of 26mm with forward bending and was able to touch his toes, which was a 35mm change in VAS score from initial evaluation. Standing trunk rotation was WFL and pain free. This demonstrated elimination of pain felt with rotation on initial evaluation. DN with IES was used in the same fashion as the initial treatment. This was followed by a thoracic stability exercise in a supine position with the hips and knees at 90 degrees. The subject then raised a 5 pound ball overhead while controlling the lumbar and thoracic spine (Figure 8A‐B). This exercise was selected to challenge the subjects' motor control in the thoracic spine with lifting tasks and could be progressed into more challenging positions (e.g. half kneeling positions and standing) as tolerance progressed. Following treatment, he had no pain with forward flexion ROM.
Figure 8.
A. Supine 90/90 overhead lift (start), B. Supine 90/90 overhead lift (finish).
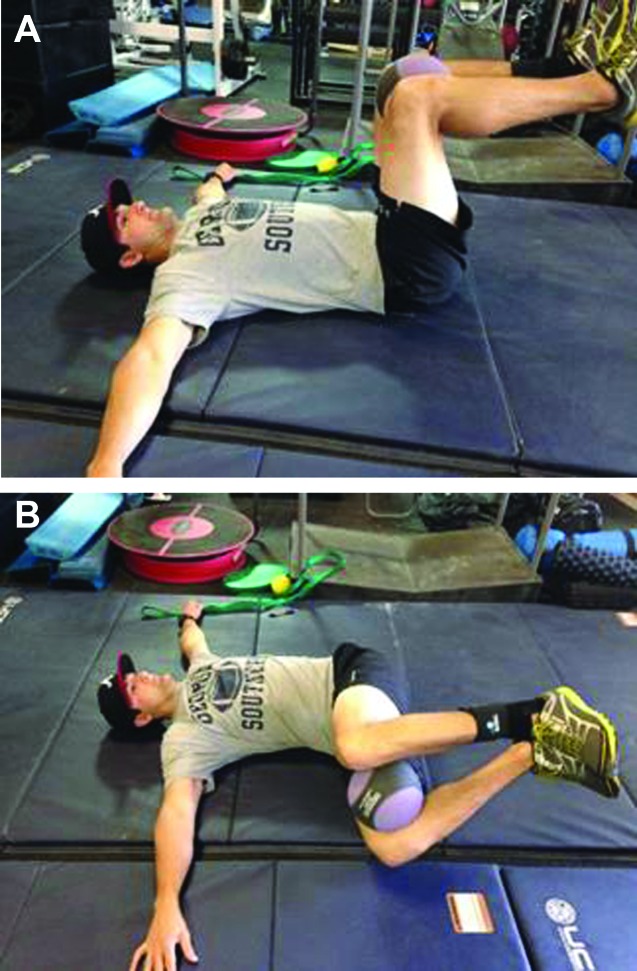
Subject 2: For this subject, the DN technique was used at the spinal levels T4 to T7 for two treatment sessions that were 48 hours apart. Both sessions were identical in set up and duration, as described above. A HEP that consisted of side lying thoracic rotation (Figure 9) and supine thoracic rotation with a stable scapula (Figure 10A‐B) was used to influence thoracic rotation mobility. The subject continued to perform his normal lifting routine that included Olympic lifting, rowing machine, and running. He completed the GROC 24 hours following his second treatment and rated himself as +5 (quite a bit better). Pain following his weightlifting workouts was reported to be minimal (20mm) with only a slight “sense of tightness”. Standing trunk rotation was WFL following the second treatment.
Figure 9.
Side lying rotation.
Figure 10.
A. Supine rotation with stable scapula (start), B. Supine rotation w/ stable scapula (finish).
Thoracic spine pain may be a more common issue than previously thought and can have large impacts in activity level and tolerance. The presence of MTrPs have been shown to be prevalent in other spinal conditions, such as WAD, and chronic low back pain,11,12 and could be a contributing factor in thoracic spine pain. MTrPs may be caused by sustained postures and repetitive tasks which would occur often in the thoracic spine. For example, individuals who work in an office setting will often have sustained sitting postures for hours throughout the day. Specifically, the subjects in this case series must carry their military gear for long periods which places the thoracic spine in a flexed position for sustained periods of time. DN has been shown to be an efficient treatment for MTrPs and was chosen as an appropriate treatment in both subject cases, due to the previously published works on DN and the authors' personal experience. The authors of this case series have seen a trend towards motor control dysfunctions in the thoracic spine in subjects with TSP, shoulder and neck pain. DN is commonly used to treat the deep multifidus musculature with the theory that MTrPs could influence timing and sequencing of the multifidus firing during activity, thus establishing a motor control dysfunction.
In both of the presented cases, subject perceived symptoms were unchanging and had been both sub‐acute and chronic in nature (8 weeks and 8 years respectively). Motor control dysfunctions were indicated in both subjects due to changing symptoms with different postural positions and no signs of joint restrictions. Upon initiating treatment consisting of DN with IES and motor control exercises, symptoms were quickly and significantly reduced. Subject 1 had a decrease from a 61mm VAS score on initial evaluation to a 23mm score on follow up treatment 19 days later. Following his second treatment, he had a 0mm VAS score (no pain) with forward bending. While he rated himself as “about the same” on the GROC, his VAS scores from visit one to two indicated a moderate change.46 Subject 2 indicated feeling “quite a bit better” on the GROC following his second visit, which matched his VAS score change from 43mm to 20mm following his second treatment. This 23mm change indicated “some relief”.46
Limitations to this case series were greatly influenced by the nature of this particular military clinic. Due to the high tempo of operational military training and drop in nature of the subjects, a consistent treatment plan was not able to be implemented. Longer term follow up was also made difficult, due to training and travel demands and requirements of the subjects, leaving long term outcomes undetermined.
Increasing use of DN illuminates the need for further research in the form of RCTs in order to investigate the influence of DN with and without IES for treatment of the multifidus musculature in subjects with non‐specific cervical, thoracic, and lumbar pain.
CONCLUSION
Thoracic spine pain can be a common occurrence with many possible causes, including MTrPs. This case series demonstrated positive, short‐term effects of treating MTrPs present in the thoracic spine with DN and IES in two subjects with nonspecific, thoracic spine pain. The quick and measureable changes in pain and movement seen in these two subjects demonstrate the positive effects of DN on subjects with different chronicity of symptoms. Further research is indicated, to examine treatment of MTrPs in the thoracic spine using DN with and without IES.
Table 1.
Outcome Measures.
| Subject 1 | Initial Exam | Final Exam |
|---|---|---|
| VAS | 61mm | 26mm |
| GROC | ‐‐‐ | +5 |
| Subject 2 | Initial Exam | Final Exam |
|---|---|---|
| VAS | 43mm | 20mm |
| GROC | ‐‐‐ | +5 |
VAS = Visual Analog Scale; GROC = Global Rating of Change
Table 2.
Objective Findings
| Subject 1 | Initial Exam | Final Exam |
|---|---|---|
| Standing Flexion | WFL | WFL |
| Standing Extension | WFL | WFL |
| Standing Rotation | WFL | WFL |
| Lumbar locked Rotation | Left 55°, Right 45° | Left 55°, Right 55° |
| Subject 2 | Initial Exam | Final Exam |
|---|---|---|
| Standing Flexion | WFL | WFL |
| Standing Rotation | 25% limited bilateral, SOT | WFL |
| Lumbar locked Rotation | Left 40°, Right 40°, SOT | Left 50°, Right 50° |
SOT = Sense of Tightness
REFERENCES
- 1.Manchikanti L Singh V Pampati V Beyer CD Evaluation of prevalence of facet joint pain in chronic thoracic pain. BMC Musculoskelet Disord. 2004; 5(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briggs AM Bragge P Smith AJ Gouil D Straker LM Prevelence and associated factors for thoracic spine pain in the adult working population: a literature review. J Occup Health. 2009;51: 177‐192 [DOI] [PubMed] [Google Scholar]
- 3.Briggs AM Smith AJ Straker LM Bragge P Thoracic spine pain in the general population: prevalence inceidence and associated factors in children, adolescents and adults: a systematic review. BMC Musculoskelet Disord. 2009; 10(77). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood KB Garvey TA Gundry C Heithoff KB Magnetic resonance imaging of the thoracic spine. Evaluation of asymptomatic individuals. J Bone Joint Surg Am. 1995;77(11):1631‐1638 [DOI] [PubMed] [Google Scholar]
- 5.Dommerholt J Bron C Franssen J Myofascial trigger points: an evidence‐informed review. J Man Manip Ther. 2006; 14(4):203‐221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tough EA White AR Richards S Cambell J Variability of criteria used to diagnose myofacial trigger point pain syndrome‐ evidence from a review of literature. Clin J Pain. 2007;23(3). [DOI] [PubMed] [Google Scholar]
- 7.Tekin L Akarsu S Durmus O Cakar E Dincer U Kiralp MZ The effect of dry needling in the treatment of myofascial pain syndrome: a randomized double blinded placebo‐controlled trial. Clin Rheumatol. 2013;32:309‐315 [DOI] [PubMed] [Google Scholar]
- 8.Bron C Dommerholt J Etiology of myofascial trigger points. Curr Pain Headache Rep.2012;16:439‐444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simons DG Review of enigmatic myofascial trigger points as a common cause of enigmatic musculoskeletal pain and dysfunction. J Electromyogr Kinesiol.2004;14:95‐107 [DOI] [PubMed] [Google Scholar]
- 10.Simons DG Travell JG Simons LS Travell and Simmons' myofascial pain and dysfunction: the trigger point manual. Vol 1 Upper half of the body. Baltimore: MD: Lippincott William & Wilkins; 1999 [Google Scholar]
- 11.Inglesias‐Gonzalez J Munoz‐Garcia MT, Rodriguez‐de‐souza DP Alburguerque‐Sendin F, Fernandex‐de‐les‐Penas C Myofascial trigger points, pain, disability and sleep quality in patients with chronic nonspecific low back pain. Cross sectional study. Pain Med. 2013;141:964‐1970 [DOI] [PubMed] [Google Scholar]
- 12.Castaldo M Ge H Chiarotto A Villafane JH Arnedt‐Nielsen L Myofascial trigger points in patients with whiplash associated disorders and mechanical neck pain. Pain Medicine. 2014 [DOI] [PubMed] [Google Scholar]
- 13.Dommerholt J Dry needling‐peripheral and central considerations. J Man Manip Ther. 2011;19(4):223‐237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerwin RD Dommerholt J Shah JP An expansion of Simons' integrated hypothesis of trigger point formation. Current Pain Headache Reports. 2004;8:468–75 [DOI] [PubMed] [Google Scholar]
- 15.Hoyle JA Marras WS Sheedy JE Hart DE Effects of postural and visual stressors on myofascial trigger point development and motor unit rotation during computer work. J Electromyogr Kinesiol. 2011;21:41–8 [DOI] [PubMed] [Google Scholar]
- 16.Treaster D Marras WS Burr D Sheedy JE Hart D Myofascial trigger point development from visual and postural stressors during computer work. J Electromyogr Kinesiol. 2006;16:115–24 [DOI] [PubMed] [Google Scholar]
- 17.Kuan TS Hsieh YL Chen SM Chen JT Yen WC Hong CZ The myofascial trigger point region: correlation between the degree of irritability and the prevalence of endplate noise. Am J Phys Med Rehabil. 2007;86:183–9 [DOI] [PubMed] [Google Scholar]
- 18.Simons DG New views of myofascial trigger points: etiology and diagnosis. Arch Phys Med Rehabil. 2008;89:157–9 [DOI] [PubMed] [Google Scholar]
- 19.Bukharaeva EA Salakhutdinov RI Vyskocil F Nikolsky EE Spontaneous quantal and non‐quantal release of acetylcholine at mouse endplate during onset of hypoxia. Physiol Res. 2005;54:251–5 [PubMed] [Google Scholar]
- 20.Shah JP Danoff JV Desai MJ Parikh S Nakamura LY Phillips TM, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89:16–23 [DOI] [PubMed] [Google Scholar]
- 21.Shah JP Phillips TM Danoff JV Gerber LH An in‐vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol. 2005;99:1977–84 [DOI] [PubMed] [Google Scholar]
- 22.Sahlin K Harris RC Nylind B Hultman E Lactate content and pH in muscle obtained after dynamic exercise. Pflugers Arch: Eur J Physiol. 1976;367:143–9 [DOI] [PubMed] [Google Scholar]
- 23.Shah JP Gilliams EA Uncovering the biochemical milieu of myofascial trigger points using in vivo microdialysis: an application of muscle pain concepts to myofascial pain syndrome. J Bodyw Mov Ther. 2008;12:371–84 [DOI] [PubMed] [Google Scholar]
- 24.Chou L Kao M Lin J Probable mechanism of needling therapies for myofascial pain control. Evid Based Complement Alternat Med.Volume. 2012, Article ID 705327, 11 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mense S How do muscle lesions such as latent and active trigger points influence central nociceptive neurons? J Musculokelet Pain. 2010;18:348–53 [Google Scholar]
- 26.Falla D Farina D Neuromuscular adaptation in experimental and clinical neck pain. J Electromyogr Kinesiol. 2008;18:255–61 [DOI] [PubMed] [Google Scholar]
- 27.Falla D Farina D Neural and muscular factors associated motor impairment in neck pain. Curr Rheumatol Rep. 2007;9:497–502 [DOI] [PubMed] [Google Scholar]
- 28.Fernandez de las Penas C Cuadrado M Arendt‐Nielsen L Simons D Pareja J Myofascial trigger points and sensitization: an updated pain model for tension‐type headache. Cephalalgia. 2007;27:383–93 [DOI] [PubMed] [Google Scholar]
- 29.Curatolo M Arendt‐Nielsen L Petersen‐Felix S Central hypersensitivity in chronic pain: mechanisms and clinical implications. Phys Med Rehabil Clin N Am. 2006;17:287–302 [DOI] [PubMed] [Google Scholar]
- 30.Chen JT Chung KC Hou CR Kuan CR Chen SM Hong CZ Inhibatory effect of dry needling on the spontaneous electrical activity recorded from myofascial trigger spots of rabbit skeletal muscle. Am J Phys Med Rehabil. 2000;80:729‐735 [DOI] [PubMed] [Google Scholar]
- 31.Hong CZ Lidocaine injection versus dry needling to myofascial trigger points: the importance of the local twitch response. Am J Phys Med Rehabil. 1994;73(4):256‐263 [DOI] [PubMed] [Google Scholar]
- 32.Affaitati G Costantini R Fabrizio A Lapenna D Tafuri E Giamberardino MA Effects of treatment of peripheral pain generators in fibromyalgia patients. Eur J Pain. 2011;15:61–9 [DOI] [PubMed] [Google Scholar]
- 33.Fernandez‐Carnero J La Touche R Ortega‐Santiago R Galan‐del‐Rio F Pesquera J Ge HY, et al. Short‐term effects of dry needling of active myofascial trigger points in the masseter muscle in patients with temporomandibular disorders. J Orofac Pain. 2010;24:106–12 [PubMed] [Google Scholar]
- 34.Lucas KR Polus BI Rich PS Latent myofascial trigger points: their effects on muscle activation and movement efficiency. J Bodyw Mov Ther. 2004;8:160–166 [Google Scholar]
- 35.Lucas KR Rich PA Polus BI Muscle activation patterns in the scapular positioning muscles during loaded scapular plane elevation: the effects of latent myofascial trigger points. Clin Biomech. 2010;25:765–70 [DOI] [PubMed] [Google Scholar]
- 36.Srbely JZ Dickey JP Lee D Lowerison M Dry needle stimulation of myofascial trigger points evokes segmental antinociceptive effects. J Rehabil Med. 2010;42:463–468 [DOI] [PubMed] [Google Scholar]
- 37.Furlan A van Tulder M Cherkin D Tsukayama H Lao L Koes B Berman B: Acupuncture and dry needling for low back pain: an updated systematic review within the framework of the Cochrane Collaboration. Spine. 2005;30(8):944‐963 [DOI] [PubMed] [Google Scholar]
- 38.Kietrys DM Palombaro KM Azzaretto E Hubler R Schaller B Schlussel JM Tucker M Effectivness of dry needling for upper‐quarter myofascial pain: a systematic review and meta‐analysis. J Orthop Sports Phys ther. 2013;43(9):620‐634 [DOI] [PubMed] [Google Scholar]
- 39.Mayoral O Salvat I Martin MT Martin S Santiago J Cotarelo J Rodriguez C Efficacy of myofascial trigger point dry needling in the prevention of pain after total knee arthroplasty: a randomized, double blinded placebo controlled trial. Evid Based Complement Alternat Med. Volume 2013, Article ID 694941, 8 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casaneuva B Rivas P Rodero B Quintial C Llorea J Gonzalez‐Gay MA Short term improvement following dry needle stimulation of tender points in fibromyalgia. Rheumatol Int. 2013; April 23. [DOI] [PubMed] [Google Scholar]
- 41.Mejuto‐Vasquez MJ Salmon‐Moreno J Ortega‐Santiago R Short‐term changes in neck pain wide spread pressure pain sensitivity and cervical range of motion after the application of triggerpoint dry needling in pts with acute mechanical neck pain: a randomized clinical trial. J Orthop Sports Phys Ther. 2014;44(4):252‐261 [DOI] [PubMed] [Google Scholar]
- 42.Rainey CE The use of trigger point dry needling and intramuscular electrical stimulation for a subject with chronic low back pain: a case report. Int J Sports Phys Ther. 2013;8(2):145‐161 [PMC free article] [PubMed] [Google Scholar]
- 43.Mason JS Tansey KA Westick RB Treatment of subacute posterior knee pain in an adolescent ballet dancer utilizing trigger point dry needling: a case report. Int J Sports Phys Ther. 2014;9(1):116‐124 [PMC free article] [PubMed] [Google Scholar]
- 44.Clewley D Flynn T Koppenhaur S Trigger point dry needling as an adjunct treatment for a patient with adhesive capsulitis of the shoulder. J Orthop Sports Phys Ther. 2014;44(2):92‐101 [DOI] [PubMed] [Google Scholar]
- 45.Bijur PE Silver W Gallagher J Reliability of the visual analog scale for measurements of acute pain. Acad Emerg Med. 2001;8(21):1153‐1157 [DOI] [PubMed] [Google Scholar]
- 46.Jensen MP Chen C Brugger A Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials post‐operative pain. J Pain. 2003;4(7):407‐414 [DOI] [PubMed] [Google Scholar]
- 47.Kelly AM The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med. 2001;18:205‐207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Childs JD Piva SR Fritz JM Responsiveness of the numeric pain rating scale in patients with low back pain. Spine. 2005;30:1331‐1334 [DOI] [PubMed] [Google Scholar]
- 49.Farrar JT Berling JA Strom BL Clinically important changes in acute pain outcome measures: a validation study. J Pain Syndrom Manag. 2003;25:406‐411 [DOI] [PubMed] [Google Scholar]
- 50.Jaeschke R Singer J Guyatt GH Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10: 407‐415 [DOI] [PubMed] [Google Scholar]
- 51.Fritz J Irrgang J A comparison of a modified oswestry low back pain disability questionnaire and the quebec back pain disability scale. Phys Ther. 2001;81:776‐788 [DOI] [PubMed] [Google Scholar]
- 52.Cook G Movement: Functional Movement Systems: Screening, Assessment and Corrective Strategies. 2010, Aptos, CA: Target Publications [Google Scholar]
- 53.Glaws KR Juneau CM Becker LC Di Stasi SL Hewett TE Intra‐ and inter‐rater reliability of the Selective Functional Movement Assessment (SFMA). Int J Sports Phys Ther. Apr 2014; 9(2):195–207 [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson KD Kim K Yu B Saliba SA Grindstaff TL Reliability of thoracic spine rotation range of motion measurements in healthy adults. J Athl Train. 2012;47(1):52‐60 [DOI] [PMC free article] [PubMed] [Google Scholar]