Abstract
Purpose
The primary aim of the present study was to analyze the association between high-risk clinicopathologic characteristics and the BRAFV600E mutation.
Methods
From March 2010 to September 2012, we performed analysis of the BRAF mutation (assessing V600E point mutation of BRAF gene, exon 15, on chromosome 7q34 by real-time polymerase chain reaction kit) from 499 papillary thyroid carcinoma (PTC) patients who underwent thyroidectomy. We analyzed the relation between the mutation and known clinicopathologic risk factors of PTC.
Results
BRAF mutations were found in 353 of 499 patients (70.7%). On univariate analysis, BRAF mutations were more frequently detected in patients with central lymph node metastasis (78.5% vs. 66.7%, P = 0.007) and classic PTC type (71.3% vs. 16.7%, P = 0.011). Patients with one or more aggressive pathologic feature such as lymph node metastasis, multifocality, and extrathyroidal extension showed higher BRAF mutation rate (73.5% vs. 62.3%, P = 0.022). BRAF mutation group showed more aggressive pathologic features, which is considered as higher necessity of radioactive iodine ablation (relative risk, 1.617; P = 0.035).
Conclusion
This study found that BRAF mutation is associated with classic PTC and central lymph node metastasis and higher necessity of radioactive iodine ablation.
Keywords: BRAF mutation, Papillary thyroid cancer, Radioactive iodine
INTRODUCTION
The incidence of thyroid cancer has increased more than two folds in the United States over the past three decades [1]. Due to a steady increase in the incidence of thyroid cancer in South Korea, thyroid cancer has become the most common cancer in this population [2]. The overall 5-year patient survival rate for PTC is 95% to 97% [3]. Considering this excellent prognosis, it is very important to identify patients who need aggressive treatment to reduce recurrence and the others who might present good outcome after limited surgery such as unilateral lobectomy or omission of central neck lymph node dissection. Unilateral lobectomy is accepted as the one of surgical options for patients with these features: age between 15 and 45 years old, no prior radiation, no distant metastases, no cervical lymph node metastases, no extrathyroidal extension, tumor less than 4 cm in diameter, and no aggressive variant [4].
BRAF represents one of the most frequently mutated protein kinase genes in human tumors [5]. The T1779A point mutation in BRAF exon 15, which results in a V600E amino acid substitution, is the most common mutation and accounts for more than 90% of all mutations found in the BRAF gene [6]. BRAF mutation has been thought to confer a worse prognosis in cases of papillary thyroid carcinoma (PTC) due to its association with lymph node metastasis, extrathyroidal extension, capsule invasion, vascular invasion, multifocality, bilateral tumors, older age, tumor size, aggressive subtype, impaired iodine uptake, recurrence, and death [7,8,9].
The primary aim of the present study was to evaluate the incidence of the BRAFV600E mutation in patients diagnosed with PTC and to analyze the association between high-risk clinicopathologic characteristics and the BRAFV600E mutation. This could allow for the use of the BRAF mutation in selecting candidates for limited thyroid surgery or radical operation premising postoperative radio iodine therapy as an initial surgical choice for papillary thyroid carcinoma in a Korean population.
METHODS
From March 2010 to September 2012, a total of 499 patients for whom thyroidectomy was planned for primary PTC at Kyung Hee University Hospital at Gangdong (Seoul, Korea) and Kyung Hee University Hospital (Seoul, Korea) were enrolled in this study.
Lobectomy was planned under National Comprehensive Cancer Network (NCCN) guidelines [4] when patients have age from 15 years to 45 years, no prior radiation, no distant metastases, no cervical lymph node metastases, no extrathyroidal extension, tumor less than 4 cm in diameter according to preoperative ultrasonographic findings in our institutions. Age was not absolute determinant for lobectomy in our institutions, same as described in NCCN guidelines [4]. Central neck lymph node dissection was performed electively in all patients. Modified radical neck dissection was performed with therapeutic intent.
Radioiodine treatment was recommended when post ope-rative pathology suggest any of followings: gross or microscopic extrathyroidal extension, high risk histology (tall cell, columnar, insular, and solid variants, as well as poorly differentiated thyroid cancer), cervical neck lymph node metastasis, multifocality or vascular invasion [4]. Age and sex were not absolute determinant of radioactive iodine ablation (RIA) in our institutions. To assess clinical value of BRAF mutation to predict aggressive tumor characteristics, which premise wider resection of thyroid such as total thyroidectomy, or prophylactic central neck lymph node dissection, and postoperative radioiodine therapy, statistical analysis was performed between existence of aggressive features and BRAF mutation. The incidence of aggressive features on pathology, such as lymph node metastasis, multifocality and extrathyroidal extension was assessed. Variant type of PTC is generally considered as one of aggressive feature of thyroid cancer. However, high risk histology include tall cell, columnar, insular, and solid variants, as well as poorly differentiated thyroid cancer. In our data variant PTC were composed of 6 follicular variant PTC and 1 diffuse sclerosing PTC, which have heterogeneous results in their clinical outcomes and benefits of RIA. Therefore aggressive pathologic features were limited to lymph node metastasis multifocality and extrathyroidal extension in this study.
Informed consent was obtained for the BRAF mutation test. The study protocols were approved by our Institutional Review Boards (IRB No.: KHNMC 2013-01-110, KMC IRB 1403-04).
DNA extraction and mutation analysis
Preparation of genomic DNA
We performed BRAFV600E mutation analysis on paraffin embedded sections of primary tumors obtained from thyroidectomy. Areas of tumor were identified on hematoxylin and eosin stained slides, marked by pathologists and microdissected using a fine needle from 10-µm-thick unstained sections. Genomic DNA was extracted from the tumor tissue using a high pure polymerase chain reaction (PCR) template preparation kit (Roche, Mannheim, Germany), according to the manufacturer's instructions. The DNA was diluted to a concentration of 50 ng/µL for the test. Genomic DNA from the formalin-fixed paraffin-embedded (FFPE) sections was extracted from 5 µm × 10 µm sections using the QIAmp DNA FFPE tissue kit (Qiagen, Valencia, CA, USA) as per the manufacturer's instructions.
PNAcqPCR
The BRAFV600E mutation was tested using a peptide nucleic acid (PNA) Clamp BRAF Mutation Detection kit according to the manufacturer's instructions. Briefly, PCR was performed on a total volume of 20 µL containing 50 ng of DNA, 13 µL of real-time SYBR green PCR master mix and each of the primers and PNA probes for codon 600. The PCR control lacked a PNA probe and contained the wild-type template. The PCR cycling conditions were 94℃ for 5 minutes, followed by 40 cycles of four temperature steps (94℃ for 30 seconds, 70℃ for 20 seconds, 63℃ for 30 seconds, and 72℃ for 30 seconds), and a final extension at 72℃ for 5 minutes. The PNA probe, designed to hybridize completely to the wild-type BRAF allele, inhibited the amplification of the wild-type BRAF allele. In contrast, the PNA/mutant-type allele hybrid was unstable due to base pair mismatch, which is why extension by the polymerase occurred. The threshold cycle (Ct) was automatically calculated from the PCR amplification plots, where fluorescence was plotted against the number of cycles. Delta-Ct values were calculated as the Ct values of the samples minus those of the controls. The higher delta-Ct value showed that the mutant was efficiently amplified. A cutoff value of 2.0 was used for determining the presence of mutant DNA.
Statistics
All statistical analyses were performed using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). The chi-square test and Fisher exact test were used for analysis of the relationship between the BRAF mutation and clinicopathologic factors. Multinomial logistic regression was adopted for multivariate analysis.
RESULTS
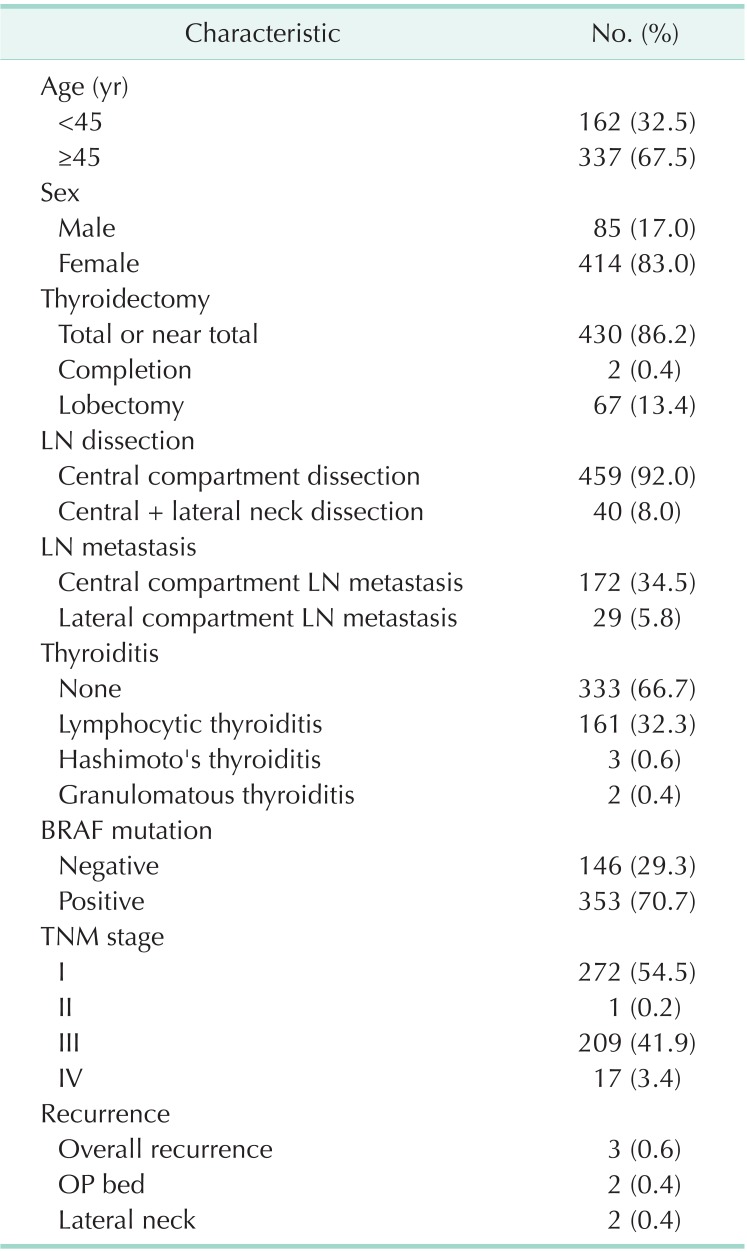
Among 499 patients, 85 males and 414 females patients were included. The mean age of patients at surgery was 50.2 ± 11.8 (18 to 82) years. Total or near total thyroidectomy was performed for 430 patients (86.2%), whereas the remaining patients underwent more limited thyroidectomy, such as completion thyroidectomy (2 patients) or lobectomy (67 patients). Central neck lymph node dissection alone was performed for 459 patients (92.0%), and modified radical neck dissection, including central neck lymph node dissection, was performed on 40 patients (8.0%). Among the 499 patients, central neck lymph node metastasis was found in 172 patients (34.5%). Lateral neck lymph node metastasis was found in 29 patients (5.8%) (Table 1).
Table 1.
Clinical and pathological characteristics (n = 499)
LN, lymph node; OP, operation.
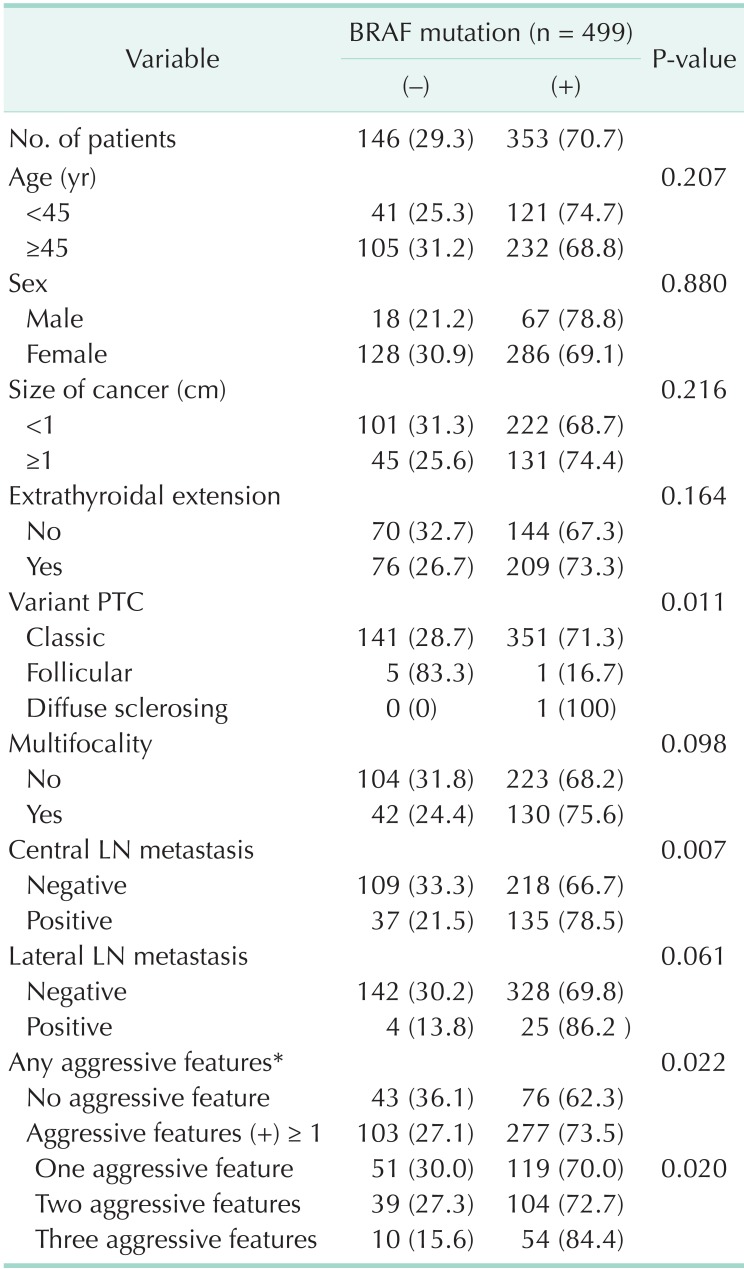
BRAF mutations were found in 353 of 499 patients (70.7%). On univariate analysis, BRAF mutations were more frequently detected in patients with central lymph node metastasis (78.5% vs. 66.7%, P = 0.007). Classic PTC showed higher BRAF positivity than follicular variants of PTC (71.3% vs. 16.7%, P = 0.011). BRAF mutation was not associated with age, sex, tumor size or extrathyroidal extension (Table 2).
Table 2.
BRAF mutation and clinicopathologic factors: univariate analysis
Values are presented as number (%).
PTC, papillary thyroid carcinoma; LN, lymph node.
*Aggressive features: any one of followings; LN metastasis, multifocality, extrathyroidal extension.
Patients who have one or more aggressive pathologic features were categorized as candidates for RIA. Patients with one or more aggressive pathologic feature such as lymph node metastasis, multifocality, and extrathyroidal extension showed higher BRAF mutation rate (73.5% vs. 62.3%, P = 0.022). As number of aggressive factor increases, BRAF mutation rate became higher with statistical significance (P = 0.020) (Table 2).
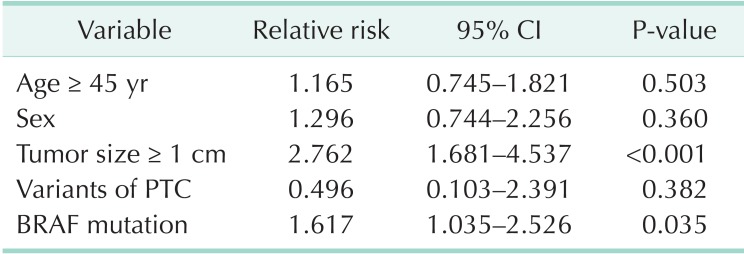
On multivariate analysis, RIA candidacy was associated with larger tumor size (relative risk [RR], 2.762; 95% confidence interval [CI], 1.681-4.537; P < 0.001) and BRAF mutation (RR, 1.617; 95% CI, 1.035-2.526; P = 0.035). Age, sex, and variant PTC were not significant contributors (Table 3).
Table 3.
Multivariate analysis for aggressive clinicopathologic features which predict greater need for radioactive iodine: aggressive features include lymph node metastasis, multifocality, extrathyroidal extension
CI, confidence interval; PTC, papillary thyroid carcinoma.
DISCUSSION
The prevalence of BRAF mutation in PTC in the present study was high at 70.7%. This result is in agreement with previous studies performed in the Korean population [10,11,12,13,14].
Many studies of PTC have shown a correlation between one or more poor prognostic factors and BRAF mutation. BRAF mutation has also been correlated with the aggressiveness of PTC, as cancers with a BRAF mutation often have advanced pathologic (TNM) staging, extrathyroidal extension, lymph node metastases, tumor persistence and recurrence [9,15,16]. In addition, BRAF mutations are correlated with lymphocytic thyroiditis, which is also associated with PTC. This may suggest a mechanism for increased invasiveness and immune system tolerance in BRAF-mutated PTCs [17]. Data available to date support the idea that BRAF mutation is an independent prognostic factor predicting a poor prognosis in PTC. In contrast, some studies failed to find a significant association between the BRAFV600E mutation and high-risk clinicopathologic characteristics [18,19,20].
The prevalence of the BRAFV600E mutation is highly variable, ranging from 29% to 83% in PTC in Western countries [9]. However, the prevalence of the BRAFV600E mutation in PTC is much higher (52%-83%) in Korea than has been observed in other countries (30%-49%) [10,11,12,13,14]. There are inconsistent reports suggesting that BRAF mutations may not predict a poor prognosis for PTC in Korea because of the relatively high incidence of that mutation in these countries [10,12]. The relatively low number of BRAFV600E-negative tumors within the Korean population might have weakened the statistical power of the corresponding analyses [10,12]. This discrepancy may be due to a lack of any prospective studies that would reduce the selection bias of observational studies, small sample size, a lack of multivariate analysis, and heterogeneous histologic subtypes of PTC. However, Kim et al. [11] recently reported the results of a prospective study analyzing a patient population in Korea. They suggested that the BRAFV600E mutation is associated with high-risk clinicopathologic characteristics in patients with PTC. They concluded that the BRAFV600E mutation may be a potential prognostic factor in Korean PTC patients [11]. The result of our study was largely consistent with Kim et al. [11]
In this study we found that BRAF mutation is associated with the classic-type PTC and central lymph node metastasis (Table 2). Furthermore, more patients presented with one or more aggressive tumor features in the BRAF mutation positive group (Tables 2, 3). Patients who have one or more aggressive pathologic features might benefit from RIA. This is important in clinical practice because the decision of whether to perform a total thyroidectomy or limited surgery is made in advance of the final pathology results. Although traditional prognostic factors such as age, gender, extratumoral extension, metastasis, size, and TNM stage are associated with the aggressiveness of the tumor, some important factors are only revealed after surgery, consequently limiting the completeness of the initial surgical choice. In considering RIA as a treatment plan, clinicians review the pathologic results after the initial surgery and decide whether or not to proceed with RIA. The necessity for RIA should reflect the aggressiveness of the tumor. What is important is that total thyroidectomy is the premise for a successive RIA. Therefore, the optimal initial treatment of choice for patients with one or more aggressive features is total thyroidectomy. The treatment of choice for patients with no aggressive features could be total thyroidectomy or less aggressive surgery, such as unilateral thyroidectomy. Since lymph node metastasis and needing to undergo RIA are both associated with BRAF mutation, knowing the BRAF mutation status of a patient prior to performing surgery might be helpful in deciding the extent of initial surgery, thus avoiding a second surgery. Furthermore, preoperative assessment of BRAF mutation in fine needle aspiration is proven to be feasible clinically [21]. In our study, the BRAF mutation is related to classic PTC and aggressive tumor features. Therefore, preoperative knowledge of BRAF mutation status could be useful in the diagnosis of PTC, and planning initial surgical therapy.
To understand clinical value of BRAF mutation in predicting prognosis of papillary thyroid cancer, reviewing recently published data by Xing et al. [22] is helpful. The only mortality data available is from a retrospective study of 1,849 subjects with PTC who were evaluated and treated at 13 centers. This study confirmed the association between specific BRAF gene mutations and multiple aggressive features in PTC [22]. The data demonstrated a strong association between BRAF gene mutations and thyroid cancer mortality in unadjusted analyses. The effect was attenuated, but persistent, after adjustment for age and sex. Disappointingly, Xing et al. [22] failed to find significantly higher mortality rates in the BRAFV600E mutation group on multivariate analysis after adjusting for the clinical and histopathologic features of aggressive thyroid tumors. As a result, the BRAFV600E mutation is a mediator for the features of clinically aggressive tumors that account for the vast majority of PTC mortality. This provides a biological rationale for targeted tyrosine kinase inhibitor therapy for tumors with BRAF mutation in advanced disease. Simultaneously, these study results suggest that BRAFV600E testing does not add predictive value for PTC-related mortality beyond the information collected in the process of PTC tumor staging [23].
It appears that the BRAF mutation has clinical value in the diagnosis of PTC, planning initial surgical therapy, and in the utilization of tyrosine kinase inhibitors. Our study supports the association between BRAF mutations and the aggressiveness of PTC. BRAF mutations are associated with lymph node metastasis and needing to undergo RIA in our study. This association might be useful in tailoring the initial surgical management of patients with PTC. However, further well designed prospective study is required to confirm clinical value of BRAF mutation in deciding surgical plan.
Footnotes
This study was presented at the 65th Annual Autumn Congress of Korean Surgical Society in November 21, 2013, also has been awarded as the best poster presentation.
No potential conflict of interest relevant to this article was reported.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States,1973-2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Information Center [Internet] Goyang: National Cancer Information Center; [cited 2011 Jun 20]. Available from: http://www.cancer.go.kr. [Google Scholar]
- 3.Previous version: SEER cancer statistics review, 1975-2009 (vintage 2009 populations) [Internet] Bethesda: National Cancer Institute; [cited 2013 May]. Available from: http://seer.cancer.gov/csr/1975_2009_pops09/ [Google Scholar]
- 4.Di Benedetto G, Fabozzi A, Rinaldi C, Rinaldi CR. BRAF test and cytological diagnosis with a single fine needle cytology sample. Acta Cytol. 2013;57:337–340. doi: 10.1159/000350618. [DOI] [PubMed] [Google Scholar]
- 5.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 6.Garnett MJ, Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell. 2004;6:313–319. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Barollo S, Pennelli G, Vianello F, Watutantrige Fernando S, Negro I, Merante Boschin I, et al. BRAF in primary and recurrent papillary thyroid cancers: the relationship with (131)I and 2-[(18)F] fluoro-2-deoxy-D-glucose uptake ability. Eur J Endocrinol. 2010;163:659–663. doi: 10.1530/EJE-10-0290. [DOI] [PubMed] [Google Scholar]
- 8.Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R, et al. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 9.Tufano RP, Teixeira GV, Bishop J, Carson KA, Xing M. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: a systematic review and meta-analysis. Medicine (Baltimore) 2012;91:274–286. doi: 10.1097/MD.0b013e31826a9c71. [DOI] [PubMed] [Google Scholar]
- 10.Ahn D, Park JS, Sohn JH, Kim JH, Park SK, Seo AN, et al. BRAFV600E mutation does not serve as a prognostic factor in Korean patients with papillary thyroid carcinoma. Auris Nasus Larynx. 2012;39:198–203. doi: 10.1016/j.anl.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Kim SJ, Lee KE, Myong JP, Park JH, Jeon YK, Min HS, et al. BRAF V600E mutation is associated with tumor aggressiveness in papillary thyroid cancer. World J Surg. 2012;36:310–317. doi: 10.1007/s00268-011-1383-1. [DOI] [PubMed] [Google Scholar]
- 12.Nam JK, Jung CK, Song BJ, Lim DJ, Chae BJ, Lee NS, et al. Is the BRAF(V600E) mutation useful as a predictor of preoperative risk in papillary thyroid cancer? Am J Surg. 2012;203:436–441. doi: 10.1016/j.amjsurg.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Lim JY, Hong SW, Lee YS, Kim BW, Park CS, Chang HS, et al. Clinicopathologic implications of the BRAF(V600E) mutation in papillary thyroid cancer: a subgroup analysis of 3130 cases in a single center. Thyroid. 2013;23:1423–1430. doi: 10.1089/thy.2013.0036. [DOI] [PubMed] [Google Scholar]
- 14.Min HS, Lee C, Jung KC. Correlation of immunohistochemical markers and BRAF mutation status with histological variants of papillary thyroid carcinoma in the Korean population. J Korean Med Sci. 2013;28:534–541. doi: 10.3346/jkms.2013.28.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Namba H, Nakashima M, Hayashi T, Hayashida N, Maeda S, Rogounovitch TI, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88:4393–4397. doi: 10.1210/jc.2003-030305. [DOI] [PubMed] [Google Scholar]
- 16.Kim TH, Park YJ, Lim JA, Ahn HY, Lee EK, Lee YJ, et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer. 2012;118:1764–1773. doi: 10.1002/cncr.26500. [DOI] [PubMed] [Google Scholar]
- 17.Smith RA, Salajegheh A, Weinstein S, Nassiri M, Lam AK. Correlation between BRAF mutation and the clinicopathological parameters in papillary thyroid carcinoma with particular reference to follicular variant. Hum Pathol. 2011;42:500–506. doi: 10.1016/j.humpath.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 18.Fugazzola L, Mannavola D, Cirello V, Vannucchi G, Muzza M, Vicentini L, et al. BRAF mutations in an Italian cohort of thyroid cancers. Clin Endocrinol (Oxf) 2004;61:239–243. doi: 10.1111/j.1365-2265.2004.02089.x. [DOI] [PubMed] [Google Scholar]
- 19.Ito Y, Yoshida H, Maruo R, Morita S, Takano T, Hirokawa M, et al. BRAF mutation in papillary thyroid carcinoma in a Japanese population: its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocr J. 2009;56:89–97. doi: 10.1507/endocrj.k08e-208. [DOI] [PubMed] [Google Scholar]
- 20.Puxeddu E, Moretti S, Elisei R, Romei C, Pascucci R, Martinelli M, et al. BRAF (V599E) mutation is the leading genetic event in adult sporadic papillary thyroid carcinomas. J Clin Endocrinol Metab. 2004;89:2414–2420. doi: 10.1210/jc.2003-031425. [DOI] [PubMed] [Google Scholar]
- 21.Cohen Y, Rosenbaum E, Clark DP, Zeiger MA, Umbricht CB, Tufano RP, et al. Mutational analysis of BRAF in fine needle aspiration biopsies of the thyroid: a potential application for the preoperative assessment of thyroid nodules. Clin Cancer Res. 2004;10:2761–2765. doi: 10.1158/1078-0432.ccr-03-0273. [DOI] [PubMed] [Google Scholar]
- 22.Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493–1501. doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cappola AR, Mandel SJ. Molecular testing in thyroid cancer: BRAF mutation status and mortality. JAMA. 2013;309:1529–1530. doi: 10.1001/jama.2013.3620. [DOI] [PubMed] [Google Scholar]