Abstract
Aminoacyl–tRNA synthetases are modular enzymes composed of a central active site domain to which additional functional domains were appended in the course of evolution. Analysis of bacterial genome sequences revealed the presence of many shorter aminoacyl–tRNA synthetase paralogs. Here we report the characterization of a well conserved glutamyl–tRNA synthetase (GluRS) paralog (YadB in Escherichia coli) that is present in the genomes of >40 species of proteobacteria, cyanobacteria, and actinobacteria. The E. coli yadB gene encodes a truncated GluRS that lacks the C-terminal third of the protein and, consequently, the anticodon binding domain. Generation of a yadB disruption showed the gene to be dispensable for E. coli growth in rich and minimal media. Unlike GluRS, the YadB protein was able to activate glutamate in presence of ATP in a tRNA-independent fashion and to transfer glutamate onto tRNAAsp. Neither tRNAGlu nor tRNAGln were substrates. In contrast to canonical aminoacyl–tRNA, glutamate was not esterified to the 3′-terminal adenosine of tRNAAsp. Instead, it was attached to the 2-amino-5-(4,5-dihydroxy-2-cyclopenten-1-yl) moiety of queuosine, the modified nucleoside occupying the first anticodon position of tRNAAsp. Glutamyl–queuosine, like canonical Glu–tRNA, was hydrolyzed by mild alkaline treatment. Analysis of tRNA isolated under acidic conditions showed that this novel modification is present in normal E. coli tRNA; presumably it previously escaped detection as the standard conditions of tRNA isolation include an alkaline deacylation step that also causes hydrolysis of glutamyl–queuosine. Thus, this aminoacyl–tRNA synthetase fragment contributes to standard nucleotide modification of tRNA.
Faithful translation of the mRNA genetic information into proteins relies on the correct attachment of an amino acid to its corresponding tRNA(s) by enzymes collectively known as aminoacyl–tRNA synthetases (aaRSs). These essential enzymes are structurally conserved (1, 2) and easily detected in the analysis of genome sequences (e.g., ref. 3). Prokaryotes usually have one gene for each aaRS, although the presence of two functional paralogous genes for a synthetase (e.g., lysS and lysU for Escherichia coli lysyl–tRNA synthetase; ref. 4) is well known. Analysis of the ever larger number of bacterial genome sequences revealed the occurrence of many aaRS paralogs containing regional deletions (compared to the full-length genes also present in the genome). Given the modular nature of the aaRS proteins (5), the presence of synthetase gene pieces in a genome was not surprising, because they are likely remnants of a complex evolutionary history (2, 3, 6, 7). Although these truncated aaRSs were considered to be pseudogenes (8), more recent attention to particular aaRSs paralogs revealed their involvement in important biological processes. For instance, HisZ, a histidyl–tRNA synthetase-like protein lacking the anti-codon domain, was shown to be essential for histidine biosynthesis in a number of bacteria (9). Likewise, an archaeal asparaginyl–tRNA synthetase paralog, lacking the anticodon-binding domain, is able to carry out tRNA-independent asparagine synthesis (10). The function of aaRS paralogs is not restricted to amino acid biosynthesis. Truncated alanyl–, prolyl–, and threonyl–tRNA synthetase-like proteins were shown to possess esterase function and be capable of specific hydrolysis of misacylated tRNA in trans (11, 12), a mechanism that should increase the fidelity of the translation process.
Here we report the characterization of another widely distributed aaRS paralog, a glutamyl–tRNA synthetase (GluRS)-related protein encoded by yadB in E. coli.
Materials and Methods
Oligonucleotides, DNA Sequencing, Radiochemicals, and Bacterial Strains. Oligonucleotides synthesis and DNAs sequencing was performed by the Keck Foundation Biotechnology Resource Laboratory at Yale University. Uniformly labeled [14C]Glu (254 mCi/mmol; 1 Ci = 37 GBq), sodium [32P]pyrophosphate (1–60 Ci/mmol), and [γ-32P]ATP (6,000 Ci/mmol) were from Amersham Biosciences. [14C]Asp (208 mCi/mmol) was from American Radiolabeled Chemicals, St. Louis.
E. coli strains W3110, W3110ΔyadB, DH5α, and the queuosine-deficient JE7350 (13) were used for tRNA preparations.
Cloning of the E. coli yadB Gene. The E. coli yadB gene (GenBank accession no. AE000123) was obtained by PCR from E. coli W3110 genomic DNA using the Expand High Fidelity PCR System (Roche Molecular Biochemicals). Oligonucleotide primers introduced an NdeI site at the 5′ end and a BamHI site at the 3′ end of the gene. The PCR product was cloned into the pCR2.1–TOPO vector (Invitrogen). After sequence confirmation, the DNA was digested with NdeI and BamHI and cloned into the pET15b vector (Novagen) digested with the same enzymes to form plasmid pDJSyadB.
Inactivation of yadB in E. coli. The deletion of the yadB gene was carried out by using the Red disruption system (14). Confirmation of the deletion was done by PCR using oligonucleotides complementary to the upstream and downstream sequence of yadB and complementary to the kanamycin gene. The sizes of the PCR products expected are 850 bp for the PCR using primers upstream of yadB and internal to the kanamycin gene, 1,280 bp for the PCR using primers downstream of yadB and internal to the kanamycin gene, and 471 bp as the amplified fragment of the kanamycin gene. Fragments of these lengths were observed on gel analysis (data not shown).
Purification of YadB. The plasmid pDJSyadB was transformed into E. coli BL21(DE3) (Invitrogen). The expression of yadB was performed by incubating the cells at 37°C until an absorbance of 0.6–0.8 A600 was reached, at which point 1 mM IPTG was added and incubated for 3 h at 25°C. Purification was performed with an Amersham Biosciences Äkta FPLC system using a TALON superflow Co2+-column (BD Biosciences/Clontech), followed by gel filtration on Superdex 200.
Preparation of E. coli tRNAs. Unfractionated tRNA was prepared from freshly grown or frozen E. coli cells as described (15). E. coli tRNA was fractionated by reverse-phase HPLC (Varian Pro-Star, Palo Alto, CA): 5 mg of unfractionated E. coli tRNA in 0.1 ml of sterile water was injected on a ProSphere 300 C4 column (250 mm × 10 mm, Alltech). The column was washed with 5 volumes of buffer A [20 mM NH4OAc/10 mM Mg(OAc)2/400 mM NaCl] at a 5 ml/min flow rate. RNA was eluted in a 300 ml (0–55%) gradient of buffer B [20 mM NH4OAc/10 mM Mg(OAc)2/400 mM NaCl/60% EtOH]. Fractions were collected (1 ml) and ethanol precipitated in the presence of glycogen (20 μg/ml), washed with cold 70% ethanol, dried, and resuspended in sterile water.
Purification of tRNAAsp. E. coli tRNAAsp was purified from unfractionated tRNA by affinity chromatography on immobilized Thermus thermophilus EF-Tu (16) as follows. Unfractionated E. coli tRNA was aminoacylated with pure E. coli aspartyl–tRNA synthetase (AspRS) and the aminoacyl–tRNA was separated from the uncharged tRNA by the formation of a ternary complex with the EF-Tu-GTP immobilized on a nickel nitrilotriacetic–agarose column. The eluted Asp–tRNAAsp was deacylated for 1 h at 37°C in 0.2 mM Tris-acetate, pH 9.0. This tRNAAsp could be charged to 1,100 pmol/A260.
Cloning and in Vitro Transcription of the E. coli tRNAAsp Gene. The tRNAAsp gene suited for in vitro T7 RNA polymerase transcription was cloned by annealing of two complementary DNA oligonucleotides and direct ligation into pUC18 plasmid by using BamHI and HindIII restriction sites. The clone was transformed into E. coli DH5α, sequenced, and amplified. The 3′ end of the transcription template was digested overnight with BstNI at 60°C. In vitro T7 RNA polymerase run-off transcription was conducted according to standard procedure (17). tRNAAsp transcripts were purified on a denaturing 12% polyacrylamide gel and recovered by soaking into 1M NaOAc pH6.0, followed by desalting on a Nap-5 Column (Amersham Biosciences).
Aminoacylation Assay. In vitro acylations with E. coli YadB and AspRS enzymes were carried out at 37°C in a 70-μl reaction mixture containing 100 mM Hepes-KOH, pH 7.2, 30 mM KCl, 12 mM MgCl2, 2 mM ATP, 2 mM DTT, 25 μM radioactive amino acid ([14C]Glu or [14C]Asp) plus 75 μM unlabeled amino acid, and 40–60 μg of unfractionated tRNA or 0.7–1.3 μg of pure tRNA. Enzyme concentration was 900 nM (YadB) or 225 nM (AspRS). Reactions were started by amino acid addition. Aliquots (15 μl) were taken at different times, spotted on Whatman 3MM filter paper discs, and washed twice with 5% TCA.
ATP-32PPi Exchange. Reactions were conducted in 100 mM Hepes-KOH, pH 7.2, 30 mM KCl, 12 mM MgCl2, 2 mM DTT, 2 mM KF, 2 mM sodium [32PPi] (2 cpm/pmol), varying concentrations of ATP (0–5 mM), varying concentrations of glutamate (0–50 mM), 150 nM YadB in a final volume of 0.2 ml at 37°C. To detect possible tRNA-dependent glutamate activation by YadB, unfractionated E. coli tRNA was added to the reaction mixture in a variety of concentrations (0.1–2 μM of estimated tRNAAsp). [32P]ATP formation was followed by specific absorption on acid-washed Norit [200 μl of a 1% suspension (wt/vol) of Norit in a solution of 0.4 M sodium pyrophosphate solution containing 15% (vol/vol) perchloric acid] and filtration on Whatman GF/C filters followed by washing with 15 ml of water and 5 ml of ethanol.
Analysis of Aminoacyl–tRNA. As an additional test to determine aminoacyl–tRNA formation with YadB, periodate oxidation followed by β elimination was used (18) with the following modifications. After aminoacylation with YadB or AspRS (positive control), half of the reaction was deacylated with 0.2 mM Tris-acetate, pH 9.0. After ethanol precipitation, the RNA of both samples was treated in the dark with 2.5 mM NaIO4 in 50 mM sodium acetate, pH 5.0, for 1 h at 37°C. The reaction was stopped by adding 50 mM glucose and incubated at 0°C for 1.5 h. The tRNA was precipitated and further processed as described (18). tRNAs were detected by Northern blotting. For this purpose, the portion of the gel containing the tRNAs was electroblotted onto a Hybond-N+ membrane (Amersham Bioscience) by using a Hoefer Electroblot apparatus (Amersham Pharmacia) at 20 V for 1 h with 40 mM Tris-acetate, pH 4.2 and 2 mM EDTA as transfer buffer. Membranes were then baked at 72°C for 2 h, and tRNAs detected by hybridization with a 5′-[32P]-oligo-deoxyribonucleotide probe complementary to nucleotides 32–51 of tRNAAsp.
Digestion of tRNA to Nucleosides. tRNA was hydrolyzed to nucleosides by using nuclease P1 and Antarctic (acid) phosphatase (New England BioLabs); enzyme amounts are per 20 μg tRNA. Typically, 1/10 vol 10× Antarctic phosphatase buffer (pH 5) was added to the tRNA solution in water, followed by 2 units of nuclease P1 (2 units/μl; Boehringer). After incubation for 2 h at 37°C, 1 unit of acid phosphatase (1 unit/μl) was added, and the reaction was continued a further 2 h.
Liquid Chromatography/Electrospray Ionization-Mass Spectrometry of Digested tRNA. tRNA hydrolyzates were analyzed directly, without cleanup. Digests of bulk W3110 tRNA were analyzed on an HP1090 liquid chromatograph coupled directly to a Micromass Quattro II mass spectrometer. Nucleosides were separated on a Develosil C30 column by using a multilinear gradient essentially as described (19), except that the flow rate was 0.3 ml/min and the concentration of buffer A was 5 mM. The ion source and nebulizer temperatures were 140°C and 275°C, respectively. Data were collected by selected ion recording of m/z 410 (MH+ for queuosine), and m/z 539 (expected MH+ ion for glutamyl–queuosine). Purified tRNAAsp was analyzed by using a Waters CapLC liquid chromatograph coupled without solvent stream splitting to a Micromass QTof2 mass spectrometer. Both instruments were controlled by using a Micromass MASSLYNX v. 3.3 data system. Nucleosides were separated on a Luna C18(2) column (150 × 0.5; Phenomenex) at a flow rate of 17 μl/min using a multilinear gradient essentially as described (19) with buffer A, 5 mM ammonium acetate (pH 5.3), and buffer B, 40% (aq.) CH3CN. Ion source and nebulizer temperatures were 80°C and 135°C, respectively; the eluate was conducted directly into the mass spectrometer without splitting. Data were collected over a mass range of m/z 100–600.
Results
The GluRS Paralog YadB. Analysis of bacterial genome sequences revealed the presence of a widely occurring GluRS paralog (named yadB in E. coli). YadB genes are found in >40 organisms that belong to the proteobacterial, cyanobacterial, and actinobacterial groups. The E. coli yadB gene encodes a protein of 308 aa. The known YadB proteins constitute a well conserved group (>45% amino acid identity) related to bacterial GluRS. Sequence alignment of the bacterial YadB orthologs and comparison with bacterial GluRS revealed the presence of the conserved Rossmann fold class I defining signature sequences (HVGG and KLSK for E. coli GluRS) and a well conserved amino acid binding pocket. The crucial glutamate binding site residues Arg-5, Ser-9, Glu-41, Asn-191, and Arg-205 of T. thermophilus GluRS (20) were strictly conserved in the YadB proteins, indicating that glutamate probably binds in a similar fashion in both GluRS and YadB. However, YadB is only 65% as large as GluRS; it lacks the C-terminal portion (171 aa for E. coli GluRS) that serves as the anticodon binding domain of this protein.
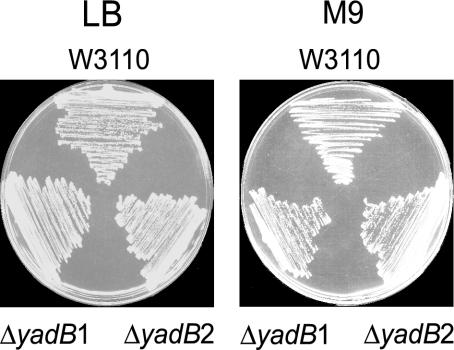
YadB Is Dispensable in E. coli. To assess the essentiality of the E. coli YadB protein, we used deletion and insertion strategy (14) to construct a yadB deletion in E. coli strain W3110, which was confirmed by PCR analysis of the resultant DNA (see Materials and Methods). This deletion strain was viable (Fig. 1) and grew in both rich (LB) and minimal (M9 glucose) medium at 37°C (and also at 42°C, data not shown). Thus, under the conditions tested, yadB is not essential. During the course of this investigation, a similar result was obtained by a genomic deletion strategy (21).
Fig. 1.
Growth of the W3110ΔyadB strain. Growth at 37°C of E. coli strain W3110 and two independent isolates of W3110ΔyadB on LB and M9 plates.
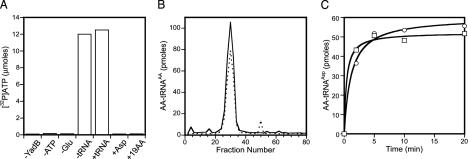
YadB Activates Glutamate in a tRNA-Independent Manner. The enzymatic functions of the recombinant N-terminal His6–YadB protein, purified to homogeneity by two-step column chromatography, was tested in amino acid activation. ATP-PPi exchange was performed in the presence and absence of unfractionated E. coli tRNA with either glutamate, aspartate, or with a mixture of 19 amino acids (except glutamate). YadB is capable of activating glutamate specifically (Fig. 2A); however, in contrast to GluRS, YadB can carry out this process in the absence of tRNA (a similar finding was published very recently, ref. 22). This observation is consistent with the fact that the amino acid-binding site is conserved in GluRS and YadB, but the lack of the anticodon binding domain in YadB should prohibit the major conformational rearrangements observed in the active site of the T. thermophilus GluRS upon tRNA binding (19). Determination of the kinetic parameters in ATP-PPi exchange experiments revealed a much lower glutamate affinity of YadB than of E. coli GluRS (7.5 mM vs. 0.66 mM, ref. 23), whereas the KM for ATP was similar for both enzymes (440 μM vs. 420 μM, ref. 23). The turnover rate for glutamate activation was also in the same range as the one observed for GluRS (11 s-1 vs. 1.77 s-1, ref. 24).
Fig. 2.
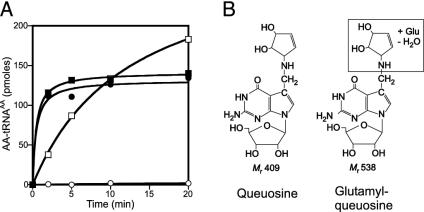
Glutamate activation and transfer onto tRNA by YadB. (A) YadB-dependent amino acid activation measured by ATP-PPi exchange. The lanes are: -YadB; -ATP; -Glu; -tRNA, Glu in the absence of tRNA; +tRNA, Glu in the presence of tRNA; +Asp, Asp alone; +19 AA, all amino acids except Glu. (B) Charging of tRNA fractions from reverse-phase HPLC with aspartate using E. coli AspRS (dotted line) and with glutamate using YadB (solid line). (C) Charging of purified tRNAAsp with aspartate using E. coli AspRS (□) and with glutamate using YadB (○).
YadB Transfers Glutamate to tRNAAsp. Given that YadB lacks a large part of the tRNA binding domain (compared to GluRS), we wanted to see whether YadB was capable of transferring activated glutamate onto tRNA. Surprisingly, ≈3% of unfractionated E. coli tRNA could be glutamylated under standard conditions. Because some GluRS enzymes can charge tRNAGlu and tRNAGln (e.g., refs. 25 and 26), we purified and tested these tRNA species; however, neither tRNA was a substrate for YadB (data not shown). To identify the tRNA substrate for YadB, E. coli tRNA was fractionated by HPLC reverse-phase chromatography (27). Assaying the column fractions (Fig. 2B) showed a coincidence of glutamylation by YadB with aspartylation by AspRS, suggesting that tRNAAsp might also be a YadB substrate and be glutamylated. This was confirmed by charging of EF-Tu affinity chromatography (16) purified tRNAAsp; this tRNA proved to be an equally good substrate for both pure YadB and pure E. coli AspRS (Fig. 2C). As expected for the labile ester linkage between glutamate and tRNA, Glu–tRNAAsp could be deacylated at pH 9.0 (data not shown). An independent report of Glu–tRNAAsp formation by YadB has recently appeared (28).
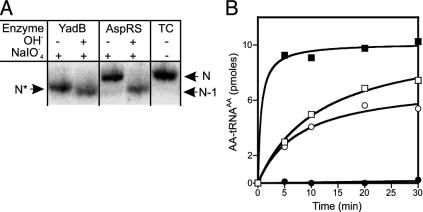
Glutamate Is Not Transferred to the tRNA's 3′-Terminal Adenosine. The nature of Glu–tRNAAsp was further examined by electrophoretic mobility shifts on a sequencing gel (18). Periodate oxidation of the vicinal cis diols of the tRNA's terminal ribose, followed by mild base treatment, leads to elimination of the terminal adenosine (29). However, if the tRNA's terminal adenosine is aminoacylated, periodate oxidation is blocked. After subsequent deacylation, the originally charged tRNA retains its length, whereas the originally free tRNA species will be 1 nt shorter and can be separated by a sequencing gel (e.g., ref. 18). Comparison of the electrophoretic mobilities (Fig. 3A) of periodate-treated Glu–tRNAAsp, Asp–tRNAAsp, and deacylated tRNA showed that, as expected, Asp–tRNAAsp was 1 nt longer than deacylated tRNA. However, the oxidation product of Glu–tRNAAsp, revealed only a very small mobility difference compared to that of oxidized deacylated tRNA, indicating that glutamate is not attached to the tRNA's 3′ end. This result was confirmed by checking the acceptor activities of periodate-treated aminoacyl–tRNA. Glu–tRNAAsp was subjected to periodate treatment, deacylated, and recharged either by E. coli AspRS with aspartate or by YadB with glutamate. Periodate-treated tRNA could be successfully reaminoacylated with glutamate by YadB, but could no longer be aspartylated by AspRS (Fig. 3B). Conversely, periodate-treated Asp–tRNAAsp could only be recharged with aspartic acid (data not shown). The results of both experiments show that glutamate is not esterified to the 3′-terminal adenosine, but to some other nucleotide in tRNAAsp.
Fig. 3.
Analysis of periodate-treated Glu–tRNAAsp and Asp–tRNAAsp. (A) Glu–tRNAAsp and Asp–tRNAAsp was treated with periodate and then lysine, deacylated, and subjected to gel electrophoresis for size determination. tRNA was visualized by hybridization with 32P-labeled probes against tRNAAsp. N, position of intact tRNAAsp; N-1, tRNAAsp without the terminal adenosine; N*, YadB-generated Glu–tRNAAsp lacking the terminal adenosine; tRNA control. (B) Glu–tRNAAsp was treated with periodate, deacylated and then recharged with either glutamate and YadB (○) or aspartate and E. coli AspRS (•). Untreated tRNA is aminoacylated by YadB (□) and AspRS (▪).
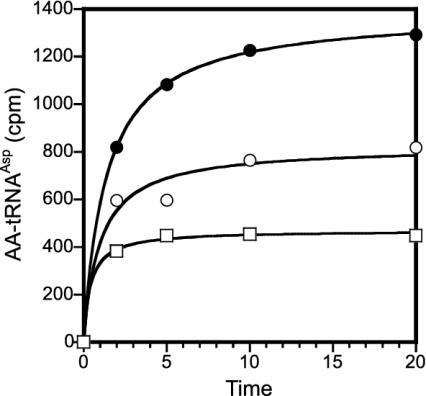
Pure E. coli tRNAAsp Can Be Acylated with Aspartate and Glutamate Simultaneously. The results above suggested that it might be possible to charge aspartate and glutamate simultaneously to tRNAAsp. This was indeed the case; Fig. 4 shows that simultaneous acylation with [14C]Asp and [14C]Glu in the presence of the E. coli AspRS and YadB enzymes led to a tRNA molecule carrying both amino acids, aspartate attached at the tRNA's acceptor end and glutamate bound to an unknown position in tRNAAsp.
Fig. 4.
Simultaneous acylation of purified E. coli tRNAAsp with aspartate and glutamate. Charging with [14C]Asp and AspRS (□), [14C]Glu using YadB (○), and [14C]Asp and AspRS in the presence of [14C]Glu and YadB (•). Accounting for the different specific activities of Asp and Glu (see Materials and Methods), aminoacylation at the 20 min time point is >90%.
The Hypermodified Nucleoside Queuosine Is the Possible Site of Glutamate Attachment. Given the alkali lability of the glutamyl–tRNA linkage and the sensitivity of the glutamylation activity to periodate-treated tRNA, we surmised that the site of glutamate attachment may have chemical features similar to the ribose at the 3′ end of tRNA. Additionally, the in vitro transcript of tRNAAsp, lacking all of the posttranscriptional modifications, could not be glutamylated by YadB, whereas it was a very good substrate for the AspRS (data not shown). This strongly suggested that glutamate might be acylated to a modified nucleoside present in tRNAAsp. A modified nucleoside in tRNAAsp that would have the appropriate properties is queuosine, the first anticodon nucleoside (position 34) in tRNAAsp (30). Queuosine, 7-{5-[(4S,5R-dihydroxy-2-cyclopenten-1-yl)amino]methyl}-7-deazaguanosine (Fig. 5B) (31) presents both a possible functional amino group and a cyclopentene moiety bearing vicinal cis diols. These hydroxyl groups are reactive, as some mammalian tRNAs contain modified versions of queuosine in which the 5-hydroxyl is mannosylated or galactosylated (32). To determine whether queuosine is the “acceptor” for glutamate, we prepared tRNA from an E. coli mutant strain deficient in the first step of queuosine formation (13). This Q-deficient tRNA could not be glutamylated by YadB (Fig. 5A), strongly suggesting that queuosine provides the site for glutamate attachment.
Fig. 5.
Glutamylation and aspartylation of queuosine-deficient tRNA and structures of queuosine and glutamyl–queuosine. (A) tRNA isolated from the queuosine-deficient E. coli JE7350 strain and wild-type tRNA was acylated in vitro. (▪) wild-type tRNA, [14C]Asp, and AspRS; (•) Q-deficient tRNA, [14C]Asp, and AspRS; (□) wild-type tRNA, [14C]Glu, and YadB; (○) Q-deficient tRNA, [14C]Glu, and YadB. (B) Queuosine and glutamyl–queuosine.
In addition to tRNAAsp, queuosine is also found in tRNAAsn, tRNAHis, and tRNATyr. We have not yet tested the ability of these tRNAs to be glutamylated by YadB.
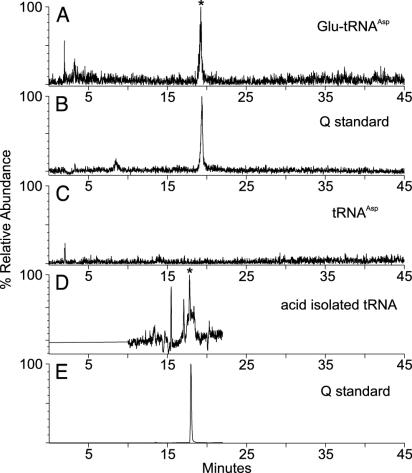
YadB Glutamylates the Nucleoside Queuosine in tRNAAsp. Therefore we decided to prepare pure Glu–tRNAAsp from the W3110ΔyadB deletion strain in vitro and subject the RNA hydrolyzate to electrospray ionization liquid chromatography MS analysis. The calculated molecular mass of glutamyl–queuosine is 538.24 (MH+ ion, m/z 539.24). As shown in Fig. 6A, the glutamylated tRNAAsp contains a nucleoside eluting at 19.2 min whose measured mass is 539.2, consistent with an assignment as glutamyl–queuosine. The control tRNA lacks this species (Fig. 6C). In a separate tandem mass spectrometry analysis of this component (from unfractionated tRNA; data not shown), an m/z 163 ion, assigned as loss of the cyclopentenediolamine side chain from the 7-deazaguanine moiety of queuosine, is likewise produced from the glutamyl–queuosine nucleoside. These data establish that the 2-amino-5-(4,5-dihydroxy-2-cyclopenten-1-yl) moiety of queuosine is glutamylated (Fig. 5B). However, the nature of the functional group (OH- or NH-) and the position of glutamate attachment, as well as the nature of the glutamate carboxyl involved (α or ε), remains to be established.
Fig. 6.
Liquid chromatography/electrospray ionization-mass spectrometry analysis of nucleoside mixtures derived from purified W3110ΔyadB tRNAAsp before and after YadB-catalyzed glutamylation (A–C) and from unfractionated W3110 tRNA isolated under acidic conditions (D and E). Reconstructed ion chromatograms for m/z 539.2, the MH+ ion expected for glutamyl–queuosine (A); m/z 410.2, the MH+ ion expected for queuosine (B); m/z 539.2 from tRNAAsp (C). Selected ion recording for m/z 539.2, the MH+ ion expected for glutamyl–queuosine (D) and m/z 410.2, the MH+ ion expected for queuosine (E). The glutamyl–queuosine peak is marked with an asterisk (A and D). Because this figure contains two different experimental runs (A–C, and D and E), two queuosine standards (B and E) are included.
Glutamyl–Queuosine Is a Normal Constituent of E. coli tRNA. Like the glutamyl–tRNA linkage at the 3′ terminus of tRNA, glutamyl–queuosine could be hydrolyzed by mild alkaline treatment. This raised the question whether glutamyl–queuosine is present in vivo in normal tRNA, as this modification may have previously escaped detection because the standard conditions of tRNA isolation (in preparation for modified nucleoside analysis) include an alkaline deacylation step. We therefore used acidic conditions to isolate tRNA from the wild-type W3110 E. coli strain. Analysis of this unfractionated tRNA (Fig. 6D) revealed the presence of glutamyl–queuosine. Therefore we conclude that this modified nucleoside is present in normal E. coli tRNA, and that YadB contributes to posttranscriptional nucleotide modification of tRNA.
Discussion
It should not be unexpected that an aaRS paralog acylates tRNA at a position different from the acceptor helix to which the amino acid is normally attached. YadB has lost most of the GluRS tRNA binding region, and as such may not have sufficient binding surface to recognize an entire tRNA. However, tRNA modifying enzymes usually recognize much less of the tRNA molecule. For instance, the minimal requirement of tRNA–guanine transglycosylase, the enzyme involved in queuosine base insertion into tRNA, is a UGU sequence in a 7-base loop (33, 34). Most importantly, the orientation in which the tRNA interacts with YadB is very different from that with GluRS, because the anticodon, not the acceptor helix, must be close to the enzyme's active site.
Several other modified nucleosides are known that contain “intact” amino acid building blocks. Lysidine in the first anti-codon position of tRNAIle is important for both codon and aaRS recognition; this modified nucleoside is formed by direct attachment of lysine to cytidine in the presence of ATP (35). N6-threonylcarbamoyladenosine and its derivatives are also amino acid containing nucleosides; however, their ATP-dependent biosynthesis (36, 37) requires introduction of an additional carbonyl group.
The biological function(s) of queuosine are relatively unknown; however, the presence of this modified nucleoside correlates with modulation of translational fidelity (e.g., ref. 38). What might be the role of glutamyl–queuosine? The fact that yadB is absent from many bacterial genomes indicates that this modified nucleoside does not have a central universal importance. On the other had, even queuosine is not essential under normal growth conditions in E. coli, as viable queuosine-deficient strains exist. However, such strains show a marked reduction of viability when the cells are kept under unsuitable conditions for growth (13). The high KM for glutamate of YadB might indicate that this reaction only takes place when glutamate concentration rises in the cell. Interestingly, a yadB ortholog is present in Shigella flexnerii, where the intracellular concentration of the virulence-related transcriptional regulator VirF was shown to be dependent on the presence of queuosine (39). Thus, it would be interesting to determine the effect of the glutamate modification. In any case, differences in the modification states of mannosyl–queuosine and galactosyl–queuosine in mammalian tRNAs from normal and cancer cells (40) suggest that hypermodifications of queuosine are relevant.
Acknowledgments
We thank Hiroaki Nakano for help with the yadB deletion and Steven Pomerantz for assistance with the liquid chromatography MS analyses. This work was supported by grants from the National Institute of General Medical Sciences (to J.A.M. and D.S.) and the Department of Energy (to D.S.).
Abbreviations: aaRS, aminoacyl–tRNA synthetase; GluRS, glutamyl–tRNA synthetase; AspRS, aspartyl–tRNA synthetase.
See Commentary on page 7493.
References
- 1.Arnez, J. G. & Moras, D. (1997) Trends Biochem. Sci. 22, 211-216. [DOI] [PubMed] [Google Scholar]
- 2.O'Donoghue, P. & Luthey-Schulten, Z. (2003) Microbiol. Mol. Biol. Rev. 67, 550-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woese, C. R., Olsen, G. J., Ibba, M. & Söll, D. (2000) Microbiol. Mol. Biol. Rev. 64, 202-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leveque, F., Plateau P, Dessen P. & Blanquet, S. (1990) Nucleic Acids Res. 18, 305-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schimmel, P. & Ripmaster, T. (1995) Trends Biochem. Sci. 20, 333-334. [DOI] [PubMed] [Google Scholar]
- 6.Rould, M. A., Perona, J. J., Söll, D. & Steitz, T. A. (1989) Science 246, 1135-1142. [DOI] [PubMed] [Google Scholar]
- 7.Ribas de Pouplana, L. & Schimmel, P. (2000) Cell. Mol. Life Sci. 57, 865-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamour, V., Quevillon, S., Diriong, S., N′Guyen, V. C., Lipinski, M. & Mirande, M. (1994) Proc. Natl. Acad. Sci. USA 91, 8670-8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sissler, M., Delorme, C., Bond, J., Ehrlich, S. D., Renault, P. & Francklyn, C. (1999) Proc. Natl. Acad. Sci. USA 96, 8985-8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy, H., Becker, H. D., Reinbolt, J. & Kern, D. (2003) Proc. Natl. Acad. Sci. USA 100, 9837-9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahel, I., Korencic, D., Ibba, M. & Söll, D. (2003) Proc. Natl. Acad. Sci. USA 100, 15422-15427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong, F. C., Beuning, P. J., Silvers, C. & Musier-Forsyth, K. (2003) J. Biol. Chem. 278, 52857-52864. [DOI] [PubMed] [Google Scholar]
- 13.Noguchi, S., Nishimura, Y., Hirota, Y. & Nishimura, S. (1982) J. Biol. Chem. 257, 6544-6550. [PubMed] [Google Scholar]
- 14.Datsenko, K. A. & Wanner, B. L. (2000) Proc. Natl. Acad. Sci. USA 97, 6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raczniak, G. Becker, H. D., Min, B. & Söll, D. (2001) J. Biol. Chem. 276, 45862-45867. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro, S., Nock, S. & Sprinzl, M. (1995) Anal. Biochem. 228, 330-335. [DOI] [PubMed] [Google Scholar]
- 17.Sampson, J. R. & Uhlenbeck, O. C. (1988) Proc. Natl. Acad. Sci. USA 85, 1033-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi, H., Gabriel, K., Schneider, J., Otten, S. & McClain, W. H. (2003) RNA 9, 386-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pomerantz, S. C. & McCloskey, J. A. (1990) Methods Enzymol. 193, 796-824. [DOI] [PubMed] [Google Scholar]
- 20.Sekine, S., Nureki, O., Dubois, D. Y., Bernier, S., Chênevert, R., Lapointe, J., Vassylyev, D. G. & Yokoyama, S. (2003) EMBO J. 22, 676-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerdes, S. Y., Scholle, M. D., Campbell, J. W., Balazsi, G., Daugherty, M. D., Somera, A. L., Kyrpides, N. C., Anderson, I., Gelfand, M. S., Bhattacharya, A., et al. (2003) J. Bacteriol. 185, 5673-5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campanacci, V., Dubois, D. Y., Becker, H. D., Kern, D., Spinelli, S., Valencia, C., Pagot, F., Salomoni, A., Grisel, S., Vincentelli, R., et al. (2004) J. Mol. Biol. 337, 273-283. [DOI] [PubMed] [Google Scholar]
- 23.Ravel, J. M., Wang, S.-F., Heinemeyer, C. & Shive, W. (1965) J. Biol. Chem. 240, 432-438. [PubMed] [Google Scholar]
- 24.Kern, D. & Lapointe, J. (1979) Biochemistry 18, 5809-5818. [DOI] [PubMed] [Google Scholar]
- 25.Skouloubris, S., Ribas de Pouplana, L., De Reuse, H. & Hendrickson, T. L. (2003) Proc. Natl. Acad. Sci. USA 100, 11297-11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salazar, J. C., Ahel, I., Orellana, O., Tumbula-Hansen, D., Krieger, R., Daniels, L. & Söll, D. (2003) Proc. Natl. Acad. Sci. USA 100, 13863-13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cayama, E., Yepez, A., Rotondo, F., Bandeira, E., Ferreras, A. C. & Triana-Alonso, F. J. (2000) Nucleic Acids Res. 28, E64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubois, D. Y., Blaise, M., Becker, H. D., Campanacci, V., Keith, G., Giegé, R., Cambillau, C., Lapointe, J. & Kern, D. (2004) Proc. Natl. Acad. Sci. USA 101, 7530-7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitfeld, P. R. (1954) Biochem. J. 58, 390-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekiya, T., Mori, M., Takahashi, N. & Nishimura, S. (1980) Nucleic Acids Res. 8, 3809-3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasai, H., Ohashi, Z., Harada, F., Nishimura, S., Oppenheimer, N. J., Crain, P. F., Liehr, J. G., Von Minden, D. L. & McCloskey, J. A. (1975) Biochemistry 14, 4198-4208. [DOI] [PubMed] [Google Scholar]
- 32.Kasai, H., Nakanishi, K., Macfarlane, R. D., Torgerson, D. F., Ohashi, Z., McCloskey, J. A., Gross, H. J. & Nishimura, S. (1976) J. Am. Chem. Soc. 98, 5044-5046. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi, S., Ueda, T., Hori, H., Yamazaki, N., Okada, N. & Watanabe, K. (1994) J. Biol. Chem. 269, 32221-32225. [PubMed] [Google Scholar]
- 34.Curnow, A. W. & Garcia, G. A. (1995) J. Biol. Chem. 270, 17264-17267. [DOI] [PubMed] [Google Scholar]
- 35.Soma, A., Ikeuchi, Y., Kanemasa, S., Kobayashi, K., Ogasawara, N., Ote, T., Kato, J., Watanabe, K., Sekine, Y. & Suzuki, T. (2003) Mol. Cell 12, 689-698. [DOI] [PubMed] [Google Scholar]
- 36.Körner, A. & Söll, D. (1974) FEBS Lett. 39, 301-306. [DOI] [PubMed] [Google Scholar]
- 37.Elkins, B. N. & Keller, E. B. (1974) Biochemistry 13, 4622-4628. [DOI] [PubMed] [Google Scholar]
- 38.Iwata-Reuyl, D. (2003) Bioorg. Chem. 31, 24-43. [DOI] [PubMed] [Google Scholar]
- 39.Durand, J. M., Dagberg, B., Uhlin, B. E. & Björk, G. R. (2000) Mol. Microbiol. 35, 924-935. [DOI] [PubMed] [Google Scholar]
- 40.Costa, A., Païs de Barros, J. P., Keith, G., Baranowski, W. & Desgrès, J. (2004) J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 801, 237-247. [DOI] [PubMed] [Google Scholar]