Abstract
Purpose
The evaluation and extent of lymph node (LN) retrieval is clinically relevant for staging because lymphatic invasion is the most common mechanism leading to up-staging of carcinoma. However, the optimal number of LN retrievals for early gastric cancer (EGC) is unclear. With the aim of clarification, we analyzed our database to investigate the optimal number of retrieved LNs in EGC.
Methods
Three hundred twenty-six gastric cancer patients who underwent curative gastrectomy with D2 LN dissection at Ewha Womans University Hospital (Dongdaemun and Mokdong) were analyzed according to sex, age, tumor location, size of tumor, macroscopic type, histological classification, depth of invasion, LNs metastasis, TNM stage and type of surgery.
Results
In LN negative cases, patients with 15-25 retrieved LNs had a 5- and 10-year survival rate of 88% and 54%, respectively, whereas retrieval of ≥26 LNs was associated with 5- and 10-year survival rate of 90% and 75%, respectively (P = 0.105). In LN positive cases, the 5- and 10-year survival rate was 50% and 30% for the 15-25 group, and 77% and 67% for the ≥26 group, respectively (P = 0.044).
Conclusion
LN metastasis is an independent factor of survival and the number of retrieved LNs significantly relate to the long-term survival benefit in node metastatic EGC. Also, our data suggest that the retrieval of at least 15 LNs may not be sufficient to warrant recommendation for more curative surgery, and that qualified LN dissection should be considered if LN metastasis is in doubt, even in EGC.
Keywords: Lymph node, Stomach neoplasms, Harvest
INTRODUCTION
In gastric cancer, curative surgery requires tumor resection with enough negative resection margin along with its corresponding lymph nodes (LNs). The evaluation and extent of LN retrieval is clinically relevant for staging because number of LN invasion is the most common mechanism leading to up-staging of carcinoma and one of the most important prognostic factors [1,2]. However, the optimal number of LNs that should be retrieved in the staging of gastric cancer is debatable. In advanced gastric cancer, retrieval of more than 25 LNs has been associated with an overall survival advantage in advanced gastric cancer patients [3,4]. Smith et al. [5] reported that the stage subgroup-specific survival depended strongly on the total number of LNs examined and culminated in the highest survival for counts of 40 or more LNs, a strong incentive in favor of extended lymphadenectomy. While there is no universally accepted minimum number of LNs necessary for accurate staging of gastric cancer, retrieval of at least 15 LNs is recommended to avoid stage migration in National Comprehensive Cancer Network (NCCN) guidelines version 2. 2013 [3,5,6,7,8].
The number of LNs that should be retrieved after gastrectomy has varied according to the institution and country [8]. In many Asian centers, surgeons do the sampling and submission of individual LNs for histological analysis, whereas surgeons in the West submit en bloc resected specimens and rely upon pathologists to retrieve LNs. Therefore, LN enumeration can depend upon both the surgeon and pathologist [9]. However, studies from Asia and the United States have concentrated on the LNs in cases of advanced cancer [10,11] with no adequate data concerning the optimal number of retrieved LNs in early gastric cancer (EGC) [12,13].
METHODS
We retrospectively reviewed the database of patients who underwent gastrectomy due to gastric carcinoma at Ewha Womans University Hospital, Seoul, Korea, from January 1995 to December 2009. Among 576 EGC patients, we enrolled 326 EGC patients who underwent curative gastrectomy with D2 LN dissection. All patients with fewer than 15 retrieved LNs and endoscopic/laparoscopic treatment were excluded. Sex, age, tumor location, size of tumor, macroscopic type, histological classification, depth of invasion, LN metastasis, TNM stage, and type of surgery were analyzed. Histologic classification was divided into differentiated and undifferentiated subgroups. The depth of tumor invasion and TNM stage were classified according to the 7th edition of the American Joint Committee on Cancer/International Union Against Cancer [14]. LN metastasis was grouped as negative and positive involvement of tumor. Patients were stratified into two groups by number of retrieved LNs using the cut-point of 25 LNs: 15 < LN < 25 vs. LN >26. Subgroup analysis of patient demographic, clinical, and pathologic factors was also performed according to the number of retrieved LNs. Most surgeries in our data were done by a single surgeon, and the LNs from the specimens were sampled by general surgeons.
Statistical analyses
Chi-square analysis and Student t-test were used to compare the subgroup parameters. All deaths including non-cancer-related mortality were regarded as events. The numerical data were expressed as the mean and standard deviation. Survival analyses were performed using the Kaplan-Meier method and comparisons across different strata were made with the log-rank test. Multivariate analysis was performed using the Cox proportional hazards regression model for the analysis of prognosis. The statistical analyses were performed using the IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). The results were evaluated with a confidence interval of 95%, and P-values below 0.05 were considered to be statistically significant.
RESULTS
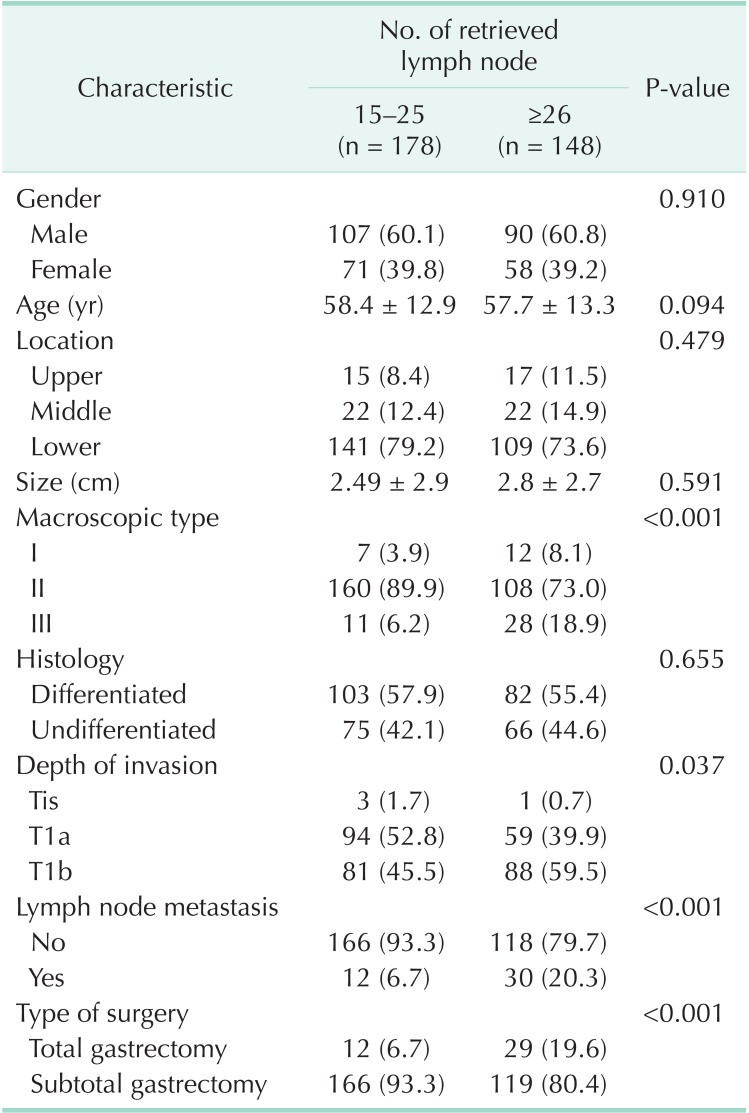
A total of 326 patients with gastric carcinoma were enrolled. Their mean follow-up period was 113.4 ± 56 months (range, 2-168 months). The characteristics of patients according to number of retrieved LNs are shown in Table 1. The mean age at diagnosis was 58.4 ± 12.9 years in males and 57.7 ± 13.3 years in females. Overall, 197 (60.4%) were male and 129 (39.5%) were female. No statistical differences between patients with 15-25 and ≥26 retrieved LNs were evident according to gender, age, location, size, and histology. The elevated tumor (type I) and depressed tumor (type III) showed higher LN yields (P < 0.001). The greater the degree of local invasion of the tumor, the more retrieved LNs were identified (P = 0.037). Likewise, the LN involvement of tumors was associated with higher LN retrieval (P < 0.001). The overall mean number of retrieved LNs per patient was 36.37 ± 18.2 (35.02 ± 17.2 in node negative patients, 43.05 ± 21.8 in node positive patients). Tumor location (P = 0.479) and size (P = 0.591) were not related with LN retrieval, but total gastrectomy was more common in patients with >25 retrieved LNs (P < 0.001).
Table 1.
Characteristics of early gastric cancer patients according to lymph node retrieval (n = 326)
Values are presented as number (%) or mean ± standard deviation.
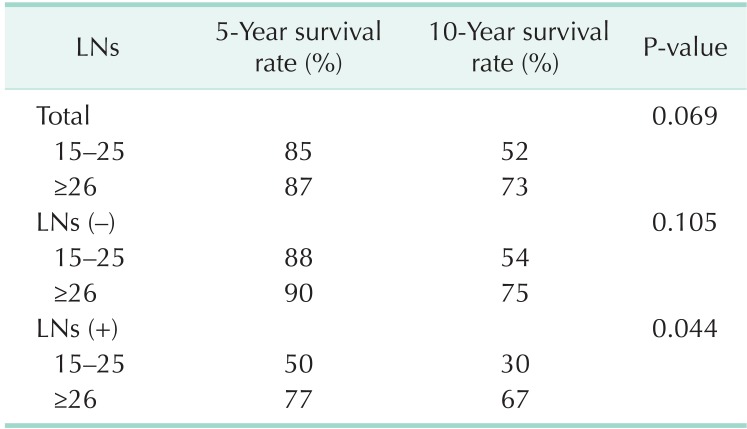
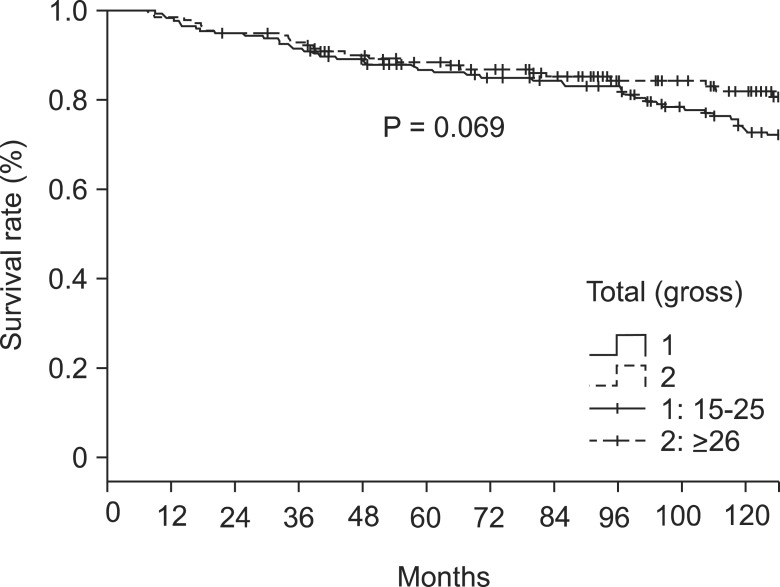
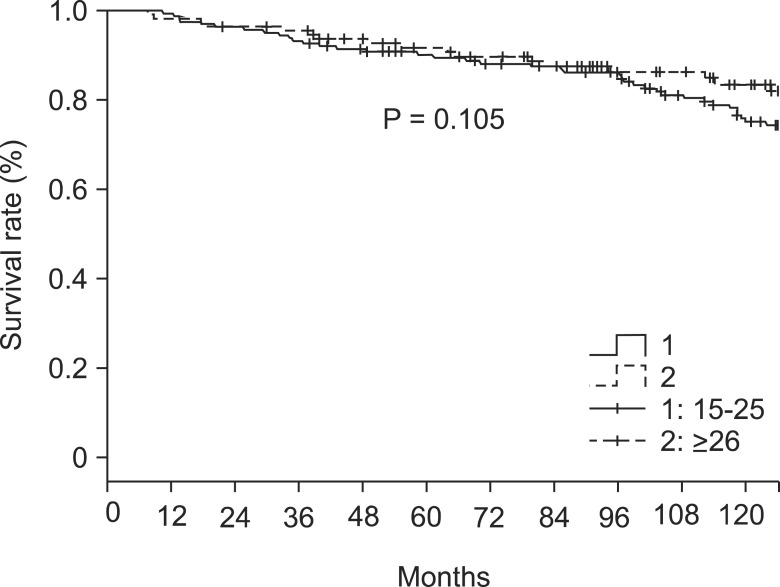
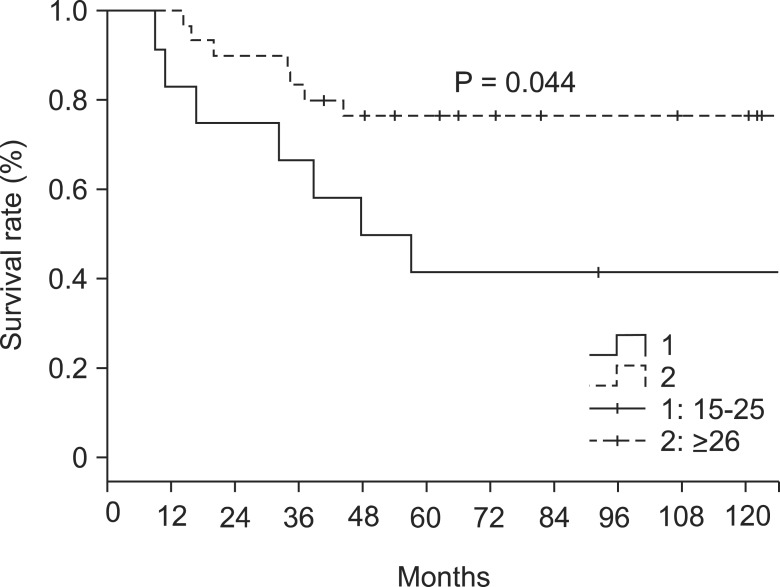
The overall 5- and 10-year survival rates were 86% and 59%, respectively. The respective rates in patients with 15-25 retrieved LNs were 85% and 52%. In patients with ≥26 retrieved LNs the respective rate was 87% and 73% (P = 0.069) (Table 2, Fig. 1). In LN negative cases, the respective survival rates in the 15-25 group were 88% and 54%, and the respective rates in the ≥26 group were 90% and 75% (P = 0.105) (Table 2, Fig. 2). In LN positive cases, the respective rates in the 15-25 group were 50% and 30%, and the respective rates in the ≥26 group were 77% and 67% (P = 0.044) (Table 2, Fig. 3).
Table 2.
Overall 5-year and 10-year survival rate according to the LNs retrieval
LN, lymph node.
Fig. 1.
Survival rate according to the number of lymph nodes retrieval in whole cases.
Fig. 2.
Survival rate according to the number of lymph nodes retrieval in node negative cases.
Fig. 3.
Survival rate according to the number of lymph nodes retrieval in node positive cases.
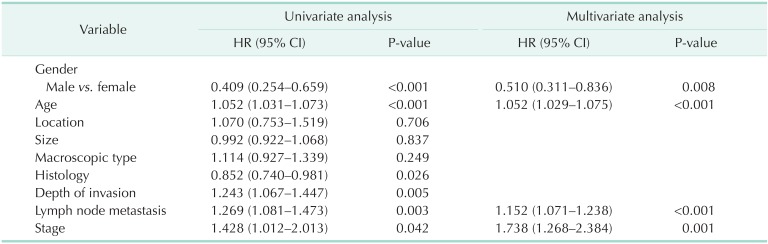
In the univariate analysis, gender, age, histology, depth of invasion, LN metastasis, and stage correlated significantly with overall survival, while location, size, and macroscopic type did not. Multivariate regression analysis (Table 3) revealed that independent variables showing predictive ability were gender (P = 0.008), age (P < 0.001), LN metastases (P < 0.001), and stage (P = 0.001).
Table 3.
Prognostic factors of early gastric cancer patients
HR, hazard ratio; CI, confidence interval.
DISCUSSION
LN involvement of gastric cancer is one of the strongest prognostic parameters after gastrectomy for survival and recurrence. Precise evaluation of the extent of LN metastasis offers the ability to more accurately predict oncologic outcomes for the individual patient. However, the appropriate degree of curative LN dissection differs between Western and Eastern countries. In a Japanese classification, the extent of LN dissection was represented by D0-D3 using the LN station system. The system is complicated and is hard to use due to variations in each category [15]. Instead, the number of the examined LNs has been used as a simpler indicator of the extent of LN dissection. However, the absence of a fixed cutoff number of retrieved LNs for standard treatment in gastric carcinoma is a complication. The optimal extent of regional LNs during the gastrectomy for gastric adenocarcinoma continues to be debated. Baiocchi et al. [3] and Chen et al. [4] proposed that the trend towards superior survival outcome could be followed after the retrieval of more than 25 LNs. Smith et al. [5] presented that the stage subgroup-specific survival depends strongly on the total number of LNs examined and culminates in the highest survival at counts of 40 LNs. Bouvier et al. [16] suggested that staging is not reliable when fewer than 10 LNs are examined. Although a universally accepted minimum number of LNs necessary for accurate staging of gastric cancer has not been recognized, retrieval of at least 15 LNs is recommended to avoid stage migration in NCCN guidelines version 2. 2013 [7].
Presently, the mean number of retrieved LNs per patient was 36.37 ± 18.2 overall, 35.02 ± 17.2 in LN negative patients, and 43.05 ± 21.8 in LN positive patients. These values were higher than other similar studies [3,10,16]. A higher number of retrieved LNs is expected to reduce the bias of stage migration and permit analysis without bias of reliability [5,17,18,19]. Presently, the higher number of retrieved LNs were identified in depressed lesions (type III, P < 0.001) and more invasive tumors (P = 0.037). A tumor invading into the submucosa with depressed lesion might be considered as an advanced carcinoma because of difficult differentiation of depth. In these cases, surgeons endeavor to perform extended LN dissection for curative surgery. Therefore, our data might show a higher number of retrieved LNs in cases of depressed lesions and deeper lesions. But, a direct comparison of our data to previous studies is not possible because of the dissimilarity of the studies.
The presence of LN metastasis was associated with a higher number of retrieved LNs (P < 0.001). This may similarly reflect the aforementioned depth of invasion. In other words, if the LN metastasis was in doubt in the operation field, more aggressive LN dissection would be performed. Extended LN dissection would enable surgeons to improve the overall survival in node metastasis cases and to prevent stage migration [1,17,18]. We recorded a greater prevalence of total gastrectomy in the ≥26 group compared with the 15-25 group, although there was no significant difference of location and size between groups. There is no precise reason to explain the result, but only that it might be attributed to the depth of invasion and LN metastasis.
Survival analysis according to the number of retrieved LNs did not differ significantly, (P = 0.258) with the exception of LN-positive cases, where overall survival was significantly different between the 15-25 group (lower survival) and ≥26 group (higher survival) (P = 0.044) (Fig. 3). From this result, we could assume higher LN retrieval might more completely remove micrometastatic LNs, which were not identified as metastatic LNs in the pathologic report.
Univariate analysis revealed statistical significance in gender, age, histology, depth of invasion, LN metastasis, and stage on survival. In multivariate analysis, LN metastasis (P < 0.001) and stage (P = 0.001) were independent predictive factors affecting survival, as in previous studies [13,20,21].
This study has several limitations. It was retrospective and small in size. Also, lacking in data concerning chemotherapy, which might result in better survival in node-positive patients.
In conclusion, LN metastasis is an independent factor of survival and the number of retrieved LNs significantly relate to the long-term survival benefit in node metastatic EGC. The data suggest that the retrieval of at least 15 LNs may not be sufficient to recommend more curative surgery, and that qualified LN dissection should be considered if LN metastasis is in doubt, even in EGC.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Yoshikawa T, Sasako M, Sano T, Nashimoto A, Kurita A, Tsujinaka T, et al. Stage migration caused by D2 dissection with para-aortic lymphadenectomy for gastric cancer from the results of a prospective randomized controlled trial. Br J Surg. 2006;93:1526–1529. doi: 10.1002/bjs.5487. [DOI] [PubMed] [Google Scholar]
- 2.Hundahl SA. Staging, stage migration, and patterns of spread in gastric cancer. Semin Radiat Oncol. 2002;12:141–149. doi: 10.1053/srao.2002.30816. [DOI] [PubMed] [Google Scholar]
- 3.Baiocchi GL, Tiberio GA, Minicozzi AM, Morgagni P, Marrelli D, Bruno L, et al. A multicentric Western analysis of prognostic factors in advanced, node-negative gastric cancer patients. Ann Surg. 2010;252:70–73. doi: 10.1097/SLA.0b013e3181e4585e. [DOI] [PubMed] [Google Scholar]
- 4.Chen XZ, Yang K, Zhang B, Hu JK, Zhou C. Is retrieval of >25 lymph nodes a superior criterion for locally advanced gastric cancer surgery? Ann Surg. 2011;254:834–835. doi: 10.1097/SLA.0b013e318235dfda. [DOI] [PubMed] [Google Scholar]
- 5.Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114–7124. doi: 10.1200/JCO.2005.14.621. [DOI] [PubMed] [Google Scholar]
- 6.Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the "different disease" hypothesis. Cancer. 2000;88:921–932. [PubMed] [Google Scholar]
- 7.NCCN clinical practice guidelines in oncology (NCCN Guidelines): gastric cancer. V.2.2013 [Internet] Fort Wathing ton: National Comprehensive Cancer Network; c2012. [cited 2013 Dec 10]. Available from: http://www.nccn.org. [Google Scholar]
- 8.Baxter NN, Tuttle TM. Inadequacy of lymph node staging in gastric cancer patients: a population-based study. Ann Surg Oncol. 2005;12:981–987. doi: 10.1245/ASO.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Hanna GB, Amygdalos I, Ni M, Boshier PR, Mikhail S, Lloyd J, et al. Improving the standard of lymph node retrieval after gastric cancer surgery. Histopathology. 2013;63:316–324. doi: 10.1111/his.12167. [DOI] [PubMed] [Google Scholar]
- 10.Bunt AM, Hermans J, van de Velde CJ, Sasako M, Hoefsloot FA, Fleuren G, et al. Lymph node retrieval in a randomized trial on western-type versus Japanese-type surgery in gastric cancer. J Clin Oncol. 1996;14:2289–2294. doi: 10.1200/JCO.1996.14.8.2289. [DOI] [PubMed] [Google Scholar]
- 11.Bunt AM, Hermans J, Boon MC, van de Velde CJ, Sasako M, Fleuren GJ, et al. Evaluation of the extent of lymphadenectomy in a randomized trial of Western-versus Japanese-type surgery in gastric cancer. J Clin Oncol. 1994;12:417–422. doi: 10.1200/JCO.1994.12.2.417. [DOI] [PubMed] [Google Scholar]
- 12.Kunisaki C, Makino H, Akiyama H, Otsuka Y, Ono HA, Kosaka T, et al. Clinical significance of the metastatic lymphnode ratio in early gastric cancer. J Gastrointest Surg. 2008;12:542–549. doi: 10.1007/s11605-007-0239-3. [DOI] [PubMed] [Google Scholar]
- 13.Cheong JH, Hyung WJ, Shen JG, Song C, Kim J, Choi SH, et al. The N ratio predicts recurrence and poor prognosis in patients with node-positive early gastric cancer. Ann Surg Oncol. 2006;13:377–385. doi: 10.1245/ASO.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 14.Sobin LH, Wittekind C. TNM classification of malignant tumors. 7th ed. Chichester, UK: Blackwell Publishing Ltd.; 2009. [Google Scholar]
- 15.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 16.Bouvier AM, Haas O, Piard F, Roignot P, Bonithon-Kopp C, Faivre J. How many nodes must be examined to accurately stage gastric carcinomas? Results from a population based study. Cancer. 2002;94:2862–2866. doi: 10.1002/cncr.10550. [DOI] [PubMed] [Google Scholar]
- 17.Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, Li AF, et al. Stage migration influences on stage-specific survival comparison between D1 and D3 gastric cancer surgeries. Eur J Surg Oncol. 2005;31:153–157. doi: 10.1016/j.ejso.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Roukos D, Lazaros A, Kappas A, Encke A. Extended lymph node dissection in gastric cancer induces substantial stage migration and increases stage-specific survival without improvement of overall survival. J Clin Oncol. 1996;14:2408–2410. doi: 10.1200/JCO.1996.14.8.2408. [DOI] [PubMed] [Google Scholar]
- 19.Sun Z, Zhu GL, Lu C, Guo PT, Huang BJ, Li K, et al. The impact of N-ratio in minimizing stage migration phenomenon in gastric cancer patients with insufficient number or level of lymph node retrieved: results from a Chinese mono-institutional study in 2159 patients. Ann Oncol. 2009;20:897–905. doi: 10.1093/annonc/mdn707. [DOI] [PubMed] [Google Scholar]
- 20.Deng J, Liang H, Sun D, Pan Y. The prognostic analysis of lymph node-positive gastric cancer patients following curative resection. J Surg Res. 2010;161:47–53. doi: 10.1016/j.jss.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Jung da H, Kim JH, Lee YC, Huh CW, Youn YH, Park H, et al. Is the 7th TNM edition suitable for biological predictor in early gastric cancer? Hepatogastroenterology. 2013;60:1225–1230. doi: 10.5754/hge11579. [DOI] [PubMed] [Google Scholar]