Abstract
Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) occurring in inflammatory bowel diseases, including ulcerative colitis (UC) and Crohn's disease, has been reported, although it is extremely rare. An 18-year-old man with a two-years history of UC underwent colon endoscopy, and was found to have active total UC ranging from anus to cecum. Six biopsies were obtained. The microscopic examinations showed severe infiltrations of atypical small lymphocytes. They showed hyperchromatic nuclei and increased nucleocytoplasmic ratio and scattered immunoblastic cells. Centrocyte-like atypical lymphocytes, monocytoid cells, and plasma cells were seen in some places. Vague germinal centers were present, and apparent lymphoepithelial lesions were seen. No crypt abscesses were seen, and there were few neutrophils. No apparent other findings of UC were seen. Immunohistochemically, the atypical lymphocytes were positive for vimentin, CD45, CD20, CD79α, CD138, κ-chain, λ-chain, and p53 and Ki-67 antigen (labeling index = 63%). They were also positive for CD45RO, CD3, and CD15, but these positive cells were very scant compared with CD20 and CD79α. They were negative for CD10, CD30, CD56, cytokeratin (CK) AE1/3, CK CAM5.2, CK34BE12, CK5, CK6, CK7, CK8, CK14, CK18, CK19, CK20, EMA, chromogranin, synaptophysin, NSE, S100 protein, CEA, CA19-9, p63, and HMB45. Without clinical information, the appearances are those of MALT lymphoma. However, with clinical information, making the diagnosis of MALT lymphoma was hesitated. It is only mentioned herein that atypical lymphocytic infiltrations indistinguishable from MALT lymphoma occurred in an 18-year-old male patient with a two-year history of UC.
Keywords: Histopathology, immunohistochemistry, MALT lymphoma, Ulcerative colitis
Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) is defined as an extranodal lymphoma composed of morphologically heterogenous small B-cells, including marginal (centrocyte-like) cells, cell resembling monocytoid cells, small lymphocytes, and scattered immunoblasts, and centroblast-like cells.[1,2] This entity was first described by Isaacson and White in 1983.[2] There is a plasma cell differentiation in a proportion of cases. The infiltrate is in the marginal zone of reactive B-cell follicles and extends into the interfollicular region. In epithelial tissues, the neoplastic cells typically infiltrate the epithelium, thus forming lymphoepithelial lesions.
MALT lymphoma most commonly involves gastrointestinal (GI) tract (50%), followed in order by salivary glands, lung (14%), head and neck (15%), ocular adnexa (12%), skin (11%), thyroid (4%), and breast (4%). In the GI tract, the majority of MALT lymphoma occurs in the stomach,[3,4,5,6,7,8,9,10,11,12,13,14] where Helicobactor pyroli is regarded as the causative agent.[1] Elimination of H. pylori commonly cures the gastric MALT lymphoma.
Primary gastrointestinal lymphoma comprises 10%–15% of all non-Hodgkin's lymphomas and encompasses 30%–40% of the total extranodal lymphomas. Approximately 60%–75% of cases of gastrointestinal lymphoma occur in the stomach, followed by colon, cecum, jejunum, ileum, and rectum.[3,4,5,6,7,8,9,10,11,12,13,14] Lymphoid neoplasms may consist of mature B, T, and less commonly extranodal NK/T cells. Of these, the two most frequently encountered histologic subtypes are MALT lymphoma and diffuse large B-cell lymphoma (DLBCL). Enteropathy-associated T-cell lymphoma, type I in particular, usually arises in a background of celiac disease. T-cell gene rearrangement confirms the clonality. NK/T cell neoplasms are invariably associated with Epstein–Barr virus infection. There were a few reports of MALT lymphoma occurring in patients with inflammatory bowel diseases, including ulcerative colitis (UC) and Crohn's disease.[15,16] Herein reported is a case of lymphoid infiltrates of colon indistinguishable from MALT lymphoma occurring in a young patient with UC.
CASE REPORT
An 18-year-old boy with 2 years history of UC was referred to our hospital from a clinic for endoscopy. The boy was diagnosed as UC of total colitis type at a large general hospital 2 years earlier. Our hospital performed colonic endoscopy, which revealed erosions, ulcers, and edema continuously from the anus to the cecum. The terminal ileum was not involved. Six biopsies were obtained from various sites of the colorectum.
The microscopic examinations of all the six biopsies showed severe infiltration of atypical small lymphocytes [Figure 1A, B]. They showed hyperchromatic nuclei and increased nucleocytoplasmic ratio. Immunoblastic cells were scattered. Centrocyte-like atypical lymphocytes (CCLs), monocytoid cells, and plasma cell differentiations were seen in some places. Vague germinal centers were present, and apparent lymphoepithelial lesions (LELs) were seen [Figure 1A, Bb]. No crypt abscesses were seen, and there were few neutrophils. No apparent features of UC such as crypt abscess, deletion of goblet cells, abnormal branching of the crypts and cryptal atrophy were seen. No Crohn's granuloma was seen.
Figure 1.
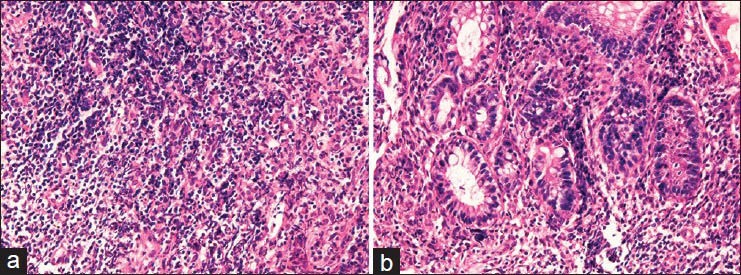
Histological findings. (A) Low power view of the one specimen of colon biopsies. Severe atypical lymphoid infiltrates are seen. (B) High power view. It is evident that the lymphoid infiltrates are composed of atypical small lymphocytes reminiscent of centrocytes-like features. Monocytoid atypical cells, plasmacytoid atypical cells, and immunoblastic cells were seen. Mitotic figures were also seen. Germinal centers and lymphepithelial lesions are seen. (A): hematoxylin and eosin (H and E), ×100. (B) (H and E), ×200
An immunohistochemical study was performed by the use of Dako Envision method (Dako Corp, Glostrup, Denmark), as previously reported.[17,18,19,20,21,22] Immunohistochemically, the infiltrations were positive for vimentin, CD45, CD20 [Figure 2A], CD79α [Figure 2B], CD138 [Figure 2C], κ-chain [Figure 2D], λ-chain (significantly weaker than κ-chain), p53 [Figure 2E], and Ki-67 antigen (labeling index = 63%) [Figure 2F]. They were also positive for CD45RO, CD3, and CD15, but these positive cells were very scant compared with CD20 and CD79α. The infiltrates were negative for CD10, CD30, CD56, cytokeratin (CK) AE1/3, CK CAM5.2, CK34BE12, CK5, CK6, CK7, CK8, CK14, CK18, CK19, CK20, EMA, chromogranin, synaptophysin, NSE, S100 protein, CEA, CA19-9, p63, and HMB45.
Figure 2.
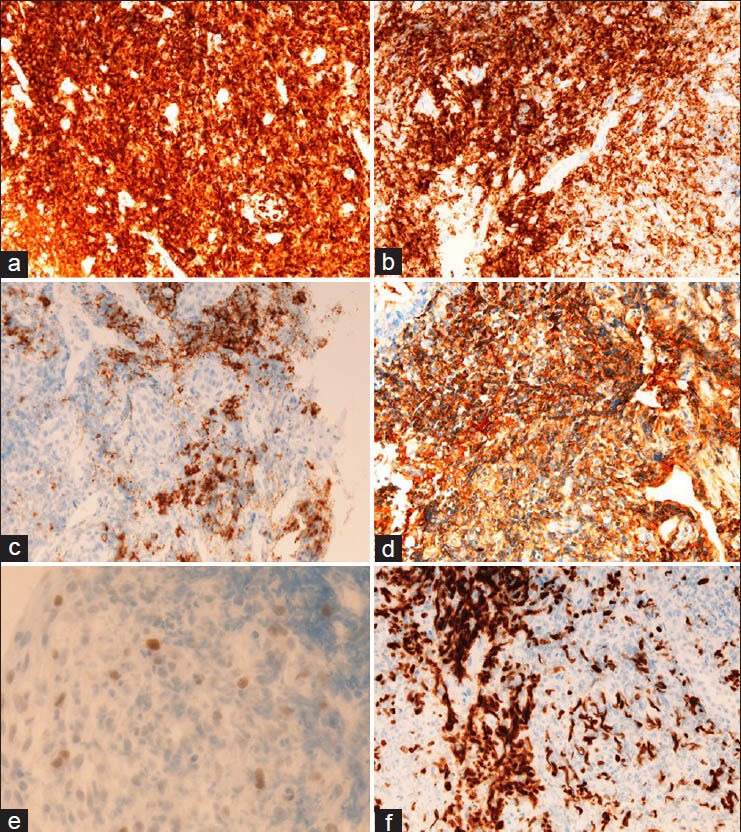
Immunohistochemical findings. The colonic infiltrations are positive for CD20 (A), CD79α (B), CD138 (C), κ-chain (D), p53 (E), and Ki-67 antigen (labeling index = 63%) (F). It is apparent that the atypical infiltrates are composed largely of B-cells. A–F, ×200
Without clinical information, the appearances are those of MALT lymphoma. However, with clinical information, making the diagnosis of MALT lymphoma was hesitated. The pathological diagnosis made by the author was atypical lymphoid infiltrates indistinguishable from MALT lymphoma in an adolescent male patient. The patient was planned to be followed up without therapy of MALT but with treatment of UC with salazosulfapyridine and steroids.
DISCUSSION
The lymphoid infiltrates in the present case were indistinguishable from MALT lymphoma, histologically and immunohistochemically. The most important thing is to distinguish between UC inflammation and MALT lymphoma. In the present case, no crypt abscesses were seen, and few neutrophilic infiltrations were seen. Other features of UC including decrease of goblet cells were not seen. The immunohistochemical study showed that the lymphoid infiltrates are mainly of B-cell, and T-cells were scant. The plasma cell differentiation was confirmed by positive CD138 immunostaining. The lymphoepithelial lesions were highlighted by CD20 and CK immunostaining. The lymphocytes atypia was obvious, and centrocyte-like lymphocytes and monocytoid lymphocytes were seen. There were vague germinal centers consisting of non-atypical cells. The immunohistochemistry showed p53 protein and high Ki-67 labeling index (63%). Although no obvious light chain restriction was seen, these histological and immunohistochemical features are those of MALT lymphoma. However, light chain restriction may be present in the present case, depending on the pathologist's investigated. MALT lymphoma in UC has been rarely reported.[23]
If pathologists diagnose these lesions without clinical information, the pathological diagnosis may be MALT lymphoma. However, the patient was an 18-year-old adolescent man with 2 years history of UC. This clinical information made the pathologist to hesitate to make the diagnosis of MALT lymphoma. The pathologist and clinicians discussed the treatment of this patient. It was decided that although the lesion may be MALT lymphoma, the patient was stringently followed up by frequent biopsies. It was also decided that no treatment of MALT lymphoma be performed now, but the patient was treated by drugs and steroids for UC.
In general, MALT lymphoma is an ambiguous entity and it shows inflammatory characteristics. Before the acceptance of the paper of Isaacson and Wright,[24] the gastric MALT lymphoma were called “reactive lymphoid hyperplasia” or “pseudolymphoma.” Such designations of inflammatory nature did not cause any problems. The discovery of H. pyroli highlighted the MALT, which are cured by eradication of H. pylori. However, in general pathology, neoplasms with such cure or regression after the causative agents are not malignant by definition. MALT lymphoma does not metastasize in any location. MALT lymphoma is well known to infrequently transform into DLBCL. Therefore, it is conceivable that MALT lymphoma encompasses broad ranges of lymphoid diseases from simple inflammation to authentic low-grade B-cell neoplasm. Therefore, the term MALT lymphoma may be inappropriate. In any way, much more studies of so-called MALT lymphoma are mandatory.
In conclusion, the author found atypical lymphoid infiltrates of the colon indistinguishable from MALT lymphoma in an 18-year-old male patient with UC.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Isaacson PG, Chott A, Nakamura S, Muller-Hermelink HK, Harris NL, Swerdlow SH. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. pp. 214–7. [Google Scholar]
- 2.Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue: A distinctive type of B-cell lymphoma. Cancer. 1983;52:1410–6. doi: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Terada T. Gastrointestinal malignant lymphoma: A pathologic study of 37 cases in a single Japanese institution. Am J Blood Res. 2012;2:194–200. [PMC free article] [PubMed] [Google Scholar]
- 4.Bautista-Quach MA, Ake CD, Chen M, Wang J. Gastrointestinal lymphomas: Morphology, immunophenotype and molecular features. J Gastrointest Oncol. 2012;3:209–25. doi: 10.3978/j.issn.2078-6891.2012.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terada T. One patient with double lymphomas: Simultaneous gastric MALT lymphoma and ileal diffuse large B-cell lymphoma. Int J Clin Exp Pathol. 2012;5:260–3. [PMC free article] [PubMed] [Google Scholar]
- 6.Terada T. Histopathologic study of the rectum in 1,464 consecutive rectal specimens in a single Japanese hospital: II. malignant lesions. Int J Clin Exp Pathol. 2013;6:385–94. [PMC free article] [PubMed] [Google Scholar]
- 7.Terada T. A clinicopathologic study of esophageal 860 benign and malignant lesions in 910 cases of consecutive esophageal biopsies. Int J Clin Exp Pathol. 2013;6:191–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Terada T. Malignant tumors of the small intestine: A histopathologic study of 41 cases among 1,312 consecutive specimens of small intestine. Int J Clin Exp Pathol. 2012;5:203–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Terada T. Esophageal cancers: A clinicopathologic and immunohistochemical study of 223 cases. Gastroenterol Res. 2009;2:148–51. doi: 10.4021/gr2009.05.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terada T. An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: II. Expresssion of MUC1, MUC2, MUC5AC, and MUC6 in normal mucosa and in 42 cases. Int J Clin Exp Pathol. 2013;6:613–21. [PMC free article] [PubMed] [Google Scholar]
- 11.Terada T. An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: I. Cytokeratin profile in 42 cases. Int J Clin Exp Pathol. 2013;6:703–10. [PMC free article] [PubMed] [Google Scholar]
- 12.Terada T. An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: III. Expressions of EMA, CEA, CA19-9, CDX-2, p53, Ki-67 antigen, TTF-1, vimentin, and p63 in normal mucosa and in 42 cases. Int J Clin Exp Pathol. 2013;6:630–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Terada T. Histopathologic study of the stomach using computer database in 10,000 consecutive gastric specimens: II. Malignant lesions [Google Scholar]
- 14.Int J Clin Exp Pathol(accepted)Terada T. Histopathologic study using computer database of the stomach in 10,000 consecutive gastric specimens: I. Benign conditions. Int [Google Scholar]
- 15.Lenzen R, Borchard F, Lübke H, Strohmeyer G. Colitis ulcerosa complicated by malignant lymphoma: Case report and analysis of published works. Gut. 1995;36:306–10. doi: 10.1136/gut.36.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luigi Adani G, Marcello D, Mazzetti J, Carrella G, Jorizzo F, Donini A. Malignant lymphoma complicating ulcerative colitis. Ann Ital Chir. 2000;71:603–7. [PubMed] [Google Scholar]
- 17.Luigi Adani G, Marcello D, Mazzetti J, Carrella G, Jorizzo F, Donini A. Malignant lymphoma complicatingulcerative colitis. Ann Ital Chir. 2000;71:603–7. [PubMed] [Google Scholar]
- 18.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–6. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 19.Terada T, Takeuchi T, Taniguchi M. Hepatobiliary cystadenocarcinoma with cystadenoma elements of the gall bladder in an old man. Pathol Int. 2003;53:790–5. doi: 10.1046/j.1440-1827.2003.01559.x. [DOI] [PubMed] [Google Scholar]
- 20.Terada T, Tanigichi M. Intraductal oncocytic papillary neoplasm of the liver. Pathol Int. 2004;54:116–23. doi: 10.1111/j.1440-1827.2004.01594.x. [DOI] [PubMed] [Google Scholar]
- 21.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;206:271–5. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]
- 22.Terada T. Extramuscular subcutaneous fibrolipoma containing foci of striated muscle cells: A hitherto unreported condition. Int J Clin Exp Pathol. 2013;6:113–5. [PMC free article] [PubMed] [Google Scholar]
- 23.Terada T. Vascular leiomyoma of the lung arising from pulmonary artery. Int J Clin Exp Pathol. 2013;6:97–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Mangla V, Pal S, Dash NR, Das P, Ahuja V, Sharma A, et al. Rectal MALT lymphoma associated with ulcerative colitis. Trop Gastroenterol. 2012;33:225–8. doi: 10.7869/tg.2012.55. [DOI] [PubMed] [Google Scholar]


