Abstract
Self-assembled monolayers (SAMs) of alkanethiols on gold have been employed as model substrates to investigate the effects of surface chemistry on cell behavior. However, few studies were dedicated to the substrates with a controlled wettability in studying stem cell fate. Here, mixed hydroxyl (-OH) and methyl (-CH3) terminated SAMs were prepared to form substrates with varying wettability, which were used to study the effects of wettability on the adhesion, spreading, proliferation and osteogenic differentiation of mesenchymal stem cells (MSCs) from human and mouse origins. The numbers of adhered human fetal MSCs (hMSCs) and mouse bone marrow MSCs (mMSCs) were maximized on -OH/-CH3 mixed SAMs with a water contact angle of 40~70° and 70~90°, respectively. Hydrophilic mixed SAMs with a water contact angle of 20~70° also promoted the spreading of both hMSCs and mMSCs. Both hMSCs and mMSCs proliferation was most favored on hydrophilic SAMs with a water contact angle around 70°. In addition, a moderate hydrophilic surface (with a contact angle of 40~90° for hMSCs and 70° for mMSCs) promoted osteogenic differentiation in the presence of biological stimuli. Hydrophilic mixed SAMs with a moderate wettability tended to promote the expression of αvβ1 integrin of MSCs, indicating that the tunable wettability of the mixed SAMs may guide osteogenesis through mediating the αvβ1 integrin signaling pathway. Our work can direct the design of biomaterials with controllable wettability to promote the adhesion, proliferation and differentiation of MSCs from different sources.
Introduction
The design of biomedical materials and devices will benefit from an understanding of molecular and cellular interactions at material surfaces. It is widely accepted that biomaterial surfaces affect protein adsorption and the subsequent activation of cells.1 Surface wettability plays an important role in regulating cell behaviors, which has been extensively studied.2, 3 However, most researches are conducted on specific materials and the conclusions may not be applied to other systems. Moreover, it is challenging to eliminate the influences of other surface parameters and obtain precisely controlled surface wettability. Self-assembled monolayers (SAMs) of alkanethiols on gold are chemically well-defined and can serve as controllable model surfaces. The use of SAMs is an efficient approach to tailoring the surface and interfacial properties, enabling their application in electrochemical, physical, bioanalytical and bioorganic chemistry.4 A host of thiols with different chemical functional groups have been extensively studied.5, 6 Our previous work also proved that neural stem cells, MCF-7 breast cancer cell line and mesenchymal stem cells (MSCs) on SAMs were greatly affected by the type of chemical groups terminated on the surfaces.7–10
Mixed SAMs composed of multiple components are as important as uniform ones because they provide a convenient approach to tuning the surface properties such as wettability and charges by adjusting the ratio of the different constituents.11 Besides, SAMs with mixed functional groups are closer to the native matrix found in biological systems. Mixed SAMs can simplify material surface effects in complex cell-material interactions to clarify how surface properties of biomaterials contribute to the activity of cells. Y. Arima and H. Iwata examined the adhesion of human umbilical vein endothelial cells and HeLa cells onto mixed SAMs with different wettability.9 However, few reports have investigated the effect of mixed SAMs (nonionic) with the difference in only wettability on phenotype expression of stem cells, especially regarding their osteogenic differentiation. MSCs are multi-potent stem cells and can differentiate into several lineages including adipocyte, osteocyte and chondrocyte.12 In recent years, MSCs have been widely studied as seeding cells for regenerative medicine applications, especially for bone repair.13 They possess the cellular totipotency with low variability from different adult donors.14 However, the adhesion, spreading, proliferation, and differentiation of MSCs within a dynamic 3-dimensional (3D) microenvironment are influenced by many complex factors and interactions. It is challenging to discriminate effects from confounding surface properties and elucidate the mechanisms of cell response.
In this study, surfaces with a wide range of wettability were prepared from alkanethiols solutions with mixed functional groups of hydrophilic hydroxyl (-OH) and hydrophobic methyl (-CH3). A change in the ratio of -OH and -CH3 terminated alkanethiols will lead to tuning of the wettability of the mixed SAMs. Moreover, comparison of the response of MSCs from different species to the same materials with different wettability has not been fully studied. Hence, we explored the response of hMSCs and mMSCs to -OH/-CH3 mixed SAMs with varying wettability. This study will provide a fundamental guidance on the design of biomaterial surfaces for bone regeneration.
Materials and methods
Materials
The starting materials used in this study included 1-dodecanethiol CH3(CH2)10CH2SH, ≥98%, Sigma-Aldrich, USA) and 11-Mercapto-1-undecanol (HSCH2(CH2)9CH2OH, 99%, Sigma-Aldrich, USA). Gold substrates were prepared using an ANELVA L-400EK electron beam evaporator (Canon Anelva Corporation, Kanagawa, Japan). The titanium (10 nm) and gold 40 nm) films were sequentially deposited onto silicon wafers polished/etched, crystal orientation 100). The wafers were diced into pieces (1cm×1cm) using a DS820 automatic dicing saw Heyan Technology Corporation, Shenyang, China).
Monolayers formation
Pure alkanethiol solutions were prepared in ethanol with a final concentration of 1 mM. Surfaces with a wide range of wettability were prepared by mixing the pure solutions (alkanethiols terminated with -OH or -CH3) with different volume ratios (-OH/-CH3=10/0, 9/1, 7/3, 5/5, 3/7, 0/10). Gold slides were cleaned by nitrogen plasma treatment using a HPC plasma cleaner system (Mycro Technologies Corporation, USA) for 2 min. Then they were rinsed 3 times with ethanol and highly purified water, and dried by a stream of nitrogen gas. The cleaned gold slides were immersed in the alkanethiol solutions for 24 h at room temperature. After incubation, the modified substrates were rinsed and dried again as mentioned.
Surface characterization of SAMs
Static water contact angles were measured by a contact angle meter (OCA15, Data Physics, Germany) at room temperature. The measurement process was repeated 5 times at different spots of each surface. The chemical functional groups were investigated by Fourier transform infrared attenuated total internal reflection (FTIR-ATR) (Vector 33, Bruker, Germany) within the wavenumber range of 4000-400 cm−1. Elemental compositions of the SAMs surfaces were characterized by X-ray photoelectron (XPS) (AXIS-ULTRA DLD, KRATOS, UK) with a Al Kα source. The photoelectrons were analyzed at a take-off angle of 55°. All the spectra were fitted using an XPS peak-fitting program (XPSPEAK Version 4.1).
Cell culture
Human fetal MSCs (hMSCs, Cyagen Biosciences Inc., USA) were maintained and expanded in growth medium (GM, Cyagen Biosciences Inc., USA) consisting of hMSCs basal medium, pre-selected fetal bovine serum (FBS), penicillin-streptomycin and glutamine. Early passages (≤5) of hMSCs were collected by addition of 0.25% trypsin/1 mM EDTA solution (Cyagen Biosciences Inc., USA) and used for analyzing cell adhesion, proliferation and differentiation. Mouse bone marrow MSCs (mMSCs, CRL-12424, ATCC, USA) were propagated in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, USA) with high glucose and supplemented with 10% FBS (Gibco, USA). Cells in passages from 3 to 4 were collected and used for the cell assays. In all experiments, medium was changed every 2 days until end-point assay. All SAMs substrates were placed in a non-treated 24-well plate, sanitized in 75% (v/v) ethanol for 3 h and then equilibrated in sterilized phosphate buffered saline (PBS). The cell suspensions were then added to the plates and incubated at 37 °C in a humidified incubator with 5% CO2.
Cell adhesion, spreading and proliferation
Cells (5×104 cells cm−2) were seeded onto the samples and cultured for 12 h. Then, the cell/substrate constructs were transferred to a new cell culture plate, washed 3 times with PBS and fixed with 4% formaldehyde for 30 min. After rinsed twice with PBS, cells were immersed first in 0.1% Triton X-100 PBS for 10 min to increase permeability, then in Phalloidin-FITC probe (AAT Bioquest® Inc, USA) for 60 min and finally in DAPI (Beyitime, China) for 10 min. The morphology of cells on each surface was observed by laser scanning confocal microscopy (Leica TCS SP5, Germany) (n=2). The number of adhered cells was evaluated by a colorimetric assay as reported (n=5).15
In order to characterize the cell spreading, the cells were fixed with 4% formaldehyde for 30 min, stained with fluorescein isothiocyanate (FITC, Santa Cruz Biotechnology, Inc., USA) for 30 min, then rinsed in PBS, and finally imaged using an inverted fluorescence microscope (40FLAXZOSKOP, ZEISS, Germany). Eight 10× images from different fields were chosen for statistics analysis of cell contact areas using image processing software (Image J, National Institutes of Health, USA).
Cell proliferation was evaluated by Cell Counting Kit-8 (CCK-8, Dojindo, Japan) assay following the manufacturer’s instructions (n=5). A suspension of hMSCs (1×104 cells cm−2) was added onto the samples. After 1, 3 and 5 days of culture, cell/sample constructs were transferred to a new plate. Then 300 µL of CCK-8 working solution was added into each well, and incubated at 37 °C for 1 h. Then 100 µL of the solution was pipetted to a 96-well plate. The absorbance at 450 nm was quantified by a microplate reader (Thermo3001, Thermo, USA).
Osteogenic differentiation and quantitative RT-PCR
The cells (5×104 cells cm−2) were cultured onto the samples in corresponding osteogenic differentiation medium. The hMSCs osteogenic medium was supplemented with hMSCs growth medium, 0.1 µM dexamethasone (Sigma-Aldrich, USA), 10 mM β-glycerophosphate (Calbiochem, USA) and 50 µM ascorbic acid Sigma-Aldrich, USA). The mMSCs osteogenic medium was composed of H-DMEM, 10% FBS, 0.1 µM dexamethasone, 10 mM β-glycerophosphate and 50 µM ascorbic acid. Cells were cultured in osteogenic medium for 7 or 14 days and then transferred to a new culture plate for alkaline phosphatase (ALP) staining and RT-PCR, respectively.
ALP staining was conducted in duplicate following the manufacturer’s instructions using the BCIP/NBT Phosphatase Subst rate (1-Component) (KPL, USA). The stained surface was observed by a digital three-dimensional video microscope (HIRO X KH-7700, Japan). Total RNA was extracted from two plates and each plate had two parallel samples for each kind of surf ace (n=4) using HiPure Total RNA Kits (Magentec, China), and reverse transcribed into cDNA using PrimeScript® RT reagent Kit with gDNA Eraser (TaKaRa Biotechnology, Japan) according to the manufacturer’s protocol. The RNA concentration was quantified by a NanoDrop2000 spectrophotometer (Thermo Scientific, USA). RT-PCR reactions were performed using th e SYBR Green System (Invitrogen, USA). Samples were held at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The relative quantification of target ge nes was analyzed using as β-actin as a reference. The level of expression of target genes were calculated by the 2−ΔΔCt method using the following primer and probe sequences (Invitrogen). Human-specific primers: β-actin {5’-GCATCCCCCAAAGTTCACAA-3’ (forward) and 5’-AGGACTGGGCCATTCTCCTT-3’ (reverse)}; Runx-2{5’-AGAAGGCACAGACAGAAGCTTGA-3’ (forward) and 5’-AGGAATGCGCCCTAAATCACT-3’ (reverse); ALP {5’-AGCACTCCCACTTCATCTGGAA-3’ (forward) and 5’-GAGACCCAATAGGTAGTCCACATTG-3’ (reverse)}; Osteocalcin {5’-CAGCGAGGTAGTGAAGAGA-3’ (forward) and 5’-GACTGGTGTAGCCGAAAG-3’(reverse)}; Collagen Ι {5’-CAGCCGCTTCACCTACAGC-3’ (forward) and 5’-TTTTGTATTCAATCACTGTCTTGCC-3’ (reverse)}; αv Integrin {5’-AAACTCGCCAGGTGGTATGTGA-3’ (forward) and 5’-CTGGT GCACACTGAAACGAAGA-3’ (reverse)}; β1 Integrin {5’-AGCTGAAGACTATCCCATTGACCTC-3’ (forward) and 5’-TGGTGTTGTGCTAATGTAAGGCATC-3’ (reverse)}. Mouse-specifi c primers: β-actin {5’-TGACAGGATGCAGAAGGAGA-3’ (f orward) and 5’-GCTGGAAGGTGGACAGTGAG-3’ (reverse)}; Runx-2 {5’-CACTGGCGGTGCAACAAGA-3’ (forward) and 5’-TTTCATAACAGCGGAGGCATTTC-3’ (reverse)}; ALP {5’ TGCCTACTTGTGTGGCGTGAA-3’ (forward) and 5’-TCACCCGAGTGGTAGTCACAATG-3’ (reverse)}; Osteocalcin {5’-AGCAGCTTGGCCCAGACCTA-3’ (forward) and 5’-TAGCGCCGGAGTCTGTTCACTAC-3’ (reverse)}; Collagen Ι {5’-TTCTGCTGCTAATGTTCTTGACC-3’ (forward) and 5’-GGGATGAAGTATTGTGTCTTGGG-3’ (reverse)}; αv Integrin {5’-TGAACTGCACGGCAGATACAGAG-3’ (forward) and 5’-ATCCCGCTTGGTGATGAGATG-3’ (reverse)}; β1 Integrin {5’-ATCATGCAGGTTGCGGTTTG-3’ (forward) and 5’-GGTGACATTGTCCATCATTGGGTA-3’ (reverse)}.
Statistical analysis
Quantitative experimental results were expressed as mean ± standard deviation. Statistical comparison was determined by analysis of variance (ANOVA) followed by post-hoc test and a p<0.05 was considered to be statistically significant.
Results
Surface characterization of SAMs
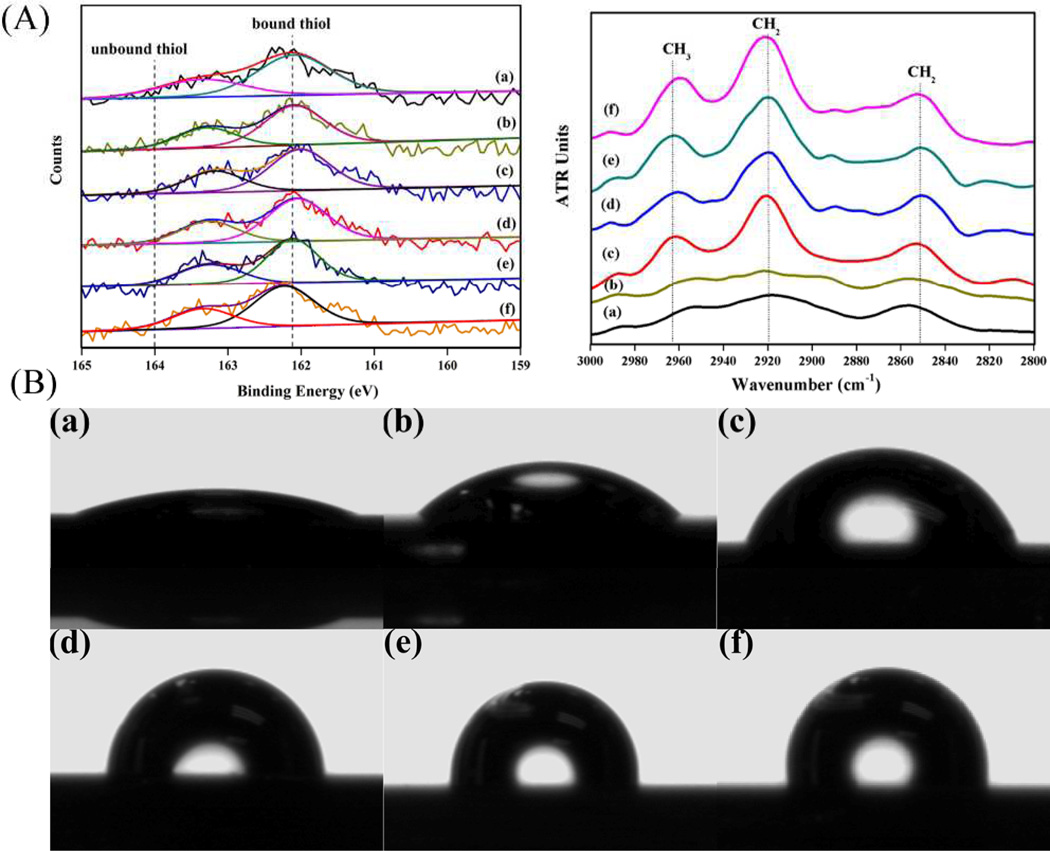
XPS and FTIR-ATR measurements were performed to prove that the -OH and -CH3 functional groups were successfully introduced onto the gold substrates. It was observed that the high-resolution spectra of sulphur can be well fitted with a doublet structure.16 In addition, all samples were free of unbound thiol molecules because no peaks were found in the binding energy region above 164 eV.17 This demonstrated that the physically adsorbed thiols were completely rinsed away. From the FTIR-ATR spectra in the interested region of 2800–3000 cm−1, the typical absorption band located at 2965 cm−1 was assigned to the asymmetric stretching vibration of -CH3.18 The intensity gradually became stronger while the -CH3 composition was increased in the mixed SAMs. In addition, the peaks of the asymmetric (νa) and symmetric (νs) stretching modes of -CH2- appeared at 2919 and 2851 cm−1, respectively,18, 19 indicating crystalline-like packing of the alkyl chains and high degree of ordering of the monolayers.6
The surface wettability can be tuned by mixing various ratios of thios terminated with -OH and -CH3 constituting the SAMs. The result of water contact angle on each surface showed that -OH group lead to well hydrophilic surface and -OH/-CH3 (9/1, 7/3, 5/5 v/v) mixed groups created moderately wettable surfaces, while -OH/-CH3 (3/7 v/v) and -CH3 groups produced hydrophobic substrata (Table1 and Fig.1 B). It can be seen that a widespread range of wettability from highly hydrophilic to highly hydrophobic was obtained. The water contact angle increased gradually with the incorporation of -CH3 on the surface. In Table 1, O/C ratios calculated by XPS data (Fig. 1A) decreased with the increase of -CH3 in the solution. The composition of –OH groups in the mixed SAMs (X-OH, surface) were calculated with the O(1s) peak intensity using the following equation.20
where AO1s and AAu4f were defined as the normalized area for O1s and Au4f photoeletrons, respectively; and XOH,solution=1 and XOH,solution=0 denoted -OH and -CH3 terminated thiols, respectively. It was showed that the percentage of incorporated -OH on surface was not equal to the nominal ratio of the thiol solution. The -CH3 terminated thiols were adsorbed preferentially over -OH terminated ones.
Table 1.
Water contact angles and the percentage of surface -OH on OH/CH3 mixed SAMs.
| SAMs -OH/-CH3 (v/v solution) |
Water contact angle θ (°) |
-OH (surface) % |
O(1s)/C(1s) |
|---|---|---|---|
| -OH | 22.8±1.1 | 100 | 0.1505±0.021 |
| 9/1 | 46±1.6 | 82.65±0.37 | 0.1405±0.026 |
| 7/3 | 73.0±0.5 | 39.56±0.52 | 0.1167±0.003 |
| 5/5 | 89.4±1.6 | 27.97±0.73 | 0.0673±0.029 |
| 3/7 | 98.1±1.8 | 16.56±0.28 | 0.0401±0.017 |
| -CH3 | 107.1±0.7 | 0 | 0.0448±0.010 |
Fig. 1.
(A) XPS S(2p) spectra and FTIR-ATR of -OH/-CH3 mixed SAMs; (B) Water contact angles on -OH/-CH3 mixed SAMs. (a) -OH; (b) -OH/-CH3 (9/1 v/v); (c) -OH/-CH3 (7/3 v/v); (d) -OH/-CH3 (5/5 v/v); (e) -OH/-CH3 (3/7 v/v); (f) -CH3.
Cell adhesion, spreading and proliferation
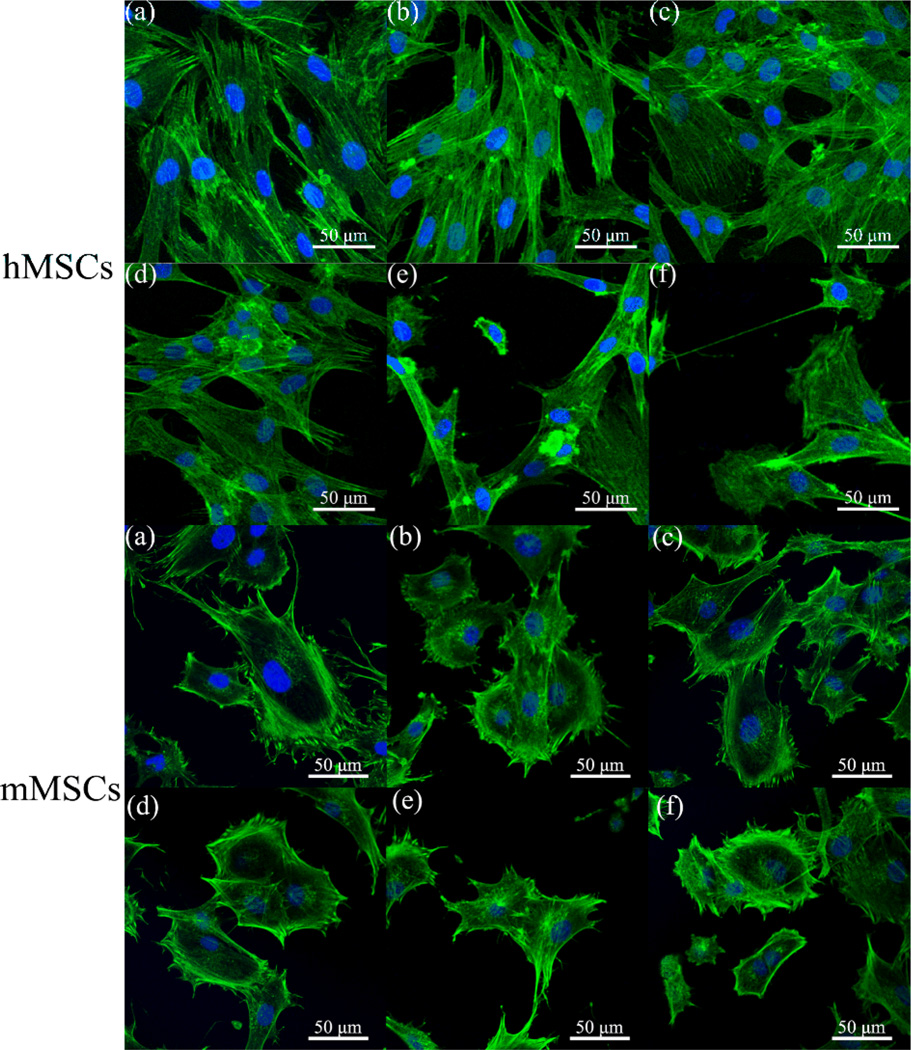
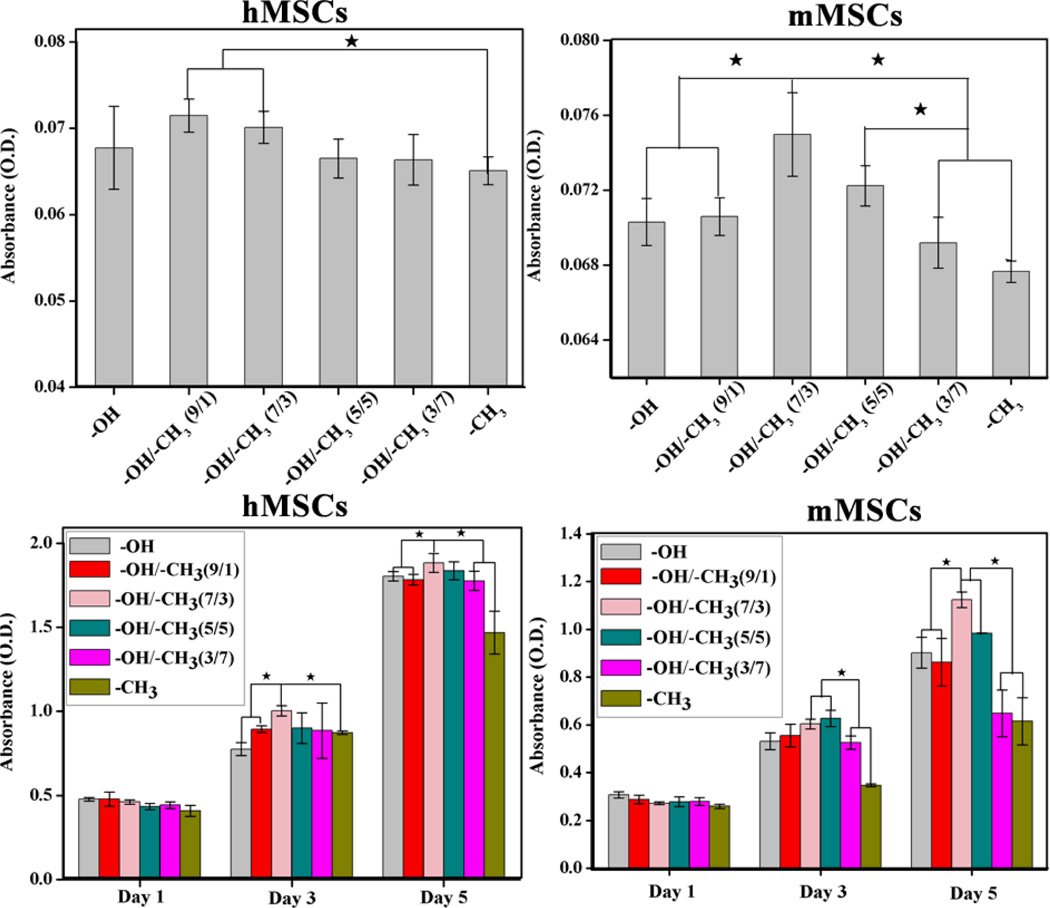
The morphologies of MSCs on -OH/-CH3 mixed SAMs after 12 h were shown in Fig. 2. The hMSCs spread well on the hydrophilic substrates (Fig.2. a, b and c) and exhibited a typical fibroblast-like morphology, while those on hydrophobic surfaces contracted a little into a compressed shape (e and f). Similarly, mMSCs adhered and spread well on -OH/-CH3 (7/3 v/v) but exhibited poor spreading on -CH3 SAMs. This result suggested that hMSCs and mMSCs responded well to hydrophilic surfaces in terms of overall cell spreading and morphology. It is consistent with the quantitative analysis of the cell spreading area, which showed that hydrophilic mixed SAMs with a contact angle of ~20–70° (especially, the -OH/-CH3 (7/3 v/v) SAMs) tended to promote the spreading of both hMSCs and mMSCs Fig. S1 and S2). In order to quantify the cell adhesion, cell cytoplasm was stained with crystal violet and the colorimetric readings, reflecting the number of adherent cells on various SAMs, were collected. As shown in Fig. 3 (A), the number of adherent hMSCs reached maximum on -OH/-CH3 (9/1 v/v, and 7/3 v/v). A maximum number was observed for the mMSCs on -OH/-CH3 (7/3 v/v). The MSCs proliferation on different -OH/-CH3 mixed SAMs was analyzed using CCK-8 assay after 1, 3 and 5 days of culture. As the culture time was extended, the cells on all samples proliferated well (Fig. 3B). We found that both hMSCs and mMSCs proliferated slower on -OH/-CH3 (3/7) and -CH3 terminated SAMs when compared with other samples. The hMSCs seemed to grow best on -OH/-CH3 (7/3 v/v) SAMs, while the mMSCs on -OH/-CH3 (7/3 v/v) and (5/5 v/v) SAMs. Namely, the hydrophilic mixed SAMs with moderate wettability promote the adhesion, spreading and proliferation of both hMSCs and mMSCs.
Fig. 2.
The morphology of MSCs on different -OH/-CH3 mixed SAMs after 12 h of culture was examined by laser scanning confocal microscopy. Cells were fixed and stained for F-actin with AlexaFluor 488 phalloidin (green). Cell nuclei were counterstained with DAPI (blue). (a) -OH; (b) -OH/-CH3 (9/1 v); (c) -OH/-CH3 (7/3 v/v); (d) -OH/-CH3 (5/5 v/v); (e) OH/-CH3 (3/7 v/v); (f) -CH3.
Fig. 3.
(A) The number of adhered cells on different -OH/-CH3 mixed SAMs after 3 h of culture by a colorimetric assay at 595 nm. (B) Cell proliferation of MSCs on different -OH/-CH3 mixed SAMs after 1, 3 and 5 day of culture ★ indicated that there was significant difference (p<0.05).
ALP staining and gene expression of osteogenic differentiation
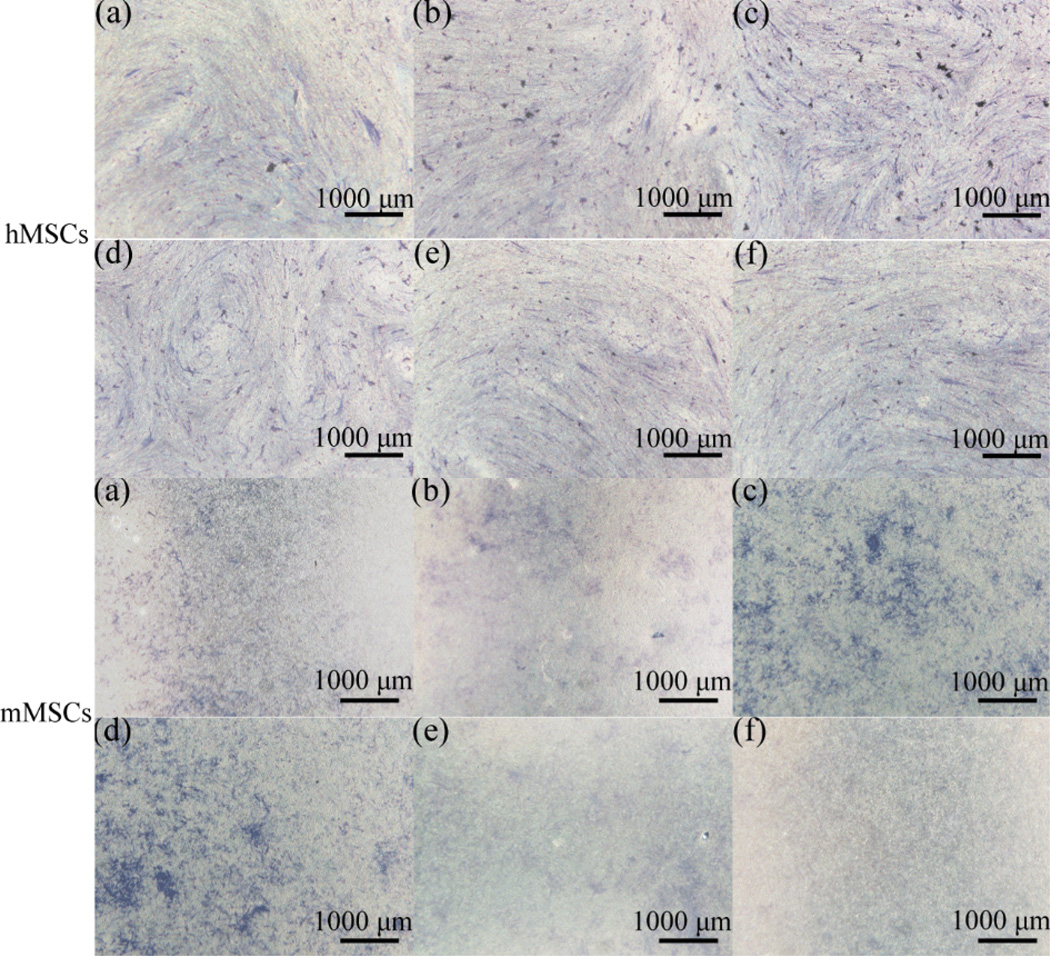
ALP staining of MSCs on different -OH/-CH3 mixed SAMs after 7 days were shown in Fig. 4. The formation of dark purple NBT-formazan was a qualitative indicator. The more blue-purple areas implied higher ALP activity and enhanced osteogenic differentiation. It was seen that the ALP activity of hMSCs and mMSCs was increased on -OH/-CH3 (9/1 v/v), (7/3 v/v) and (5/5 v) SAMs, confirming the improvement in osteogenesis by hydrophilic surfaces.
Fig. 4.
ALP staining of MSCs on different -OH/-CH3 mixed SAMs after 7 days of culture. (a) -OH; (b) -OH/-CH3 (9/1 v/v); (c) OH/-CH3 (7/3 v/v); (d) -OH/-CH3 (5/5 v/v); (e) -OH/-CH3 (3/7 v); (f) -CH3.
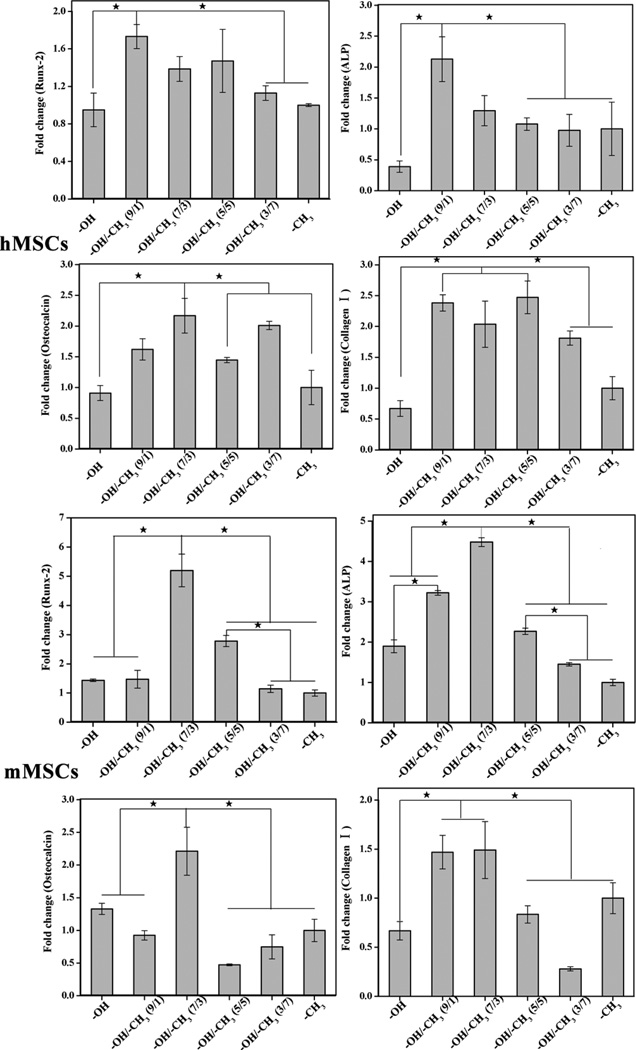
Runx-2, ALP, Osteocalcin and collagen I are well-accepted makers for osteogenic differentiation. Among them, Runx-2, ALP, and osteocalcin are osteo-specific markers whereas collagen I is non-osteo-specific. Runx-2, a member of the runt homology domain transcription factor family, was essential for osteoblastic differentiation and expressed early in the embryo in mesenchyme.21 High expression of ALP as a maker for bone metabolism indicated active bone formation.22 Osteocalcin and collagen I were included in the formation of mineralized bone-like nodules and reflected in late bone differentiation.23, 24 The expression of these osteogenic genes in MSCs after 7 days of culture were shown in Fig. 5. The expression of the makers in hMSCs on different -OH/-CH3 mixed SAMs did not show a regular trend. Higher expression of runx-2 was observed on -OH/-CH3 (9/1 v/v) SAMs. ALP and collagen Ι were expressed at a higher level on -OH/-CH3 (7/3 v/v) mixed SAMs than on other SAMs. As for mMSCs, the gene expression of all four osteogenic markers, including runx-2, ALP, osteocalcin and collagen Ι, was higher on OH/CH3 (7/3 v/v) mixed SAMs than on other SAMs. In addition to the favorite OH/CH3 (7/3 v/v) mixed SAMs, OH/CH3 9/1 v/v) mixed SAMs also upregulated ALP and collagen Ι in comparison to the other SAMs. Runx-2 and osteocalcin were upregulated on OH/CH3 (5/5 v/v) and OH terminated SAMs, respectively. The difference in the expression of the osteogenic genes in both hMSCs and mMSCs among different SAMs was similar between on Day 7 and Day 14 (Fig. S3). These results indicated that the surface wettability influenced the overall osteogenic differentiation of MSCs. Overall, expression of four osteogenic genes indicated that the differentiation of MSCs on different -OH/-CH3 mixed SAMs can be affected by the wettability and the cell origins. The surface with water contact angle about 40° could upregulate the gene expression of the early stage differentiation markers (Runx-2 and ALP) of hMSCs. However, the surface with contact angle of 70~90° upregulated the expression of the later stage osteogenic markers of osteocalcin and collagen I. As for mMSCs, the expression of the four osteogenic genes consistently suggested that the surface with water angle about 70° was most favorable for osteogenic expression of mMSCs.
Fig. 5.
Relative gene expression of Runx-2, ALP, Osteocalcin and Collagen Ι using RT-PCR of MSCs on different -OH/-CH3 mixed SAMs against -CH3 after 7 days of culture ★ indicated that there was significant difference (p<0.05).
Discussion
As proved above, a highly oriented -OH/-CH3 mixed SAMs were formed on the gold surface and surfaces with a graded range of wettability could be achieved. Co-adsorption of two or more thiols was not in complete thermodynamic equilibrium but rather competitive adsorption.25 Many factors could mediate the process co-adsorption, such as concentration of thiols, head groups, chain lengths and solvents. In our study, the adsorption of -CH3 terminated thiols was favorable on the surfaces probably because OH terminated thiols could form hydrogen bonds with the solvent whereas -CH3 terminated thiols had a higher activity in solution.20 In principle, the incorporation of -OH terminated thiols onto the surface did not increase linearly with the increase the proportion of –OH terminated thiols in solution.
We believe that the different wettability of the mixed SAMs mediated the signaling pathway to direct the fate of the MSCs. It well known that the integrins receptors mediate cell-matrix interactions and play a central role in cell adhesion, spreading, migration, proliferation and differentiation.26–30 The αvβ1 integrin enables the cells to bind to extracellular matrix proteins such as fibronectin and vitronectin, forming focal adhesion and triggering the intracellular signals with the actin cytoskeleton.26 We found that both hMSCs and mMSCs on the surface with moderate wettability presented a higher level of gene expression of αvβ1 integrin than those on the more hydrophobic surfaces (Fig. S4), which was consistent with the results of increased cell adhesion, spreading, proliferation and osteogenic differentiation of both hMSCs and mMSCs on hydrophilic mixed SAMs (Figs. 2–5 and S2). Therefore, αvβ1 integrin may be one of the signaling pathways triggered by surface hydrophilicity of -OH/-CH3 mixed SAMs for directing the fate of both hMSCs and mMSCs including their adhesion, spreading, proliferation and osteogenic differentiation.
Our results show that the morphologies of MSCs adhered onto the different -OH/-CH3 mixed SAMs were quite different (Fig. 2). On the hydrophobic surface, hMSCs and mMSCs both exhibited relatively round morphology. The quantification of the spreading area revealed that both hMSCs and mMSCs showed the largest contact areas on -OH/-CH3 (7/3 v/v) SAMs among all substrates (Figs. S1 and S2). The cell spreading data is consistent with the data on the adhesion, proliferation and differentiation in that the moderate hydrophilic SAMs tend to favor cell adhesion, proliferation and differentiation of both hMSCs and mMSCs. It is also likely that the mixed SAMs with different wettability direct the stem cell fate by modulating the adsorption of the extracellular protein. Cells adhesion and the formation of actin organization were found to be largely mediated by the interaction between integrin receptor and adsorbed matrix protein such as fibronectin, vitronectin, and fibrinogen.31 Keselowsky et al found that the density and conformation of fibronectin may all play a part in the amount of integrin receptor.32 It was also found that the -CH3 surface would have more adsorbed fibronectin than -OH surface due to the hydrophobic interaction.33 However, there was a strong preference for cell adhesion on –OH surfaces.34 Therefore, MSCs were able to organize their cytoskeleton and spread well on the hydrophilic -OH/-CH3 mixed SAMs.
A trend was found in the expression of osteocalcin (highest on 7/3) and collagen I (highest on 9/1 and 5/5) as well as Runx-2 and ALP (highest on 9/1) for hMSCs (Fig. 5). There was also a similar trend in the expression of osteocalcin, ALP and Runx-2 (highest on 7/3) as well as Rux-2 (highest on 9/1 and 7/3) for mMSCs (Fig. 5). Namely, different mixed SAMs differentially enhanced lineage specification and commitment although overall the moderate hydrophilic surfaces promoted the osteogenic differentiation of MSCs. This indicates that the modulation of these lineage specific markers over time during differentiation is a complex process and thus difficult to quantify. Indeed, we found that -OH/-CH3 mixed SAMs with moderate wettability promoted the expression of αvβ1 integrin (Fig. S4), which might trigger the integrin signaling pathway to promote the osteogenic differentiation. This is in agreement with the earlier finding35 that β1 integrin was critical for osteogenic differentiation of MSCs. A closer look at Fig. S4 showed a similar trend in the expression of αv integrin (highest on 7/3) and β1 integrin (highest on 9/1 and 5/5) for hMSCs as well as in the expression of αv integrin (highest on 7/3) and β1 integrin (highest on 9/1) for mMSCs, suggesting that different mixed SAMs differentially enhance the expression of integrins as well. Hence, it is likely that different wettability arising from the different mixed SAMs influences the expression of integrins differently to direct the osteogenic differentiation of for both hMSCs and mMSCs.
Hence, the adsorption of adhesive ligands of the intergrin family cell-surface receptors on these SAMs is worth further investigation in order to understand the stem cell fate directed by the mixed SAMs. In addition, activities of stem cells from different sources are regulated by many cues in their respective local tissue microenvironment. Mechanisms of cellular senescence in human and mouse cells are distinct.36 Yet so far, the comparison between hMSCs and mMSCs for the regulation of differentiation by surface chemistry is not clear. To understand the molecular mechanism and signaling pathway of the differentiation in human cells, numerous studies have been done using mouse or rat cells.37–39 However, osteogenic gene expression in our studies clearly differs between mouse and human cells, and thus needs to be further elucidated.
Conclusions
Surface hydrophilicity of hydroxyl-methyl mixed SAMs, as model substrates with controlled wettability, increased gradually with the incorporation of hydroxyl terminated groups on the surface of the monolayer. Such control over the wettability of the substrate surface has allowed us to systematically study the effect of wettability on the fate of MSCs from two different origins. The adhesion and proliferation of hMSCs and mMSCs were enhanced on hydrophilic surfaces with contact angles of 40~70° and 70~90°, respectively. Hydrophilic mixed SAMs with a moderate wettability (water contact angle of 20~70°) also promoted the spreading of both hMSCs and mMSCs. The surfaces of moderate wettability promoted osteogenic differentiation depending on the origins (40~90° for hMSCs and 70° for mMSCs) in the presence of biological stimuli. Hydrophilic mixed SAMs with a moderate wettability tended to promote the expression of αvβ1 integrin of both hMSCs and mMSCs, indicating that one of the possible mechanism for directing stem cell fate by the tunable wettability of the mixed SAMs is through mediating the αvβ1 integrin signaling pathway. The results indicated that cells behavior was affected by surface wettability and chemical functional groups, but also the cell origins. These findings can shed light into the design of biomaterials for stem cell-based regenerative medicine.
Supplementary Material
Acknowledgements
This work was financially supported by National Basic Research Program of China (Grant NO. 2012CB619100), National Natural Science Foundation of China (Grant51232002), the 111 project B13039). CBM would also like to thank the financial support from National Science Foundation (CMMI-1234957, CBET-0854414, CBET-0854465, and DMR-0847758), National Institutes of Health (5R01HL092526, 1R21EB015190), Department of Defense Peer Reviewed Medical Research Program (W81XWH-12-1-0384), Oklahoma Center for the Advancement of Science and Technology (070014 and HR11-006) Oklahoma Center for Adult Stem Cell Research (434003).
Footnotes
Electronic Supplementary Information (ESI) available: The fluorescence images and quantitative analysis of cell adhesion (Figs. S1 and S2); Osteogenic differentiation on day 14 (Fig. S3); the gene expression of αvβ1 integrin (Fig. S4) at 12 h.
Contributor Information
Chuanbin Mao, Email: cbmao@ou.edu.
Yingjun Wang, Email: imwangyj@163.com.
references
- 1.Anderson JM. Ann. Rev. Mater. Res. 2001;31:81–110. [Google Scholar]
- 2.Ponsonnet L, Reybier K, Jaffrezic N, Comte V, Lagneau C, Lissac M, Martelet C. Mater. Sci. Eng. C. 2003;23:551–560. [Google Scholar]
- 3.Van Wachem P, Beugeling T, Feijen J, Bantjes A, Detmers J, Van Aken W. Biomaterials. 1985;6:403–408. doi: 10.1016/0142-9612(85)90101-2. [DOI] [PubMed] [Google Scholar]
- 4.Hudalla GA, Murphy WL. Soft Matter. 2011;7:9561–9571. doi: 10.1039/C1SM05596H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Chem. Rev. 2005;105:1103–1170. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 6.Ulman A. Chem.Rev. 1996;96:1533–1554. doi: 10.1021/cr9502357. [DOI] [PubMed] [Google Scholar]
- 7.Ren Y-J, Zhang H, Huang H, Wang X-M, Zhou Z-Y, Cui F-Z, An Y-H. Biomaterials. 2009;30:1036–1044. doi: 10.1016/j.biomaterials.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 8.Bai B, He J, Li Y-S, Wang X-M, Ai H-J, Cui F-Z. BioMed. Res. Intl. 2013;2013 doi: 10.1155/2013/361906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan H, Zhang S, He J, Yin Y, Wang X, Chen X, Cui F, Li Y, Nie Y, Tian W. Biomed. Mater. 2013;8:035008. doi: 10.1088/1748-6041/8/3/035008. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, He J, Zhang S, Wang XM, Liu HY, Cui FZ. J. Tissue Eng. Regen. Med. 2013;7:112–117. doi: 10.1002/term.498. [DOI] [PubMed] [Google Scholar]
- 11.Arima Y, Iwata H. Biomaterials. 2007;28:3074–3082. doi: 10.1016/j.biomaterials.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Caplan AI. J. Orthopaed. Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 13.Caplan AI. J. Cell. Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 14.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 15.Malmström J, Christensen B, Lovmand J, Sørensen ES, Duch M, Sutherland DS. J. Biomed. Mater. Res. A. 2010;95:518–530. doi: 10.1002/jbm.a.32879. [DOI] [PubMed] [Google Scholar]
- 16.Castner DG, Hinds K, Grainger DW. Langmuir. 1996;12:5083–5086. [Google Scholar]
- 17.Martins MCL, Ratner BD, Barbosa MA. J. Biomed. Mater. Res. A. 2003;67:158–171. doi: 10.1002/jbm.a.10096. [DOI] [PubMed] [Google Scholar]
- 18.Porter MD, Bright TB, Allara DL, Chidsey CE. J. Am. Chem. Soc. 1987;109:3559–3568. [Google Scholar]
- 19.Nuzzo RG, Dubois LH, Allara DL. J. Am. Chem. Soc. 1990;112:558–569. [Google Scholar]
- 20.Bain CD, Evall J, Whitesides GM. J. Am. Chem. Soc. 1989;111:7155–7164. [Google Scholar]
- 21.Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, Javed A, Van Wijnen AJ, Stein JL, Stein GS. J. Biol. Chem. 2005;280:33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 22.Aschaffenburg R, Mullen J. J. Dairy Res. 1949;16:58–67. [Google Scholar]
- 23.Park JS, Yang HN, Jeon SY, Woo DG, Na K, Park K-H. Biomaterials. 2010;31:6239–6248. doi: 10.1016/j.biomaterials.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Frank O, Heim M, Jakob M, Barbero A, Schäfer D, Bendik I, Dick W, Heberer M, Martin I. J. Cell. Biochem. 2002;85:737–746. doi: 10.1002/jcb.10174. [DOI] [PubMed] [Google Scholar]
- 25.Folkers JP, Laibinis PE, Whitesides GM. Langmuir. 1992;8:1330–1341. [Google Scholar]
- 26.Docheva, Popov C, Mutschler W, Schieker M. J. Cell. Mol. Med. 2007;11:21–38. doi: 10.1111/j.1582-4934.2007.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosa A, Kato R, Castro Raucci L, Teixeira L, de Oliveira F, Bellesini L, de Oliveira P, Hassan M, Beloti M. J. Cell. Biochem. 2014;115:540–548. doi: 10.1002/jcb.24688. [DOI] [PubMed] [Google Scholar]
- 28.Garcia AJ. Biomaterials. 2005;26:7525–7529. doi: 10.1016/j.biomaterials.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 29.Hynes RO. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 30.Hynes RO. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 31.Hersel U, Dahmen C, Kessler H. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 32.Keselowsky BG, Collard DM, García AJ. J. Biomed. Mater. Res. A. 2003;66:247–259. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 33.Roach P, Farrar D, Perry CC. J. Am. Chem. Soc. 2005;127:8168–8173. doi: 10.1021/ja042898o. [DOI] [PubMed] [Google Scholar]
- 34.Barrias CC, Martins MCL, Almeida-Porada G, Barbosa MA, Granja PL. Biomaterials. 2009;30:307–316. doi: 10.1016/j.biomaterials.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 35.Gronthos S, Simmons P, Graves S, Robey PG. Bone. 2001;28:174–181. doi: 10.1016/s8756-3282(00)00424-5. [DOI] [PubMed] [Google Scholar]
- 36.Itahana K, Campisi J, Dimri GP. Biogerontology. 2004;5:1–10. doi: 10.1023/b:bgen.0000017682.96395.10. [DOI] [PubMed] [Google Scholar]
- 37.Zhu H, Cao B, Zhen Z, Laxmi A, Li D, Liu S, Mao CB. Biomaterials. 2011;32:4744–4752. doi: 10.1016/j.biomaterials.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Wang L, Li X, Mao CB. Sci. Rep. 2013;3:1242. doi: 10.1038/srep01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Yang LM, Zhu Y, Wang L, Tomsia AP, Mao CB. Adv. Mater. 2014 doi: 10.1002/adma.201400154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.