Abstract
Background/Aims:
Gastric cancer (GC) is a multifactorial disorder mediated by genetic, epigenetic, and environmental risk factors. GC is the most common cancer in India and it is the third prominent cause of cancer death worldwide. A single nucleotide polymorphism (SNP) in the promoter region of interstitial collagenase (MMP-1) gene appears to have an impact on the transcriptional activity and regulation of its expression. Hence, the present study is aimed to evaluate the role of interstitial collagenase gene-1607 1G/2G (rs1799750) promoter polymorphism in the etiology of GC.
Patients and Methods:
The study included 166 GC patients and 202 control subjects. Genomic DNA was isolated from whole blood samples of the subjects, and the genotyping of interstitial collagenase promoter polymorphism was carried out by polymerase chain reaction-restriction fragment length polymorphism method followed by agarose gel electrophoresis. Appropriate statistical methods were applied to test the significance of the results.
Results:
The risk factor profile of the patients revealed that male gender, age above 50 years, addiction to alcohol and smoking were the most common risk factors (P < 0.05). There was a significant difference in the distribution of 2G/2G genotype (2G/2G vs. 1G/1G, P = 0.016) and 1G/2G genotype (2G/2G + 1G/2G vs. 1G/1G, P = 0.010) in patient group compared with that of the control subjects.
Conclusion:
The present study provides indirect evidence for the role of interstitial collagenase gene 1G/2G promoter polymorphism in the etiology of GC in South Indian population.
Keywords: Gastric cancer, genotype, interstitial collagenase, Matrix metalloproteinases-1, polymorphism, polymerase chain reaction-restriction fragment length polymorphism
Gastric cancer (GC), also known as stomach cancer, can arise in any part of the stomach and may progress to the entire stomach and to other surrounding organs; the esophagus, liver, lungs, and the lymph nodes. It is a complex and multifactorial disorder controlled by environmental, genetic, and epigenetic risk factors.[1] On a global scale, GC causes approximately 8,00,000 deaths per year and it is the third leading cause of cancer death worldwide in both males and females.[2] The recent study from Tata Memorial Centre (TMC) on cancer mortality in India has rightly focused GC as the second largest cause of cancer-related deaths among Indians.[3] On the whole 90% of the stomach tumors are malignant and 95% of these tumors reported to be adenocarcinomas.[4]
Matrix metalloproteinases (MMPs) compose a vast group of zinc-dependent proteolytic endopeptidases having a metal in their integral structure and thought to be involved in degeneration of proteins in the interstitial matrix, including basement membrane and tissue remodeling via critical biological procedures.[5,6,7] MMPs perform a key role in promoting proliferation of tumor, angiogenesis, cell migration, apoptosis, and connective tissue degradation.[8]
Among the 20+ ECM proteases, interstitial collagenase also called as matrix metalloproteinase 1 (MMP-1) or collagenase-1 or fibroblast-type collagenase (FIB-CL) degrades the collagens I, II, III, VII, VIII, X, and gelatin[5] and is involved in initiation and progression of tumors by transforming the cellular microenvironment that promotes tumorigenesis.[9] The gene for interstitial collagenase is localized on chromosome 11 at q22.3 and a single nucleotide polymorphism (SNP) in the promoter region appears to control the transcriptional activity.[10] The polymorphism is due to the insertion or deletion of an extra guanine (G) at -1607 bp (rs1799750), results in the sequence 5’GGAA3’ (2G allele) from 5’GAA3’ (1G allele). This new 5’GGAA3’ sequence, is a consensus-binding site for the Ets (E 26) family of transcription factors specific to metazoans, which act as the downstream target for numerous growth factors.[11] Overexpression of collagenase gene had been manifested in malignant tissues and proposed to be associated with invasion and metastasis of tumors.[12,13] The 1G/2G polymorphism was found to enhance the risk to develop various cancers[14] such as lung cancer, colorectal cancer, ovarian cancer, renal cell carcinoma, cervical cancer, and is associated with an overall weak prognosis in colorectal[15] and esophageal cancers.[16] Thus, interstitial collagenase can be regarded as a candidate gene for susceptibility to GC. To explore whether the collagenase-1 gene promoter polymorphism is involved in the gastric carcinogenesis, we carried out a case–control study investigating the association between the polymorphism and the risk for GC in South Indian population.
PATIENTS AND METHODS
The present study included endoscopically and histopathologically confirmed 166 GC patients and 202 healthy control subjects (with no family history of gastric ulcer or cancer) referred to Department of Gastroenterology, Gandhi Hospital, Secunderabad. The information on demographic features such as age, gender, dietary habits, weight, consanguinity, familial incidence of cancer, and addiction to smoking and alcohol was obtained from all the patients and controls using a structured questionnaire. Helicobacter pylori infectivity status was tested in antral biopsies from all the patients by rapid urease test following Vaira et al. method.[17] The study was approved for ethical clearance by Ethics Committee of the Institute. The ethical standards of experiments were in accordance with the guidelines provided by ICMR.
Five milliliters of blood was collected from both patients and control subjects in EDTA-coated vacutainers. Genomic DNA was isolated from whole blood samples following Lahiri et al. salting out method.[18]
Analysis of -1607 1G/2G (rs1799750) promoter polymorphism of the interstitial collagenase gene was carried out by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. The PCR primers (Genei, Bangalore) used for amplifying a fragment of 269 bp for the polymorphism at -1607 (1G > 2G) were: Forward primer: 5ʹ-TGA CTT TTA AAA CAT AGT CTA TGT TCA-3ʹ Reverse primer: 5ʹ-TCT TGG ATT GAT TTG AGA TAA GTC ATA GC-3ʹ. The PCR reaction was performed in a 20 μL final volume consisting 20 ng of genomic DNA, 1.5 mM MgCl2, 1 × Taq buffer, dNTPs (2.5 m M each dATP, dGTP, dTTP, dCTP) (Genei, Bangalore), forward and reverse primers (0.4 pM each) and recombinant Taq DNA polymerase 1 U/μL (Genei, Bangalore). The PCR conditions were initial denaturation of 5 min at 94°C, denaturation of 30 s at 94°C, annealing of 30 s at 58°C, extension of 30 s at 72°C, followed by 34 cycles and a final extension step at 72°C for 5 min.
The restriction endonuclease Alu I (Fermentas™) (for -1607 1G>2G) (rs1799750) was used to digest the PCR product. A 10 μL of PCR aliquot was incubated at 37°C overnight for digestion in a 20 μL reaction comprising 10 units of restriction endonuclease and 2 μL reaction buffer.
After overnight digestion of amplicons with Alu I the RFLP products were resolved on a 3% agarose gel stained with ethidium bromide. The 1G allele has the AGCT recognition site, but this recognition site is destroyed by the insertion of a guanine in the 2G allele.[19] The 1G/1G homozygous alleles at -1607 were distinguished by 241 and 28 bp DNA bands as the 1G allele has the recognition site for Alu I, whereas the 2G/2G homozygous alleles were characterized by a single DNA band with a size of 269 bp size. The 1G/2G heterozygote displayed the presence of three bands (269, 241, and 28 bp). The genotypes were determined based on the appearance of bands with help of 100 bp ladder. Ten percent of the samples were taken randomly, and the assay was performed again with no bias observed in genotyping. The replicative study revealed similar findings with 100% concordant results.
Statistical Analyses
The evaluation of case and control differences in the distribution of alleles and genotypes was carried out by χ2 test of association.[20] Odd's ratios (ORs) and corresponding 95% confidence intervals (CIs) were determined using Javastat 2-way contingency table analysis[20] to measure the strength of association between interstitial collagenase gene polymorphism and GC. All statistical tests were two-tailed, and P values < 0.05 were considered to be statistically significant.[20]
RESULTS
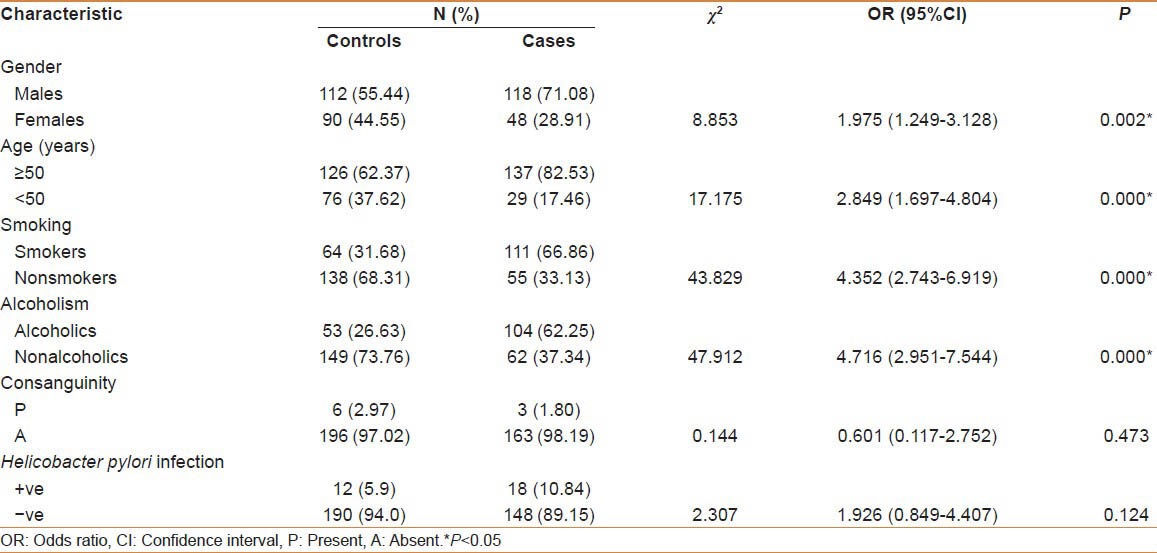
The present case-control study included a total of 166 patients and 202 control subjects. The demographic characteristics of the study population have been indexed in table 1. All the GC patients were of South Indian origin. The study subjects were classified based on gender, age, addiction to smoke and alcohol, family history, H. pylori infection, and other organisms. The risk factor profile exhibited that male gender, age above 50 years, and addiction to smoking and alcohol were the most common risk factors in patients compared with the control subjects (P < 0.05), indicating the role of these factors in the etiology of the disease. Among the patients 2.4% of them revealed familial incidence of GC. No significant difference was noticed between cases and controls with regard to consanguinity (P = 0.473) and H. pylori infection (P = 0.124).
Table 1.
Demographic characteristics among gastric cancer cases and controls
The distribution of genotype and allele frequencies of interstitial collagenase gene 1G/2G promoter polymorphism in patients and controls was given in table 2. The frequency of 1G/1G, 1G/2G, and 2G/2G genotypes in patients were 3.61%, 68.67%, and 27.71%, whereas in the control subjects the distribution was 10.89%, 64.35%, and 24.75%, respectively. The allelic frequencies were found to be 37.95% for 1G and 62.04% for 2G in patient group whereas 43.06% and 56.93% in controls, respectively.
Table 2.
Distribution of genotype, allelic frequencies and odd's risk estimates in patients compared with control subjects
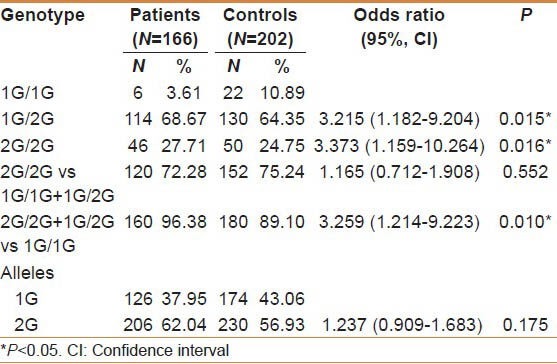
There was a significant difference in the distribution of 2G/2G genotype [(2G/2G vs 1G/1G, χ2 = 5.206; P = 0.016; OR = 3.373 (95% CI; 1.159−10.264)] in patient group compared with the control subjects with threefold increased risk. The 2G allele as a single copy in heterozygote (1G/2G) condition also increase the risk [1G/2G vs 1G/1G, χ2 = 5.532; P = 0.015; OR = 3.215 (95%CI; 1.182−9.204)] in patients compared with the control subjects. When the 2G/2G and 1G/2G genotypes were combined, there was a threefold enhanced GC risk for individuals with 2G allele. [2G/2G + 1G/2G vs 1G/1G, χ2 = 5.876; P = 0.010; OR = 3.259 (95%CI; 1.214−9.223)].
Table 3 stratifies the genotype distribution based on epidemiological factors such as age and gender. Patients above 50 years have shown the disease with 1G/1G (P = 0.021) and 1G/2G genotypes (P = 0.003), indicating the possible association of 1G allele with an advanced age. Moreover, higher GC risk was observed in men with 2G/2G genotype (OR = 6.021, P = 0.000) suggesting gender-specific effect of the polymorphism.
Table 3.
Distribution of genotype frequency in patients and control subjects with regard to Age and gender
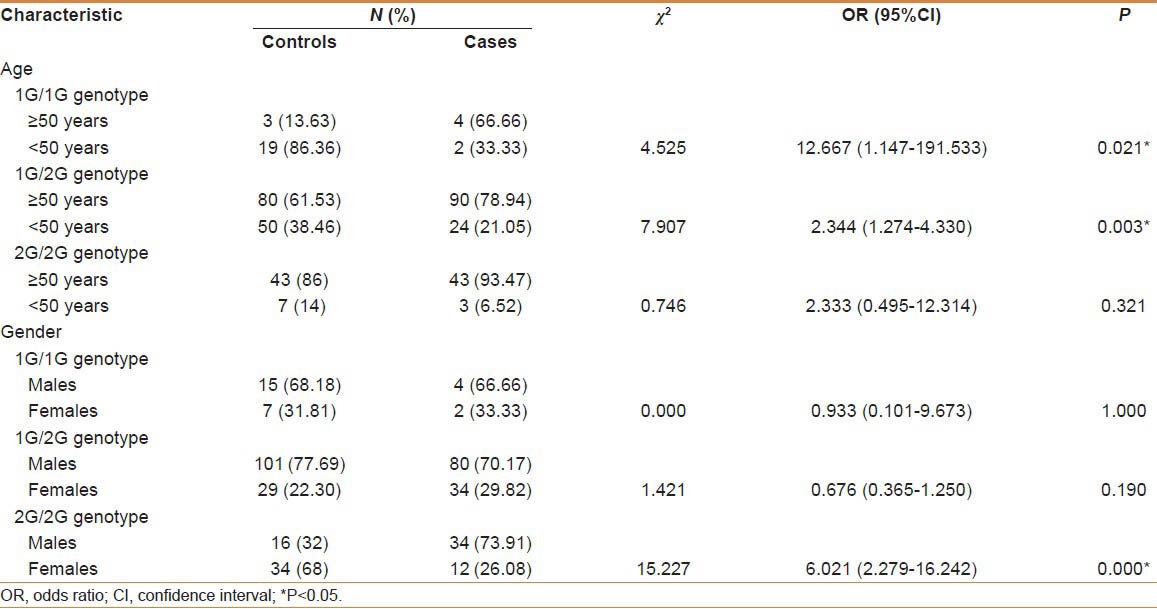
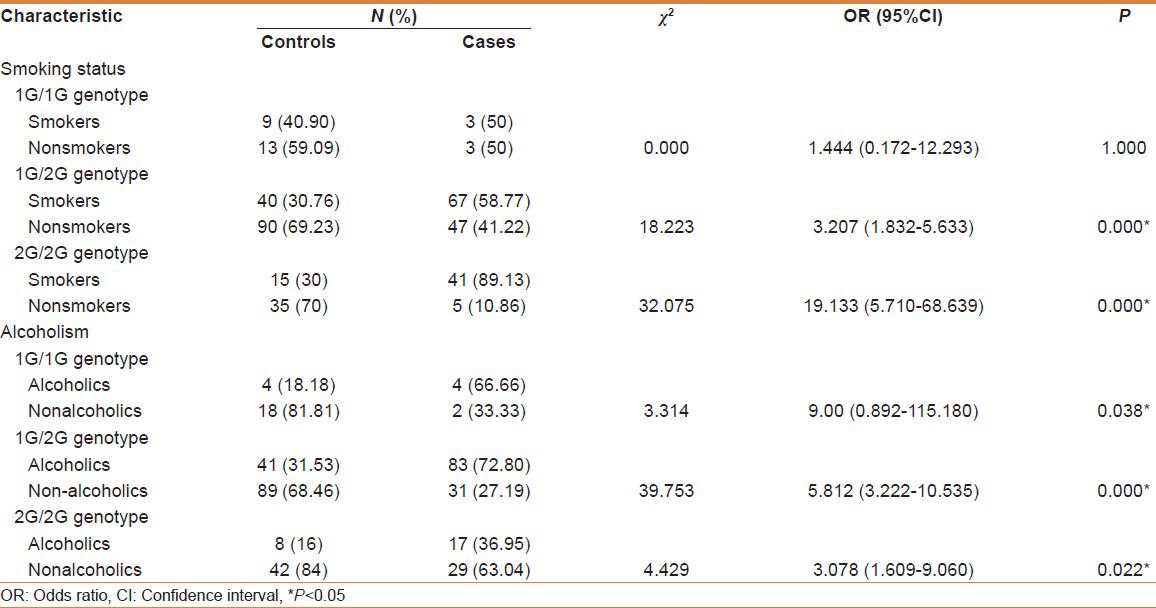
Table 4 exhibits the genotype distribution based on addictions. Patients who are smokers developed the disease even with a single 2G allele revealing a strong association of 2G allele with smoking. All the patients displayed a significant association of alcoholism with the disease irrespective of the genotype (P < 0.05). Possible association was revealed in patients of 1G/2G genotype with H. pylori infection in developing cancer (P = 0.022). Comparisons were also made among patients and control subjects with respect to consanguinity and familial incidence and found no significant association.
Table 4.
Distribution of genotype frequency in patients and control subjects with regard to addictions
DISCUSSION
GC, the fifth most frequent cancer in the world and third most in India, is defined as a multifactorial disorder resulting from various genetic, epigenetic predisposition, and environmental risk factors.[21] MMPs are zinc dependent metalloproteases, degenerate the extracellular matrix collagens, and thought to be very critical in tissue repair and remodeling during development and inflammation. MMPs are important in altering the cell cycle check point controls, thus promoting genomic imbalance by influencing cell adhesion[22] and contribute to initiation and development of tumors by altering the cellular microenvironment that help tumor formation.[23]
The present study revealed the significance of advanced age, male gender, smoking, and alcoholism in the patient group compared with the control subjects. It is evident that most of the patients belong to age 50 years or more showcasing GC incidence to be higher in advanced age group compared with the early age group and is in agreement with the study of Neugut et al.[24] It is clearly observed that risk of developing GC is higher in males compared with females and the same was reported by Parkin et al.[25] This may be assigned to environmental risk triggering factors such as alcohol and smoking, which were most common in males. The cytoprotective effect of estrogen hormone may be one of the factors for lowered frequency of GC in women. Smoking and alcoholism were identified as potential risk factors in the development of GC. Earlier studies also highlighted the role of smoking and alcoholism in enhancing the risk for GC, which is correlated with the present findings. It is interpreted based on the fact that cigarette smoke may enhance the risk of developing GC via the formation of nitroso amine, a potent carcinogen, while consuming alcohol had impact on tumor volume doubling time (TVDT), which in turn stimulates the tumor growth by promoting angiogenesis.[26,27,28]
Extracellular matrix-degrading interstitial collagenase is thought to be involved in tumor aggression, metastasis, and may also aid in initiation and development of tumor by changing the cellular microenvironment that facilitates tumor formation.[23] The 2G allele of 1G/2G polymorphism was found to increase the risk for the development of various cancers such as lung cancer, colorectal cancer, ovarian cancer, cervical cancer, and renal cell carcinoma.[14] The results were conflicting regarding the association studies from different ethnicities. This is the first research study to investigate the role of interstitial collagenase gene promoter polymorphism in GC from south Indian population. It is postulated that this polymorphism may influence the expression and activity of the enzyme, promoting extracellular matrix deterioration and invasion, leading to progression of cancer.
There is a statistically significant difference in the distribution of 2G/2G genotype and 1G/2G genotype in GC patients compared with control individuals. A significantly elevated risk to GC was observed in combination of the 2G/2G genotype and 1G/2G genotypes than individuals with 1G/1G genotype. The present study thus suggests that even a single dose of 2G allele may contribute to the risk of the disease.
CONCLUSION
The present study provides the first molecular epidemiological evidence from South Indian cohort for the association of 1G/2G polymorphism with a risk to GC and thus suggesting the indirect role of interstitial collagenase gene promoter polymorphism in altering cellular microenvironment and facilitation of tumor development. These findings may ultimately help in identifying the individuals at high risk for the disease. However, a large confirmatory study involving other populations is warranted to understand the population specificity and the relative contribution of this polymorphism in the disease phenotype.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Koo SH, Jeong TE, Kang J, Kwon KC, Park JW, Noh SM. Prognostic implications for gastric carcinoma based on loss of heterozygosity genotypes correlation with clinicopathologic variables. Cancer Genet Cytogenet. 2004;153:26–31. doi: 10.1016/j.cancergencyto.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization; [Last accessed on 2014 Mar 08]. Cancer Factsheet. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=stomach) [Google Scholar]
- 3.Stomach- Cancer incidence in India. [Last accessed on2014 Feb 26]. Available from: http://www.dnaindia.com/health/report-stomach-cancer-second-biggest-killer-in-india-1670749 .
- 4.Schwartz GK. Invasion and metastases in gastric cancer: In vitro and in vivo models with clinical correlations. Semin Oncol. 1996;23:316–24. [PubMed] [Google Scholar]
- 5.Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, et al. Matrix metalloproteinases: A review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 6.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circ Res. 2002;90:251–62. [PubMed] [Google Scholar]
- 7.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ Res. 2003;92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 8.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–4. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 9.Su L, Zhou W, Asomaning K, Lin X, Wain JC, Lynch TJ, et al. Genotypes and haplotypes of matrix metalloproteinase 1, 3 and 12 genes and the risk of lung cancer. Carcinogenesis. 2006;27:1024–9. doi: 10.1093/carcin/bgi283. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhary AK, Singh M, Bharti AC, Asotra K, Sundaram S, Ravi M. Genetic polymorphisms of matrix metalloproteinases and their inhibitors in potentially malignant and malignant lesions of the head and neck. J Biomed Sci. 2010;17:10. doi: 10.1186/1423-0127-17-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutter JL, Mitchell TI, Buttice G, Meyers J, Gusella JF, Ozelius LJ, et al. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcription. Cancer Res. 1998;58:5321–5. [PubMed] [Google Scholar]
- 12.Hewitt RE, Leach IH, Powe DG, Clark IM, Cawston TE, Turner DR. Distribution of collagenase and tissue inhibitor of metalloproteinases (TIMP) in colorectal tumors. Int J Cancer. 1991;49:666–72. doi: 10.1002/ijc.2910490507. [DOI] [PubMed] [Google Scholar]
- 13.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–70. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 14.Hirata H, Okayama N, Naito K, Inoue R, Yoshihiro S, Matsuyama H, et al. Association of a haplotype of matrix metalloproteinase (MMP)-1 and MMP-3 polymorphisms with renal cell carcinoma. Carcinogenesis. 2004;25:2379–84. doi: 10.1093/carcin/bgh254. [DOI] [PubMed] [Google Scholar]
- 15.Murray GI, Duncan ME, O’Neil P, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med. 1996;2:461–2. doi: 10.1038/nm0496–461. [DOI] [PubMed] [Google Scholar]
- 16.Murray GI, Duncan ME, O’Neil P, McKay JA, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in oesophageal cancer. J Pathol. 1998;185:256–61. doi: 10.1002/(SICI)1096-9896(199807)185:3<256::AID-PATH115>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 17.Vaira D, Holton J, Cairns S, Polydorou A, Falzon M, Dowsett J, et al. Urease test for Campylobacter pylori: Care in interpretation. J Clin Pathol. 1988;41:812–3. doi: 10.1136/jcp.41.7.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y, Spitz MR, Lei L, Mills GB, Wu X. A single nucleotide polymorphism in the Matrix Metalloproteinase-1 promoter enhances lung cancer susceptibility. Cancer Res. 2001;61:7825–9. [PubMed] [Google Scholar]
- 20.Java stat 2-way Contingency Table Analysis. [Last accessed on 2014 Feb 26]. Available from: http://statpages.org/ctab2×.2html .
- 21.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–77. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 22.Tlsty TD. Cell-adhesion-dependent influences on genomic instability and carcinogenesis. Curr Opin Cell Biol. 1998;10:647–53. doi: 10.1016/s0955-0674(98)80041-0. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y, Spitz MR, Lei L, Mills GB, Wu X. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter enhances lung cancer susceptibility. Cancer Res. 2001;61:7825–9. [PubMed] [Google Scholar]
- 24.Neugut AI, Hayek M, Howe G. Epidemiology of gastric cancer. Semin Oncol. 1996;23:281–91. [PubMed] [Google Scholar]
- 25.Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 26.Sung NY, Choi KS, Park EC, Park K, Lee SY, Lee AK, et al. Smoking, alcohol and gastric cancer risk in Korean men: The National Health Insurance Corporation Study. Br J Cancer. 2007;97:700–4. doi: 10.1038/sj.bjc.6603893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuhashi T, Yamada N, Shinzawa H, Takahashi T. Effect of alcohol on tumor growth of hepatocellular carcinoma with type C cirrhosis. Intern Med. 1996;35:443–8. doi: 10.2169/internalmedicine.35.443. [DOI] [PubMed] [Google Scholar]
- 28.Tan W, Bailey AP, Shparago M, Busby B, Covington J, Johnson JW, et al. Chronic alcohol consumption stimulates VEGF expression, tumor angiogenesis and progression of melanoma in mice. Cancer Biol Ther. 2007;6:1211–7. doi: 10.4161/cbt.6.8.4406. [DOI] [PubMed] [Google Scholar]


