Abstract
Cirrhosis affects millions of people throughout the world. Two of the most serious complications of liver cirrhosis are ascites and spontaneous bacterial peritonitis (SBP). The development of ascites is related to the severity of portal hypertension and is an indicator of increased mortality. Although sodium restriction and diuretic therapy have proven effective, some patients may not respond appropriately or develop adverse reactions to diuretic therapy. In such cases, interventions such as transjugular intrahepatic portosystemic shunt (TIPS) placement are warranted. SBP is a complication of ascites that confers a very high mortality rate. Recognition and prompt treatment of this condition is essential to prevent serious morbidity and mortality. Initiation of prophylaxis in SBP remains controversial. Given the burden of liver cirrhosis on the health care system, ascites and SBP will continue to provide challenges for the primary care provider, hospitalist, internist, and gastroenterologist alike.
Keywords: Cirrhosis, portal hypertension, serum-to-ascites albumin gradient (SAAG), transjugular intrahepatic portosystemic shunt (TIPS)
Ascites is the accumulation of lymphatic fluid within the peritoneal cavity.[1,2,3] It is one of the major complications of decompensated liver disease, along with variceal hemorrhage and hepatic encephalopathy,[1] and is the most common cause of hospitalization in the cirrhotic patient.[4] The development of ascites is a marker of prognosis in liver cirrhosis, as it indicates a reduction in 1- and 5-year survival rates by 15% and 23.5%, respectively.[5] One of the most serious sequelae of ascites is spontaneous bacterial peritonitis (SBP).[4] SBP is the most common source of infection in liver cirrhosis, accounting for approximately 25% of bacterial infections.[6,7] Mortality due to SBP ranges between 30% and 90% within the first year of diagnosis.[8] This article is intended to review the pathogenesis, evaluation, and management of ascites and SBP in the setting of liver cirrhosis.
ASCITES
Pathogenesis of ascites
Ascites may develop from a variety of causes including cirrhosis, malignancy, tuberculosis, Budd–Chiari syndrome, or congestive heart failure (CHF).[9] Liver cirrhosis accounts for nearly 85% of cases of ascites.[1,2,5] In cirrhosis, portal hypertension (PHTN) is the necessary predecessor to the development of ascites. The degree of PHTN is assessed by measuring the hepatic–venous pressure gradient (HVPG), as calculated by subtracting the wedged hepatic pressure from the free hepatic pressure. The threshold HVPG after which fluid retention occurs is above 12 mmHg.[10,11,12]
Alongside portal hypertension, additional changes occur that lead to the development of ascites. Initially, there is dilation and pooling of blood within the splanchnic vessels, leading to a decrease in effective end-arterial volume, which is compensated for by an increase in heart rate and cardiac output.[13,14] However, as liver decompensation worsens, systemic arterial vasodilation occurs in response to several factors, including release of bacterial-derived endotoxin, syntheses of the vasodilator nitric oxide, and altered vascular response to vasoconstricting agents.[15,16,17] This leads to a reduction in systemic vascular resistance to a point where the compensatory rise in cardiac output does not maintain effective end-arterial blood volume. Subsequently, stimulation of baroreceptors occurs, causing activation of the renin–angiotensin–aldosterone (RAA) axis, and leading to retention of sodium and water. Ultimately, the increased volume of blood in the splanchnic vessels leads to elevated hydrostatic pressure and increased capillary permeability, causing leakage of fluid into the peritoneum.[18,19]
In combination with increased hydrostatic pressure, reduction in oncotic pressure also causes ascites fluid accumulation.[20,21] Plasma oncotic pressure, which drives fluid from the interstitial space back into blood vessels, is mediated by albumin, a large negatively charged protein synthesized by the liver.[21] As such, hypoalbuminemia from decompensated liver disease leads to a decrease in oncotic pressure that further promotes fluid leakage from the splanchnic vessels.[20,21] The lymphatic system usually drains excess fluid that accumulates in the interstitial space,[20] but accumulation of greater than 900 mL of fluid into the peritoneal cavity overwhelms the lymphatic system's absorptive capacity, leading to the development of ascites [Figure 1].
Figure 1.
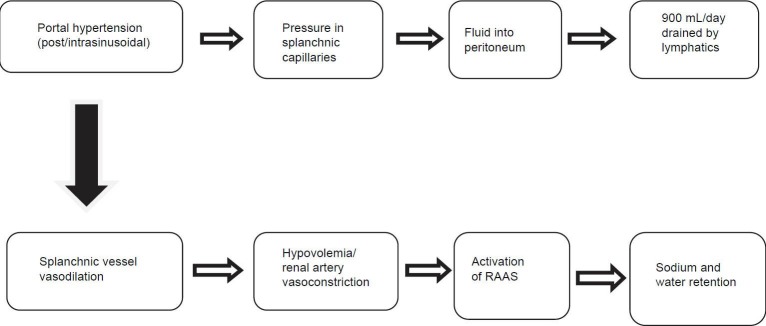
Pathogenesis of ascites
DIAGNOSTIC EVALUATION
History and physical examination
The evaluation of ascites begins with a history and physical examination. The most common presenting symptom is increasing abdominal girth. Other symptoms include weight gain, shortness of breath from diaphragmatic compression, or early satiety. The patient should also be asked about risk factors for other causes of ascites, such as CHF or nephrotic syndrome.[22] A history of pregnancy or hypercoagulable state may predispose to acute portal vein thrombosis or Budd–Chiari syndrome. A patient receiving chemotherapy prior to bone marrow transplantation may have developed sinusoidal obstructive syndrome. Finally, diseases such as hereditary hemorrhagic telangiectasia can cause ascites through development of nodular regenerative hyperplasia and resultant PHTN.
The classic physical examination finding seen in ascites is the presence of flank dullness, which is usually present when patients have more than 1500 mL of fluid within the peritoneal cavity.[23] Patients with flank dullness should also be tested for shifting dullness, which is another common finding that has 83% sensitivity and 56% specificity for detecting ascites.[23] One group of patients who may be hard to assess for ascites on examination are obese patients. These patients may complain of an enlarging abdomen, but the timeline for development of their abdominal girth should be investigated, because ascites usually develops over days to weeks, whereas the obese patient may complain of worsening abdominal distention over months to years.[22]
Imaging
Although the history and physical examination can suggest the presence of ascites, the diagnosis should be confirmed through imaging. A simple, cost-effective modality for confirming ascites is an upper abdominal ultrasound.[23] An ultrasound with venous doppler can also assess for the presence of portal vein or hepatic vein thrombosis or compression.[22]
Abdominal paracentesis
Once the diagnosis of ascites has been established, the next step is an abdominal paracentesis followed by an ascitic fluid analysis.[24,25] Ascitic fluid analysis has the benefit of allowing one to ascertain whether ascites is secondary to PHTN.[26] Complications of this procedure are rare, occurring in approximately 1% of patients, even in patients with significant coagulopathy.[27,28,29] Major complications include ascitic fluid leakage from the point of needle insertion into the skin, bleeding, and infection. Morbidity and mortality due to the paracentesis, however, is rare.[27,28,29,30,31] Contraindications to paracentesis are mainly related to severe coagulopathy with evidence of either DIC or primary fibrinolysis, or in patients with massive ileus who may be at risk for bowel perforation.[22,32]
The ideal location for performing a paracentesis is in the left lower quadrant (LLQ), two fingerbreadths medial and cephalad to the anterior superior iliac spine, because in this location the abdominal wall is thinner.[22,33,34] In contrast to the LLQ, the right lower quadrant is a less ideal location because of the presence of the cecum, which may be dilated in certain circumstances.[33] Additionally, one should avoid placing the paracentesis needle through surgical scars, because of the possibility of bowel being tethered to the skin, and avoid visible blood vessels, due to risk of puncture and hemorrhage. If the fluid is difficult to localize, the use of ultrasonography prior to performing the paracentesis is useful to visualize the fluid location and determine if there is risk of injuring the bowel.
Ascitic fluid analysis
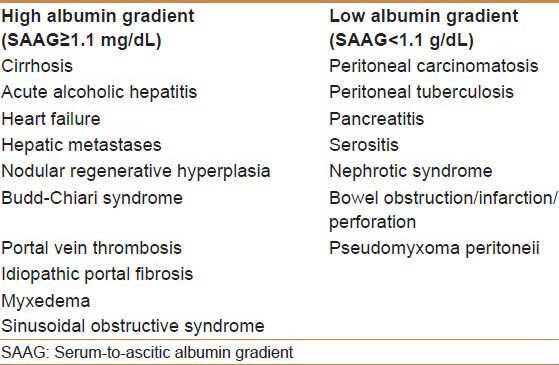
One of the first measures to calculate from the ascitic fluid analysis is the serum-to-ascites albumin gradient (SAAG). The SAAG is measured by subtracting the serum albumin level from the ascites fluid albumin level. A SAAG greater than 1.1, known as high-SAAG ascites, has a sensitivity of 97% in indicating portal-hypertensive ascites.[35,36] As conditions other than cirrhosis, such as heart failure or Budd–Chiari syndrome, can lead to ascites with a SAAG greater than 1.1, ascitic fluid total protein should be tested. An ascites fluid protein of greater than 2.5 g/dL or 25 g/L indicates hepatic vein outflow obstruction from heart failure or Budd–Chiari syndrome.[37] Other tests that may be indicated to determine the etiology of ascites may include fungal culture, acid–fast bacillus smear and culture, and cytology. These tests may be useful but should only be requested when there is high clinical suspicion of tuberculosis, fungal peritonitis, or malignancy, due to increased cost and poor yield. The potential etiologies of high- and low-SAAG ascites are depicted in Table 1.
Table 1.
Differential diagnosis of ascites based on the serum-to-ascitic albumin gradient
Ascitic fluid should additionally be tested for cell count with differential, to assess for the presence of infection. As compared to bacterial cultures, the cell count and differential is a simple test with results available within hours. We recommend routine testing for cell count and differential, even for patients with an established cause of ascites, because infection in the peritoneum can lead to significant morbidity and mortality if not detected and treated early.[38,39]
Management of ascites
Once the diagnosis of ascites due to cirrhosis has been confirmed, treatment should be initiated. Management should be performed carefully, so as not to deplete intravascular volume. The general approach to management of ascites includes two major interventions: Reducing sodium intake and initiation of diuretic therapy. There is no limit to the amount of weight loss when a patient has ascites and concurrent peripheral edema, and even weight loss rates of up to 2 kg per day seem to be well tolerated when edema is present.[40] Patients with ascites but without significant peripheral edema should be restricted to less than 0.75 kg per day of weight loss, to decrease the risk of intravascular fluid depletion.[40] Once peripheral edema and ascites resolve, a daily maximum weight loss value of 0.5 kg is acceptable.[40]
Sodium restriction
The reduction of sodium intake is critical to controlling ascites. Patients are usually counseled to decrease their dietary sodium intake to 2000 mg or 88 mEq per day.[41] Since fluid normally follows sodium passively, fluid restriction is usually not necessary for treatment, though excessive fluid intake should be discouraged. Dietary restrictions can be difficult for patients to achieve, so proper education with the involvement of a dietitian can aid in teaching patients on how to make the necessary diet changes, and written instruction is proven to be helpful in our experience.
Diuretics
Dietary changes alone will only treat a small subset of patients, and the difficulty in maintaining a sodium-restricted diet makes diuretic therapy a requirement for most patients with cirrhotic ascites. The typical diuretic regimen for ascites consists of oral spironolactone, an aldosterone antagonist, combined with furosemide, an ascending loop and distal convoluted tubule diuretic. Oral spironolactone may also be used as monotherapy and has been shown to be more efficacious than furosemide alone, in treating ascites.[42,43] However, this regimen is only used in rare occasions due to the risk of hyperkalemia associated with spironolactone monotherapy. Furthermore, a randomized trial has shown that the combination of spironolactone and furosemide can mobilize ascites faster than monotherapy with either medication.[44] The typical dosing of the combination starts at a ratio of spironolactone to furosemide at 100:40 mg daily. This can be titrated to a maximum dosage of 400 mg of spironolactone and 160 mg of furosemide to achieve the desired effect, assuming the patient can tolerate such dosages. Amiloride and eplerenone are aldosterone antagonists that can be substituted for spironolactone when gynecomastia occurs. Muscle cramps are often seen in patients taking diuretics and can be treated effectively with magnesium oxide supplementation.
Medications to avoid in ascites
Patients with ascites from cirrhosis may have other comorbidities and are usually on multiple medications. Some of these medications may lower survival rates of patients with cirrhosis and ascites. Beta-blockers should be avoided or used with caution in patients with refractory ascites, as data indicate potentially reduced survival in these patients.[45] Nonsteroidal anti-inflammatory drugs are another class of medications that should be avoided because of the risk of gastrointestinal bleeding and precipitation of renal failure in the setting of cirrhosis.[46,47]
Refractory ascites
Ascites that is insensitive to sodium restriction and high-dose diuretic therapy, or intolerant to diuretic therapy due to resultant renal failure, or that recurs rapidly following therapeutic paracentesis is considered to be refractory ascites.[48] Refractory ascites can be further stratified into diuretic resistant, or unresponsive to diuretics, and diuretic-intractable, where the side effects preclude the use or upward titration of diuretics. The prevalence of refractory ascites has been shown to occur in approximately 10% of patients with cirrhotic ascites.[43,49]
Several therapeutic options exist for the treatment of refractory ascites including serial large-volume paracentesis (LVP), transjugular intrahepatic portosystemic shunt (TIPS), peritoneovenous shunt, or liver transplantation. Prior to consideration of these therapeutic options, an effort should be made to improve adverse reactions in patients with diuretic-intractable ascites. For instance, in patients who cannot increase diuretic dosages due to hypotension, potential interventions include discontinuing beta-blockers or other antihypertensive medications and adding oral midodrine, to raise systemic blood pressure.[50] In patients who cannot tolerate diuretics due to severe cramping, symptomatic relief of cramping should be attempted by replenishing electrolytes or prescribing magnesium oxide. In patients with hyponatremia from diuretics, fluid restriction of 1.5 liters per day should be initiated to keep the serum sodium level stable. Vasopressin receptor antagonists (Vaptans) are also under investigation regarding their efficacy in refractory ascites, although research so far has not shown any clear benefit and these medicaitons have a black-box warning regarding the potential for worsening liver failure.[51,52]
Serial LVPs are effective and safe therapeutic options, with less than 1% risk of bleeding.[53] Patients in whom more than 5 L is removed should have albumin (6–8 g/L) administered to avoid the complication of postparacentesis circulatory dysfunction (PCD), a condition of decreased plasma volume, which could lead to renal failure.[53,54,55] Trials involving multiple plasma volume expanders have been conducted with drugs such as albumin, dextran, and polygeline, but albumin has consistently shown to be the most effective medication currently available to avoid PCD.[53,54]
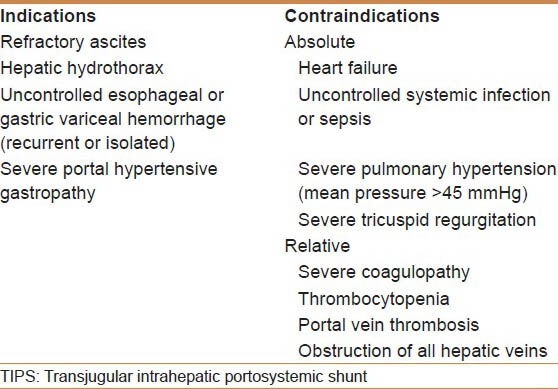
The TIPS procedure is another option available to treat refractory ascites. TIPS is considered when a patient meets certain criteria [Table 2], which can include intolerance of LVP or requiring frequent LVP. Certain criteria also exist, which make patients poor candidates for TIPS, including low cardiac ejection fraction, pulmonary hypertension, or portal vein thrombosis [Table 2]. Therefore, we recommend a cardiac echocardiography and ultrasound with portal vein Doppler in all patients being evaluated for TIPS procedure. Hepatic encephalopathy is one of the most significant complications associated with TIPS, and develops in up to 30% of patients following this procedure, although it can usually be medically managed.[56,57] Primary risk factors for post-TIPS encephalopathy include a prior history of encephalopathy and age greater than 65 years.[57] Other complications of TIPS placement include, stent thrombosis or stenosis, although the advent of coated stents has reduced the rate of these complications.[58] The Model for End-stage Liver Disease (MELD) scoring system is a validated predictor of 3-month mortality after TIPS, and can be used as a tool to assist in the evaluation of candidacy for TIPS placement.[59] We recommend to avoid elective TIPS procedures in patients with a MELD score greater than 18.
Table 2.
Indications and contraindications for TIPS
A peritoneovenous shunt was performed more frequently in the 1970s to treat ascites.[49,60] The procedure today has been virtually abandoned because of the high rate of complications.[49,60,61] The rare indication for this procedure includes patients who are not candidates for TIPS or liver transplantation, and who cannot tolerate serial LVP.[25]
Liver transplantation is the definitive treatment option for patients with refractory ascites. Unfortunately, this option is not always available due to a greater demand for organs than supply, and that many patients are not appropriate candidates for transplantation. Therefore, patients who are on the transplant list must still have their ascites managed while awaiting transplantation.
SPONTANEOUS BACTERIAL PERITONITIS
Pathogenesis
One of the most serious complications associated with ascites is the development of spontaneous bacterial peritonitis (SBP), defined as the presence of >250 polymorphonuclear (PMN) cells/mm3, with positive ascitic fluid bacterial culture, and no evidence of an intra-abdominal infection.[22] SBP is associated with high mortality, as 1-year mortality after the first episode ranges from 30% to 90%.[8] The primary mechanism in the development of SBP is believed to be from bacterial translocation (BT) across the bowel wall.[9,62,63] The mesenteric lymph nodes are the most common site of translocation, and the bacterial species that most commonly translocate include Eschericia coli, Klebsiella pneumoniae, the streptococcal species, and other microorganisms, of the family Enterobacteriaceae.[63,64,65] Patients with decompensated cirrhosis often have a compromised host defense system due to impaired phagocytic activity of neutrophils and macrophages and deficient complement activation.[63] In this setting, bacteria may spread from the lymph nodes to extraintestinal sites such as the bloodstream, lungs or urinary tract, and subsequently seed ascites fluid.
In addition to impaired host defense, additional factors that promote BT include intestinal bacterial overgrowth and increased intestinal permeability.[66] Changes in gut microbiota may promote BT by increasing the prevalence of pathogenic strains of bacteria when testing fecal microbial composition.[67,68] Small intestinal bacterial overgrowth (SIBO), defined as >105 colony-forming units/mL in jejunal aspirate, is present in 20%–60% of patients with cirrhosis and has been linked to SBP development.[69,70,71] Liver cirrhosis often predisposes to SIBO due to a combination of reduced intestinal peristalsis, hypochlorhydria, and altered bile secretion.[67] Evidence has demonstrated that BT occurs in only 0%–11% of patients who do not have SIBO, demonstrating the relevance of SIBO in the pathogenesis of SBP.[63]
Translocation of bacteria through the intestinal mucosa is also due to increased intestinal permeability. Cirrhosis is associated with altered structure and function of the intestinal mucosa.[72,73,74] The mechanism underlying the alteration of intestinal mucosal structure and function are not fully elucidated, but animal studies have attributed it to oxidative stress.[72,73,74] Additionally, a study conducted by Chiva et al. found that cirrhosis affects enterocyte mitochondrial function and increased lipid peroxidation among cirrhotic rats, leading to enterocyte damage from oxidative stress.[72] Another study found increased levels of malondialdehyde, a marker of lipid peroxidation and oxidative stress, in cirrhotic rats.[73] Findings by Teltschik et al. further found that decreased function by the antimicrobial-producing intestinal Paneth cells predisposes to SBP development.[74]
Evaluation
Patients who develop SBP typically have large ascitic fluid volume.[75,76] Clinical manifestations of SBP include fever, abdominal pain, and hepatic encephalopathy, although these symptoms may not always be present.[64] Diagnostic paracentesis should be performed prior to the administration of antibiotics. This is necessary because even a single dose of a broad-spectrum antibiotics within 6 h prior to a paracentesis, reduces the yield of bacterial cultures in 86% of cases.[77] When the PMN count of the ascitic fluid is greater than 250 cells/mm3, the diagnosis of SBP can be made.[78] Of note, although gram-negative bacteria often cause sufficient PMN elevation to make the diagnosis of SBP, gram-positive bacteria may not cause such an increase in ascites fluid PMNs.[79] Therefore, we recommend maintaining a high index for suspecting SBP in patients with ascites who clinically appear infected, even if the ascites fluid analysis is inconsistent with SBP.
Along with an ascitic fluid analysis, bacterial culture should be sent to determine the specific bacterial species causing infection. Prompt inoculation of the ascitic fluid into blood culture bottles at the bedside will improve the yield of these cultures. However, even if the culture is negative, treatment for SBP should be administered if the ascitic fluid analysis has shown a sufficiently elevated PMN count.[80] Due to the slow growing nature of many bacterial species involved in SBP, alternative methods of testing for bacterial DNA have been developed to allow for a more rapid diagnosis.[81,82,83] However, these techniques have not been validated, are often not commercially available, and require further investigation regarding cost-effectiveness.
In patients at risk for secondary peritonitis from bowel perforation or infection of other intra-abdominal organs, we recommend checking for an elevated ascitic fluid lactate dehydrogenase, or a decreased ascites glucose level. A gram-stain of the ascitic fluid is a simple method to differentiate SBP from secondary peritonitis, as multiple organisms seen on gram-stain is inconsistent with SBP. Other potentially useful markers include ascites fluid alkaline phosphatase (ALP) and carcinoembryonic antigen (CEA).[84] An ALP > 240 U/L or CEA > 5 ng/mL may reflect secondary peritonitis of secondary origin in 80% of cases.[84]
Treatment and prophylaxis
The treatment of SBP initially starts with empiric antibiotic therapy. In patients with suspected SBP, broad-spectrum antibiotics should be administered once a diagnostic paracentesis has been performed. The antibiotics commonly used are third-generation cephalosporins, specifically ceftriaxone dosed at 2 g daily, and or cefotaxime dosed at 2 g three times daily. In one study, cefotaxime proved to be superior when compared to a combination therapy of ampicillin and tobramycin.[85] Another acceptable antibiotic group are the fluoroquinolones, such as ciprofloxacin and ornorfloxacin, which may be particularly useful when the patient has a penicillin allergy. It should be noted that patients who are on fluoroquinolone prophylaxis should not receive a fluoroquinolone for treatment, since the bacteria causing infection may have developed resistance to this class of medications. In this subset of patients where fluoroquinolone resistance may have developed, cefotaxime has still proven to be effective.[86] In cases where bacteria are resistant to cefotaxime and fluoroquinolones, the use of carbapenems has been suggested as an alternative.[87,88]
A short duration of antibiotic therapy proved to be as effective as a longer-term course. One study in which 90 patients with SBP were randomized to a 5- or 10-day courses of cefotaxime, similar cure rates of 93% to 91%, respectively, were seen.[89] We therefore recommend either intravenous ceftriaxone or cefotaxime for a total of 5 days.
In addition to antibiotics, it is imperative to provide intravenous albumin. A reduction of mortality secondary to hepatorenal syndrome of more than 15% has been reported when albumin was used.[90] Dosage of albumin should be at 1.5 gm/kg of patient body weight on day 1 of treatment and 1 g/kg of patient body weight on day 3 of SBP treatment.[90]
Prophylactic antibiotics for SBP prevention should be given to all patients who have had a previous diagnosis of SBP.[91,92,93,94,95] Antibiotic options include norfloxacin, trimethoprim–sulfomethoxazole, and ciprofloxacin. Oral norfloxacin 400 mg or trimethoprim–sulfomethoxazole one tablet double strength daily is commonly used in the outpatient setting.[93,94,95,96,97,98] Dosages of ciprofloxacin 750 mg weekly is an alternative option for SBP prophylaxis. As there is a greater risk for development of bacterial resistance, we recommend the use of daily dosing over intermittent dosing.
Primary prophylaxis with a quinolone has also been suggested as a method in preventing the occurrence of SBP.[63,99,100,101,102] Primary prophylaxis can be considered for certain patients with low protein ascites (<1.5 mg/dL).[22] A meta-analysis demonstrated significant preventative benefit of SBP prophylaxis in such patients with numbers needed to treat to prevent an episode of SBP of 8.4 at 6 months and 6.3 at 12 months.[63] One study in particular demonstrated that patients with severe liver disease, defined as serum bilirubin ≥ 3 mg/dL and Child score ≥ 9, or renal dysfunction, defined as creatinine ≥ 1.2 mg/dL, BUN ≥ 25 mg/dL or sodium ≤ 130 mEq/L, along with low-protein ascites, found that the 1 year probability of SBP in this high-risk group was reduced from 61% to 7%, with administration of primary prophylaxis.[100] Although antibiotic prophylaxis can be helpful, prolonged use of antibiotics can lead to the emergence of resistant bacterial strains. For instance, the use of norfloxacin for primary prevention has been shown to be most effective in the first 3 months of treatment followed by a gradual decrease in efficacy over time.[100,102] Considering these findings, which indicate a risk of developing infection with antibiotic resistant bacteria, we do not recommend routine use of primary prophylaxis for SBP even in the patients who have severe liver disease.
Footnotes
Source of Support: Nil
Conflict of Interest: The opinions and assertions contained herein are the sole views of the authors and are not to be construed as official or as reflecting the views of the Cedars-Sinai Medical Center or Temple University Health System.
REFERENCES
- 1.Gines P, Quintero E, Arroyo V, Teres J, Bruguera M, Rimola A, et al. Compensated Cirrhosis: Natural history and prognostic factors. Hepatology. 1987;7:122–8. doi: 10.1002/hep.1840070124. [DOI] [PubMed] [Google Scholar]
- 2.Guevara M, Cárdenas A, Uriz J, Ginès P. Prognosis in patients with cirrhosis and ascites. In: Gines P, Arroyo V, Rodes J, Schrier RW, editors. Ascites and renal dysfunction in liver disease: Pathogenesis, diagnosis and treatment. Mal-den: Blackwell; 2005. pp. 260–70. [Google Scholar]
- 3.Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–8. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Lucena MI, Andrade RJ, Tognoni G, Hidalgo R, De La Cuesta FS. Spanish Collaborative Study Group on Therapeutic Management in Liver Disease Multicenter hospital study on prescribing patterns for prophylaxis and treatment of complications of cirrhosis. Eur J Clin Pharmacol. 2002;58:435–40. doi: 10.1007/s00228-002-0474-1. [DOI] [PubMed] [Google Scholar]
- 5.Planas R, Montoliu S, Ballest B, Rivera M, Miguel M, Masnou H, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol. 2006;4:1385–94. doi: 10.1016/j.cgh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Navasa M, Rodés J. Bacterial infections in cirrhosis. Liver Int. 2004;24:277–80. doi: 10.1111/j.1478-3231.2004.0934.x. [DOI] [PubMed] [Google Scholar]
- 7.Vilstrup H. Cirrhosis and bacterial infections. Rom J Gastroenterol. 2003;12:297–302. [PubMed] [Google Scholar]
- 8.Fernandez J, Navasa M, Gomez J, Colmenero J, Vila J, Arroyo V, et al. Bacterial Infections in cirrhosis: Epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–8. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 9.Moore CM, Van Theil DH. Cirrhotic Ascites Review: Pathophysiology, diagnosis and management. World J Hepatol. 2013;5:251–63. doi: 10.4254/wjh.v5.i5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gines P, Fernandez-Esparrach G, Arroyo V, Rodes J. Pathogenesis of Ascites in Cirrhosis. Semin Liver Dis. 1997;17:175–89. doi: 10.1055/s-2007-1007196. [DOI] [PubMed] [Google Scholar]
- 11.Morali GA, Sniderman KW, Deitel KM, Tobe S, Witt-Sullivan H, Simon M, et al. Is sinusoidal portal hypertension a necessary factor for the development of hepatic ascites. J Hepatol. 1992;16:249–50. doi: 10.1016/s0168-8278(05)80128-x. [DOI] [PubMed] [Google Scholar]
- 12.Pinzani M, Rosselli M, Zuckermann M. Liver Cirrhosis. Best Pract Res Clin Gastroenterol. 2011;25:281–90. doi: 10.1016/j.bpg.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Abelmann WH. Hyperdynamic circulation in cirrhosis: A historical perspective. Hepatology. 1994;20:1356–8. [PubMed] [Google Scholar]
- 14.Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46:927–34. doi: 10.1016/j.jhep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Sola E, Gines P. Renal and Circulatory dysfunction in cirrhosis: Current management and future perspectives. J Hepatol. 2010;53:1135–45. doi: 10.1016/j.jhep.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Frances R, Munoz C, Zapater P, Uceda F, Gascón I, Pascual S, et al. Bacterial DNA activates cell mediated immune response and nitric oxide overproduction in peritoneal macrophages from patients with cirrhosis and ascites. Gut. 2004;53:860–4. doi: 10.1136/gut.2003.027425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angus PW. Role of Endothelin in systemic and portal resistance in cirrhosis. Gut. 2006;55:1230–2. doi: 10.1136/gut.2005.088633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korthius RJ, Kinden DA, Brimer GE, Slattery KA, Stogsdill P, Granger DN. Intestinal capillary filtration in acute and chronic portal hypertension. Am J Physiol. 1988;254:G339–45. doi: 10.1152/ajpgi.1988.254.3.G339. [DOI] [PubMed] [Google Scholar]
- 19.Henriksen JH, Lassen NA, Parving HH, Winkler K. Filtration as the main transport mechanism of protein exchange between plasma and the peritoneal cavity in hepatic cirrhosis. Scand J Clin Lab Invest. 1980;40:503–13. doi: 10.3109/00365518009091957. [DOI] [PubMed] [Google Scholar]
- 20.Chung C, Iwakiri Y. The lymphatic vascular system in liver diseases: Its role in ascites formation. Clin Mol Hepatol. 2013;19:99–104. doi: 10.3350/cmh.2013.19.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong F. Management of ascites in cirrhosis. J Gastroenterol Hepatol. 2012;27:11–20. doi: 10.1111/j.1440-1746.2011.06925.x. [DOI] [PubMed] [Google Scholar]
- 22.Runyon BA AASLD Practice Guidelines Committee. Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. Hepatology. 2013;57:2087–107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 23.Cattau EI, Jr, Benjamin SB, Knuff TE, Castell DO. The accuracy of the physcial exam in the diagnosis of suspected ascites. JAMA. 1982;247:1164–66. [PubMed] [Google Scholar]
- 24.Runyon BA. Care of patients with ascites. N Engl J Med. 1994;330:337–42. doi: 10.1056/NEJM199402033300508. [DOI] [PubMed] [Google Scholar]
- 25.Runyon BA. Ascites and spontaneous bacterial peritonitis. In: Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger and Fordtran's Gastrointestinal and Liver Disease. 9th ed. Philadelphia: Saunders Elsevier; 2010. pp. 1517–41. [Google Scholar]
- 26.Borzio M, Salerno F, Piantoni L, Cazzaniga M, Angeli P, Bissoli F, et al. Bacterial infection in patients with advanced cirrhosis: A multicentre prospective study. Dig Liver Dis. 2001;33:41–8. doi: 10.1016/s1590-8658(01)80134-1. [DOI] [PubMed] [Google Scholar]
- 27.McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31:164–71. doi: 10.1046/j.1537-2995.1991.31291142949.x. [DOI] [PubMed] [Google Scholar]
- 28.Runyon BA. Paracentesis of ascitic fluid: A safe procedure. Arch Intern Med. 1986;146:2259–61. [PubMed] [Google Scholar]
- 29.Grabau CM, Crago SF, Hoff LK, Simon JA, Melton CA, Ott BJ, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40:484–8. doi: 10.1002/hep.20317. [DOI] [PubMed] [Google Scholar]
- 30.Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21:525–9. doi: 10.1111/j.1365-2036.2005.02387.x. [DOI] [PubMed] [Google Scholar]
- 31.De Gottardi A, Thevenot T, Spahr L, Morard I, Bresson-Hadni S, Torres F, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: A prospective study. Clin Gastroenterol Hepatol. 2009;7:906–9. doi: 10.1016/j.cgh.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Gunawan B, Runyon BA. The efficacy and safety of epsilon-aminocaproic acid treatment in patients with cirrhosis and hyperfibrinolysis. Aliment Pharmacol Ther. 2006;23:115–20. doi: 10.1111/j.1365-2036.2006.02730.x. [DOI] [PubMed] [Google Scholar]
- 33.Thomsen TW, Shaffer RW, White B, Setnik GS. Videos in clinical medicine: Paracentesis. N Engl J Med. 2006;355:e21. doi: 10.1056/NEJMvcm062234. [DOI] [PubMed] [Google Scholar]
- 34.Sakai H, Sheer TA, Mendler MH, Runyon BA. Choosing the location for non-image guided abdominal paracentesis. Liver Int. 2005;25:984–6. doi: 10.1111/j.1478-3231.2005.01149.x. [DOI] [PubMed] [Google Scholar]
- 35.Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, Mchutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215–20. doi: 10.7326/0003-4819-117-3-215. [DOI] [PubMed] [Google Scholar]
- 36.Mauer K, Manzione NC. Usefulness of serum-ascites albumin difference in separating transudative from exudative ascites: Another look. Dig Dis Sci. 1988;33:1208–12. doi: 10.1007/BF01536667. [DOI] [PubMed] [Google Scholar]
- 37.Runyon BA. Cardiac Ascites: A characterization. J Clin Gastroenterol. 1988;10:410–2. doi: 10.1097/00004836-198808000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Jeffries MA, Stern MA, Gunaratnum NT, Fontana RJ. Unsuspected infection is infrequent in asymptomatic outpatients with refractory ascites undergoing therapeutic paracentesis. Am J Gastroenterol. 1999;94:2972–6. doi: 10.1111/j.1572-0241.1999.01445.x. [DOI] [PubMed] [Google Scholar]
- 39.Evans LT, Kim WR, Poterucha JJ, Kamath PS. Spontaneous bacterial peritonitis in asymptomatic outpatients with cirrhotic ascites. Hepatology. 2003;37:897–901. doi: 10.1053/jhep.2003.50119. [DOI] [PubMed] [Google Scholar]
- 40.Pockros PJ, Reynolds TB. Rapid diuresis in patients with ascites from chronic liver disease: The importance of peripheral edema. Gastroenterology. 1986;90:1827–33. doi: 10.1016/0016-5085(86)90249-0. [DOI] [PubMed] [Google Scholar]
- 41.Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, et al. The management of ascites in cirrhosis: Report on the consensus conference of the international Ascites club. Hepatology. 2003;38:258–66. doi: 10.1053/jhep.2003.50315. [DOI] [PubMed] [Google Scholar]
- 42.Fogel MR, Sawhney VK, Neal EA, Miller RG, Knauer CM, Gregory PB. Diuresis in the ascitic patient: A randomized controlled trial of three regimens. J Clin Gastroenterol. 1981;3(Suppl 1):S73–80. doi: 10.1097/00004836-198100031-00016. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Ayuso RM, Arroyo V, Planas R, Gaya J, Bory F, Rimola A, et al. Randomized comparative study of efficacy of furosemide versus spironolactone in nonazotemic cirrhosis with ascites. Relationship between the diuretic response and the activity of the renin-aldosterone system. Gastroenterology. 1983;84:961–8. [PubMed] [Google Scholar]
- 44.Angeli P, Fasolato S, Mazza E, Okolicsanyi L, Maresio G, Velo E, et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: Results of an open randomised clinical trial. Gut. 2010;59:98–104. doi: 10.1136/gut.2008.176495. [DOI] [PubMed] [Google Scholar]
- 45.Sersté T, Melot C, Francoz C, Durand F, Rautou PE, Valla D, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010;52:1017–22. doi: 10.1002/hep.23775. [DOI] [PubMed] [Google Scholar]
- 46.Arroyo V, Gines P, Rimola A, Gaya J. Renal function abnormalities, prostaglandins, and effects of nonsteroidal anti-inflammatory drugs in cirrhosis with ascites. An overview with emphasis on pathogenesis. Am J Med. 1986;81:104–22. doi: 10.1016/0002-9343(86)90912-5. [DOI] [PubMed] [Google Scholar]
- 47.de Lédinghen V, Mannant PR, Foucher J, Perault MC, Barrioz T, Ingrand P, et al. Non-steroidal anti-inflammatory drugs and variceal bleeding: A case-control study. J Hepatol. 1996;24:570–3. doi: 10.1016/s0168-8278(96)80142-5. [DOI] [PubMed] [Google Scholar]
- 48.Arroyo V, Gines P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Hepatology. 1996;23:164–76. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 49.Stanley MM, Ochi S, Lee KK, Nemchausky BA, Greenlee HB, Allen JI, et al. Peritoneovenous shunting compared with medical treatment in patients with alcoholic cirrhosis and massive ascites. N Engl J Med. 1989;321:1632–8. doi: 10.1056/NEJM198912143212403. [DOI] [PubMed] [Google Scholar]
- 50.Singh V, Dhungana SP, Singh B, Vijayverghia R, Nain CK, Sharma N, et al. Midodrine in patients with cirrhosis and refractory or recurrent ascites: A randomized pilot study. J Hepatol. 2012;56:348–54. doi: 10.1016/j.jhep.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 51.Dahl E, Gluud LL, Kimer N, Krag A. Meta-analysis: The safety and efficacy of vaptans (tolvaptan, satavaptan and lixivaptan) in cirrhosis with ascites or hyponatraemia. Aliment Pharmacol Ther. 2012;36:619–26. doi: 10.1111/apt.12025. [DOI] [PubMed] [Google Scholar]
- 52.Wong F, Watson H, Gerbes A, Vilstrup H, Badalamenti S, Bernardi M, et al. Satavaptan for the management of ascites in cirrhosis: Efficacy and safety across the spectrum of ascites severity. Gut. 2012;61:108–16. doi: 10.1136/gutjnl-2011-300157. [DOI] [PubMed] [Google Scholar]
- 53.Ginès A, Fernández-Esparrach G, Monescillo A, Vila C, Domènech E, Abecasis R, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. 1996;111:1002–10. doi: 10.1016/s0016-5085(96)70068-9. [DOI] [PubMed] [Google Scholar]
- 54.Planas R, Gines P, Arroyo V, Llach J, Panes J, Vargas V, et al. Dextran 70 versus albumin as plasma expanders in cirrhotic patients with tense ascites treated with total paracentesis: Results of a randomized study. Gastroenterology. 1990;99:1736–44. doi: 10.1016/0016-5085(90)90481-f. [DOI] [PubMed] [Google Scholar]
- 55.Ruiz-del-Arbol L, Monescillo A, Jimenez W, Garcia-Plaza A, Arroyo V, Rodés J. Paracentesis induced circulatory dysfunction: Mechanisms and the effect on hepatic hemodynamics in cirrhosis. Gastroenterology. 1997;113:579–86. doi: 10.1053/gast.1997.v113.pm9247479. [DOI] [PubMed] [Google Scholar]
- 56.Martinet JP, Fenyves D, Legault L, Roy L, Dufresne MP, Spahr L, et al. Treatment of refractory ascites using transjugular intrahepatic portosystemic shunt (TIPS): A caution. Dig Dis Sci. 1997;42:161–6. doi: 10.1023/a:1018861827399. [DOI] [PubMed] [Google Scholar]
- 57.Sanyal AJ, Freedman AM, Shiffman ML, Purdum PP, 3rd, Luketic VA, Cheatham AK. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: Results of a prospective controlled study. Hepatology. 1994;20:46–55. doi: 10.1016/0270-9139(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 58.Bureau C, Garcia-Pagan JC, Otal P, Pomier-Layrargues G, Chabbert V, Cortez C, et al. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: Results of a randomized study. Gastroenterology. 2004;126:469–75. doi: 10.1053/j.gastro.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 59.Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–71. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 60.Gines P, Arroyo V, Vargas V, Planas R, Casafont F, Panes J, et al. Paracentesis with intravenous infusion of albumin as compared with peritoneovenous shunting in cirrhosis with refractory ascites. N Engl J Med. 1991;325:829–35. doi: 10.1056/NEJM199109193251201. [DOI] [PubMed] [Google Scholar]
- 61.Rosemurgy AS, Zervos EE, Clark WC, Thometz DP, Black TJ, Zwiebel BR, et al. TIPS versus peritoneovenous shunt in the treatment of medically intractable ascites: A prospective randomized trial. Ann Surg. 2004;239:883–9. doi: 10.1097/01.sla.0000128309.36393.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Preda CM, Ghita R, Ghita C, Mindru C, Vlaciu L, Andrei A, et al. A retrospective study of bacterial infections in cirrhosis. Maedica (Buchar) 2011;6:185–92. [PMC free article] [PubMed] [Google Scholar]
- 63.Wiest R, Krag A, Gerbes A. Spontaneous bacterial peritonitis: Recent guidelines and beyond. Gut. 2012;61:297–310. doi: 10.1136/gutjnl-2011-300779. [DOI] [PubMed] [Google Scholar]
- 64.McHutchison JG, Runyon BA. Spontaneous Bacterial Peritoniti. In: Surawicz CM, Owen RL, editors. Gastrointestinal and Hepatic Infections. Philadelphia: WB Saunders; 1995. p. 455. [Google Scholar]
- 65.Wells CL. Relationship between intestinal microecology and the translocation of intestinal bacteria. Antonie Van Leeuwenhoek. 1990;58:87–93. doi: 10.1007/BF00422722. [DOI] [PubMed] [Google Scholar]
- 66.Weist R, Garcia-Tsao G. Bacterial Translocation (BT) in cirrhosis. Hepatology. 2005;41:422–33. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–72. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 68.Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bauer TM, Steinbruckner B, Brinkmann FE, Ditzen AK, Schwacha H, Aponte JJ, et al. Small intestinal bacterial overgrowth in patients with cirrhosis: Prevalence and relation with spontaneous bacterial peritonitis. Am J Gasteroenterol. 2001;96:2962–7. doi: 10.1111/j.1572-0241.2001.04668.x. [DOI] [PubMed] [Google Scholar]
- 70.Guarner C, Runyon BA, Young S, Heck M, Sheikh MY. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J Hepatol. 1997;26:1372–8. doi: 10.1016/s0168-8278(97)80474-6. [DOI] [PubMed] [Google Scholar]
- 71.Pardo A, Bartoli R, Lorenzo-Zuniga V, Planas R, Vinado B, Riba J, et al. Effect of cisapride on intestinal bacterial overgrowth and bacterial translocation in cirrhosis. Hepatology. 2000;31:858–63. doi: 10.1053/he.2000.5746. [DOI] [PubMed] [Google Scholar]
- 72.Chiva M, Guarner C, Peralta C, Llovet T, Gomez G, Soriano G, et al. Intestinal mucosal oxidative damage and bacterial translocation in cirrhotic rats. Eur J Gastroenterol Hepatol. 2003;15:145–50. doi: 10.1097/00042737-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 73.Ramachandran A, Prabhu R, Thomas S, Reddy JB, Pulimood A, Balasubramanian KA. Intestinal mucosal alterations in experimental cirrhosis in the rat: Role of oxygen free radicals. Hepatology. 2002;35:622–9. doi: 10.1053/jhep.2002.31656. [DOI] [PubMed] [Google Scholar]
- 74.Teltschik Z, Wiest R, Beisner J, Nuding S, Hofmann C, Schoelmerich J, et al. Intestinal bacterial translocation in cirrhotic rats is related to compromised Paneth cell antimicrobial host defence. Hepatology. 2012;55:1154–63. doi: 10.1002/hep.24789. [DOI] [PubMed] [Google Scholar]
- 75.Runyon BA. Bacterial infections in patients with cirrhosis. J Hepatol. 1993;18:271–2. doi: 10.1016/s0168-8278(05)80267-3. [DOI] [PubMed] [Google Scholar]
- 76.Such J, Runyon BA. Spontaneous bacterial peritonitis. Clin Infect Dis. 1998;27:669–74. doi: 10.1086/514940. [DOI] [PubMed] [Google Scholar]
- 77.Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology. 1990;98:127–33. doi: 10.1016/0016-5085(90)91300-u. [DOI] [PubMed] [Google Scholar]
- 78.Runyon BA, Antillon MR. Ascitic fluid pH and lactate: Insensitive and nonspecific tests in detecting ascitic fluid infection. Hepatology. 1991;13:929–35. [PubMed] [Google Scholar]
- 79.Yang CY, Liaw YF, Chu CM, Sheen IS. White count, pH and lactate in ascites in the diagnosis of spontaneous bacterial peritonitis. Hepatology. 1985;5:85–90. doi: 10.1002/hep.1840050118. [DOI] [PubMed] [Google Scholar]
- 80.Hoefs JC, Canawati HN, Sapico FL, Hopkins RR, Weiner J, Montogomerie JZ. Spontaneous bacterial peritonitis. Hepatology. 1982;2:399–407. doi: 10.1002/hep.1840020402. [DOI] [PubMed] [Google Scholar]
- 81.Bruns T, Sachse S, Straube E, Assefa S, Herrmann A, Hagel S, et al. Identification of bacterial DNA in neutrocytic and non-neutrocytic cirrhotic ascites by means of a multiplex polymerase chain reaction. Liver Int. 2009;29:1206–14. doi: 10.1111/j.1478-3231.2009.02073.x. [DOI] [PubMed] [Google Scholar]
- 82.Frances R, Benlloch S, Zapater P, Gonzalez JM, Lozano B, Munoz C, et al. A sequential study of serum bacterial DNA in patients with advanced cirrhosis and ascites. Hepatology. 2004;39:484–91. doi: 10.1002/hep.20055. [DOI] [PubMed] [Google Scholar]
- 83.Frances R, Zapater P, Gonzalez-Navajas JM, Munoz C, Cano R, Moreu R, et al. Bacterial DNA in patients with cirrhosis and noninfected ascites mimics the soluble immune response established in patients with spontaneous bacterial peritonitis. Hepatology. 2008;47:978–85. doi: 10.1002/hep.22083. [DOI] [PubMed] [Google Scholar]
- 84.Wu SS, Lin OS, Chen YY, Hwang KL, Soon MS, Keeffe EB. Ascitic fluid carcinoembryonic antigen and alkaline phosphatase levels for the differentiation of primary from secondary bacterial peritonitis with intestinal perforation. J Hepatol. 2001;34:215–21. doi: 10.1016/s0168-8278(00)00039-8. [DOI] [PubMed] [Google Scholar]
- 85.Felisart J, Rimola A, Arroyo V, Perez-Ayuso RM, Quintero E, Gines P, et al. Cefotaxime is more effective than is ampicillin-tobramycin in cirrhotics with severe infections. Hepatology. 1985;5:457–62. doi: 10.1002/hep.1840050319. [DOI] [PubMed] [Google Scholar]
- 86.Llovet JM, Rodríguez-Iglesias P, Moitinho E, Planas R, Bataller R, Navasa M, et al. Spontaneous bacterial peritonitis in patients with cirrhosis undergoing selective intestinal decontamination. A retrospective study of 229 spontaneous bacterial peritonitis episodes. J Hepatol. 1997;26:88–95. doi: 10.1016/s0168-8278(97)80014-1. [DOI] [PubMed] [Google Scholar]
- 87.Fernandez J, Acevedo J, Castro M, Garcia O, Rodriguez de Lope C, et al. Prevalence and risk factors of infections by multi-resistant bacteria in cirrhosis: A prospective study. Hepatology. 2012;55:1551–61. doi: 10.1002/hep.25532. [DOI] [PubMed] [Google Scholar]
- 88.Castellote J, Ariza X, Girbau A, Salord S, Vazquez X, Labaton T, et al. Antibiotic-resistant bacteria in spontaneous bacterial peritonitis. Is it time to change? J Hepatol. 2010;52:S69. [Google Scholar]
- 89.Runyon BA, McHutchison JG, Antillon MR, Akriviadis EA, Montano AA. Short-course versus long-course antibiotic treatment of spontaneous bacterial peritonitis. A randomized controlled study of 100 patients. Gastroenterology. 1991;100:1737–42. doi: 10.1016/0016-5085(91)90677-d. [DOI] [PubMed] [Google Scholar]
- 90.Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403–9. doi: 10.1056/NEJM199908053410603. [DOI] [PubMed] [Google Scholar]
- 91.Runyon BA. Low-Protein-Concentration ascitic fluid is predisposed to spontaneous bacterial peritonitis. Gastroenterology. 1986;91:1343–6. doi: 10.1016/0016-5085(86)90185-x. [DOI] [PubMed] [Google Scholar]
- 92.Soriano G, Teixedo M, Guarner C, Such J, Barrios J, Enriquez J, et al. Selective intestinal decontamination prevents spontaneous bacterial peritonitis. Gastroenterology. 1991;100:477–81. doi: 10.1016/0016-5085(91)90219-b. [DOI] [PubMed] [Google Scholar]
- 93.Gines P, Rimola A, Planas R, Vargas V, Marco F, Almela M, et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: Results of a double-blind, placebo-controlled trial. Hepatology. 1990;12:716–24. doi: 10.1002/hep.1840120416. [DOI] [PubMed] [Google Scholar]
- 94.Fernandez J, Ruiz del Arbol L, Gomez C, Durandez R, Serradilla R, Guarner C, et al. Norfloxacinvs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology. 2006;131:1049–56. doi: 10.1053/j.gastro.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 95.Singh N, Gayowski T, Yu VL, Wagener MM. Trimethoprim-Sulfamethoxazole for the prevention of spontaneous bacterial peritonitis in cirrhosis: A randomized trial. Ann Intern Med. 1995;122:595–8. doi: 10.7326/0003-4819-122-8-199504150-00007. [DOI] [PubMed] [Google Scholar]
- 96.Rolachon A, Cordier L, Bacq Y, Nousbaum JB, Franza A, Paris JC, et al. Ciprofloxacin and long-term prevention of spontaneous bacterial peritonitis: Results of a prospective controlled trial. Hepatology. 1995;22:1171–4. doi: 10.1016/0270-9139(95)90626-6. [DOI] [PubMed] [Google Scholar]
- 97.Saab S, Hernandez JC, Chi AC, Tong MJ. Oral antibiotic prophylaxis reduces spontaneous bacterial peritonitis occurrence and improves short-term survival in cirrhosis: A meta-analysis. Am J Gastroenterol. 2009;104:993–1001. doi: 10.1038/ajg.2009.3. [DOI] [PubMed] [Google Scholar]
- 98.Loomba R, Wesley R, Bain A, Csako G, Pucino F. Role of fluoroquinolones in the primary prophylaxis of spontaneous bacterial peritonitis: Meta-analysis. Clin Gastroenterol Hepatol. 2009;7:487–93. doi: 10.1016/j.cgh.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Terg R, Fassio E, Guevara M, Cartier M, Longo C, Lucero R, et al. Ciprofloxacin in primary prophylaxis of spontaneous bacterial peritonitis: A randomized, placebo-controlled study. J Hepatol. 2008;48:774–9. doi: 10.1016/j.jhep.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 100.Fernandez J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818–24. doi: 10.1053/j.gastro.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 101.Grange JD, Roulot D, Pelletier G, Pariente FA, Denis J, Ink O, et al. Norfloxacin primary prophylaxis of bacterial infections in cirrhotic patients with ascites: A double-blind randomized trial. J Hepatol. 1998;29:430–6. doi: 10.1016/s0168-8278(98)80061-5. [DOI] [PubMed] [Google Scholar]
- 102.Runyon BA. A pill a day can improve survival in patients with advanced cirrhosis. Gastroenterology. 2007;133:1029–31. doi: 10.1053/j.gastro.2007.07.017. [DOI] [PubMed] [Google Scholar]


