Abstract
Venomous mammals are rare, and their venoms have not been characterized. We have purified and characterized the blarina toxin (BLTX), a lethal mammalian venom with a tissue kallikrein-like activity from the submaxillary and sublingual glands of the short-tailed shrew Blarina brevicauda. Mice administered BLTX i.p. developed irregular respiration, paralysis, and convulsions before dying. Based on the amino acid sequence of purified protein, we cloned the BLTX cDNA. It consists of a prosequence and an active form of 253 aa with a typical catalytic triad of serine proteases, with a high identity with tissue kallikreins. BLTX is an N-linked microheterogeneous glycoprotein with a unique insertion of 10 residues, L106TFFYKTFLG115. BLTX converted kininogens to kinins, which may be one of the toxic pathogens, and had dilatory effects on the blood vessel walls. The acute toxicity and proteolytic activity of BLTX were strongly inhibited by aprotinin, a kallikrein inhibitor, suggesting that its toxicity is due to a kallikrein-like activity of the venom.
Venomous mammals are rare (1), and their venoms have not been characterized. Toxic constituents produced by other vertebrates, such as snake, lizard, and frog, have been well studied. Several bird species (the genus Pitohui) are also known to contain steroidal alkaloid toxin as a chemical defense (2). However, only a few members of the order Insectivora produce toxic compounds, including the Haitian solenodon (Solenodon paradoxus), the European water shrew (Neomys fodiens), the Mediterranean shrew (Neomys anomalous), and the American short-tailed shrew (Blarina brevicauda) (3–6). Mammals in other orders, such as Monotremata (platypuses and echidnas), are also suspected to have venom (7). Envenoming by a platypus causes immediate excruciating pain that evolves toward prolonged hyperalgesia. Defensin-like peptides (8) and the C-type natriuretic peptides (9) have been isolated from this venom. However, the precise mechanism of the excruciating pain caused by platypus toxin in humans remains unclear.
Among mammals, the short-tailed shrew B. brevicauda (Say, 1923) is well known to produce a potent venom in its saliva, which is toxic to mammals, such as mice, voles, rabbits, and cats (1, 3). Human accounts of bites from Blarina describe a local burning sensation around the tooth puncture marks and subsequent swelling (10). In general, soricine shrews consume large amounts of food to meet their high metabolic demands (11). Although they belong to the order Insectivora, B. brevicauda do not eat insects and invertebrates exclusively but also vertebrates, even larger than themselves, such as murid rodents and frogs (1, 11–13). Therefore, this shrew species may use its venom to paralyze and catch larger preys.
The stability of crude extracts of Blarina submaxillary glands has been studied (14, 15). Its toxicity was lost rapidly at pH >7 and gradually at room temperature. Products from precipitation of the crude extracts with ammonium sulfate (33–80% saturation) are toxic. Therefore, it has been suggested that the most toxic ingredient is a high molecular weight water-soluble protein, not a carbohydrate or nucleoprotein (1). However, because the Blarina venom loses its toxicity rapidly after the victim's death, its purification and more precise identification have not been successfully completed.
In this study, we purify and characterize the B. brevicauda venom, blarina toxin (BLTX). This venom exerted a kallikrein-like proteolytic activity similar to that of a lizard venom (16).
Materials and Methods
Chemicals. Peptidyl-4-methylcoumaryl-7-amide (MCA) substrates and kinins were obtained from Peptide Research Institute (Osaka). Human low molecular weight kininogen (LK), RNAlater, inhibitors for the enzyme assay and deglycosylation reaction, porcine pancreatic kallikrein (PPK), and thromboxane A2 analogue (U46619) were purchased from Sigma. Human high molecular weight kininogen (HK) was provided by Biogenesis (Bournemouth, U.K.). Peptide-N4-(N-acetyl-β-glucosaminyl)asparagine amidase (N-glycosidase) (EC 3.5.1.52) and O-glycopeptide endo-d-galactosyl-N-acetyl-α-galactosamino hydrolase (O-glycosidase) (EC 3.2.1.97) were supplied by Roche Diagnostics. Plasma kallikrein specific inhibitor and other chemicals were purchased from Wako Pure Chemical (Osaka).
Harvesting and Handling of Tissue Specimens. Fifty B. brevicauda shrews (28 males and 22 females) were trapped with Sherman traps (SFA type; H. B. Sherman Traps, Tallahassee, FL) baited with peanut butter and oats, within the Fresh Air Camp and the E. S. George Reserve, University of Michigan (Livingstone Country, MI), between July 22 and July 26, 2002, and September 9 and September 13, 2003. The animals were deposited in the mammal collection of one of the authors (S.D.O.) at Hokkaido University. Tissue samples were excised immediately after the animals were trapped and were stored in acetone at -20°C as described (3). Tissues were also immediately stored in RNAlater for mRNA extraction, according to the manufacturer's instructions. All animals were treated in accordance with the ethical guidelines of the International Association for the Study of Pain (17).
Purification of Venom. All procedures were performed at 4–6°C and all separation systems and columns were purchased from Amersham Biosciences. Fifteen submaxillary and sublingual gland specimens were homogenized with 0.85% NaCl and centrifuged as described (3). The supernatant was loaded twice on a Hiroad 26/60 Superdex 200pg column connected to an FPLC system and eluted with 0.85% NaCl at a flow rate of 1 ml·min-1, with monitoring of absorbance at 280 nm. The fraction with toxic activity was dialyzed against 20 mM [bis(2-hydroxyethyl)amino]tris(hydroxymethyl)methane (Bis-Tris)·HCl (pH 7.0), then applied to a Mono Q HR 5/5 column connected to the same system as above, which had been previously equilibrated with the same buffer. A linear gradient of NaCl from 0.1 to 0.2 M was applied for 100 min at a flow rate of 0.5 ml·min-1, with monitoring at 280 nm. After the active fraction was diluted 5-fold with 20 mM Bis-Tris·HCl (pH 7.0), it was applied twice to a Mini Q PE 4.6/50 column connected to a SMART system and equilibrated with 20 mM Bis-Tris·HCl (pH 7.0) containing 60 mM NaCl. After equilibrium was reached, a linear gradient of NaCl from 0.06 to 0.1 M was applied for 120 min at a flow rate of 0.3 ml·min-1, with monitoring at 280 nm. The protein concentration was measured with a Bio-Rad protein assay kit with BSA as a standard. SDS/PAGE of BLTX was performed by using a precast 10–20% polyacrylamide gradient gel (Daiichi Kagaku, Kyoto), and stained with a Silver Stain Kit, Protein (Amersham Biosciences).
2D-PAGE Analysis. BLTX and crude extracts of Blarina salivary glands (1.5 individuals) were subjected to 2D-PAGE with an IPGphor System and a Multiphor II System (Amersham Biosciences). Isoelectric-focusing electrophoresis was performed with an 18-cm Immobiline DryStrip (Amersham Biosciences) in a pI range between 5.0 and 6.0. SDS/PAGE was performed with ExcelGel SDS XL Gradient 12–14 (Amersham Biosciences). Cysteine residues were carbamidomethylated with iodoacetamide. The protein bands were stained with SYPRO Ruby (Molecular Probes), then scanned with a variable image analyzer (Typhoon 9400, Amersham Biosciences), at excitation and emission wavelengths of 532 nm and 610 nm, respectively. The spots on 2D-PAGE were analyzed with imagemaster 2d elite software according to the manufacturer's instructions.
Determination of the N-Terminal and Internal Amino Acid Sequences. Stained spots of crude salivary gland extracts were excised from a 2D gel and incubated with a sequence-grade modified trypsin (Promega) at 37°C for 19 h. The tryptic peptides mixture was separated with a Develosil RP-AQUEOUS AR-5 column (Nomura Chemical, Aichi, Japan), with monitoring at 215 nm. The amino acid sequences of these peptides were analyzed with an automatic sequencer (model 477A, Applied Biosystems) and/or with a matrix-assisted laser desorption ionization–time-of-flight/time-of-flight mass spectrum analyzer (model 4700, Applied Biosystems) by using α-cyano-4-hydroxycinnamic acid as a matrix. An aliquot of the stained spots on 2D-PAGE was used for the analysis of the N-terminal amino acid sequence.
Deglycosylation. Purified BLTX (60 ng) was denatured by boiling for 3 min in 10 μl of 1% SDS and 5% 2-mercaptoethanol solution, then digested in 50 μl of deglycosylation buffer containing 1% octyl β-d-glucopyranoside, 0.5 unit of N-glycosidase, 0.5 milliunit of O-glycosidase, 10 μM 4-amidinophenylmethanesulfonyl fluoride, 5 μg·ml-1 leupeptin, and 20 mM phosphate (pH 7.2) at 37°C for 23 h. After incubation, samples were lyophilized, SDS/PAGE was performed by using a precast 12.5% polyacrylamide gel, and the gels were stained with a Silver Stain Kit, Protein.
Acute Toxicity Assay. Lethality was tested by i.p. injection into male ddY and C57BL mice weighing between 9.9 and 10.1 g (Japan SLC, Hamamatsu, Japan). The injectates were diluted up to 0.5 ml with 0.85% NaCl. For the inhibition of acute toxicity by protease inhibitors, crude extracts of Blarina salivary glands (0.2 individuals) or purified BLTX were incubated with various inhibitors and EDTA for 30 min at 0°C before injection.
Enzyme Assays. Enzyme activity using peptidyl-MCA substrates was analyzed with a fluorescence spectrophotometer (model 650–10 MS, Hitachi, Tokyo) by measuring the released amount of 4-amino-7-methylcoumarin, with excitation and emission wavelengths of 370 nm and 460 nm, respectively, at 37°C, as described (18). For inhibition studies, BLTX was preincubated with various inhibitors for 5 min at 37°C before measurements of activity.
The conversion of kininogens to small peptides was measured by incubating 0.2 mg·ml-1 LK and HK in 50 mM Tris·HCl buffer, pH 9.0, with purified BLTX or PPK (14 ng) at 37°C, with a reaction mixture volume of 20 μl. At 0, 10, 30, and 60 min, 5 μl of the reaction mixture was withdrawn and added to 5 μl of denaturing solution, as described (16). The samples were boiled for 5 min, SDS/PAGE was performed by using a precast 10% polyacrylamide gel, and the gels were stained with a Silver Stain Kit, Protein. For the HPLC analysis of degradation products, LK and HK (10 μg) were incubated as described above, except for a concentration of BLTX of 0.4 μg in 50 μl of reaction mixture. The mixture was boiled for 10 min, diluted with 150 μl of 0.1 M ammonium acetate, and ultrafiltered through a Microcon YM-10 (cut-off Mr, 10,000; Millipore). After lyophilization of 180 μl of filtrate, the degradation products were loaded on a Develosil RP-AQUEOUS AR-5 column and eluted with 18% acetonitrile containing 0.1% trifluoroacetic acid, at a flow rate of 120–130 μl·min-1, with monitoring at 215 nm.
Measurement of Vascular Tone. Fresh bovine hearts were obtained from a local slaughterhouse. The coronary arteries were dissected free of connective tissue and cut into 3- to 4-mm-long ring segments and suspended in a tissue bath containing a Krebs–Ringer bicarbonate buffer at 35°C and bubbled with a mixture of 95% O2 and 5% CO2. Isometric tension was measured as described (19). Briefly, arterial rings were slowly attached to a basal tension of 1.5 g. After equilibration, KCl (60 mM) was repeatedly added and rinsed until stable and reproducible contractions were observed. The thromboxane mimetic U46619 (500 nM) was added to increase the tension to ≈50–75% of maximal KCl contraction. After a 20- to 30-min equilibration period, 1–100 nM bradykinin (BK) as a standard, or LK treated with BLTX for 1 h, was added, and the changes in vascular tone were recorded.
Molecular Cloning. Total RNA was isolated from submaxillary and sublingual glands of B. brevicauda with a Qiagen RNeasy kit according to the manufacturer's instructions. First-strand cDNA template was prepared from 1 μg of total RNA by using ImProm-II Reverse Transcriptase in the presence of an oligo(dT) primer. After cDNA synthesis, PCR was carried out by using the degenerate oligonucleotides KLK-His and KLK-Ser, designed from the conserved amino acid sequences within the active sites of mammal kallikreins. All of the primer sequences, including gene-specific primers, KLK-1–5, are shown in Table 1. The PCR conditions were as follows: an initial denaturing at 95°C for 5 min, followed by 33 cycles of 30 sec of denaturing at 95°C, 30 sec of annealing at 60°C, and 30 sec of extension at 72°C, and a final extension at 72°C for 7 min. Amplified products were subcloned into the pGEM-T Easy vector (Promega) and sequenced by using dye terminator reactions with an automated ABI Prism 3100 Avant Genetic Analyzer (Applied Biosystems). The 22 independent clones were sequenced, three of which were completely matched with the sequences corresponding to the amino acid sequences within BLTX. Two other tissue kallikrein isoforms were obtained from the other clones.
Table 1. Oligonucleotide primers used for PCR.
| Name | Sequence | Forward/reverse | Corresponding amino acid sequence |
|---|---|---|---|
| KLK-His | 5′-GGT(GT)(ACTG)TCAC(AG)GC(CT)GC(CT)CA(CT)TG-3′ | Forward | WV(LV)TAAHC |
| KLK-Ser | 5′-CCTGA(AG)TCTCCC(ACTG)(CT)ACA(AG)GTGTC-3′ | Reverse | D(ST)CVGDSG |
| Oligo(dT) | 5′-CGCAGGAATTTTTTTTTTTTTTT-3′ | Reverse | |
| KLK-1 | 5′-GGCAGGACCAGTCAGAACTATG-3′ | Forward | GRTSQNY |
| KLK-2 | 5′-CCCATGCCCACAAGTTTAAGG-3′ | Forward | HAHMFK |
| KLK-3 | 5′-GAAGGTCTTGTAGAAGAACGTCAG-3′ | Reverse | LTFFYKTF |
| KLK-4 | 5′-CTATCACCATGTGTTTCCTGCTCC-3′ | Forward | MCFLL |
| KLK-5 | 5′-GGGTGTTTAGCTGTGTGCCTTGA-3′ | Reverse | IKAHS* |
The terminal codon
Based on the deduced nucleotide sequence of PCR fragment, 3′-RACE was carried out with KLK-1 and the oligo(dT) primer. Hemi-nested PCR was performed by using KLK-2 and the oligo(dT) primer. For the 5′-RACE reaction, first-strand cDNA template was synthesized by using a Smart RACE cDNA Amplification Kit (BD Bioscience) according to the manufacturer's instructions and PCR was performed by using a universal primer mix(long,5′-CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT-3′; short, 5′-CTAATACGACTCACTATAGGGC-3′) and KLK-3.
The full-length BLTX cDNA was confirmed by PCR, by using KLK-4 and KLK-5. The PCR conditions were as follows: an initial denaturing at 94°C for 3 min, followed by 30 cycles of 30 sec of denaturing at 94°C and 90 sec of annealing and extension at 68°C, and a final extension at 72°C for 7 min. The amplified DNA fragments were subcloned and 10 independent clones were sequenced. All clones showed an identical sequence.
Results
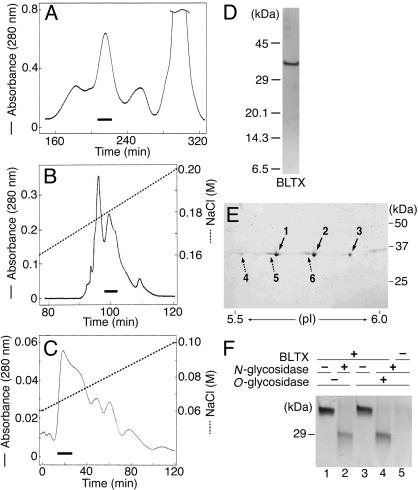
Purification of the BLTX. Instead of saliva, we used extracts from 500 mg of B. brevicauda submaxillary and sublingual glands as starting material and purified them through a series of standard chromatographic procedures. The toxicity coincided with a protein peak on analytical gel permeation and anion-exchange HPLC (Fig. 1 A–C). The purified venom, BLTX, was unstable, lost its toxicity under gel permeation chromatography at room temperature as well as by freezing and thawing, and displayed an absorbance wavelength peak at 280 nm. These properties are consistent with those of protein. The LD50 of BLTX injected i.p. was ≈1 mg·kg-1. Death occurred within 3–5 h, preceded by characteristic manifestations similar to those reported after administration of crude extracts (3, 15, 20), including rapid and irregular respiration, hypotension, hind limb paralysis, and convulsions. Purified BLTX yielded a single protein band with a molecular mass of ≈35 kDa under reducing conditions on SDS/PAGE (Fig. 1D) and of 32,400 by matrix-assisted laser desorption ionization–time-of-flight mass spectrometry.
Fig. 1.
Purification and electrophoresis of BLTX. (A) Gel-permeation chromatogram on a Hiroad 26/60 Superdex 200pg column. Eluant, 0.85% NaCl. The solid bars in A and B show toxic fractions. (B) Anion-exchange chromatogram on a Mono Q HR 5/5 column. Buffer, 20 mM Bis-Tris·HCl (pH 7.0) and a linear gradient of 0.1–0.2 M NaCl (broken line). (C) Second anion-exchange chromatogram on a Mini Q PE 4.6/50 column. Buffer, 20 mM Bis-Tris·HCl (pH 7.0) and a linear gradient of 0.06–0.10 M NaCl (broken line). The solid bar shows purified BLTX. (D) SDS/PAGE analysis of BLTX under reducing conditions. (E) 2D-PAGE analysis of BLTX. Solid and broken arrows show major and minor spots, respectively. (F) SDS/PAGE analysis of the BLTX before (lane 1) and after (lanes 2–4) deglycosylation. Lanes 2, 4, and 5, N-glycosidase treatment; lanes 3–5, O-glycosidase treatment. Lanes 2 and 4 show a single band with a molecular mass of 28 kDa.
2D-PAGE analysis of purified BLTX showed three major and three minor spots with similar molecular sizes but different pI values between 5.5 and 5.9, suggesting glycosylation of different complex oligosaccharides in each BLTX (Fig. 1E). Although BLTX consists of six components with different pI values, a single amino acid sequence of the 25 N-terminal residues analyzed was detected, starting from an Ile-Ile-Gly-Gly serine protease motif. The sequence of the N-terminal and tryptic peptide fragments (K1–K7) matched exactly those of BLTX deduced from the nucleotide sequence of the BLTX gene (Fig. 2).
Fig. 2.
Nucleotide sequence of cDNA and deduced amino acid sequence of BLTX. The amino acid sequence of the N-terminal 25 residues of the active protein determined is shadowed. Sequences of the tryptic peptides (K1–K7) are underlined. The translation-initiation site (atg) and stop codon (taa) are boxed. The putative N-glycosylation site in the BLTX sequence is highlighted in black.
cDNA Cloning and Sequence Analysis. We cloned BLTX cDNA from B. brevicauda submaxillary and sublingual glands by PCR, using degenerate oligonucleotides designed from the sequences of the N terminus, tryptic peptide fragments, and the consensus sequences of active sites of serine proteases. The isolated cDNA consisted of 971 bp and contained a putative single ORF of 846 bp with a proposed initiation codon of ATG at nucleotide 49 and a 3′ untranslated region of 77 bp with a polyadenylation signal (AATAAA) and a poly(A) tail (Fig. 2). The deduced amino acid sequence of this cDNA indicated that BLTX translated a 282-aa protein, with a prosequence and an active mature protein composed of 253 amino acids with a molecular mass of 28.1 kDa. Because of the presence of predicted N-glycosylation sites (Asn-80 and Asn-93) and the different pI values of BLTX (Fig. 1E), purified BLTX was treated with N- and/or O-glycosidase under denaturing conditions. After treatment with glycosidases, the 35-kDa BLTX band shifted to a single 28-kDa band on SDS/PAGE (Fig. 1F, lanes 2 and 4), corresponding to the molecular mass of the mature polypeptide backbone of BLTX calculated from its sequence.
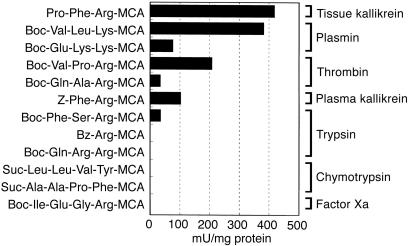
Enzymatic Properties of BLTX. The optimum pH of BLTX was 9.0, identical to that reported for tissue kallikreins (21, 22). It had a relatively narrow specificity among the substrates of serine proteases tested. It preferentially hydrolyzed Pro-Phe-Arg-MCA and Boc-Val-Leu-Lys-MCA, which are substrates of tissue kallikrein and plasmin, respectively (Fig. 3). Arg and Lys were favored in position P1, and hydrophobic amino acid residues were favored at P2. However, the substrates for plasma kallikrein, trypsin, factor Xa, and chymotrypsin were either poorly hydrolyzed or not hydrolyzed at all. The activity of BLTX was strongly inhibited by aprotinin (Ki = 3.6 × 10-10 M); moderately inhibited by secretory leukoprotease inhibitor (Ki = 4.8 × 10-7 M), the Kunitz-type soybean trypsin inhibitor, and leupeptin; and not inhibited by urinary trypsin inhibitor or α1-protease inhibitor (Table 2).
Fig. 3.
Substrate specificity of BLTX. One unit is defined as 1 μmol of 4-amino-7-methyl-coumarin released from peptidyl-MCA substrates per minute, at 37°C. Boc, t-butoxycarbonyl; Z, benzyloxycarbonyl; Bz, benzoyl; Suc, succinyl.
Table 2. Inhibitory effects of protease inhibitors and EDTA on the proteolytic activity of BLTX.
| Inhibitor* | Conc., μM | Residual activity,†% |
|---|---|---|
| None | - | 100 |
| Aprotinin | 1 | 17 |
| Leupeptin | 1 | 65 |
| Kunitz-type soybean trypsin inhibitor | 1 | 67 |
| α1-Protease inhibitor | 1 | 113 |
| Urinary trypsin inhibitor | 1 | 108 |
| Secretory leukoprotease inhibitor | 1 | 50 |
| Benzamidine | 1 | 78 |
| Phenylmethanesulfonyl fluoride | 1 | 85 |
| EDTA | 10,000 | 99 |
Substrate, Pro-Phe-Arg-MCA
Residual activity was calculated as a percentage of that of the enzyme without inhibitor
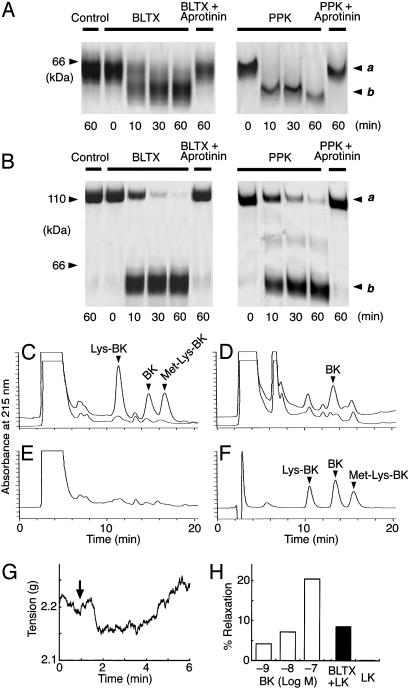
On SDS/PAGE analyses, BLTX converted human LK and HK into various peptides in a time-dependent manner (Fig. 4 A and B). PPK was used as a positive control, and the HK and LK degradation products resulting from incubation with BLTX and PPK appeared identical. Aprotinin, in a concentration of 10 μM, markedly suppressed the degradation of kininogens by BLTX. HPLC analysis of the degradation products revealed the release of BK, lysyl-BK, and methionyllysyl-BK from LK (in ≈1:2:1 ratios), and mostly BK from HK (Fig. 4 C–F). The various amino acid sequences were determined by MS-MS analysis. These products were identical to those formed by human tissue kallikrein, suggesting that BLTX exerts a tissue kallikrein-like activity. Furthermore, a prominent vasodilation by LK treated with BLTX was observed in the measurement of tension on the bovine coronary artery preconstricted by U46619. This vasodilation was equivalent to that produced by BK (Fig. 4 G and H).
Fig. 4.
Kininogen cleavage activity of BLTX. (A) SDS/PAGE analysis of the degradation of LK by BLTX or PPK at various time intervals and its inhibition by aprotinin. Band a, LK (64 kDa); band b, degraded products (59 kDa). Control, LK incubated without enzymes; + Aprotinin, LK was hydrolyzed by enzymes that have been preincubated with aprotinin (10 μM) for 5 min. (B) SDS/PAGE analysis of the degradation of HK by BLTX or PPK, as described for A. Band a, HK (110 kDa); band b, degraded products (58–63 kDa). (C) HPLC analysis of the peptides released from LK after incubation with (upper trace) and without (lower trace) BLTX. (D) HPLC analysis of the peptides released from HK as described in C. (E) A sample incubated without kininogens. (F) Standard kinins (50 ng each). (G) Transient vasodilation in bovine coronary artery produced by LK treated with BLTX. The artery was precontracted with U46619 (500 nM). Arrow indicates the time of LK injection. (H) Effect of BK-induced and the LK degradation products-induced relaxation on bovine coronary arteries. Results are expressed as percent relaxation of the U46619-treated rings, with 100% relaxation representing the basal tension.
To determine the relationship between the lethal and proteolytic activities of BLTX, we analyzed the effects of protease inhibitors on its acute toxicity (Table 3). Treatment of the crude extracts of Blarina salivary glands with aprotinin and leupeptin in maximal concentrations of 7.8 and 240 μM, respectively, abolished its acute toxicity, such that all treated animals survived. Although the administration of these inhibitors in concentrations equivalent to 50% of maximum did not completely suppress the lethal effects of BLTX, the amount of time to death after its administration was significantly lengthened. Treatment of crude toxin with a plasma kallikrein inhibitor (23) and EDTA, in concentrations of 1 and 10 mM, respectively, did not suppress its lethal properties. The lethality of purified BLTX was also inhibited by treatment with 7.8 μM aprotinin.
Table 3. Inhibitory effects of protease inhibitors and EDTA on the lethality of crude extracts of Blarina salivary glands.
| Conc., μM | Dead/alive* | Time to death, h | |
|---|---|---|---|
| Control | 5/0 | 0.5-2.0 | |
| Aprotinin | 7.8 | 0/3 | NA |
| Aprotinin | 3.9 | 3/0 | 2.0-3.5 |
| Leupeptin | 240 | 0/2 | NA |
| Leupeptin | 120 | 2/0 | 1.5-3.5 |
| EDTA | 10,000 | 3/0 | 0.5-2.0 |
| PKSI | 1,000 | 2/0 | 1.0-2.0 |
NA, not applicable; PKSI, plasma kallikrein specific inhibitor.
Mice surviving beyond 24 h were classified as “alive”
Discussion
Our study shows that the shrew Blarina, one of the smallest and most primitive mammals, produces BLTX, a multifunctional tissue kallikrein-like protease in saliva. BLTX consists of a prosequence and an active form of 253 aa with a presumed catalytic triad of serine proteases (His-44, Asp-121, and Ser-237). Its amino acid sequence bore the highest identity of 53.4% with human tissue kallikrein 1 (21) and identities of 45.1%, 33.6%, 32.4%, 30.4%, and 28.1% with, respectively, mouse tissue kallikrein 1 (22), gila toxin (GTX) (16), a lethal venom from the Mexican beaded lizard Heloderma horridum, human plasmin (24, 25), human plasma kallikrein (26), and human thrombin (27) (Fig. 5). BLTX has highly conserved flanking residues at a catalytic triad and 10 highly conserved cysteine residues that may form disulfide bonds and stabilize the catalytic pocket. It has a characteristic motif (residues 97–115) containing a unique insertion of 10 residues, L106TFFYKTFLG115. GTX, a similar toxin, also includes a nonhomologous insertion of seven residues, L123KRELFP129, in a near downstream location. In general, tissue kallikreins are acidic glycoproteins with molecular heterogeneity, and BLTX also shows microheterogeneity by glycosylation.
Fig. 5.
Structure-based sequence alignment of active forms of BLTX and related serine proteases. ♦ marks the putative active site residues. Highly conserved serine protease domains are underlined. Amino acid sequences that are identical in more than four of the seven sequences are highlighted in black. hK1, human tissue kallikrein 1; mGK1, mouse tissue kallikrein 1; huPK, human plasma kallikrein; GTX, gila toxin, a lethal venom from the Mexican beaded lizard H. horridum.
The enzymatic properties of BLTX, i.e. optimum pH, substrate and inhibitor specificities, and kininogen cleavage activity, were similar to those of tissue kallikreins. Although the potent inhibitory effect of aprotinin on the proteolytic activity of BLTX was similar to that exerted on tissue kallikrein, the efficiencies of other inhibitors, such as Kunitz-type soybean trypsin inhibitor and α1-protease inhibitor, were different from those of tissue kallikrein (28). In addition, a specific inhibitor of plasma kallikrein, had no effect on the toxicity of BLTX. These observations indicate that BLTX is a tissue kallikrein family protein with its own unique properties.
Several serine proteases have been identified in reptilian venom. Snake venom is well known to contain a thrombin-like enzyme, because of its fibrinolytic activity and interference with blood coagulation (29–31). However, the factors contributing to the lethality of snake venom are relatively small proteolytic degradation products with molecular sizes of 60–75 aa. Among lizard venoms, GTX and horridum toxin resemble mammal kallikreins with regard to their amino acid sequences and protease activities (16, 32–34). The LD50 values of GTX and horridum toxin injected i.v. are 2.5 mg/kg of body weight (16, 34), nearly equivalent or slightly higher than those of BLTX. Amounts of BLTX in submaxillary and sublingual glands were ≈160 μg per Blarina shrew weighing 10–20 g, estimated by the specific activity of BLTX and 14% in total kallikrein content in these tissues. Given the amounts of BLTX in the salivary glands, 10–20 μg of BLTX per mouse weighing 10–20 g may be an accurate LD50 value, or perhaps slightly excessive. In addition, BK generated by BLTX may play a role in catching vasodilated and paralyzed preys or in warning against the enemy. Kallikreins from other mammalian species, however, also generate BK from kininogen, although they are not lethal. These results suggest that a kallikrein activity-linked vasodilatation and undefined toxicity of BLTX may contribute to its lethality in pharmacological doses. Furthermore, it remains possible that the lethality of BLTX is enhanced by other constituents in saliva, because the lethality of horridum toxin is synergistically increased ≈8-fold when combined with GTX (16).
BLTX readily cleaved both LK and HK and produced kinins, including BK. BK is a mediator of inflammation, which increases vascular permeability, lowers blood pressure, raises the pain threshold, stimulates cell growth and differentiation (35, 36), and is degraded by kininase in vivo. Therefore, kinins produced by BLTX may be one of the main toxic agents, explaining some of the manifestations observed in mice after its injection, such as dyspnea, hypotension, and hypokinesia. In addition, tissue kallikreins degrade not only kininogens but also various proteins in vivo, including growth factors, hormones, and extracellular matrix, and produce bioactive compounds involved in apoptosis, angiogenesis, and tumor metastasis (37). These observations also suggest that proteolytic activity of BLTX is the source of one or more unknown toxic product(s) as well as of BK and related compounds.
Acknowledgments
The Blarina shrew project was conducted on property owned by the University of Michigan and in collaboration with Dr. P. Myers. We thank Drs. K. Suenaga and S. Kawada (Nagoya University), Mr. A. P. G. Dowling (University of Michigan), Ms. A. Uesugi, and Ms. F. Okabe (Hokkaido University) for collecting Blarina specimens, Drs. Y. Nakaya and N. Harada (University of Tokushima) for performing the vascular reactivity assay, Dr. T. Koyama and Mr. M. Iwatsuki (Nagoya University) for prompting stimulating discussion, and Dr. K. Nakanishi (Columbia University, New York) for critically reviewing the manuscript. The amino acid sequence analysis was performed, in part, at The Center for Analytical Instruments, National Institute for Basic Biology (Aichi, Japan). This work was supported, in part, by Grants-in-Aid for Scientific Research on Priority Area (A) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (to D.U.).
Abbreviations: BLTX, blarina toxin; MCA, 4-methyl-coumaryl-7-amide; LK, low molecular weight kininogen; HK, high molecular weight kininogen; PPK, porcine pancreatic kallikrein; Bis-Tris, [bis(2-hydroxyethyl)amino]tris(hydroxymethyl)methane; BK, bradykinin; GTX, gila toxin.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AB111919).
References
- 1.Dufton, M. J. (1992) Pharmacol. Ther. 53, 199-215. [DOI] [PubMed] [Google Scholar]
- 2.Dumbacher, J. P., Beehler, B. M., Spande, T. F., Garraffo, H. M. & Daly, J. W. (1992) Science 258, 799-801. [DOI] [PubMed] [Google Scholar]
- 3.Pearson, O. P. (1942) J. Mammal. 23, 159-166. [Google Scholar]
- 4.Rabb, G. B. (1959) Nat. Hist. Miscellanea 170, 1-3. [Google Scholar]
- 5.Pucek, M. (1959) Acta Theriolog. 3, 93-104. [Google Scholar]
- 6.Pucek, M. (1969) Bull. Acad. Pol. Sci. Serr. Sci. Biol. 17, 569-574. [Google Scholar]
- 7.Calaby, J. H. (1968) in Venomous Vertebrates, Venomous Animals and their Venoms, eds. Bücherl, W., Buckley, E. A. & Deulofen, V. (Academic, New York), Vol. 1, pp. 15-29. [Google Scholar]
- 8.Torres, A. M., de Plater, G., Doverskog, M., Birinyi-Strachan, L. C., Nicholson, G. M., Gallagher, C. H. & Kuchel, P. W. (2000) Biochem. J. 348, 649-656. [PMC free article] [PubMed] [Google Scholar]
- 9.de Plater, G., Martin, R. L. & Milburn, P. J. (1995) Toxicon 33, 157-169. [DOI] [PubMed] [Google Scholar]
- 10.Maynard, C. J. (1889) Contributions to Science Newtonville 1, 57-59. [Google Scholar]
- 11.Churchfield, S. (1990) The Natural History of Shrews (A & C Black, London), pp. 111-130.
- 12.Babcock, H. L. (1914) Science 40, 526-530. [DOI] [PubMed] [Google Scholar]
- 13.Getz, L. L., Larson, C. M. & Lindstrom, K. A. (1992) J. Mammal. 73, 591-596. [Google Scholar]
- 14.Ellis, S. & Krayer, O. (1955) J. Pharmacol. Exp. Ther. 114, 127-137. [PubMed] [Google Scholar]
- 15.Pucek, M. (1968) in Venomous Vertebrates, Venomous Animals and their Venoms, eds. Bücherl, W., Buckley, E. A. & Deulofen, V. (Academic, New York), Vol. 1., pp. 43-50. [Google Scholar]
- 16.Utaisincharoen, P., Mackessy, S. P., Miller, R. A. & Tu, A. T. (1993) J. Biol. Chem. 268, 21975-21983. [PubMed] [Google Scholar]
- 17.Zimmermann, M. (1983) Pain 16, 109-110. [DOI] [PubMed] [Google Scholar]
- 18.Kido, H., Yokogishi, Y., Sakai, K., Tashio, M., Kishino, Y., Fukutomi, A. & Katunuma, N. (1992) J. Biol. Chem. 267, 13573-13579. [PubMed] [Google Scholar]
- 19.Harada, N., Sakamoto, S., Niwa, Y. & Nakaya, Y. (2001) Am. J. Physiol. 280, H2911-H2919. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence, B. (1945) J. Mammal. 26, 393-396. [PubMed] [Google Scholar]
- 21.Fukushima, D., Kitamura, N. & Nakanishi, S. (1985) Biochemistry 24, 8037-8043. [DOI] [PubMed] [Google Scholar]
- 22.Mason, A. J., Evans, B. A., Cox, D. R., Shine, J. & Richards, R. I. (1983) Nature 303, 300-307. [DOI] [PubMed] [Google Scholar]
- 23.Wanaka, K., Okamoto, S., Bohgaki, M., Hijikata-Okunomiya, A., Naito, T. & Okada, Y. (1990) Thromb. Res. 57, 889-895. [DOI] [PubMed] [Google Scholar]
- 24.Forsgren, M., Raden, B., Israelsson, M., Larsson, K. & Heden, L.-O. (1987) FEBS Lett. 213, 254-260. [DOI] [PubMed] [Google Scholar]
- 25.Malinowski, D. P., Sadler, J. E. & Davie, E. W. (1984) Biochemistry 23, 4243-4250. [DOI] [PubMed] [Google Scholar]
- 26.Chung, D. W., Fujikawa, K., McMullen, B. A. & Davie, E. W. (1986) Biochemistry 25, 2410-2417. [DOI] [PubMed] [Google Scholar]
- 27.Degen, S. J. F. & Davie, E. W. (1987) Biochemistry 26, 6165-6177. [DOI] [PubMed] [Google Scholar]
- 28.Chao, J. (1998) in Handbook of Proteolytic Enzymes, eds. Barret, A. J., Rawlings, N. D. & Woessner, J. F. (Academic, San Diego), pp. 97-100.
- 29.Pirkle, H. (1998) Thromb. Haemostasis 79, 675-683. [PubMed] [Google Scholar]
- 30.Tu, A. T. (1996) Adv. Exp. Med. Biol. 391, 37-62. [DOI] [PubMed] [Google Scholar]
- 31.Rosing, J. & Zwaal, R. F. A. (1988) in Hemostasis and Animal Venoms, eds. Pirkle, H. & Markland, F. S., Jr. (Dekker, New York), pp. 3-27.
- 32.Hendon, R. A. & Tu, A. T. (1981) Biochemistry 20, 3517-3522. [DOI] [PubMed] [Google Scholar]
- 33.Datta, G. & Tu, A. T. (1997) J. Pept. Res. 50, 443-450. [DOI] [PubMed] [Google Scholar]
- 34.Tu, A. T. (2000) in Natural and Selected Synthetic Toxins: Biological Implications, ACS Symposium Series, eds. Tu, A. T. & Gaffield, W. (Oxford Univ. Press, New York), No. 745, pp. 283-301.
- 35.Colman, R. W. & Schmaier, A. H. (1997) Blood 90, 3819-3843. [PubMed] [Google Scholar]
- 36.Proud, D. & Kaplan, A. P. (1988) Annu. Rev. Immunol. 6, 49-83. [DOI] [PubMed] [Google Scholar]
- 37.Yousef, G. M. & Diamandis, E. P. (2002) Tumour Biol. 23, 185-192. [DOI] [PubMed] [Google Scholar]