Abstract
Resting state functional magnetic imaging (fMRI) is a novel means to examine functional brain networks. It allows investigators to identify functional networks defined by distinct, spontaneous signal fluctuations. Resting state functional connectivity (RSFC) studies examining child and adolescent psychiatric disorders are being published with increasing frequency, despite concerns about the impact of motion on findings. Here we review important RSFC findings on typical brain development and recent publications of child and adolescent psychiatric disorders. We close with a summary of the major findings and current strengths and limitations of RSFC studies.
Keywords: functional connectivity, resting state, brain development, functional MRI
Introduction
For decades, acquiring functional magnetic resonance imaging (fMRI) scans while research participants complete tasks has been the “gold standard” technique for understanding human brain function. However, over the last 5 years, publications and scientific presentations on “resting state fMRI,” “resting state functional connectivity, ” or “intrinsic functional connectivity” have become quite common. Here we briefly summarize this new brain imaging technique and its relevance to understanding the developing brain. Specifically, we review studies of typical development that have examined how resting state functional connectivity (RSFC) changes across development and in some of the most common psychiatric disorders seen in youth: autism spectrum disorders, mood and anxiety disorders, attention-deficit/hyperactivity disorder and psychotic disorders. We conclude with a summary and concerns that have been raised about this novel neuroimaging technique.
What is resting state functional connectivity?
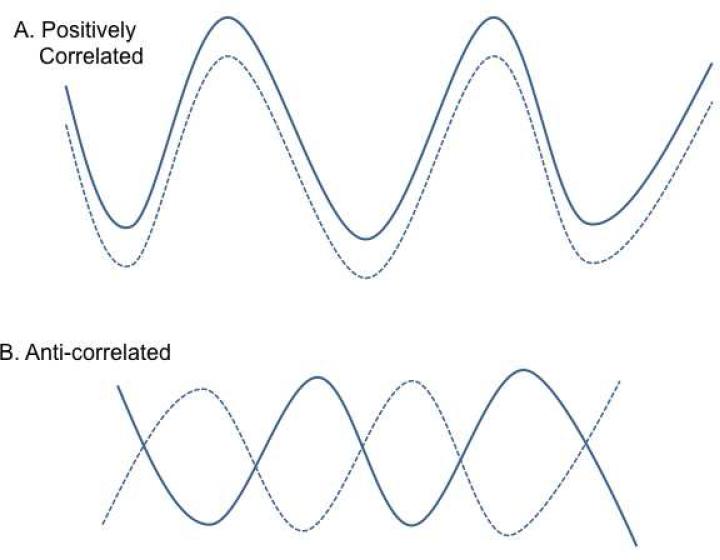
RSFC is a commonly used term in the scientific literature to describe a form of fMRI used in research studies where, in contrast to traditional fMRI scan paradigms, there is no specific task for the participants to complete; rather, the scan is acquired while the subject is “at rest.” Typically, participants are asked to lie still and to not think about anything in particular. Many studies provide instructions to stay awake with eyes closed, while others instruct participants to keep their eyes fixed on a neutral stimulus such as a cross, throughout the scan. The primary principle underlying RSFC is that the pattern of low-frequency fluctuations in the blood oxygen level-dependent (BOLD) signal is highly correlated between brain regions that form functional circuits, even in the absence of an experimental task (Figure 1) [1]. Resting state networks discussed in this paper are visually depicted in section (a) of Figure 2, while section (b) represents the structural connections that are thought to underlie the functional networks (reproduced with permission from [2]). RSFC allows for more facile comparisons across research sites and enables the study of brain function in populations typically excluded from task-based MRI studies (i.e., infants, developmentally disabled). Currently, the utility of RSFC is limited to non-clinical, research settings.
Figure 1.
The waveforms in A. and B. represent the low-frequency blood oxygen level-dependent (BOLD) signal fluctuations (x-axis) originating from 2 distinct locations in the resting brain, over time (y-axis). A. represents “positively” correlated waveforms that are often referred to as “positive resting state functional connectivity.” B. represents anti-correlated or “negative resting state functional connectivity” between 2 regions.
Typical Development
Changes During the First Two Years
RSFC has been studied in infants as young as two weeks old. The earliest infant study identified five functionally and spatially independent brain networks in 24-27 week-old-infants (n=12) [3]. Each of these networks has been described in adults, although the authors noted the absence of one of the best-characterized RSFC networks, the default mode network (DMN). The DMN is comprised of medial prefrontal, posterior cingulate, temporal and parietal cortices, which have been shown to be active, as a network, during rest or during low cognitive demand tasks [4]. Theories about the function of the DMN include “the retrieval and manipulation of episodic memories and semantic knowledge” [4], social or self-referential processing [5, 6] or stimulus independent thought [7, 8].
Another study that compared 2-4-week-olds (n=28), one-year-olds (n=26) and two-year-olds (n=21) reported that RSFC strength increased with age, with a more rapid developmental trajectory noted for sensorimotor as opposed to visual networks [9]. More recently, Gao and colleagues examined RSFC in neonates (n=51), one-year-olds (n=50) and two-year-olds (n=46) and found that two well-known networks (DMN and dorsal attention, which is thought to consciously orient attention toward stimuli [10]) evolved from isolated regions in neonates to cohesive networks at one year of age, with further expansion/strengthening of the networks at age two [11]. Whereas adult research has shown that these networks are anti-correlated (i.e., regions ossilating out of sync with each other) [12], this study found that the anti-correlation was absent at birth, but became apparent at one year and further enhanced by age two [11]. Thus, primitive RSFC networks appear to be present early in infancy, but rapidly mature over the first two years of life.
Changes from Childhood to Adulthood: Integration, Segregation, Homotopy and Anticorrelations
Several different techniques have been used to study changes in development in RSFC networks from childhood to adulthood. However, findings characterizing RSFC across development must be considered with caution, in light of recent work suggesting that head motion likely impacts and even negates some of these findings, as discussed in more detail below (“Conclusions”). In a series of studies, Fair and colleagues examined RSFC differences between children, adolescents and adults. They first examined attention control networks and found that each developmental phase was associated with an increase in long-distance connections (interpreted as improved “integration”) and a decrease in short-range connections [13]. They also found that four networks spanning the brain (DMN, fronto-parietal, cingulo-opercular, and cerebellar) showed increased integration and segregation across development [14, 15]. Utilizing a large sample (n=100) of 12-30-year-olds, Stevens and colleagues also found that increasing age was associated with within-network connectivity growth and more efficient between-network connectivity [16].
Kelly and colleagues examined RSFC development of anterior cingulate cortex (ACC) networks from childhood through adulthood [17]. Similar to the studies above, they noted a decrease in short-range (local) connectivity with age, and an increase in long-range connectivity. Further, they noted that the greatest degree of developmental change was observed in emotionally relevant regions (originating in the subgenual ACC) and a network of regions involved in social processing (originating in the perigenual ACC). RSFC has also been used to predict individual brain maturity [18, 19], with the greatest predictive power within the cingulo-opercular network, a network though to be invoved with cognitive control [20]. Brain maturity was associated with overall weakening of between-network connections, and strengthening of within-network connections [18].
Zuo and colleagues examined homotopy, or the degree to which regions in each hemisphere are connected, of RSFC networks across development [21]. In addition to age-related changes, they found sex differences in homotopy, where females had greater homotopy in posterior cingulate cortex, medial prefrontal cortex, and middle and superior frontal cortex, but males had greater homotypy in cerebellum, parahippocampus and fusiform cortex. Further, there was a sex by age interaction for dorsolateral prefrontal cortex and amygdala where females and males showed opposite developmental trajectories.
Narrowing in on specific networks, a recent study examined “task positive” (intraparietal sulcus, frontal eye fields, and middle temporal region; regions routinely exhibit task-related activations [22]; also known as “ventral attention network”) and “task negative” (similar regions to the DMN; regions routinely exhibit task-related deactivations [22]) RSFC networks in children (n=63) and adults (n=28) [23]. They found that adults showed greater connectivity between the task-positive network and dorsolateral prefrontal cortex and stronger anti-correlations between the “task negative” network and anterior insula, parietal and posterior cingulate cortices [23]. Chai and colleagues also examined the development of anti-correlated networks in 8-24 year-olds [24] and found that as age increased, anti-correlations also increased between networks (i.e., medial and dorsolateral prefrontal cortex, and between lateral parietal and supramarginal gyrus and precuneus). Authors noted that the correlation between regions in these networks was positive in childhood but negative in adults, with adolescent connectivity levels falling in the middle [24].
To summarize, across normal development, RSFC networks have been found to span longer distances in the brain and become increasingly segregated, resulting in both within and between-network efficiency. It appears that particular RSFC networks (i.e., involving subgenual ACC) are tied to specific developmental functions (i.e., emotional development). Gender differences have received little experimental attention, but do appear to be present in studies that examined them [21]. Finally, anti-correlated networks have been reproduced in several studies as markers of more advanced, adult-like development [23, 24]. All of the studies reviewed here have had the limitation of cross-sectional designs and concerns for the impact of head motion on findings. Longitudinal examinations of developing RSFC networks are needed to definitively characterize changes across development.
Resting State Functional Connectivity Studies with Clinical Samples
1. Autism Spectrum Disorders
Current RSFC research comparing individuals with autism spectrum disorders (ASD) to healthy comparison participants (number of participants ranging from 20-39 for each group) has implicated RSFC abnormalities, including both abnormally high and low RSFC, in nearly every region of the cortex and the cerebellum [25-28].
Studies characterizing specific behavioral problems associated with ASD have largely focused on social and communication deficits. Assaf and colleagues found abnormally decreased RSFC between precuneus and other DMN areas in ASD patients compared to controls (n=16 per group) [29]. This atypical connectivity was associated with social and communication deficits [29]. A similar study found that increased RSFC between posterior cingulate and temporal cortex was associated with social impairment (n=20 ASD n= 19 controls) [27]. Furthermore, Abrams and colleagues found that abnormally low RSFC between temporal cortex and regions of the brain associated with reward was associated with the severity of communication deficits among children with ASD (n=20 ASD; n=19 controls) [30]. This finding suggests that communication deficits in ASD may either be the result of, or exacerbated by, a reduced ability to associate social interaction with a sense of reward. This is important to consider in the treatment of ASD as other, more salient, rewarding stimuli may be necessary to advance a patient's social interaction skills. Indeed, this is a practice employed in an applied behavioral analysis (ABA) framework, which emphasizes the use of positive reinforcement in the promotion of social behaviors [31]. Further research examining RSFC before and after initiation of treatments would be of important clinical significance, as RSFC of these regions may normalize over time with interventions.
Regarding the developmental trajectories of RSFC among children and adolescents with ASD, findings have been inconsistent. One study found that relative to controls (n=41), ASD participants (n=39) had smaller increases in RSFC within the DMN over time [28], while another found that group differences in DMN RSFC appear to diminish with age (n=40 ASD; n=40 controls) [32]. Of note, these studies were both cross sectional, and overall functioning and treatment histories varied greatly in participants in these studies. Future research should carefully consider the influence of these factors and implement longitudinal designs to better understand the developmental trajectory of RSFC in ASD.
2. Anxiety Disorders
Studies in this area, while few, have typically taken the approach of examining the association of pediatric anxiety severity with RSFC in healthy participants. Among typically developing children (n=76), Qin and colleagues found that anxiety was associated with increased RSFC between the amygdala and regions involved in emotion perception and regulation and attention [33]. Furthermore, these findings were specific to anxiety symptoms, as other symptoms, such depressive symptoms, did not show a relationship to RSFC. Also among healthy youth (n=67), anxiety severity was related to increased DMN-insula RSFC [34]. A cross-sectional study found that life stress in infancy was associated with higher childhood cortisol and, later, with decreased amygdala-prefrontal RSFC. In turn, amygdala- prefrontal RSFC was inversely related to adolescent anxiety symptoms (n=57) [35]. Thus, anxiety symptoms in healthy samples implicate networks involving the insula, prefrontal cortex and amydgala.
Roy and colleagues scanned adolescents with generalized anxiety disorder (GAD; n=15) and found abnormal amygdala RSFC between the medial prefrontal cortex, insula and cerebellum compared to controls (n=20) [36]. Furthermore, anxiety severity was associated with amygdala RSFC with the insula and superior temporal gyrus. Clearly further research is needed to replicate these GAD findings and examine other unstudied pediatric anxiety disorders, such as separation anxiety disorder, social anxiety disorder and specific phobias.
3. Mood Disorders
A larger body of literature has used resting-state fMRI to examine the neural circuitry underlying mood disorders in children and adolescents. These studies have broadly implicated abnormalities within circuitry that has been shown to be relevant for processing emotions [37]. Specifically, this circuitry encompasses limbic regions such as amygdala and hippocampus, which are important for both immediate emotional experiences and emotional memory, and regulatory cortical regions such as the prefrontal cortex and the perigenual anterior cingulate cortex (pgACC). Additional regions in this network include the subgenual anterior cingulate cortex (sgACC), which has been strongly implicated in the pathophysiology of mood disorders through the use of several different types of imaging modalities and also post-mortem studies [38-40], and the insula, which is known to be important for processing the emotional salience of an individual's experiences. Thus, abnormalities in this circuit could broadly impact emotional processing, resulting in mood states seen in both unipolar and bipolar depressive disorders.
Major Depressive Disorder
Several studies examining depressed youth have shown abnormalities in sgACC networks, although the pattern of findings has varied. In a small study of mostly medicated adolescents with MDD (n=12) versus controls (n=14), Cullen and colleagues reported that depressed teens showed lower RSFC between sgACC and a network comprising dorsal ACC, several frontal and temporal regions, and insula [41]. These findings have been corroborated in a sample of 36 children (ages 7-11), 17 of which had a history of MDD [42]. However, other studies found greater RSFC between sgACC and frontal regions in unmedicated adolescents with MDD (n=23) compared to controls (n=36) [43] and in adolescents/young adults with MDD (n=18) compared to controls (n=20) [44]. Furthermore, while Gaffrey and colleagues found greater RSFC between the sgACC and precuneus among their children with histories of MDD [42], Connolly et al. found lower RSFC between sgACC and precuneus in their adolescent MDD group [43]. Divergence of findings in the pattern of sgACC RSFC abnormalities across studies may stem from differences in methodological steps, sample characteristics (e.g. age, pubertal status, age of MDD onset, medication status, co-morbidity), or a general difficulty in replicability of RSFC findings. Larger studies are needed to clarify the RSFC of the sgACC in pediatric MDD.
Brain regions comprising the striatum (caudate, putamen, and nucleus accumbens) are thought to play an important role in reward processing, which is known to be impaired in MDD [45]. Gabbay and colleagues examined RSFC centered around the striatum in medication-free adolescents with MDD (n=21) and healthy controls (n=21) [46]. They reported that the MDD group showed greater RSFC between striatal regions and dorsomedial prefrontal cortex and ACC. They also reported a diverse set of connections that were associated with anhedonia [46]. In contrast, Davey and colleagues found decreased ACC-caudate RSFC on older adolescents with MDD [44]. Although the directionality varied, both studies implicated abnormal fronto-striatal neural circuits in the pathophysiology of depression, especially with respect to anhedonic symptomatology.
The amygdala is another important brain region investigated in mood-disordered youth. This region is involved in the processing of negative emotion [47] and has consistently been implicated in mood disorders [48]. Luking and colleagues studied amygdala RSFC in a sample of children (ages 7-11) with either a history of preschool-onset MDD (n=13), a maternal history of MDD (n=11), both (n=13), or neither (n=14) [49]. They found that in comparison to low-risk children, the at-risk groups showed decreased negative amygdala connectivity in a network comprised of cortical regulatory regions, but greater positive amygdala connectivity with a network of limbic regions. More recently, Cullen and colleagues examined amygdala RSFC in a large sample of unmedicated adolescents with MDD (n=41) versus healthy controls [50]. They found that adolescents with MDD showed lower amygdala RSFC with hippocampus, parahippocampus and brainstem, but increased amygdala–precuneus RSFC than controls. These findings partially overlapped with the Luking at-risk findings [49], suggesting that amygdala connectivity abnormalities may be present both in childhood and adolescence, before and after onset of the disorder.
Bipolar Disorder
Three studies have examined RSFC in youth with bipolar disorder (BD). They used quite different approaches, and each revealed a different aspect of the aberrant RSFC. Dickstein and colleagues examined RSFC stemming from dorsolateral PFC, amygdala, and nucleus accumbens and found a group difference with the dorsolateral PFC analysis, in which the BD group (n=15) showed greater negative RSFC with the right superior temporal gyrus, while controls (n=15) had positive RSFC in this circuit [51]. Authors noted that the abnormal fronto-temporal circuit is also implicated in memory and learning and could represent an underlying mechanism for the cognitive deficits involved in BD.
In a more recent paper, Wu and colleagues used an automated method to examine RSFC across the entire brain [52]. They found that in comparison to controls, youth with BD (n=34) had greater levels of involvement of the dorsal ACC, within affective and executive networks, and greater wide spread connectivity within a sensorimotor network than controls (n=40). Authors speculated that the excessive involvement of ACC in both affective and executive networks could explain the association with BD and poor academic performance.
Third, Xiao and colleagues recently studied regional homogeneity (ReHo) in 15 adolescents with BD versus 15 healthy controls [53]. Relative to controls, BDs showed lower ReHo in several cortical areas, but greater ReHo in several limbic areas. Furthermore, elevated limbic ReHo was correlated with manic symptoms. Thus, three papers each using distinct methods report cortical-limbic RSFC abnormalities. Caution must be taken in reading the BD literature, however, given the concern for likely differences between depressed, manic and euthymic mood states [54]. Considering the difficulty in diagnosing these disorders in youth, additional work with larger, carefully characterized samples is needed.
4. Combined Mood and Anxiety Disorders
Depressive and anxiety disorders are often studied together due to their high comorbidity and their categorization as internalizing disorders [55]. Among children with a history of depression and anxiety (n=30), Sylvester and colleagues found reduced RSFC of the “task positive” network compared to healthy controls (n=42) [56]. Another study found that maltreatment in childhood was associated with decreased RSFC at the age of 18 between the hippocampus and subgenual ACC in both males and females with internalizing symptoms (n=64) [57]. Decreased RSFC between the amygdala and subgenual ACC was also found, but only in females [57]. RSFC in this study was found to mediate the relationship between childhood maltreatment and later internalizing symptoms, suggesting that childhood maltreatment may lead to disrupted connectivity of the fear circuit, which may lead to increased levels of internalizing symptoms.
5. Attention-Deficit/Hyperactivity Disorder (ADHD)
Of all psychiatric disorders diagnosed in youth, RSFC has been best studied in ADHD. A multi-site data sharing effort termed ADHD-200 [58], has recently resulted in publications with very large samples. For example, ADHD brains (n=757) exhibited altered RSFC between default network and ventral attention networks [59]. Specifically, diminished anti-correlation was observed between posterior cingulate cortex (DMN) and anterior insula and supplementary motor area (ventral attention network). Additionally, the DMN was hypoconnected within-network and had abnormal interconnections between several other networks. This paper replicates similar DMN-related findings from other papers with smaller sample sizes [60-62]. Given that these regions are involved in directing and sustaining attention, it is logical that they are found to be abnormal in a disorder with inattention as one of its hallmark symptoms.
Clinically meaningful applications of RSFC in ADHD have also begun to appear. Methylphenidate, a primary treatment for ADHD has been found to influence RSFC of multiple regions including dorsolateral prefrontal, parietal and visual cortices [63] and DMN and the task positive network [64] in pediatric samples. Similarities between RSFC networks have been identified between disorders with shared symptoms (e.g., autism and ADHD) [65], providing neurobiological substantiation for disorder-crossing traits. Clinically observed symptoms have been linked to specific RSFC networks. For example, youth with ADHD and high parent ratings of emotional lability have been found to have abnormal amygdala RSFC, even after accounting for hyperactivity [66]. Finally, RSFC has been used to test models to diagnose ADHD using brain activity alone, although with mixed success [67, 68]. Research geared toward clinical applications of RSFC is still in it's infancy, but it appears that such studies are likely to emerge in other psychiatric disorders affecting youth, as more publications emerge.
6. Psychotic Disorders
The use of fMRI in individuals with psychotic disorders is limited by the cognitive dysfunction associated with these illnesses, making performance on in-scanner experimental tasks difficult to interpret [69, 70]. Resting-state fMRI circumvents the issue of in-scanner task performance and has proven to be a useful tool in schizophrenia research, revealing new information about the functional organization and connectivity of the brain [71-73].
There is increasing interest in studying individuals at ultra-high risk (UHR) for psychosis or those in their first-episode of psychosis (FEP), as they consist of younger individuals with fewer confounding factors associated with chronically ill psychotic populations such as antipsychotic drug exposure or medical comorbidities. Many studies in these early populations have examined RSFC of the DMN, finding abnormal coherence within DMN structures such as the medial PFC, lateral temporal cortex, precuneus, posterior cingulate cortex, and parietal cortex [74-76]. Studies have also examined RSFC between DMN structures and other brain regions, finding abnormal connectivity with “task positive” networks [75] as well as the dorsolateral prefrontal cortices (DLPFC) [77]. Some of these studies have also examined inter-hemispheric connectivity, finding decreased RSFC in frontal and temporal regions as well associations between aberrant connectivity and positive and negative symptoms, as well as cognitive dysfunction [74].
A study by Lui et al. utilized resting-state fMRI to investigate the effects of antipsychotic medication on regional and neural network function in treatment naïve FEP subjects (n=34), examining RSFC both before and six weeks after the initiation of second generation antipsychotic medication. Findings revealed that after this short duration of antipsychotic treatment, participants exhibited increased spontaneous regional neural activity in association with symptom improvement, as well as an attenuation of connectivity across widely distributed neural networks. These results may help explain the beneficial effects of antipsychotic medication, hypothetically because of improvements in neurons’ ability to function more synchronously with other regions. Importantly, this study highlights the promise that resting state fMRI may hold for strategic development of novel therapeutic agents, as well as for study of biomarkers for patient response to medication in psychotic illness [78].
Conclusions
RSFC has been used to study the major child and adolescent psychiatric disorders, albeit with mixed conclusions. The field is still in its infancy and all findings reviewed here require replication. However, as the methodology continues to advance, RSFC is a promising tool for populations who struggle to comply with tasks and is well suited for large-scale, multi-site, and longitudinal studies, like those characterizing child and adolescent development. Despite these advantages, aspects of RSFC have been met with skepticism among some researchers [70, 79], as discussed in more detail below.
Limitation 1: What are people actually doing at rest?
Interpretation of RSFC findings are subject to the limitation that investigators cannot be sure what their subjects are thinking about or feeling while they are resting. Of particular concern for studies with psychiatric populations, anxiety may be induced by the scanner experience and may be difficult to account for without a “control” task, as in task-based fMRI. Concerns have also been raised that research participants may sleep through scans and may not be aware of when they drift off, even if investigators inquire.
Limitation 2: Head Motion
Two influential papers documented the serious impact of head motion during the MRI scan on RSFC results [80, 81]. Then, Satterhwaite and colleagues called the validity and replicability of previous developmental connectivity studies into question [82]. They noted that prior work on age-related increases in long-distance, within network connectivity and decreases in short-distance, between-network connectivity, is the opposite pattern seen for head motion. They reported that increased head motion is associated with younger age and that when connectivity analyses are conducted with rigorous correction of head motion effects, the developmental findings are still present, but substantially tempered. This publication highlighted the importance of correcting for head motion in developmental studies of RSFC.
In sum, clinicians will likely encounter publications or presentations utilizing RSFC, an innovative fMRI technique. RSFC refers to spontaneous brain fluctuations, organized into functional communication networks that are quantifiable when subjects are at rest. RSFC can reveal novel information about how brain networks develop and how that development can go awry in various psychiatric disorders. The RSFC literature is still in an early stage, as illustrated by the fact that vast networks of abnormalities have been implicated for most disorders. Few findings have been replicated; this may be due to the fact that many of the initial studies have included small samples and the methodologies to process and analyze the data have varied widely. RSFC is a technique that is quite susceptible to motion artifact, leading to some recent skepticism of the initial findings from pediatric studies, given the large degree of motion in children and adolescent participants. However, recent work has been incorporating more robust techniques to address motion. Moving forward, studies with larger samples that incorporate uniform methods across studies will be most useful for advancing the field. There is a strong interest in gaining insight into how RSFC changes across normal and abnormal development, thus longitudinal research is needed. RSFC is likely to continue to serve as an important neuroimaging modality as findings are reproduced, clinical interventions are tested and analytic techniques improve.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Kathryn Cullen received grants from the NIH, NARSAD, the Center for Translational Science Institute at the University of Minnesota and the Academic Center at the University of Minnesota. Cullen received travel expenses covered from the National Institutes of Mental Health grant. Michael Francis, Leslie Hulvershorn and Melinda Westlund have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
References
Papers of particular interest, published recently, have been highlighted as:
*Of importance
**Of major importance
- 1.Biswal B, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 2.Fransson P, et al. Resting-state networks in the infant brain. Proc Natl Acad Sci U S A. 2007;104(39):15531–6. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin W, et al. Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain. AJNR Am J Neuroradiol. 2008;29(10):1883–9. doi: 10.3174/ajnr.A1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao W, et al. The synchronization within and interaction between the default and dorsal attention networks in early infancy. Cereb Cortex. 2013;23(3):594–603. doi: 10.1093/cercor/bhs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raichle ME, et al. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fair DA, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104(33):13507–12. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fair DA, et al. The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A. 2008;105(10):4028–32. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fair DA, et al. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30(8):2356–66. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly AM, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19(3):640–57. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- 11*.Dosenbach NU, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358–61. doi: 10.1126/science.1194144. [This study showed that RSFC can reliably be used to predict “brain age.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, et al. Decoding lifespan changes of the human brain using resting- state functional connectivity MRI. PLoS One. 2012;7(8):e44530. doi: 10.1371/journal.pone.0044530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuo XN, et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 2010;30(45):15034–43. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barber AD, et al. Developmental changes in within- and between-network connectivity between late childhood and adulthood. Neuropsychologia. 2013;51(1):156–67. doi: 10.1016/j.neuropsychologia.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chai XJ, et al. Selective development of anticorrelated networks in the intrinsic functional organization of the human brain. J Cogn Neurosci. 2014;26(3):501–13. doi: 10.1162/jocn_a_00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maximo JO, et al. Approaches to local connectivity in autism using resting state functional connectivity MRI. Front Hum Neurosci. 2013;7:605. doi: 10.3389/fnhum.2013.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paakki JJ, et al. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Res. 2010;1321:169–79. doi: 10.1016/j.brainres.2009.12.081. [DOI] [PubMed] [Google Scholar]
- 18.Lynch CJ, et al. Default mode network in childhood autism: posteromedial cortex heterogeneity and relationship with social deficits. Biol Psychiatry. 2013;74(3):212–9. doi: 10.1016/j.biopsych.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiggins JL, et al. Using a self-organizing map algorithm to detect age-related changes in functional connectivity during rest in autism spectrum disorders. Brain Res. 2011;1380:187–97. doi: 10.1016/j.brainres.2010.10.102. [Study of the difference in developmental changes of RSFC between youth with ASD and healthy controls.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assaf M, et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53(1):247–56. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21**.Abrams DA, et al. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci U S A. 2013;110(29):12060–5. doi: 10.1073/pnas.1302982110. [RSFC and ASD study that integrates anomalous RSFC involving reward circuitry with communication deficits, which corresponds well with current treatment approaches for ASD.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovaas OI. Behavioral treatment and normal educational and intellectual functioning in young autistic children. J Consult Clin Psychol. 1987;55(1):3–9. doi: 10.1037//0022-006x.55.1.3. [DOI] [PubMed] [Google Scholar]
- 23.Anderson JS, et al. Functional connectivity magnetic resonance imaging classification of autism. Brain. 2011;134(Pt 12):3742–54. doi: 10.1093/brain/awr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin S, et al. Amygdala Subregional Structure and Intrinsic Functional Connectivity Predicts Individual Differences in Anxiety During Early Childhood. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dennis EL, et al. Anxiety modulates insula recruitment in resting-state functional magnetic resonance imaging in youth and adults. Brain Connect. 2011;1(3):245–54. doi: 10.1089/brain.2011.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burghy CA, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15(12):1736–41. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27*.Roy AK, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(3):290–299. e2. doi: 10.1016/j.jaac.2012.12.010. [First study examining amygdala RSFC among youth with GAD compared to controls and associating amygdala RSFC with anxiety severity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hulvershorn LA, Cullen K, Anand A. Toward dysfunctional connectivity: a review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging Behav. 2011;5(4):307–28. doi: 10.1007/s11682-011-9134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drevets WC, Ongur D, Price JL. Reduced glucose metabolism in the subgenual prefrontal cortex in unipolar depression. Mol Psychiatry. 1998;3(3):190–1. doi: 10.1038/sj.mp.4000380. [DOI] [PubMed] [Google Scholar]
- 30.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13(8):663–81. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 32.Cullen KR, et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci Lett. 2009;460(3):227–31. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaffrey MS, et al. Subgenual cingulate connectivity in children with a history of preschool-depression. Neuroreport. 2010;21(18):1182–8. doi: 10.1097/WNR.0b013e32834127eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Connolly CG, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry. 2013;74(12):898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davey CG, et al. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol Med. 2012;42(10):2071–81. doi: 10.1017/S0033291712000323. [DOI] [PubMed] [Google Scholar]
- 36.Phillips ML, et al. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54(5):504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 37*.Gabbay V, et al. Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry. 2013;52(6):628–41. e13. doi: 10.1016/j.jaac.2013.04.003. [This study found abnormal frontal-striatal RSFC in adolescents with depression. This abnormality was related to symptoms of anhedonia.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 39.Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–44. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 40.Luking KR, et al. Functional connectivity of the amygdala in early-childhood- onset depression. J Am Acad Child Adolesc Psychiatry. 2011;50(10):1027–41. e3. doi: 10.1016/j.jaac.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cullen KR, et al. Abnormal Amygdala Resting -State Functional Connectivity in Adolescent Depression. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2014.1087. in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dickstein DP, et al. Fronto-temporal spontaneous resting state functional connectivity in pediatric bipolar disorder. Biol Psychiatry. 2010;68(9):839–46. doi: 10.1016/j.biopsych.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu M, et al. Altered affective, executive and sensorimotor resting state networks in patients with pediatric mania. J Psychiatry Neurosci. 2013;38(4):232–40. doi: 10.1503/jpn.120073. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Xiao Q, et al. Altered regional homogeneity in pediatric bipolar disorder during manic state: a resting-state fMRI study. PLoS One. 2013;8(3):e57978. doi: 10.1371/journal.pone.0057978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hulvershorn LA, et al. Neural activation during facial emotion processing in unmedicated bipolar depression, euthymia, and mania. Biological psychiatry. 2012;71(7):603–10. doi: 10.1016/j.biopsych.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry. 1999;56(10):921–6. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- 47.Sylvester CM, et al. Resting state functional connectivity of the ventral attention network in children with a history of depression or anxiety. J Am Acad Child Adolesc Psychiatry. 2013;52(12):1326–1336. e5. doi: 10.1016/j.jaac.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48**.Herringa RJ, et al. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci U S A. 2013;110(47):19119–24. doi: 10.1073/pnas.1310766110. [A study examining the effects of childhood maltreatment on later internalizing symptoms (i.e., depression and anxiety) and RSFC in adolescence. This study suggests important environmental effects on developmental psychopathology.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49*.The ADHD-200 Consortium: A Model to Advance the Translational Potential of Neuroimaging in Clinical Neuroscience. Frontiers in systems neuroscience. 2012;6:62. doi: 10.3389/fnsys.2012.00062. [A description of an innovative RSFC data sharing project.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Sripada C, et al. Disrupted network architecture of the resting brain in attention- deficit/hyperactivity disorder. Human brain mapping. 2014 doi: 10.1002/hbm.22504. [A product of the ADHD-200 Consortium, a large, multi-site examination of RSFC in ADHD that replicated prior findings of smaller samples.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun L, et al. Abnormal functional connectivity between the anterior cingulate and the default mode network in drug-naive boys with attention deficit hyperactivity disorder. Psychiatry research. 2012;201(2):120–7. doi: 10.1016/j.pscychresns.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Chabernaud C, et al. Dimensional brain-behavior relationships in children with attention-deficit/hyperactivity disorder. Biological psychiatry. 2012;71(5):434–42. doi: 10.1016/j.biopsych.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang H, et al. Abnormal spontaneous brain activity in medication-naive ADHD children: a resting state fMRI study. Neuroscience letters. 2011;502(2):89–93. doi: 10.1016/j.neulet.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 54.An L, et al. Methylphenidate normalizes resting-state brain dysfunction in boys with attention deficit hyperactivity disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(7):1287–95. doi: 10.1038/npp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Querne L, et al. Effects of Methylphenidate on Default-Mode Network/Task- Positive Network Synchronization in Children With ADHD. Journal of attention disorders. 2014 doi: 10.1177/1087054713517542. [DOI] [PubMed] [Google Scholar]
- 56.Di Martino A, et al. Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biological psychiatry. 2013;74(8):623–32. doi: 10.1016/j.biopsych.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hulvershorn LA, et al. Abnormal amygdala functional connectivity associated with emotional lability in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(3):351–361. e1. doi: 10.1016/j.jaac.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown MR, et al. ADHD-200 Global Competition: diagnosing ADHD using personal characteristic data can outperform resting state fMRI measurements. Frontiers in systems neuroscience. 2012;6:69. doi: 10.3389/fnsys.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bohland JW, et al. Network, anatomical, and non-imaging measures for the prediction of ADHD diagnosis in individual subjects. Frontiers in systems neuroscience. 2012;6:78. doi: 10.3389/fnsys.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Venkataraman A, et al. Whole brain resting state functional connectivity abnormalities in schizophrenia. Schizophr Res. 2012;139(1-3):7–12. doi: 10.1016/j.schres.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21(4):424–30. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 62.Biswal B, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 63.Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 64.Peltier SJ, Polk TA, Noll DC. Detecting low-frequency functional connectivity in fMRI using a self-organizing map (SOM) algorithm. Hum Brain Mapp. 2003;20(4):220–6. doi: 10.1002/hbm.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shim G, et al. Altered resting-state connectivity in subjects at ultra-high risk for psychosis: an fMRI study. Behav Brain Funct. 2010;6:58. doi: 10.1186/1744-9081-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lui S, et al. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry. 2009;166(2):196–205. doi: 10.1176/appi.ajp.2008.08020183. [DOI] [PubMed] [Google Scholar]
- 67.Guo J, et al. Regional homogeneity abnormalities in patients with transient ischaemic attack: A resting-state fMRI study. Clin Neurophysiol. 2014;125(3):520–5. doi: 10.1016/j.clinph.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 68.Guo W, et al. Abnormal default-mode network homogeneity in first-episode, drug-naive schizophrenia at rest. Prog Neuropsychopharmacol Biol Psychiatry. 2014;49:16–20. doi: 10.1016/j.pnpbp.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 69.Alonso-Solis A, et al. Altered default network resting state functional connectivity in patients with a first episode of psychosis. Schizophr Res. 2012;139(1-3):13–8. doi: 10.1016/j.schres.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou Y, et al. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett. 2007;417(3):297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- 71.Mwansisya TE, et al. The diminished interhemispheric connectivity correlates with negative symptoms and cognitive impairment in first-episode schizophrenia. Schizophr Res. 2013;150(1):144–50. doi: 10.1016/j.schres.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 72.Rder CH, et al. Systematic review of the influence of antipsychotics on the blood oxygenation level-dependent signal of functional magnetic resonance imaging. Current medicinal chemistry. 2013;20(3):448–61. doi: 10.2174/092986713804870891. [DOI] [PubMed] [Google Scholar]
- 73*.Lui S, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67(8):783–92. doi: 10.1001/archgenpsychiatry.2010.84. [An interesting study of the effect of second generation antipsychotic medication on resting state functional connectivity in medication naïve individuals with first- episode psychosis.] [DOI] [PubMed] [Google Scholar]
- 74.Leibenluft E, Pine DS. Resting state functional connectivity and depression: in search of a bottom line. Biological psychiatry. 2013;74(12):868–9. doi: 10.1016/j.biopsych.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Power JD, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–8. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77**.Satterthwaite TD, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60(1):623–32. doi: 10.1016/j.neuroimage.2011.12.063. [Influential paper which called into question the concern for head motion, particularly in developmental RSFC studies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lui S, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67(8):783–92. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- 79.Leibenluft E, Pine DS. Resting state functional connectivity and depression: in search of a bottom line. Biological psychiatry. 2013;74(12):868–9. doi: 10.1016/j.biopsych.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 80.Power JD, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–54. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–8. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Satterthwaite TD, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60(1):623–32. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]