Abstract
Objective:
A review of published data addressing hepatic histopathological, metabolical, and functional changes following gastric banding, sleeve gastrectomy, gastric bypass surgery, and biliopancreatic with duodenal switch surgeries on nonalcoholic fatty liver disease (NAFLD). NAFLD is currently the most common chronic liver disease. Owing to the strong relationship between obesity and NAFLD, the idea of weight reduction as a method to treat NAFLD has rapidly emerged. Bariatric surgery has proved to be the most efficient method for weight reduction; hence, their beneficial effects on NAFLD have been evaluated by several studies. A literature review of published data was performed during the years 2012-2014 using PubMed with the following key words: Bariatric, NAFLD, steatosis, sleeve gastrectomy, gastric bypass, gastric banding, biliopancreatic diversion with duodenal switch, obesity, and insulin resistance (IR). Exclusion criteria were non-English articles and inherited NAFLD, pregnancy-induced NAFLD, and children. The majority of published data are in favor of indicating that bariatric surgeries improve the histologic and metabolic changes associated with NAFLD. The suggested mechanisms are: The reversal of IR, reduction of inflammatory markers, and improved histological features of NAFLD. Accordingly, bariatric surgeries are potentially one of the future methods in treating patients with morbid obesity and NAFLD. However, some questions remain unanswered, such as whether timing of surgery, type of surgery most effective, and whether bariatric surgeries are capable of curing the disease. Long-term and well-designed prospective studies are needed to address these issues.
Keywords: Bariatric, gastric bypass, gastric banding, insulin resistance biliopancreatic diversion with duodenal switch, nonalcoholic fatty liver disease, obesity, steatosis, sleeve gastrectomy
Increased consumption of high-density food and declining physical activity have led to an epidemic of obesity.[1] Obesity affects health adversely, thereby increasing comorbid metabolic disorders such as type 2 diabetes mellitus (T2DM), hypertension, hyperlipidemia, and steatohepatitis.[2] This hepatic pathology is part of a wide spectrum of liver pathologies known as nonalcoholic fatty liver disease (NAFLD),[3,4,5,6,7] which is becoming the most common type of chronic liver disease.[8,9,10]
OBESITY
Definition
Obesity is defined by the World Health Organization (WHO) as an abnormal or excessive fat accumulation that may impair health, with body mass index (BMI) greater than or equal to 30 kg/m2.[11] Obesity is associated with metabolic alterations including insulin resistance (IR), hyperinsulinemia, dyslipidemia, hypertension, endothelial dysfunction, pro-atherogenic and chronic inflammatory status.[12,13,14,15]
Prevalence
WHO and International Obesity Task Force reported that 312 million adults worldwide are obese. WHO also reported that an estimate of more than 1.4 billion adults, were overweight. Of these overweight adults, over 200 million men and nearly 300 million women are obese. Overall, more than one in 10 of the world's adult population are obese,[16] with Middle East, Pacific Islands, Southeast Asia, and China being the areas at greatest risk.[17] In Saudi Arabia, obesity is increasing with an overall prevalence of 35.5%; in comparison, females were significantly more obese than males with a prevalence of 44% and 26.4%, respectively.[18]
NONALCOHOLIC FATTY LIVER DISEASE
Definition
NAFLD is a spectrum of diseases that is associated with fatty infiltration of the liver that starts with simple fat accumulation (steatosis), which may progress into hepatic inflammation, termed as nonalcoholic steatohepatitis (NASH), with or without accompanying hepatic fibrosis/cirrhosis, with some patients eventually developing hepatocellular carcinoma.[19,20,21] It was first reported by Ludwig in 1979, who described it as an alcoholic-like liver disease in nonalcoholic people.[22] Until the last decade little information existed about the pathogenesis, etiology, or progression of the disease.
Kleiner proposed the NASH activity score (NAS), which is designed by the Pathology Committee of the NASH Clinical Research Network to grade the active histological features of NASH. The score is the sum of all features: Steatosis (0-3), lobular inflammation (0-3), and ballooning (0-2); ranging from 0 to 8. Score less than 3 is not NASH, whereas a score equal to or more than 5 is a definite NASH.[23]
Prevalence
Due to the modern lifestyle we are living, NAFLD prevalence is increasing in today's population,[24] being the most common chronic liver disease.[25,26] It is estimated to occur in approximately 30% of the general population in western countries,[27] and it ranges from 65% to 92.3% in morbidly obese patients (BMI > 35 kg/m2),[3,4,5,6] with up to 25% of them having NASH.[7] Moreover, the estimated prevalence of NAFLD in Saudi Arabia is 7%-10% of the general population.[26]
NAFLD Pathophysiology
The exact cause of NAFLD is still unknown; however, many theories have been proposed. The most quoted theory was the “2-hit” hypothesis, which states that NAFLD is initiated with hepatocyte accumulation of triglycerides (TGs) resulting in steatosis. This makes hepatocytes more prone to the second hit, played by the inflammatory cytokines, adipokines, mitochondrial dysfunction, and oxidative stress, which lead to steatohepatitis and/or fibrosis.[28,29] It is important to note that TGs themselves are not hepatotoxic[30]; however, they are considered as markers for increased hepatic exposure to potentially toxic-free fatty acids (FFAs).[31]
More recently, a third hit “inadequate hepatocyte proliferation” was proposed to the hypothesis. Normally the loss of hepatocytes stimulates its multiplication. However, the presence of inflammatory mediators and radical oxygen species may hinder hepatocyte replication. This results in further damage and increased number of hepatic progenitor described as oval cells,[32] which have the ability to differentiate into hepatocyte-like cells. The degree of oval cell activation and intermediate hepatocyte-like cells have shown positive correlation with fibrosis stage. In addition, these cells are also implicated in the pathogenesis of hepatocellular carcinoma that could be a possible consequence of NAFLD.[31,32]
Most attention has been focused recently on the effect of IR on the development and progression of NAFLD. IR is a condition in which a certain amount of insulin does not produce the expected biological effect on insulin-sensitive tissue.[33] In IR, there is an increased influx of FFAs into the liver, which undergoes either β-oxidation or esterification with glycerol to form TGs, resulting in an additional source of fat in the liver. FFAs are known to have a negative effect on insulin action on targeted peripheral tissue.[34] There is also evidence that FFAs can directly lead to hepatotoxicity via oxidative stress and activation of inflammatory pathways such as TNF-α[35] and leptin, which are produced by macrophages and adipocyte, respectively.[36,37] Some studies have suggested that the toxic effect of unesterified FFAs could be prevented by hepatic TG accumulation.[31,38]
Obese patients are in a chronic inflammatory state, which is correlated with IR as elevation of both tumor necrosis factor-α (TNF-α) and monocyte chemotactic protein-1 (MCP-1) which causes impairment of adipocyte insulin sensitivity.[39,40,41] In addition to IR and hyperinsulinemia being caused by obesity, there is also ground for a considerable possibility that IR contributes to the development of obesity. The latter happens by increasing the circulating insulin leading to weight gain.[42,43] Inflammation and activation of several immune pathways in obese patients affect hepatic lipid metabolism, leading to hepatic injury.[28,29,44] Adipose tissue inflammation starts by recruitment and stimulation of monocytes in the adipose tissue by chemokines such as MCP-1 and osteopontin.[14,39,45] The hypertrophied visceral adipocytes in morbidly obese patients release chemokines that lead to further macrophage infiltration into the adipose tissue. This will result in the production of proinflammatory cytokines, and these inflammatory modifications create what is known as “adipocyte dysregulation.”[2,46]
Recently, dysregulated adipocytokines were divided into “offensive” and “defensive” adipocytokines. Offensive adipocytokines include plasminogen activator inhibitor-1,[47] TNF-α,[40,48] interleukin-6 (IL-6),[49] MCP-1,[41] and angiotensinogen.[50] Examples of defensive adipocytokines are Adiponectin[51,52,53] and leptin.[54,55,56] The dysregulation of adipocytokines such as these contributes to obesity-related metabolic disorders including NAFLD.[2] However, the mechanisms by which TG accumulation leads to abnormal expression of adipocytokines and development of the metabolic syndrome have not been fully clarified.
BARIATRIC SURGERIES
Due to the strong association of NAFLD with obesity,[3,4,5,6,7,57,58,59,60] weight loss proved to have a beneficial effect on NAFLD.[5,60,61] In patients who have failed dietary manipulation and weight reduction exercise programs, bariatric surgeries have proved to be the most effective way for durable, marked, and sustained weight loss.[62,63,64,65,66]
Types of Bariatric Surgeries
There are 3 principles of bariatric operations, categorized in respect to their mechanism: (1) Restrictive procedures, which decrease the stomach size to limit the intake of solids; (2) malabsorptive procedures, which limit the absorption of nutrients by shortening the small intestine; thus decreasing the surface area that is exposed to food; and (3) combined, restrictive, and malabsorptive.[67] Laparoscopic adjustable gastric banding (LAGB), sleeve gastrectomy (SG), and gastric bypass (GBP) have become the most preferred procedures worldwide.[68] Restrictive and combined procedures have shown very promising effects on liver function and histology[7] [Figure 1].
Figure 1.
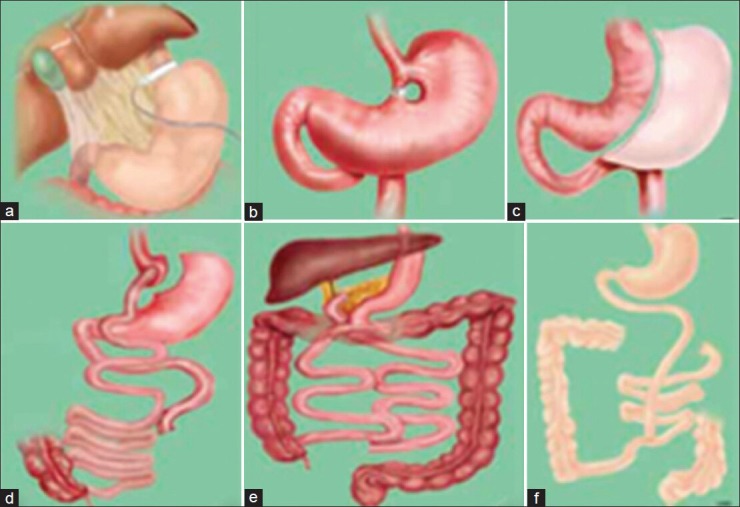
Bariatric surgical procedure. (a) Laproscopic adjustable gastric banding; (b) Vertical banding gastroplasty; (c) Laproscopic adjustable gatseric banding; (d) Roux-en-Y gastric bypass; (e) Biliopancreaatic diversion; (f) Jejunoileal bypass
Gastric Banding
In 1978, Wilkinson and Peloso reported the first gastric banding procedure. At that time it was neither adjustable nor laparoscopic.[69,70] The techniques of adjustable banding were proposed in the early 1980s, and with the emergence of laparoscopy in the mid-1990s the band insertion was done laparoscopically.[70] LAGB involves making a small proximal gastric pouch by inserting a gastric band around the superior end of the stomach [Figure 1a]. This band is linked to an injection port in the skin through a tube, which gives its adjustability.[70] LAGB accounts for 42.3% of bariatric procedures.[68] Irrespective of its purely restrictive principles in weight reduction, LAGB showed significant weight loss with excess weight loss (EWL) of 58.8 ± 30.0%, 56.8 ± 35.0%, and 58.4 ± 46.6%, at 1, 3, and 5 years, respectively, with a failure rate (%EWL\50%) of 40.4%, 43.5%, 46.3%, and 55% at 1, 3, 5, and 7 years, respectively.[71,72]
Sleeve Gastrectomy
In 1993, Hess and Marceau introduced SG as a restrictive component of biliopancreatic diversion (BPD; a malabsorptive procedure). Initially it was not intended as a standard single procedure.[73] In 2008, it became a common procedure, making up to 5.4% of the total number of performed bariatric surgeries.[68] In this procedure, the surgeon removes 75% of the stomach, resulting in a sleeve-like structure extending from the esophagus until the duodenum [Figure 1c].[73] Although SG is a restrictive procedure, it results in marked reduction of Ghrelin production; a pleiotropic hormone secreted from neuroendocrine P/D1 cells of the stomach fundus; a hormone involved in appetite and its reduction decreases hunger and improves satiety.[74,75] SG results in EWL of 55.81% at 1 year and 67.42% at 2 years.[76,77]
Gastric bypass
Currently, GBP surgery is considered to be the most effective surgical intervention in morbidly obese patients.[20,78] It accounts for 49.3% of bariatric surgeries.[68] Here the surgeon splits the stomach into two pouches; a smaller proximal and a larger distal pouch, and connects both ends to the anatomically manipulated small intestines [Figure 1d]. GBP is considered as a restrictive procedure with mild malabsorptive effect. GBP shows tremendous systemic beneficial effects starting with ghrelin level reduction,[79] marked sustained weight loss,[20,78,80,81] with an EWL of 64% 1 year after the surgery,[82] and 64.9% 7 years later.[83]
Biliopancreatic Diversion with Duodenal Switch
In 1976, Scopinaro reported the first BPD procedure.[84] Hess[85] and Marceau[73,86] created duodenal switch by uniting the Demeester method[87] with Scopinaro's to avoid duodenogastric reflux in the original BPD.[84] BPD with or without duodenal switch (DS) comprises SG with redirection of the small intestine forming two distinct routes (the shorter route collects food from the stomach, the longer route transfers bile from the liver) with a common canal [Figure 1e]. Unlike GBP, BPD with DS (BPD/DS) is mainly malabsorptive with slight restrictive effect.[85] BPD/DS accounts for 0.8% of all bariatric surgeries,[68] as it is a complex procedure with long operative time and higher risk of complications.[88] However, BPD/DS provides the greatest and most sustained weight loss with an EWL of 85% in 1 year[88] and 75% in 10 years.[89]
Complications and Side Effects
Although LAGB is considered as the safest of all bariatric surgeries, it has its own complications. These include pouch enlargement,[90] band slip,[91] and band erosion[92] with rates of 12%, 3.2%, and 1.66%, respectively, as well as esophageal dysmotility, which was a poorly appreciated complication affecting 68.8% of patients in the long term.[93] SG has minimal complications, with staple line leak as the most common complication (4%).[94,95] All bariatric surgeries share the need for postoperative multivitamin and multimineral supplements to minimize the risk of deficiencies. The most common deficiencies encountered were of iron and vitamin B12.[96,97,98] Malabsorptive procedures result in substantial weight loss, but have the highest complication rates and serious side effects with a total complication rate of 23% for GBP and 25% for BPD.[99,100,101,102,103,104,105,106]
With GBP, the most commonly reported complications were anastomotic stricture (8.9%), intestinal obstruction (7.3%), gastrointestinal bleeding (4%), staple line leakage (1.6%),[107] and dumping syndrome.[67] BPD complications include hepatic failure (explained by rapid weight loss).[100] Complications also comprise gastric leak (0.07%),[89] marginal ulcers (0.3%),[108] and duodenal stump leak (0.02%),[89] but no dumping syndrome.[85] Furthermore, it has been reported that patients with protein depletion may require revisions and reversals of their surgeries with rates of 3.7% and 0.61%, respectively.[89] Unfortunate mortality was reported in 0.1% of patients undergoing LAGB or SG, in 0.5% of those who had undergone GBP, and in 1.1% in those who had PBD.[109]
Effects of Bariatric Surgeries
Besides the previously mentioned benefits (EWL) and complications of bariatric surgeries, the following text focuses mainly on the metabolic, inflammatory, histologic, and liver function changes.
Metabolic Changes
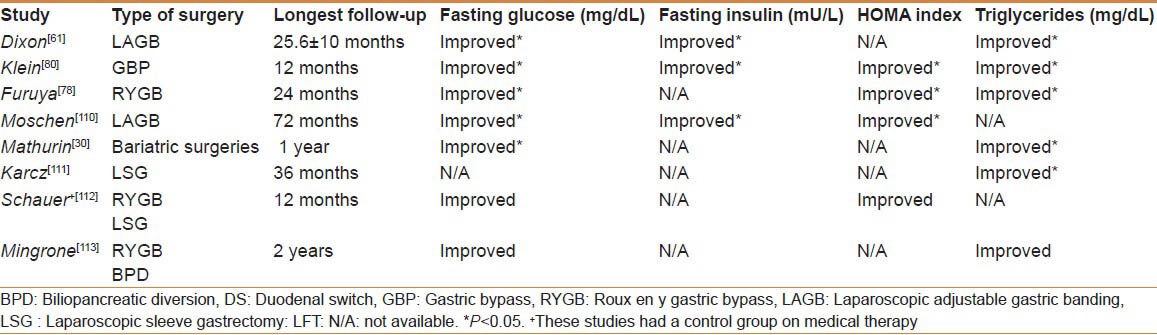
Consistently reported outcome of bariatric surgeries have shown improvement in many metabolic aspects, studies have proved that bariatric surgeries along with medical therapy achieved glycemic control in significantly more than medical therapy alone. In a 2-year prospective study on 18 patients undergoing GBP, by Furuya et al., 8 had T2DM at baseline and 2 after surgery (P < 0.05), 11 had hyperlipidemia at baseline and 3 after surgery (P < 0.05), and IR (measured by HOMA) was closer to normal after surgery (P < 0.05)[78]. Moschen el al showed complete resolution of T2DM (P < 0.05), and they also reported a decrease in HOMA index, as it was 5.5 before, and 2.4 1 year later (P < 0.05).[110] Karcz et al. reported that 23 patients had T2DM prior to SG, 12 months later the median hemoglobin A1c levels dropped and remained within normal range 2 years after.[111] Mathurin et al. assessed IR using the quantitative insulin sensitivity check index (QUICKI), which was 3.2 at baseline and declined to 2.84 a year after surgery and remained consistent for 5 years (P < 0.05) along with a significant decline in serum TGs (P < 0.05). A decline in IR has been proved to be an early indicator of an improvement in liver histology (steatosis and ballooning).[30] A randomized clinical trial by Schauer et al.[112] showed that bariatric surgery in 99 patients (RYGB and LSG) has resulted in a significantly lower glycemic control measured by the percentage of glycosylated hemoglobin (P = 0.02) and HOMA-IR (P < 0.01), when compared with intensive medical therapy (41 patients). Another study by Mingrone et al. targeted patients with DM and then randomly assigned to either medical therapy or bariatric surgery (RYGB, BPD).[113]. The surgical group patients (60 patients) had a significantly better glycemic (P < 0.001) and a better lipid control (P < 0.001) [Table 1].
Table 1.
Comparison between the metabolic effects after bariatric surgeries between selected studies
Inflammatory Changes
It has been shown that weight loss due to bariatric surgeries is associated with a significant reduction in hepatic expression of several factors involved in hepatic inflammation such as MCP-1 and interleukin-8 (IL-8).[80] Weight loss has also been shown to regulate hepatic fibrogenesis by decreasing several inflammatory and fibrogenesis factors, including transforming growth factor-β1, tissue inhibitor of metalloproteinase 1, α-smooth muscle actin, and collagen-α1,[80] which inhibits the activity of matrix metalloproteinases.[80] Along the same lines, Moschen et al. showed significant systemic reduction in C-reactive protein (mg/dL) from 0.86 to 0.42, TNF-α (pg/mL) from 2.36 to 0.8, and leptin serum levels from 27.4 to 15.15 (ng/mL) after 1-year of surgery (P < 0.05); however, the hepatic leptin expression [messenger ribonucleic acid (mRNA), and protein level] was not affected.[110] Serum levels of adiponectin rose significantly from 7.46 to 8.95 μg/mL (P < 0.05) 1 year after LAGB. Adiponectin protein expression (done via immunohistochemistry) in liver biopsies increased significantly (P < 0.05) after LAGB. This study supports the hypothesis that weight loss in morbidly obese patients is associated with increased levels of anti-inflammatory adipocytokines and decreased levels of pro-inflammatory adipocytokines. Also, Moschen et al. considered the effects of weight loss on the hepatic or adipose tissue expression of IL-6, adiponectin, and TNF-α in 20 morbidly obese patients undergoing LAGB, with 6 months follow up. For IL-6, they reported significant reduction in serum, subcutaneous tissues (25.9-fold), and hepatic mRNA expression (P < 0.05). Nevertheless, serum TNF-α was undetectable at both baseline and follow up, hepatic TNF-α expression remained the same, but subcutaneous TNF-α had a 2.1-fold reduction (P < 0.05). In addition, their results showed noticeable increase in subcutaneous adiponectin expression.[114]
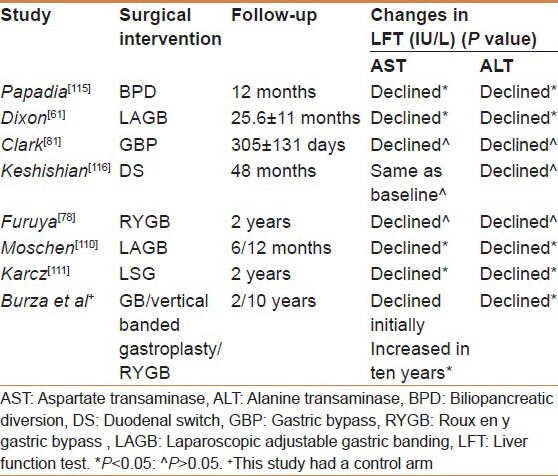
Biochemical Liver Changes
Although it is not ideal to use liver enzymes as an accurate reflection of NAFLD status, most of the studies used aspartate transaminase (AST) and alanine transaminase (ALT) to evaluate NAFLD effect on liver function at baseline and after bariatric surgeries. Papadia et al. intended to assess the risk factors for acute liver damage after BPD. They included 99 patients, AST levels were elevated 2 months after BPD (P < 0.05), this elevation was followed by a significant drop 10 months later (P < 0.05).[115] Keshishian et al. reported a transient elevation of AST (130%) and ALT (160%) 6 months after BPD/DS.[116] However, these levels were normalized in 1 year, and persisted for 3 years. Moschen et al. stated that 7 out of 30 patients had an elevated ALT at baseline; 3 remained elevated at 6 months, but in 12 months from the procedure; only 1 patient continued having elevated ALT.[110] In the Swedish obese subjects paper, a recent prospective controlled study, they examined the long-term effect of bariatric surgery on transaminase levels in 2 and 10 years.
At 2 years, results showed lower serum ALT and AST levels and no change in the control group but at 10 years ALT levels continued to drop, whereas AST increased.[117] It is worth mentioning that limited studies looked into albumin as a reflection of liver function, with no reports yet of significant changes.[20] None of the reviewed studies reported results of protein C and S, or coagulation profile [Table 2].
Table 2.
Comparison between the biochemical liver changes after bariatric surgeries
Histological Changes
Steatosis
A prospective study by Clark et al. aimed to evaluate liver histological effect before and after GBP surgery in 16 patients. They showed an improvement in histological features of NAFLD (based on Brunt criteria) with regression of steatosis in 13 (81%) of the patients (P < 0.05).[81] No patient had an increase in steatosis after 305 ± 131 days from an open GBP surgery. This study was limited by the small sample size, and nonprotocolized criteria of the second biopsy.[81] Similarly, Furuya et al., showed that (33%) of their patients displayed variable degrees of steatosis prior to surgery, which disappeared in 89% after 2 years (P < 0.05)[78]. Moschen et al. found reduction of liver steatosis in 14 out of 18 patients (P < 0.001) using the modified classification system from Kleiner.[110] Keshishian et al., reported the effect of DS 697 patients who were followed with a median of 6, 12, 18 months, and annually for 4 years. The histology results were only available in 78 out of 697 patients. These 78 patients had a second liver biopsy with a time interval ranging from 6 months to 3 years; depending on the need for a second operative procedure. Based on subjective assessment, the severity of steatosis had more than 50% reduction compared with baseline readings.[116] Mathurin et al. studied 381 patients who underwent bariatric surgeries with protocol liver biopsies at three intervals, first intraoperatively, second 1 year after, and a third 5 years later. They used the NAFLD activity score (NAS) to evaluate the NAFLD histological changes, and the 5-grade scale for fibrosis assessment.[30] They revealed significant reduction of liver steatosis from 37% before surgery to 16% in 5 years (P < 0.01). They reported that patients with persistent steatosis had higher BMI, IR, TG, ALT, and gamma glutamyl transferase than patients without steatosis.[30]
Ballooning and Inflammation
Most studies did not show improvement in inflammation such as the one by Mathurin et al (P > 0.05).[30] “However, some showed changes. Clark et al. studied 16 paired liver biopsies, 15 showed inflammation in the initial biopsy, and 12 revealed a significant reduction (P < 0.01) after a mean follow up of 305 ± 131 (SD) days from the surgery.[81] Many studies have shown that hepatocyte ballooning improves after bariatric surgeries such as Mathurin et al. (CI 95%)[30] Clark et al. (P < 0.05),[81] Furuya et al.,[78] and Moschen et al. (P < 0.05).[110]
Fibrosis
Most of the studies showed significant improvement of fibrosis. Clark et al. reported 14 patients with some degree of perisinusoidal fibrosis at the time of GBP. Of these, 6 patients showed improvement in the fibrosis score by one point; however, 8 patients had no change in the second biopsy. Similarly, of the 13 patients with portal fibrosis at baseline, 6 had improvement in their fibrosis score by one point, whereas the remaining 7 had no change. None of the patients showed development or progression at the time of follow up (P = 0.01, 0.01, and 0.003, respectively).[81] Likewise, Furuya et al., reported that fibrosis disappeared in 75% patients (P < 0.05), with no worsening at the time of the second biopsy.[78] Moschen et al., did not detect any changes in liver fibrosis 6 months after the LAGB surgery (P = 0.27).[110] On the other hand, Mathurin et al. showed worsening of the extent of fibrosis from baseline to 1 and 5 years in 20% of patients, whereas there was no change in 80% of patients (P = 0.01).[30] At 5 years, the progression of fibrosis was detected in patients with higher IR, BMI, and NAS. This unexpected effect may be attributed to the very high starting body weight of the patients included in this study.
Nonalcoholic Steatohepatitis
Dixon et al. studied the improvement of liver histology including NASH and cirrhosis with LAGB-induced weight loss.[61]. They evaluated 197 patients, 36 had a second biopsy, which was not protocolized with a mean follow up of 25.6 ± 11 months, 23 had NASH. Over all change was significant (P < 0.05)-19 cases (82%) had resolution or remission of NASH, 2 (9%) had improvement without resolution, and 2 (9%) remained unchanged.[61] Similar results were obtained by Keshishian et al.[116] and Moschen et al.[110]
Mathurin et al. had 99 patients with NASH at baseline, 5 years later only 30 patients remained with NASH (P < 0.01).[30] Nonetheless, this marked improvement was observed in the first year of follow up, with no significant change from 1 to 5 years (P > 0.05).[30]
CONCLUSION
This review demonstrates the effect of bariatric surgery on the metabolic, biochemical, and histopathological parameters of NAFLD. Due to the complexity of the disease, additional clinical studies with stronger methodology, longer follow up, and with a larger sample size are needed to confirm the sustainability of these effects. Moreover, the significant morbidities as well as the rare mortality rates from bariatric surgeries do need to be considered and clearly explained to the patient.
ACKNOWLEDGMENT
This study is supported by a grant (11-MED1910-02) from the National Plan for Science, Technology and Innovation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Dixon JB. Surgical treatment for obesity and its impact on non-alcoholic steatohepatitis. Clin Liver Dis. 2007;11:141–54. doi: 10.1016/j.cld.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Kamada Y, Takehara T, Hayashi N. Adipocytokines and liver disease. J Gastroenterol. 2008;43:811–22. doi: 10.1007/s00535-008-2213-6. [DOI] [PubMed] [Google Scholar]
- 3.Gholam PM, Kotler DP, Flancbaum LJ. Liver pathology in morbidly obese patients undergoing Roux-en-Y gastric bypass surgery. Obes Surg. 2002;12:49–51. doi: 10.1381/096089202321144577. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira CP, Faintuch J, Rascovski A, Furuya CK, Jr, Bastos Mdo S, Matsuda M, et al. Lipid peroxidation in bariatric candidates with nonalcoholic fatty liver disease (NAFLD): Preliminary findings. Obes Surg. 2005;15:502–5. doi: 10.1381/0960892053723493. [DOI] [PubMed] [Google Scholar]
- 5.Frantzides CT, Carlson MA, Moore RE, Zografakis JG, Madan AK, Puumala S, et al. Effect of body mass index on nonalcoholic fatty liver disease in patients undergoing minimally invasive bariatric surgery. J Gastrointest Surg. 2004;8:849–55. doi: 10.1016/j.gassur.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Beymer C, Kowdley KV, Larson A, Edmonson P, Dellinger EP, Flum DR. Prevalence and predictors of asymptomatic liver disease in patients undergoing gastric bypass surgery. Arch Surg. 2003;138:1240–4. doi: 10.1001/archsurg.138.11.1240. [DOI] [PubMed] [Google Scholar]
- 7.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: Predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 8.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 9.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 10.Sanyal AJ. NASH: A global health problem. Hepatol Res. 2011;41:670–4. doi: 10.1111/j.1872-034X.2011.00824.x. [DOI] [PubMed] [Google Scholar]
- 11.Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 12.Targher G, Bertolini L, Padovani R, Zenari L, Zoppini G, Falezza G. Relation of nonalcoholic hepatic steatosis to early carotid atherosclerosis in healthy men: Role of visceral fat accumulation. Diabetes care. 2004;27:2498–500. doi: 10.2337/diacare.27.10.2498. [DOI] [PubMed] [Google Scholar]
- 13.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–50. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 14.Bertola A, Deveaux V, Bonnafous S, Rousseau D, Anty R, Wakkach A, et al. Elevated expression of osteopontin may be related to adipose tissue macrophage accumulation and liver steatosis in morbid obesity. Diabetes. 2009;58:125–33. doi: 10.2337/db08-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angel A. Pathophysiologic changes in obesity. Can Med Assoc J. 1978;119:1401–6. [PMC free article] [PubMed] [Google Scholar]
- 16.State-specific prevalence of obesity among adults--United States, 2005. Morb Mortal Wkly Rep. 2006;55:985–8. [PubMed] [Google Scholar]
- 17.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world: A growing challenge. N Engl J Med. 2007;356:213–5. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 18.Al-Nozha MM, Al-Mazrou YY, Al-Maatouq MA, Arafah MR, Khalil MZ, Khan NB, et al. Obesity in Saudi Arabia. Saudi Med J. 2005;26:824–9. [PubMed] [Google Scholar]
- 19.Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002;17(Suppl):S186–90. doi: 10.1046/j.1440-1746.17.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 20.Mattar SG, Velcu LM, Rabinovitz M, Demetris AJ, Krasinskas AM, Barinas-Mitchell E, et al. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann Surg. 2005;242:610–7. doi: 10.1097/01.sla.0000179652.07502.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: A follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–8. [PubMed] [Google Scholar]
- 23.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 24.Kirovski G, Schacherer D, Wobser H, Huber H, Niessen C, Beer C, et al. Prevalence of ultrasound-diagnosed non-alcoholic fatty liver disease in a hospital cohort and its association with anthropometric, biochemical and sonographic characteristics. Int J Clin Exp Med. 2010;3:202–10. [PMC free article] [PubMed] [Google Scholar]
- 25.Paschos P, Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13:9–19. [PMC free article] [PubMed] [Google Scholar]
- 26.Al-hamoudi W, El-Sabbah M, Ali S, Altuwaijri M, Bedewi M, Adam M, et al. Epidemiological, clinical, and biochemical characteristics of Saudi patients with nonalcoholic fatty liver disease: A hospital-based study. Ann Saudi Med. 2012;32:288–92. doi: 10.5144/0256-4947.2012.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: The Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 28.Day CP. From fat to inflammation. Gastroenterology. 2006;130:207–10. doi: 10.1053/j.gastro.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Day CP, James OF. Steatohepatitis: A tale of two “hits”? Gastroenterology. 1998;114:842–5. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 30.Mathurin P, Hollebecque A, Arnalsteen L, Buob D, Leteurtre E, Caiazzo R, et al. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 2009;137:532–40. doi: 10.1053/j.gastro.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 31.Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:370–9. doi: 10.1055/s-0028-1091981. [DOI] [PubMed] [Google Scholar]
- 32.Roskams T, Yang SQ, Koteish A, Durnez A, DeVos R, Huang X, et al. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163:1301–11. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–29. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 34.Bevilacqua S, Bonadonna R, Buzzigoli G, Boni C, Ciociaro D, Maccari F, et al. Acute elevation of free fatty acid levels leads to hepatic insulin resistance in obese subjects. Metabolism. 1987;36:502–6. doi: 10.1016/0026-0495(87)90051-5. [DOI] [PubMed] [Google Scholar]
- 35.Sherman ML, Datta R, Hallahan DE, Weichselbaum RR, Kufe DW. Regulation of tumor necrosis factor gene expression by ionizing radiation in human myeloid leukemia cells and peripheral blood monocytes. J Clin Invest. 1991;87:1794–7. doi: 10.1172/JCI115199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hedman MH, Rolandsson O, Hagg E, Mincheva-Nilsson L, Lindahl B. Association between insulin resistance and GAD65-autoantibody levels-a pilot study in an adult non-diabetic population. Autoimmunity. 2004;37:33–6. doi: 10.1080/08916930310001607943. [DOI] [PubMed] [Google Scholar]
- 37.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–37. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–74. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 39.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95:2111–9. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:7265–70. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–81. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sigal RJ, El-Hashimy M, Martin BC, Soeldner JS, Krolewski AS, Warram JH. Acute postchallenge hyperinsulinemia predicts weight gain: A prospective study. Diabetes. 1997;46:1025–9. doi: 10.2337/diab.46.6.1025. [DOI] [PubMed] [Google Scholar]
- 44.Bertola A, Bonnafous S, Anty R, Patouraux S, Saint-Paul MC, Iannelli A, et al. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PloS One. 2010;5:e13577. doi: 10.1371/journal.pone.0013577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valenti L, Fracanzani AL, Fargion S. The immunopathogenesis of alcoholic and nonalcoholic steatohepatitis: Two triggers for one disease? Semin Immunopathol. 2009;31:359–69. doi: 10.1007/s00281-009-0152-9. [DOI] [PubMed] [Google Scholar]
- 46.Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D, et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–61. doi: 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- 47.Shimomura I, Funahashi T, Takahashi M, Maeda K, Kotani K, Nakamura T, et al. Enhanced expression of PAI-1 in visceral fat: Possible contributor to vascular disease in obesity. Nat Med. 1996;2:800–3. doi: 10.1038/nm0796-800. [DOI] [PubMed] [Google Scholar]
- 48.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 49.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: Depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 50.Hainault I, Nebout G, Turban S, Ardouin B, Ferre P, Quignard-Boulange A. Adipose tissue-specific increase in angiotensinogen expression and secretion in the obese (fa/fa) Zucker rat. Am J Physiol Endocrinol Metab. 2002;282:E59–66. doi: 10.1152/ajpendo.2002.282.1.E59. [DOI] [PubMed] [Google Scholar]
- 51.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: An adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–9. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 52.Pagano C, Soardo G, Esposito W, Fallo F, Basan L, Donnini D, et al. Plasma adiponectin is decreased in nonalcoholic fatty liver disease. Eur J Endocrinol. 2005;152:113–8. doi: 10.1530/eje.1.01821. [DOI] [PubMed] [Google Scholar]
- 53.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 54.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 55.Farooqi IS, Keogh JM, Kamath S, Jones S, Gibson WT, Trussell R, et al. Partial leptin deficiency and human adiposity. Nature. 2001;414:34–5. doi: 10.1038/35102112. [DOI] [PubMed] [Google Scholar]
- 56.Unger RH. The physiology of cellular liporegulation. Annu Rev Physiol. 2003;65:333–47. doi: 10.1146/annurev.physiol.65.092101.142622. [DOI] [PubMed] [Google Scholar]
- 57.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71–9. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 58.Spaulding L, Trainer T, Janiec D. Prevalence of non-alcoholic steatohepatitis in morbidly obese subjects undergoing gastric bypass. Obes Surg. 2003;13:347–9. doi: 10.1381/096089203765887633. [DOI] [PubMed] [Google Scholar]
- 59.Moretto M, Kupski C, Mottin CC, Repetto G, Garcia Toneto M, Rizzolli J, et al. Hepatic steatosis in patients undergoing bariatric surgery and its relationship to body mass index and co-morbidities. Obes Surg. 2003;13:622–4. doi: 10.1381/096089203322190853. [DOI] [PubMed] [Google Scholar]
- 60.Silverman EM, Sapala JA, Appelman HD. Regression of hepatic steatosis in morbidly obese persons after gastric bypass. Am J Clin Pathol. 1995;104:23–31. doi: 10.1093/ajcp/104.1.23. [DOI] [PubMed] [Google Scholar]
- 61.Dixon JB, Bhathal PS, Hughes NR, O’Brien PE. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–54. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- 62.Padwal RS. Characteristics of patients undergoing bariatric surgery in Canada. Obes Res. 2005;13:2052–4. doi: 10.1038/oby.2005.253. [DOI] [PubMed] [Google Scholar]
- 63.Trus TL, Pope GD, Finlayson SR. National trends in utilization and outcomes of bariatric surgery. Surg Endosc. 2005;19:616–20. doi: 10.1007/s00464-004-8827-8. [DOI] [PubMed] [Google Scholar]
- 64.Dixon JB, Anderson M, Cameron-Smith D, O’Brien PE. Sustained weight loss in obese subjects has benefits that are independent of attained weight. Obes Res. 2004;12:1895–902. doi: 10.1038/oby.2004.235. [DOI] [PubMed] [Google Scholar]
- 65.MacDonald KG J, Long SD, Swanson MS, Brown BM, Morris P, Dohm GL, et al. The gastric bypass operation reduces the progression and mortality of non-insulin-dependent diabetes mellitus. J Gastrointest Surg. 1997;1:213–20. doi: 10.1016/s1091-255x(97)80112-6. [DOI] [PubMed] [Google Scholar]
- 66.Kashyap SR, Daud S, Kelly KR, Gastaldelli A, Win H, Brethauer S, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 2010;34:462–71. doi: 10.1038/ijo.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karmali S, Johnson Stoklossa C, Sharma A, Stadnyk J, Christiansen S, Cottreau D, et al. Bariatric surgery: A primer. Can Fam Physician. 2010;56:873–9. [PMC free article] [PubMed] [Google Scholar]
- 68.Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide 2008. Obes Surg. 2009;19:1605–11. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 69.Wilkinson LH, Peloso OA. Gastric (reservoir) reduction for morbid obesity. Arch Surg. 1981;116:602–5. doi: 10.1001/archsurg.1981.01380170082014. [DOI] [PubMed] [Google Scholar]
- 70.Steffen R. The history and role of gastric banding. Surg Obes Relat Dis. 2008;4:S7–13. doi: 10.1016/j.soard.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Schouten R, Wiryasaputra DC, van Dielen FM, van Gemert WG, Greve JW. Long-term results of bariatric restrictive procedures: A prospective study. Obes Surg. 2010;20:1617–26. doi: 10.1007/s11695-010-0211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boza C, Gamboa C, Perez G, Crovari F, Escalona A, Pimentel F, et al. Laparoscopic adjustable gastric banding (LAGB): Surgical results and 5-year follow-up. Surg Endosc. 2011;25:292–7. doi: 10.1007/s00464-010-1176-x. [DOI] [PubMed] [Google Scholar]
- 73.Marceau P, Biron S, Bourque RA, Potvin M, Hould FS, Simard S. Biliopancreatic Diversion with a New Type of Gastrectomy. Obes Surg. 1993;3:29–35. doi: 10.1381/096089293765559728. [DOI] [PubMed] [Google Scholar]
- 74.Rindi G, Necchi V, Savio A, Torsello A, Zoli M, Locatelli V, et al. Characterisation of gastric ghrelin cells in man and other mammals: Studies in adult and fetal tissues. Histochem Cell Biol. 2002;117:511–9. doi: 10.1007/s00418-002-0415-1. [DOI] [PubMed] [Google Scholar]
- 75.Inui A, Asakawa A, Bowers CY, Mantovani G, Laviano A, Meguid MM, et al. Ghrelin, appetite, and gastric motility: The emerging role of the stomach as an endocrine organ. FASEB J. 2004;18:439–56. doi: 10.1096/fj.03-0641rev. [DOI] [PubMed] [Google Scholar]
- 76.Sabbagh C, Verhaeghe P, Dhahri A, Brehant O, Fuks D, Badaoui R, et al. Two-year results on morbidity, weight loss and quality of life of SG as first procedure, SG after failure of gastric banding and gastric banding. Obes Surg. 2010;20:679–84. doi: 10.1007/s11695-009-0007-4. [DOI] [PubMed] [Google Scholar]
- 77.Moon Han S, Kim WW, Oh JH. Results of laparoscopic sleeve gastrectomy (LSG) at 1 year in morbidly obese Korean patients. Obes Surg. 2005;15:1469–75. doi: 10.1381/096089205774859227. [DOI] [PubMed] [Google Scholar]
- 78.Furuya CK, Jr, de Oliveira CP, de Mello ES, Faintuch J, Raskovski A, Matsuda M, et al. Effects of bariatric surgery on nonalcoholic fatty liver disease: Preliminary findings after 2 years. J Gastroenterol Hepatol. 2007;22:510–4. doi: 10.1111/j.1440-1746.2007.04833.x. [DOI] [PubMed] [Google Scholar]
- 79.Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33:786–95. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klein S, Mittendorfer B, Eagon JC, Patterson B, Grant L, Feirt N, et al. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1564–72. doi: 10.1053/j.gastro.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 81.Clark JM, Alkhuraishi AR, Solga SF, Alli P, Diehl AM, Magnuson TH. Roux-en-Y gastric bypass improves liver histology in patients with non-alcoholic fatty liver disease. Obes Res. 2005;13:1180–6. doi: 10.1038/oby.2005.140. [DOI] [PubMed] [Google Scholar]
- 82.Campos GM, Rabl C, Roll GR, Peeva S, Prado K, Smith J, et al. Better weight loss, resolution of diabetes, and quality of life for laparoscopic gastric bypass vs banding: Results of a 2-cohort pair-matched study. Arch Surg. 2011;146:149–55. doi: 10.1001/archsurg.2010.316. [DOI] [PubMed] [Google Scholar]
- 83.Suter M, Donadini A, Romy S, Demartines N, Giusti V. Laparoscopic Roux-en-Y gastric bypass: Significant long-term weight loss, improvement of obesity-related comorbidities and quality of life. Ann Surg. 2011;254:267–73. doi: 10.1097/SLA.0b013e3182263b66. [DOI] [PubMed] [Google Scholar]
- 84.Scopinaro N, Gianetta E, Civalleri D, Bonalumi U, Bachi V. Bilio-pancreatic bypass for obesity: II. Initial experience in man. Br J Surg. 1979;66:618–20. doi: 10.1002/bjs.1800660906. [DOI] [PubMed] [Google Scholar]
- 85.Hess DS, Hess DW. Biliopancreatic diversion with a duodenal switch. Obes Surg. 1998;8:267–82. doi: 10.1381/096089298765554476. [DOI] [PubMed] [Google Scholar]
- 86.Marceau P, Hould FS, Simard S, Lebel S, Bourque RA, Potvin M, et al. Biliopancreatic diversion with duodenal switch. World J Surg. 1998;22:947–54. doi: 10.1007/s002689900498. [DOI] [PubMed] [Google Scholar]
- 87.DeMeester TR, Fuchs KH, Ball CS, Albertucci M, Smyrk TC, Marcus JN. Experimental and clinical results with proximal end-to-end duodenojejunostomy for pathologic duodenogastric reflux. Ann Surg. 1987;206:414–26. doi: 10.1097/00000658-198710000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nelson D, Beekley A, Carter P, Kjorstad R, Sebesta J, Martin M. Early results after introduction of biliopancreatic diversion/duodenal switch at a military bariatric center. Am J Surg. 2011;201:678–84. doi: 10.1016/j.amjsurg.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 89.Hess DS, Hess DW, Oakley RS. The biliopancreatic diversion with the duodenal switch: Results beyond 10 years. Obes Surg. 2005;15:408–16. doi: 10.1381/0960892053576695. [DOI] [PubMed] [Google Scholar]
- 90.Moser F, Gorodner MV, Galvani CA, Baptista M, Chretien C, Horgan S. Pouch enlargement and band slippage: Two different entities. Surg Endosc. 2006;20:1021–9. doi: 10.1007/s00464-005-0269-4. [DOI] [PubMed] [Google Scholar]
- 91.Sarker S, Herold K, Creech S, Shayani V. Early and late complications following laparoscopic adjustable gastric banding. Am Surg. 2004;70:146–9. [PubMed] [Google Scholar]
- 92.Niville E, Dams A, Vlasselaers J. Lap-Band erosion: Incidence and treatment. Obes Surg. 2001;11:744–7. doi: 10.1381/09608920160558704. [DOI] [PubMed] [Google Scholar]
- 93.Naef M, Mouton WG, Naef U, vander Weg B, Maddern GJ, Wagner HE. Esophageal dysmotility disorders after laparoscopic gastric banding-an underestimated complication. Ann Surg. 2011;253:285–90. doi: 10.1097/SLA.0b013e318206843e. [DOI] [PubMed] [Google Scholar]
- 94.Kuesters S, Marjanovic G, Karcz WK. Redo operations after bariatric and metabolic surgery. Zentralbl Chir. 2009;134:50–6. doi: 10.1055/s-0028-1098805. [DOI] [PubMed] [Google Scholar]
- 95.Albanopoulos K, Alevizos L, Linardoutsos D, Menenakos E, Stamou K, Vlachos K, et al. Routine abdominal drains after laparoscopic sleeve gastrectomy: A retrospective review of 353 patients. Obes Surg. 2011;21:687–91. doi: 10.1007/s11695-010-0343-4. [DOI] [PubMed] [Google Scholar]
- 96.Pournaras DJ, le Roux CW. After bariatric surgery, what vitamins should be measured and what supplements should be given? Clin Endocrinol (Oxf) 2009;71:322–5. doi: 10.1111/j.1365-2265.2009.03564.x. [DOI] [PubMed] [Google Scholar]
- 97.Bloomberg RD, Fleishman A, Nalle JE, Herron DM, Kini S. Nutritional deficiencies following bariatric surgery: What have we learned? Obes Surg. 2005;15:145–54. doi: 10.1381/0960892053268264. [DOI] [PubMed] [Google Scholar]
- 98.Skroubis G, Sakellaropoulos G, Pouggouras K, Mead N, Nikiforidis G, Kalfarentzos F. Comparison of nutritional deficiencies after Roux-en-Y gastric bypass and after biliopancreatic diversion with Roux-en-Y gastric bypass. Obes Surg. 2002;12:551–8. doi: 10.1381/096089202762252334. [DOI] [PubMed] [Google Scholar]
- 99.Holzbach RT. Hepatic effects of jejunoileal bypass for morbid obesity. Am J Clin Nutr. 1977;30:43–52. doi: 10.1093/ajcn/30.1.43. [DOI] [PubMed] [Google Scholar]
- 100.Grimm IS, Schindler W, Haluszka O. Steatohepatitis and fatal hepatic failure after biliopancreatic diversion. Am J Gastroenterol. 1992;87:775–9. [PubMed] [Google Scholar]
- 101.Hocking MP, Davis GL, Franzini DA, Woodward ER. Long-term consequences after jejunoileal bypass for morbid obesity. Dig Dis Sci. 1998;43:2493–9. doi: 10.1023/a:1026698602714. [DOI] [PubMed] [Google Scholar]
- 102.Luyckx FH, Desaive C, Thiry A, Dewe W, Scheen AJ, Gielen JE, et al. Liver abnormalities in severely obese subjects: Effect of drastic weight loss after gastroplasty. Int J Obes Relat Metab Disord. 1998;22:222–6. doi: 10.1038/sj.ijo.0800571. [DOI] [PubMed] [Google Scholar]
- 103.Luyckx FH, Lefebvre PJ, Scheen AJ. Non-alcoholic steatohepatitis: Association with obesity and insulin resistance, and influence of weight loss. Diabetes Metab. 2000;26:98–106. [PubMed] [Google Scholar]
- 104.DeWind LT, Payne JH. Intestinal bypass surgery for morbid obesity. Long-term results. JAMA. 197615;236:2298–301. [PubMed] [Google Scholar]
- 105.O’Leary JP. Hepatic complications of jejunoileal bypass. Semin Liver Dis. 1983;3:203–15. doi: 10.1055/s-2008-1040686. [DOI] [PubMed] [Google Scholar]
- 106.Parikh MS, Laker S, Weiner M, Hajiseyedjavadi O, Ren CJ. Objective comparison of complications resulting from laparoscopic bariatric procedures. J Am Coll Surg. 2006;202:252–61. doi: 10.1016/j.jamcollsurg.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 107.Papasavas PK, Caushaj PF, McCormick JT, Quinlin RF, Hayetian FD, Maurer J, et al. Laparoscopic management of complications following laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Endosc. 2003;17:610–4. doi: 10.1007/s00464-002-8826-6. [DOI] [PubMed] [Google Scholar]
- 108.Keshishian A, Zahriya K, Hartoonian T, Ayagian C. Duodenal switch is a safe operation for patients who have failed other bariatric operations. Obes Surg. 2004;14:1187–92. doi: 10.1381/0960892042387066. [DOI] [PubMed] [Google Scholar]
- 109.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: A systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 110.Moschen AR, Molnar C, Wolf AM, Weiss H, Graziadei I, Kaser S, et al. Effects of weight loss induced by bariatric surgery on hepatic adipocytokine expression. J Hepatol. 2009;51:765–77. doi: 10.1016/j.jhep.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 111.Karcz WK, Krawczykowski D, Kuesters S, Marjanovic G, Kulemann B, Grobe H, et al. Influence of Sleeve Gastrectomy on NASH and Type 2 Diabetes Mellitus. J Obes. 2011;2011:765473. doi: 10.1155/2011/765473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–76. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–85. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- 114.Moschen AR, Molnar C, Geiger S, Graziadei I, Ebenbichler CF, Weiss H, et al. Anti-inflammatory effects of excessive weight loss: Potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 2010;59:1259–64. doi: 10.1136/gut.2010.214577. [DOI] [PubMed] [Google Scholar]
- 115.Papadia F, Marinari GM, Camerini G, Adami GF, Murelli F, Carlini F, et al. Short-term liver function after biliopancreatic diversion. Obes Surg. 2003;13:752–5. doi: 10.1381/096089203322509336. [DOI] [PubMed] [Google Scholar]
- 116.Keshishian A, Zahriya K, Willes EB. Duodenal switch has no detrimental effects on hepatic function and improves hepatic steatohepatitis after 6 months. Obes Surg. 2005;15:1418–23. doi: 10.1381/096089205774859290. [DOI] [PubMed] [Google Scholar]
- 117.Burza MA, Romeo S, Kotronen A, Svensson PA, Sjoholm K, Torgerson JS, et al. Long-term effect of bariatric surgery on liver enzymes in the Swedish Obese Subjects (SOS) study. PloS One. 2013;8:e60495. doi: 10.1371/journal.pone.0060495. [DOI] [PMC free article] [PubMed] [Google Scholar]


