Abstract
Roux-en-Y gastric bypass (RYGB) is one of the most commonly performed bariatric procedures around the world. Although RYGB is the gold standard for treating morbid obesity, it carries the risk of rare but serious long-term complications from malnutrition. We report a case of laparoscopic reversal of RYGB. A female patient reported prolonged incapacitating postprandial pain that consequently made her avoid proper oral intake. Therefore, she became seriously malnourished at 30 months after RYGB and requested reversal of RYGB into normal anatomy. The operation was successfully performed via laparoscopy. Operating time was 120 minutes, and intraoperative blood loss was 20 mL. The patient was discharged without any complications directly related to surgical procedures, although her hospital stay was prolonged by the treatment of asymptomatic septicemia of unknown origin. Laparoscopic reversal of RYGB into normal anatomy is technically feasible and might be performed safely after thorough preoperative evaluation in carefully selected patients.
Keywords: Gastric bypass, Bariatric surgery, Morbid obesity, Malnutrition, Reoperation
INTRODUCTION
Roux-en-Y gastric bypass (RYGB) is one of the most commonly performed bariatric procedures around the world. Numerous previous studies have reported that it has the drastic effect of sustained weight reduction, with a reported percent of excess weight loss (%EWL) ranges from 56.7% to 66.5% at mid-term follow-up, and remarkable resolution of obesity-related comorbidities, including diabetes, cardiovascular diseases, and dyslipidemia [1].
Although RYGB is the gold standard for treating morbid obesity, this procedure requires multidisciplinary follow-up because of infrequent but serious long-term complications. Restoration of the normal intestinal integrity is sometimes required, and the most commonly reported indications for RYGB reversal into a normal anatomy include dumping syndrome with or without postprandial hyperinsulinemic hypoglycemia, cachexia, and malnutrition. Weight problems, either too much or too little, can also be other indications.
Here, we report a case of laparoscopic conversion of RYGB into normal anatomy in a patient who presented with malnutrition after RYGB.
SURGICAL TECHNIQUE
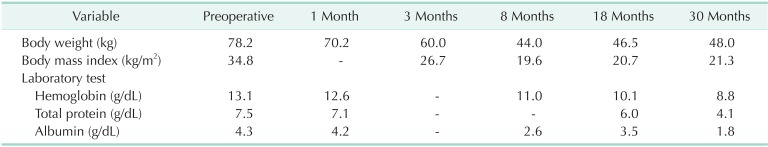
In February 2011, a laparoscopic RYGB was performed for a 53-year-old female patient with a body mass index (BMI) of 34.8 kg/m2. She had hypertension treated with oral medication, obesity-induced arthropathy, and a history of sling surgery for urinary incontinence before the RYGB procedure. All laboratory test results at the preoperative evaluation were within the normal range, except for the finding of dyslipidemia. Gastrojejunal anastomosis was established using a linear stapler, and the entry hole was hand-sewn closed during the surgery. After RYGB, the patient reported intermittent epigastric discomfort that was well managed with proton-pump inhibitors. The clinical course and the changes in laboratory test results after RYGB are shown in Table 1. At 18 months postoperatively, the patient showed mild anemia (hemoglobin, 10.1 g/dL), attributable to iron and vitamin B12 deficiency, along with calcium deficiency; oral iron and calcium supplementation and cobalamin injection were prescribed to correct these deficiencies. Thirty months after the RYGB, the patient visited the Emergency Department with general weakness and persistent diarrhea. Her BMI at this time was 21.3 kg/m2, and laboratory tests showed anemia (hemoglobin, 8.8 g/dL) and hypoalbuminemia (serum albumin, 1.8 g/dL). Serum iron, vitamin B12, calcium, and other micronutrient levels were also markedly decreased. Her fasting blood glucose level and insulin levels were within normal range, and there was no evidence of hyperinsulinemic hypoglycemia. She was referred to the hematologic department and conservatively managed with oral replacement therapy of iron, folic acid, protein, and multivitamins for 2 months. Furthermore, she was encouraged to ingest proper foods thorough dietary counseling. However, the deficiencies were not easily reversed, and she reported persistent postprandial abdominal pain that made her avoid proper oral intake. She requested conversion to normal anatomy and hence underwent laparoscopic RYGB reversal in November 2013.
Table 1.
Clinical course after Roux-en-Y gastric bypass
The patient was admitted 10 days before revisional surgery for gradual correction of malnourishment with total parenteral nutrition to prevent postoperative complications, such as pulmonary edema, related to acute nutritional resuscitation.
Surgical procedures
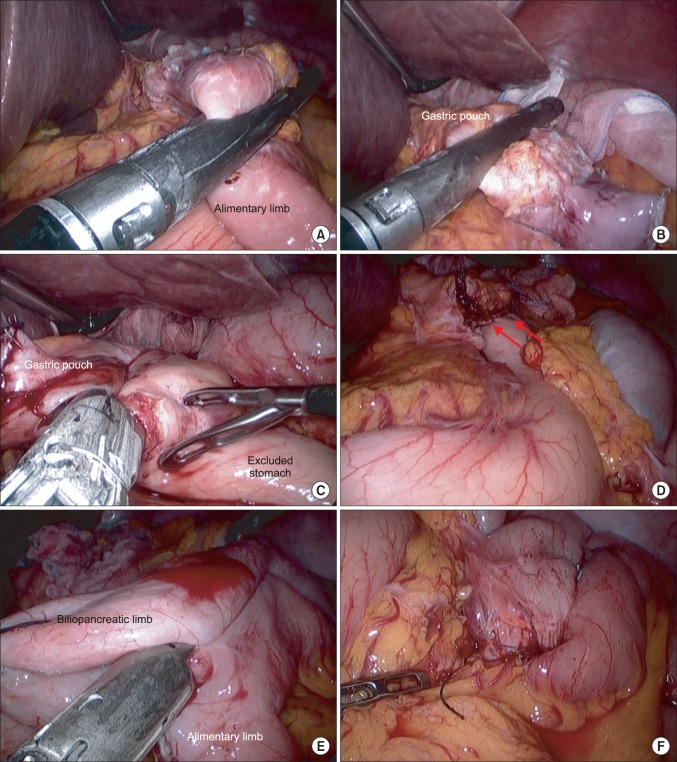
Six trocars were used to perform the surgery: one 11-mm port for a camera at the umbilicus, one 12-mm port for stapling in the right lower quadrant, and 4 additional 5-mm ports for assistance and liver retraction. Detailed sequential procedures of the surgery are shown in Fig. 1. The procedure began with localization of the old jejunojejunostomy site; then, the small bowel was completely inspected to identify the alimentary and biliopancreatic limbs and to roughly measure the bypassed length of the small bowel. The length of the alimentary limb was estimated at approximately 120 cm and, interestingly, the blind loop at the distal end of the biliopancreatic limb was approximately 70 cm long. Careful adhesiolysis was carried out until the old gastrojejunostomy and surrounding structures were clearly visualized. The alimentary limb was divided from the old gastrojejunostomy with a linear stapler (Fig. 1A). After adhesion around the gastric pouch was cleared, the old gastrojejunostomy was dismantled on the gastric side, leaving the small proximal gastric pouch (Fig. 1B). The gastric continuity was restored through a linear stapled side-to-side anastomosis between the new gastric pouch and the excluded stomach (Fig. 1C). The stapler entry hole was hand-sewn closed, and the anastomosis site was reinforced. Anastomotic leakage was assessed using a methylene blue-stained saline injection via the orogastric bougie tube. The proximal end of the alimentary limb and the distal end of the biliopancreatic limb were then anastomosed by a linear stapler without dismantling the old jejunojejunostomy (Fig. 1E). A closed drain was placed in the abdominal cavity near the gastrogastric anastomosis, and the removed specimen was retrieved via an extended umbilical incision.
Fig. 1.
Details of the Roux-en-Y gastric bypass reversal. (A) Division of the alimentary limb right below the old gastrojejunostomy. (B) Transection of the gastrojejunostomy near the distal end of the gastric pouch. (C) Construction of the gastrogastric anastomosis between the gastric pouch remnant and excluded stomach using a linear stapler. (D) Newly established gastrogastrostomy (red arrows). (E) Entero-entero anastomosis between the proximal end of the old alimentary limb and the distal end of the old biliopancreatic limb. (F) Newly established enteroenterostomy.
Surgical outcomes
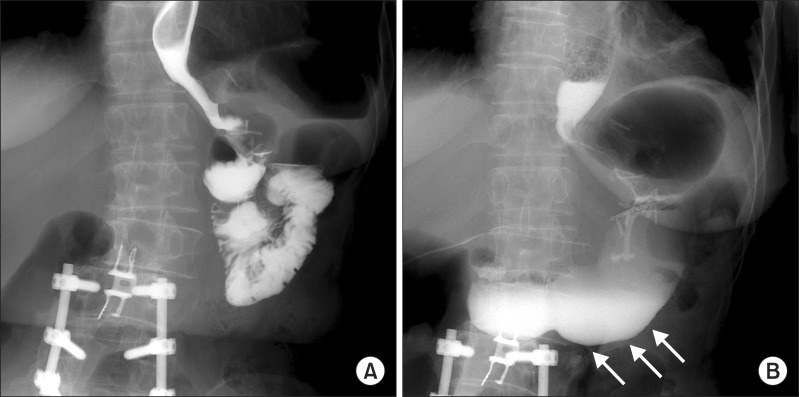
The operating time was 120 minutes, and the estimated amount of intraoperative blood loss was 20 mL. The contrast upper gastrointestinal study on the first postoperative day showed that the contrast drained well into the excluded stomach without passage disturbance (Fig. 2). The patient presented with a fever of unknown origin on the second postoperative day; therefore, empirical antibiotic therapy was started. The blood culture showed septicemia from Escherichia coli, but she did not have any further subjective symptoms. She was transferred to the infection department on the seventh postoperative day for 2 weeks of antibiotic therapy and was then discharged on the 21st day postoperatively without any further events. The previous symptoms related to food intake improved during the postoperative period, and her serum albumin level reached 2.6 g/dL at discharge. Her hemoglobin level was still low (7.8 mg/dL), and she was prescribed oral supplementation of iron, vitamin B1, and folic acid at discharge.
Fig. 2.
Pre- and postoperative contrast upper gastrointestinal (UGI) studies. (A) UGI image before the reversal of the Roux-en-Y gastric bypass (RYGB). (B) UGI image after the RYGB reversal. Contrast passage into the old excluded stomach is noted after the reversal operation (white arrows).
DISCUSSION
As growing numbers of bariatric procedures, including RYGB, are performed worldwide, the number of patients requiring revisional procedure is expected to increase. Since Himpens et al. [2] first reported a case of laparoscopic restoration of an RYGB into normal anatomy, several reports have demonstrated the feasibility of conversion of RYGB into normal anatomy [3,4].
The reported indications of RYGB reversal include poor dietary compliance, severe dumping syndrome, hyperinsulinemic hypoglycemia, recalcitrant marginal ulcers, malnutrition and cachexia, and failure to lose weight [3,4]. Among these, severe dumping syndrome and postprandial hypoglycemia seem to be the most common. Nesidioblastoma, which represents extreme neuroglycopenia resulting from endogenous hyperinsulinemic hypoglycemia, is increasingly recognized in patients undergoing RYGB. In these patients, restoration of the normal anatomy could serve as a less aggressive alternative instead of other procedures such as pancreatectomy.
Nutritional deterioration after RYGB is well-documented in previous studies [5,6,7]. It results from reduced oral intake or excessive losses secondary to reconfiguration of the gastrointestinal tract. Specific micronutrients appear to be malabsorbed postoperatively and present as deficiencies when adequate vitamin and mineral supplementation is not administered. Retrospective analyses of patients who have undergone RYGB reveal micronutrient deficiencies, including iron, vitamin B12, and folic acid deficiencies, which, in turn, frequently results in anemia in 20%-49% of these patients [6]. Several reports have also shown that thiamin and zinc deficiency or bone loss can develop; these metabolic consequences can be potentially disastrous.
On the other hand, protein deficiency is not common after RYGB unless malabsorptive distal RYGB was performed [8]. A minimal amount of macronutrient malabsorption is thought to occur after conventional RYGB. Investigators have reported that only a few patients presented with protein malnutrition after RYGB, and this usually resulted from patient noncompliance with nutrition instructions [6,9]. Protein deficiency after RYGB is usually associated with various coexistent circumstances that lead to decreased dietary intake, including anorexia, prolonged vomiting, diarrhea, food intolerance, depression, and fear of weight regain, rather than being solely attributable to malabsorptive components of bariatric surgery alone.
In the current case, the reason for malnutrition also seems to be attributable not only to the prolonged bypass limb but also to inadequate food intake resulting from postprandial pain. The patient reported prolonged incapacitating postprandial pain that consequently made her avoid proper oral intake. Meanwhile, the intraoperative findings showed that the length of her alimentary limb was approximately 130 cm long and, interestingly, the blind loop at the end of the biliopancreatic limb was 70 cm long, which was much longer than the usual RYGB. Together, these factors made the length of the bypassed small bowel more than 200 cm, which could have caused malnutrition considering the short stature of the patient (150 cm), along with her poor dietary habits. Because it is essential to preserve at least a 3-m-long common channel to prevent malnutrition after RYGB, surgeons should be very careful to determine the length of the alimentary and biliopancreatic limbs to achieve a proper level of malabsorption while avoiding malnutrition, especially in patients of short stature.
Most of the previous reports on RYGB reversal into normal anatomy included disassembling the old gastrojejunostomy and jejunojejunal anastomosis and their total re-creation to restore normal gastrointestinal integrity. The surgeon in the current case, who originally was a gastric cancer surgeon, was extremely inexperienced in bariatric surgery at the time of the primary surgery and simply adopted uncut Roux-en-Y reconstruction for gastric cancer surgery to RYGB. Therefore, sufficient distance between gastrojejunostomy and jejunojejunostomy was preserved in order to avoid bile reflux into the remnant stomach when staple line dehiscence occurs. However, intraoperative findings at reversal showed that the previously uncut staple line was found split apart, far from being recanalized; this consequently resulted in a long "true" blind loop at the distal end of the biliopancreatic limb. At reversal, we established a new jejunojejunal anastomosis between the distal end of the blind loop and the cut end of the proximal alimentary limb, and left the old jejunojejunostomy in situ. Consequently, this formed an open-circuit system with double passage of food intake consisting of both the old and new jejunojejunostomies. Although the jejunojejunostomy was not completely reversed to normal anatomical integrity, the nutritional deficiencies caused by the long bypass were expected to be normalized with food exposure to the duodenum and the very proximal jejunum and partially to the previously bypassed jejunal segments via an open loop. The operative procedure per se became much simpler by not dismantling the old jejunojejunostomy; therefore, the number of new anastomoses and the operating time could be reduced.
The patient showed immediate relief of the postprandial discomfort after the surgery, and the meals provided by our clinical dietitian were well tolerated. Protein deficiency was markedly improved at discharge, but anemia was still present. The patient needs to be followed to elucidate whether all the micronutrient deficiencies, including iron and vitamin B12 deficiencies, are normalized over time.
CONCLUSION
Laparoscopic reversal to normal anatomy after RYGB is technically feasible. However, revisional bariatric surgery including an RYGB reversal should be performed only by sufficiently experienced bariatric surgeons because perioperative management of these patients can be complicated.
ACKNOWLEDGEMENTS
This research was supported by the Soonchunhyang University Research Fund.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Himpens J, Dapri G, Cadiere GB. Laparoscopic conversion of the gastric bypass into a normal anatomy. Obes Surg. 2006;16:908–912. doi: 10.1381/096089206777822179. [DOI] [PubMed] [Google Scholar]
- 3.Vilallonga R, van de Vrande S, Himpens J. Laparoscopic reversal of Roux-en-Y gastric bypass into normal anatomy with or without sleeve gastrectomy. Surg Endosc. 2013;27:4640–4648. doi: 10.1007/s00464-013-3087-0. [DOI] [PubMed] [Google Scholar]
- 4.Dapri G, Cadiere GB, Himpens J. Laparoscopic reconversion of Roux-en-Y gastric bypass to original anatomy: technique and preliminary outcomes. Obes Surg. 2011;21:1289–1295. doi: 10.1007/s11695-010-0252-6. [DOI] [PubMed] [Google Scholar]
- 5.Poitou Bernert C, Ciangura C, Coupaye M, Czernichow S, Bouillot JL, Basdevant A. Nutritional deficiency after gastric bypass: diagnosis, prevention and treatment. Diabetes Metab. 2007;33:13–24. doi: 10.1016/j.diabet.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Allied Health Sciences Section Ad Hoc Nutrition Committee. Aills L, Blankenship J, Buffington C, Furtado M, Parrott J. ASMBS Allied Health Nutritional Guidelines for the surgical weight loss patient. Surg Obes Relat Dis. 2008;4(5 Suppl):S73–S108. doi: 10.1016/j.soard.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Dalcanale L, Oliveira CP, Faintuch J, Nogueira MA, Rondo P, Lima VM, et al. Long-term nutritional outcome after gastric bypass. Obes Surg. 2010;20:181–187. doi: 10.1007/s11695-009-9916-5. [DOI] [PubMed] [Google Scholar]
- 8.Kellum JM, Chikunguwo SM, Maher JW, Wolfe LG, Sugerman HJ. Long-term results of malabsorptive distal Roux-en-Y gastric bypass in superobese patients. Surg Obes Relat Dis. 2011;7:189–193. doi: 10.1016/j.soard.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Skroubis G, Sakellaropoulos G, Pouggouras K, Mead N, Nikiforidis G, Kalfarentzos F. Comparison of nutritional deficiencies after Roux-en-Y gastric bypass and after biliopancreatic diversion with Roux-en-Y gastric bypass. Obes Surg. 2002;12:551–558. doi: 10.1381/096089202762252334. [DOI] [PubMed] [Google Scholar]