Abstract
Background and Aim:
Ischemia/reperfusion (I/R) injury in the liver occurs after a prolonged period of ischemia followed by restoration of hepatic blood perfusion. During the surgery of abdominal aorta, I/R injury causes damage to lower extremities and many organs, especially liver. The antioxidant and tumor necrosis factor-alpha (TNF-α) suppression effects of topiramate (TPM) have been reported in several studies. We evaluated the potential protective effect of TPM on cellular damage in liver tissue during I/R injury.
Materials and Methods:
Thirty male Wistar albino rats were divided into three groups: Control, I/R, and I/R plus TPM (I/R + TPM) groups. Laparotomy without I/R injury was performed in the control group. After laparotomy, cross-ligation of infrarenal abdominal aorta was applied for 2 h in I/R groups that was followed by 2 h of reperfusion. TPM (100 mg/kg/day) was orally administrated to the animals in the I/R + TPM group for seven consecutive days before I/R procedure.
Results:
The I/R group's TNF-α and interleukin-6 (IL-6) levels were significantly higher than those of the control (P = 0.010; P = 0.002) and I/R + TPM groups (P = 0.010; P = 0.002, respectively). Asymmetric dimethyl arginine (ADMA) levels of I/R group were higher than the control (P = 0.015) and I/R + TPM groups. I/R caused serious histopathological damage to liver tissue; however, TPM led to very low histopathological changes.
Conclusion:
Our data demonstrated that TPM treatment prominently decreases the severity of liver I/R injury. TPM pretreatment may have preventive effects on liver injury via I/R during intra-abdominal surgery.
Keywords: Asymmetric dimethyl arginine, carbonic anhydrase II, ischemia reperfusion, liver, topiramate
The liver is the main organ having the key enzymes that regulate oxidative stress.[1,2] Ischemia/reperfusion (I/R) injury in the liver occurs after a prolonged period of ischemia followed by restoration of hepatic blood perfusion.[3] During the surgery of abdominal aorta, I/R injury causes damage not only to lower extremities but also to many organs and tissues, including lungs, kidneys, heart, and especially liver.[4] Various pathologic responses are involved in liver I/R injury, including oxidative stress, inflammation, and mitochondrial damage.[5] The other pathologic responses are Kupffer cell activation, endothelial cell damage, abnormal release of nitric oxide (NO), vasoconstriction, and inadequate vasodilatation due to diminished NO.[6,7] Hepatic I/R triggers a cascade of inflammatory mediators. Activated Kupffer cells release pro-inflammatory cytokines, including tumor necrosis factor (TNF-a) and interleukin (IL-6). These cytokines increase the inflammatory responses in the I/R liver, which results in hepatocellular dysfunction due to hepatic necrosis and apoptosis.[8]
Topiramate (TPM) is a sulfamate-substituted monosaccharide drug that is used to treat epilepsy and migraine.[9] TPM is an effective antioxidant that has been demonstrated to decrease TNF-α and oxidative stress.[10,11,12] Arginase I is a cytosolic enzyme found mainly in the liver. It is the key enzyme in urea cycle and has arginine as substrate.[13] It competes with NO synthetase (NOS) enzyme for arginine.[14] So inhibition of arginase leads to the elevation of NO level, on the contrary its overexpression leads to reduction of NO level, which increases asymmetric dimethyl arginine (ADMA).[15,16] Carbonic anhydrase (CA)-II is a zinc metalloenzyme, which reversibly catalyzes the hydration reaction of CO2 to form carbonic acid (H2CO3).[17] This enzyme is found in many tissues, including liver. Overexpression of CA-II may lead to the formation of carbonate radicals.[18] A previous study has shown TPM to be a very potent inhibitor of human CA-II.[19]
In this study, we aimed to evaluate whether TPM prevents cellular damage during I/R and whether it has an effect on arginase-I and CA-II enzymes.
MATERIALS AND METHODS
In this study, we used 30 Wistar albino male rats weighing 250–300 g (12–15 weeks old). The rats were divided into three groups: An I/R group (n = 10), a TPM-preconditioned I/R group (n = 10), and a control group (n = 10). The study was approved by the institutional Ethics Committee (approval numbers: 2012/19)
Rats included in the control groups received saline solution. After the exploration of their abdominal aorta neither ischemia nor treatment was given to the rats of the control group. After the laparotomy of I/R group rats, infrarenal abdominal aorta (IAA) was clamped for 120 min then reperfusion was carried out for 120 min. TPM (100 mg/kg/day, Topamax®, Johnson and Johnson) was orally (via gastric gavage) given to I/R + TPM group for seven consecutive days before I/R.[20] By the end of these seven days, 120 min of ischemia and 120 min of reperfusion was applied to I/R + TPM group.
Aortic ischemia/reperfusion
The I/R model was designed similar to the previous studies.[15] Intraperitoneal injection of 50 mg/kg ketamine hydrochloride (Ketalar, 50 mg/kg intramuscularly; Eczacibasi, Istanbul, Turkey) was used to anesthetize the rats. The rats were placed supine on a heated mat in standard room conditions, then the surgery was performed. Ten milliliters per hour of sterile saline was injected subcutaneously into the neck of the rats to replace surgical fluid losses from evaporation. After performing a midline laparotomy, a minimum dissection of the retroperitoneum was made to expose the infrarenal aorta; then a microvascular clamp was placed across the IAA. After that the abdomen was closed and the wound was covered with plastic wrap to prevent the loss of heat and fluid. After 120 min, the microvascular clamp on the IAA was removed and the lower limb reperfusion was maintained for 120 min. Aortic occlusion and reperfusion was confirmed by observing the loss and reappearance of the pulsation on the distal aorta. After ending the reperfusion, a median sternotomy was performed and the blood samples were drawn from the right ventricles of all rats for biochemical analyses.
Tissue homogenates
The sample was homogenized in phosphate buffered solution (PBS), pH 7.4, and centrifuged at 10,000 g for 20 min and then the aliquot of the supernatant was put into tubes and frozen at −80°C. The parameters were examined within one month.
Measurement of protein
Tissue homogenate protein assay is a turbidimetric procedure that uses benzethonium chloride as a protein denaturing agent. A protein in the fine suspension is quantitated turbidimetrically at 404 nm (Architect c 16000, Abbott Laboratories, Illinois, USA).
Tumor necrosis factor-α
The level of TNF-α was measured by enzyme-linked immunosorbent assay (ELISA) method. We used commercially available rat TNF-α ELISA kit (eBioscience, Vienna, Austria). The ELISA method was applied according to the instructions provided by the manufacturer. Absorbance was measured at a wavelength of 450 ηm using ELISA reader. The levels of TNF-α are expressed as pg/mL. The intra-assay and interassay coefficients of variation were <5% and <10%, respectively. The limit of detection (LOD) for the TNF-α assay was 11 pg/mL. When dividing the obtained values by protein levels, the final results were obtained as μmol/g protein. When dividing the values we have gotten by protein levels, the final results were obtained as μmol/g protein.
Interleukin-6
The level of IL-6 was measured by ELISA method. We used commercially available rat IL-6 ELISA kit (Invitrogen, CA, USA). The ELISA method was applied according to the instructions provided by the manufacturer. Absorbance was measured at a wavelength of 450 ηm using ELISA reader. The levels of IL-6 are expressed as pg/mL. The intra-assay and interassay coefficients of variation were 3.5% and 6.3%, respectively. The LOD for the IL-6 assay was <5 pg/mL. When dividing the values we obtained by protein levels, the final results were expressed as μmol/g protein.
Asymmetric dimethyl arginine
The concentration of ADMA was measured using ELISA method. We used commercially available ADMA Elisa kit (DLD diagnostika GMBH, Hamburg, Germany). The ELISA method was applied according to the instructions provided by the manufacturer. Absorbance was measured at a wavelength of 450 ηm using ELISA reader. The levels of ADMA are presented as μmol/L. The intra-assay and interassay coefficients of variation were 6.1% and 9.4%, respectively. The LOD for the ADMA assay was 0.05 μmol/L. When dividing the values we obtained by protein levels, the final results were expressed as μmol/g protein.
Biochemical parameters
The blood samples (10 mL) were collected into tubes. The blood was separated by centrifugation at 3000 rpm for 10 min after standing at room temperature for 15 min. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were checked by using the determined commercial kits (Architect c 16000, Abbott Laboratories, Illinois, USA).
Histologic evaluation
The liver tissue obtained from all the rat groups were given label code numbers and names of the group and then left in 10% neutral formaldehyde solution. After waiting in the fixative for 24 h, they were washed by streaming water for 4–6 h. After that they were dealt with ethanol–xylene (50%–100%) before being automated by tissue tracking (Citadel 2000, Thermo Fisher Scientific Shandon, England) and embedded in paraffin. Tissues were cut into 3–4 μm thick sections. The light microscopic examination was performed with different magnifications and appropriate views were photographed. Immunohistochemical staining steps started by cutting the tissues into 3–4 μm thick sections and followed by leaving them for 20 min in xylene before the application of alcohol series (50%–100%) then leaving them in 3% H2O2 solution for 10 min. After PBS washing, they were heated in citrate buffer solution by 800 W power for 4 × 5 min, then stand in secondary blocker substance for 20 min.
Each slide was allowed to stand for 75 min in different dilutions of primary antibody (liver arginase 1 μg/mL and carbonic anhydrase-II 1/250–/500] before being stained by anticarbonic anhydrase II (cod: ab124687, Abcam plc, Cambridge, UK) and anti-liver arginase antibody (Cod: ab91279, Abcam plc, Cambridge, UK). Diaminobenzidine solution was used as a chromogen and for counterstain, Mayer's hematoxylin was applied for 3–5 min. We used PBS as a negative controller. Preparations were photographed after being covered with appropriate covering materials. Immunohistochemical staining preparations were categorized into four items according to the tissues percentage of immunopositive reaction areas as mild (+), moderate (++), severe (+++), and very severe (++++). The blocked tissues were cut into 4–5 μm thick sections before being stained by hematoxylin and eosin (H and E). All histologists slides were reviewed by two histologist who were blinded for the rat groups.
Statistical analyses
The results are presented as mean ± SD. The Kruskal–Wallis test was used to compare the groups. A Bonferroni adjusted Mann–Whitney U test was used to compare the two groups. Statistically significant differences were obtained at a P value of less than 0.05.
RESULTS
Biochemical parameters
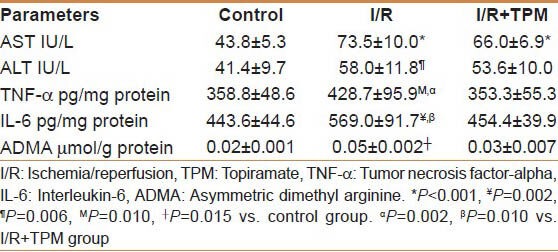
The I/R group's TNF-α levels (428.7 ± 95.9 pg/mg protein) were significantly higher than those of the control (358.8 ± 48.6 pg/mg protein, P = 0.010) and I/R + TPM groups (353.3 ± 55.3 pg/mg protein, P = 0.010). I/R group's IL-6 levels (569.0 ± 91.7 pg/mg protein) were significantly higher than those of the control (443.6 ± 44.6 pg/mg protein, P = 0.002) and I/R + TPM groups (454.4 ± 39.9 pg/mg protein, P = 0.002). I/R group's ADMA levels (0.05 ± 0.002 μmol/g protein) were significantly higher than those of the control (0.02 ± 0.001 μmol/g protein, P = 0.015) and I/R + TPM groups (0.03 ± 0.007 μmol/g protein) but were not statistically significant. The control group's AST levels (43.8 ± 5.3 IU/L) were significantly higher than those of the I/R (73.5 ± 10.0 IU/L, P < 0.001) and I/R + TPM groups (66.0 ± 6.9 IU/L, P < 0.001). The control group's ALT levels (41.4 ± 9.7 IU/L) were significantly higher than those of the I/R (58.0 ± 11.8 IU/L, P = 0.006) and I/R + TPM groups (53.6 ± 10.0 IU/L) but were not statistically significant. All the results of biochemical parameters are shown in table 1.
Table 1.
Biochemical results of the three groups
Histologic evaluation
No textural or cellular deformity has been found in the histopathological examination of the liver tissue of the control group animals stained by immunohistochemical and H and E methods. The morphologic structures of the cells and tissues were observed to have normal histologic appearance [Figure 1a and Table 2].
Figure 1.
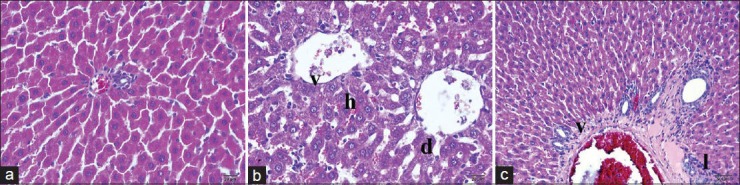
Histopathologic examination of liver tissue by light microscopy. (a) Control group, (b) ischemia/reperfusion (I/R) applied group, (c) I/R+ topiramate applied group. d, Dilatation; h, hepatization; I, lymphocyte accumulation; v, vacuolization; H&E stain
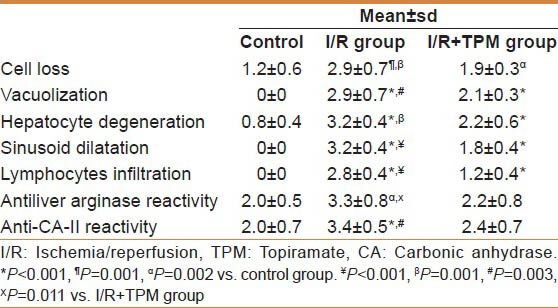
Table 2.
Histopathological results of the three groups
Hepatocellular necrosis, cellular damage with intensive degenerations, and cellular loss were observed in the histopathological examination of the I/R group animals stained by H and E method [Figure 1b]. In the acute phase of reperfusion, there was intensive vasoconstriction related to degenerations and vacuolization of muscles of the vessel walls. Many histopathologic changes have been demonstrated in the portal region and adjacent sinusoids including an increase in leukocytes, scattered platelet aggregation in the sinusoids, endothelial cells swelling related to intracellular edema, and cellular shedding. Additionally, there were projections and recess deformities on the surface of endothelial and Kupffer cells, intensive vacuolization of vessel wall myocytes, and cellular degenerations. Eosinophil granulocytes and neutrophil granulocyte infiltrations were detected around the large vessels of the portal area. There were partial dilatations of the sinusoidal walls and Kupffer cells were rounded, flat, and bulging with protrusion into the lumen. There were intensive necrotic cells and shedding related to hepatocyte degenerations in both central vein and portal regions. In acute phase, there was an increase in the number of neutrophil granulocytes in the connective tissue surrounding the areas of severe congestion in liver capillaries [Figure 1b and Table 2].
The histopathological examination after H and E stain method in ischemic reperfusion and TPM applied group revealed lower tissue and cellular deformity than ischemic reperfusion group [Table 1 and Figure 1c]. The intensive sinusoidal dilatations of I/R group were lower in the control group. However, the two groups have similar elongated sinusoidal structures. When compared with the I/R group, Kupffer cells settling the sinusoidal walls were observed to be more flat and stained deep basophilic. Vacuolization of the hepatocytes were lower in this group. However, they were observed around the portal region [Figure 1c]. The immunopositivity of CA-II in the hepatic tissues of the control and application groups was more intensively positive in sinusoidal walls and in the surroundings of the central vein. Although there was a decrease in immunoreactivity in progress to portal regions, it had a continuous course. Although immunoreactivity of the control group showed homogeneous distribution, it showed a decrease in the homogeneity and positivity when moving away from the central vein in the application group [Figure 2]. The immunopositivity of antiliver arginase in the hepatic tissues of the control group has low immunoreactivity in the surroundings of central vein. Although there was a decrease in immunoreactivity in progress to portal regions, it was lower in the periphery of the portal region. There was an extreme decrease in the antiliver arginase immunoreactivity [20% (+)] in necrotic and degenerative hepatocytes [Figure 3]. Histopathological results are shown in table 2.
Figure 2.
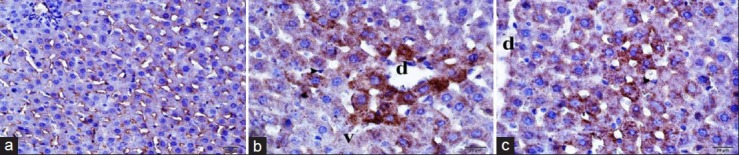
Histopathologic examination of kidney tissue stained by immunoperoxidase method by light microscopy. (a) Control group, (b) ischemia/reperfusion (I/R)applied group, (c) I/R+ topiramate applied group. d, Degeneration; v, vacuolization; arrow, Kupffer cells; immunoperoxidase–stained anticarbonic anhydrase II antibody
Figure 3.
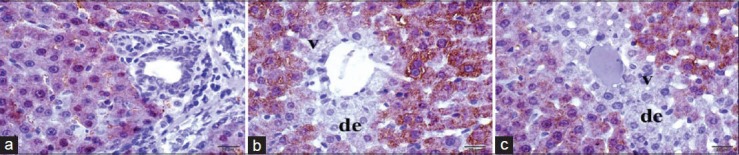
Histopathologic evaluation of kidney tissue stained by immunoperoxidase method by light microscopy. (a) Control group, (b) I/R applied group, (c) I/R+TPM applied group. de, Degeneration; v, vacuolization; immunoperoxidase–stained antiliver arginase antibody
DISCUSSION
In our study, TNF α, IL 6, and ADMA levels in the hepatic tissue from the I/R group were higher than both the control and TPM applied groups. TNF-α and IL-6 levels were found to be similar in the TPM applied group and the control group. In the I/R group, the levels of AST and ALT were high. AST, ALT, and tissue ADMA levels of TPM applied group were lower than those of the I/R group. The examined tissue stained by H and E revealed intensive cellular damage in I/R group, contrary to TPM applied group, which had lower cellular damage. In the tissues stained immunohistochemically, arginase and CPS-1 enzyme activities of I/R group were strongly higher than TPM applied group. Although the inflammatory cytokine levels of I/R group were obviously higher with intense histopathological damage, TPM group had lower histopathological damage with inflammatory cytokines level similar to the control group.
NO as a vasodilator may maintain perfusion and prevent endothelial injury. It can accept other electrons as a free-radical scavenger. However, an excessive production of NO, especially by NOS is cytotoxic and may produce toxic peroxynitrite via its reaction with superoxide.[21] NO is synthesized from arginine. The extensive production of ADMA could deteriorate the balance between NO and endothelin leading to vasoconstriction that affects the liver functions under I/R.[22] It has been reported that ADMA as an inhibitor may have a role in overproduction of NO mainly by regulating NOS activity.[23] Similar results have reported that in various pathological conditions there was an increase in ADMA level and a decrease in arginine level.[24] NO inhibits the proliferation of liver tissues and adhesion of monocytes and leukocytes to endothelium. Additionally, it acts as an inhibitor by lowering vascular production of superoxide radicals leading to decreased low-density lipoprotein oxidation.[6,7] ADMA itself may lead to severe endothelial dysfunction. The release of ADMA from an ischemic organ during the reperfusion stage competes with arginine for the active binging site in the center of NOS.[25] ADMA level is increased in response to oxidative stress during I/R. In our study, increased oxidative stress might lead to an increase in the ADMA level. Increased ADMA level may decrease NO-related endothelial vasodilatation by inhibiting NOS synthase. Additionally, excessive leukocyte accumulation in the endothelium may occur due to low NO level.
Arginase-I is found primarily in the cytosol of hepatocytes and catabolizes the hydrolysis of l-arginine to produce l-ornithine and urea. Arginine is a substrate for both NO synthase and arginase enzymes. There is a balance between arginase and NOS.[26] Decreased arginase enzyme-related activities increased NO level, whereas increased arginase-related activities were associated with decreased NO level.[15] Arginase inhibitors have been shown to have a preventive effect in experimental I/R models. The inhibition of arginase during ischemia increases NO level, which leads to vasodilatation and lower ischemic damage.[27] In our study, arginase enzyme activities and ADMA level were high in the I/R group. High levels of arginase and ADMA inhibit NOS enzyme ending in low NO level that may lead to severe vasoconstriction. Arginase level in the TPM group was similar to the control group, and ADMA level was lower. TPM treatment during I/R inhibits arginase, which may maintain the balance of NO level and prevent the increase of ADMA. CA is a ubiquitous metalloenzyme, which catalyzes the hydration reaction of carbon dioxide to bicarbonate.[17] CA has 12 active and highly homologous catalytic isoforms in the human body. CA-II is abundant in many tissues, including the liver. It is responsible for catalyzing the reversible reaction between carbon dioxide and bicarbonate.[28] Overexpression of CA-II increases Cl− /HCO3 − exchange activity, which increases intracellular bicarbonate, which leads to carbonate radicals.[29,30,31] A previous study has shown that the reaction of nitrogen dioxide and carbonate radicals with carbohydrates and proteins could form a number of nitration and nitrosation products in the endothelial cells of the vessels during reperfusion injury.[32] A previous study has shown peroxymonocarbonate, which was released from a spontaneous reaction of bicarbonate with H2O2, to be an intermediate metabolite of superoxide dismutase and peroxidase and a precursor of carbonate radical anions.[33] Our study has shown that high CA-II enzyme activity in I/R group indicates a high carbonate radical leading to liver tissue damage. TPM treatment may decrease carbonate radical by preventing overexpression of CA-II enzyme.
During the early phase of I/R, hepatocytes are damaged by lipid peroxidation, protein oxidation, mitochondrial dysfunction, and DNA damage.[34] Previous studies have shown that TNF-α and IL-6, which are responsible for apoptotic pathway activation, to be elevated during I/R.[35] In our study, intensive release of TNF-α and IL-6 was observed in I/R group, whereas these cytokines were suppressed in TPM group and their levels were similar to those in the control group. The current study has shown that increased production of TNF-α and IL-6 during I/R may lead to strong oxidative stress and severe cellular damage. TNF-α suppressive effect of TPM is known. Our study has shown TPM to prevent liver tissue damage by reducing cytokine release during I/R. ALT and AST levels may increase due to liver tissue damage. The lower damage seen in TPM applied group may be related to the antioxidant effect of TPM that may decrease the production of pro-inflammatory cytokine and prevent I/R-induced liver tissue damage. Additionally, it may decrease I/R injury by the inhibition of cytokine release and maintenance of NO balance.
In our study, there were intensive histopathological changes observed in the liver tissue of I/R group such as cell loss, degeneration, vacuolization, sinusoidal dilatation, and lymphocytic infiltration. Histopathological damage of TPM applied group was obviously lower than I/R group. Neutrophil and macrophage infiltration has an important role in I/R damage. In this study, we did not use special stain for macrophages as this study is a pilot study and the present findings support the preventive effect of TPM against I/R injury. Additionally, in this study there was no intensive infiltration of neutrophils. I/R injury can be caused by many mechanisms.[36] In addition, lymphocytes also are known to cause severe liver damage.[37] Our results confirmed that TPM significantly reduce lymphocyte accumulation in I/R injury. Also, elevated levels of cytokines and peroxynitrite that lead to reactive oxygen species formation are in the forefront in the present study.
CONCLUSION
Overproduction of cytokines during I/R leads to liver tissue damage. High or low levels of NO can lead to tissue damage. TPM treatment decreases liver damage during I/R injury through a preventive effect by maintaining the balance of tissue NO level and antioxidant effect by decreasing cytokines release.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–6. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 2.Mato JM, Corrales FJ, Lu SC, Avila MA. S-Adenosylmethionine: A control switch that regulates liver function. FASEB J. 2002;16:15–26. doi: 10.1096/fj.01-0401rev. [DOI] [PubMed] [Google Scholar]
- 3.Aydogan MS, Erdogan MA, Polat A, Yücel A, Ozgül U, Parlakpinar H, et al. Protective effects of melatonin and β-d-Glucan against liver injury in rats a comparative study. Adv Clin Exp Med. 2013;22:621–7. [PubMed] [Google Scholar]
- 4.Kaçmaz A, User EY, Sehirli AO, Tilki M, Ozkan S, Sener G. Protective effect of melatonin against ischemia/reperfusion-induced oxidative remote organ injury in the rat. Surg Today. 2005;35:744–50. doi: 10.1007/s00595-005-3027-2. [DOI] [PubMed] [Google Scholar]
- 5.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;26:173–9. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- 6.Peralta C, Brenner C. Endoplasmic reticulum stress inhibition enhances liver tolerance to ischemia/reperfusion. Curr Med Chem. 2011;18:2016–24. doi: 10.2174/092986711795590039. [DOI] [PubMed] [Google Scholar]
- 7.Peralta C, Jimenez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: Effects on the liver sinusoidal milieu. J Hepatol. 2013;59:1094–106. doi: 10.1016/j.jhep.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Hanaoka J, Shimada M, Utsunomiya T, Morine Y, Imura S, Ikemoto T, et al. Beneficial effects of enteral nutrition containing with hydrolyzed whey peptide on warm ischemia/reperfusion injury in the rat liver. Hepatol Res. 2014;44:114–21. doi: 10.1111/hepr.12097. [DOI] [PubMed] [Google Scholar]
- 9.Sommer BR, Mitchell EL, Wroolie TE. Topiramate: Effects on cognition in patients with epilepsy, migraine headache and obesity. Ther Adv Neurol Disord. 2013;6:211–27. doi: 10.1177/1756285613481257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazýroðlu M, Yürekli VA. Effects of antiepileptic drugs on antioxidant and oxidant molecular pathways: Focus on trace elements. Cell Mol Neurobiol. 2013;33:589–99. doi: 10.1007/s10571-013-9936-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Himmerich H, Bartsch S, Hamer H, Mergl R, Schönherr J, Petersein C, et al. Impact of mood stabilizers and antiepileptic drugs on cytokine production in-vitro. J Psychiatr Res. 2013;47:1751–9. doi: 10.1016/j.jpsychires.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 12.El-Abhar HS, Schaalan MF. Topiramate-induced modulation of hepatic molecular mechanisms: An aspect for its anti-insulin resistant effect. PLoS One. 2012;7:e37757. doi: 10.1371/journal.pone.0037757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sin YY, Ballantyne LL, Mukherjee K, St Amand T, Kyriakopoulou L, Schulze A, et al. Inducible arginase 1 deficiency in mice leads to hyperargininemia and altered amino acid metabolism. PLoS One. 2013;8:e80001. doi: 10.1371/journal.pone.0080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estevez AG, Sahawneh MA, Lange PS, Bae N, Egea M, Ratan RR. Arginase 1 regulation of nitric oxide production is key to survival of trophic factor-deprived motor neurons. J Neurosci. 2006;26:8512–6. doi: 10.1523/JNEUROSCI.0728-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cure E, Cure MC, Tumkaya L, Kalkan Y, Aydin I, Kirbas A, et al. Adalimumab Ameliorates Abdominal Aorta Cross Clamping Which Induced Liver Injury in Rats. Biomed Res Int 2014. 2014:907915. doi: 10.1155/2014/907915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Böger RH. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr. 2004;134:2842–7. doi: 10.1093/jn/134.10.2842S. [DOI] [PubMed] [Google Scholar]
- 17.Al-Samir S, Papadopoulos S, Scheibe RJ, Meißner JD, Cartron JP, Sly WS, et al. Activity and distribution of intracellular carbonic anhydrase II and their effects on the transport activity of anion exchanger AE1/SLC4A1. J Physiol. 2013;591:4963–82. doi: 10.1113/jphysiol.2013.251181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medinas DB, Cerchiaro G, Trindade DF, Augusto O. The carbonate radical and related oxidants derived from bicarbonate buffer. IUBMB Life. 2007;59:255–62. doi: 10.1080/15216540701230511. [DOI] [PubMed] [Google Scholar]
- 19.Dodgson SJ, Shank RP, Maryanoff BE. Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia. 2000;41:35–9. doi: 10.1111/j.1528-1157.2000.tb06047.x. [DOI] [PubMed] [Google Scholar]
- 20.Armagan A, Kutluhan S, Yilmaz M, Yilmaz N, Bülbül M, Vural H, et al. Topiramate and vitamin e modulate antioxidant enzyme activities, nitric oxide and lipid peroxidation levels in pentylenetetrazol-induced nephrotoxicity in rats. Basic Clin Pharmacol Toxicol. 2008;103:166–70. doi: 10.1111/j.1742-7843.2008.00271.x. [DOI] [PubMed] [Google Scholar]
- 21.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laleman W, Omasta A, Van de Casteele M, Zeegers M, Vander Elst I, Van Landeghem L, et al. A role for asymmetric dimethylarginine in the pathophysiology of portal hypertension in rats with biliary cirrhosis. Hepatology. 2005;42:1382–90. doi: 10.1002/hep.20968. [DOI] [PubMed] [Google Scholar]
- 23.Trocha M, Merwid-Lad A, Szuba A, Chlebda E, Piesniewska M, Sozanski T, et al. Effect of simvastatin on nitric oxide synthases (eNOS, iNOS) and arginine and its derivatives (ADMA, SDMA) in ischemia/reperfusion injury in rat liver. Pharmacol Rep. 2010;62:343–51. doi: 10.1016/s1734-1140(10)70274-7. [DOI] [PubMed] [Google Scholar]
- 24.Dimitrow PP, Undas A, Bober M, Tracz W, Dubiel JS. Plasma biomarkers of endothelial dysfunction in patients with hypertrophic cardiomyopathy. Pharmacol Rep. 2007;59:715–20. [PubMed] [Google Scholar]
- 25.Martin-Sanz P, Olmedilla L, Dulin E, Casado M, Callejas NA, Perez-Pena J, et al. Presence of methylated arginine derivatives in orthotopic human liver transplantation: Relevance for liver function. Liver Transpl. 2003;9:40–8. doi: 10.1053/jlts.2003.50008. [DOI] [PubMed] [Google Scholar]
- 26.Steppan J, Nyhan D, Berkowitz DE. Development of Novel Arginase Inhibitors for Therapy of Endothelial Dysfunction. Front Immunol. 2013;4:278. doi: 10.3389/fimmu.2013.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeyabalan G, Klune JR, Nakao A, Martik N, Wu G, Tsung A, et al. Arginase blockade protects against hepatic damage in warm ischemia-reperfusion. Nitric Oxide. 2008;19:29–35. doi: 10.1016/j.niox.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boone CD, Pinard M, McKenna R, Silverman D. Catalytic Mechanism of α-Class Carbonic Anhydrases: CO2 Hydration and Proton Transfer. Subcell Biochem. 2014;75:31–52. doi: 10.1007/978-94-007-7359-2_3. [DOI] [PubMed] [Google Scholar]
- 29.Sterling D, Brown NJ, Supuran CT, Casey JR. The functional and physical relationship between the DRA bicarbonate transporter and carbonic anhydrase II. Am J Physiol Cell Physiol. 2002;283:1522–9. doi: 10.1152/ajpcell.00115.2002. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann KM, Samardzic D, Heever KV, Rowlett RS. Co (II)-substituted Haemophilus influenzae β-carbonic anhydrase: Spectral evidence for allosteric regulation by pH and bicarbonate ion. Arch Biochem Biophys. 2011;511:80–7. doi: 10.1016/j.abb.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aljuhani N, Michail K, Karapetyan Z, Siraki AG. The effect of bicarbonate on menadione-induced redox cycling and cytotoxicity: Potential involvement of the carbonate radical. Can J Physiol Pharmacol. 2013;91:783–90. doi: 10.1139/cjpp-2012-0254. [DOI] [PubMed] [Google Scholar]
- 32.Queliconi BB, Marazzi TB, Vaz SM, Brookes PS, Nehrke K, Augusto O, et al. Bicarbonate modulates oxidative and functional damage in ischemia-reperfusion. Free Radic Biol Med. 2013;55:46–53. doi: 10.1016/j.freeradbiomed.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medinas DB, Toledo JC, Jr, Cerchiaro G, do-Amaral AT, de-Rezende L, Malvezzi A, et al. Peroxymonocarbonate and carbonate radical displace the hydroxyl-like oxidant in the Sod1 peroxidase activity under physiological conditions. Chem Res Toxicol. 2009;22:639–48. doi: 10.1021/tx800287m. [DOI] [PubMed] [Google Scholar]
- 34.El-Bahy AA, Kassem LA, Heikal OA, Mahran LG. Antiapoptotic effect of DDB against hepatic ischemia-reperfusion injury. J Toxicol Sci. 2011;36:145–54. doi: 10.2131/jts.36.145. [DOI] [PubMed] [Google Scholar]
- 35.Ocuin LM, Zeng S, Cavnar MJ, Sorenson EC, Bamboat ZM, Greer JB, et al. Nilotinib protects the murine liver from ischemia/reperfusion injury. J Hepatol. 2012;57:766–73. doi: 10.1016/j.jhep.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant Rev (Orlando) 2009;23:1–10. doi: 10.1016/j.trre.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


