Summary
Cryptogenic, or unexplained, stroke is present in about 30%–40% of ischemic stroke patients. Pursuing a stroke mechanism is important in such patients to better choose therapy to reduce the stroke recurrence risk. Intracranial vessel imaging and cardiac evaluation with transesophageal echocardiogram and outpatient cardiac monitoring may help identify the stroke mechanism. This article highlights the diagnostic yield of various tests in identifying a stroke mechanism in stroke patients whose initial diagnostic evaluation is negative, and the implications for treatment.
A 57-year-old man with an unremarkable medical history presented to the emergency room 5 days after the acute onset of left arm and hand weakness and numbness. He denied other neurologic symptoms. Blood pressure was 160/78 mm Hg and heart rate 78 and regular. General physical examination results were normal. He had mild dysarthria. Mental status and cranial nerve examination results were otherwise normal. He had mild weakness and reduced dexterity in the left hand, but cerebellar and sensory examinations, including graphesthesia and stereognosis, had normal results. ECG showed normal sinus rhythm. MRI showed a small cortical infarct in the right precentral gyrus (figure 1). Magnetic resonance angiography showed no major carotid or intracranial arterial stenosis. Inpatient telemetry did not show atrial fibrillation. Transthoracic echocardiography with agitated saline contrast suggested an intracardiac shunt, and transesophageal echocardiography confirmed the diagnosis of a patent foramen ovale (PFO) associated with an atrial septal aneurysm. A subsequent 21-day outpatient cardiac monitor did not show evidence of atrial fibrillation. He was treated with antiplatelet and statin therapy and referred for evaluation for participation in a randomized trial of interventional closure for PFO.
Figure 1. MRI.
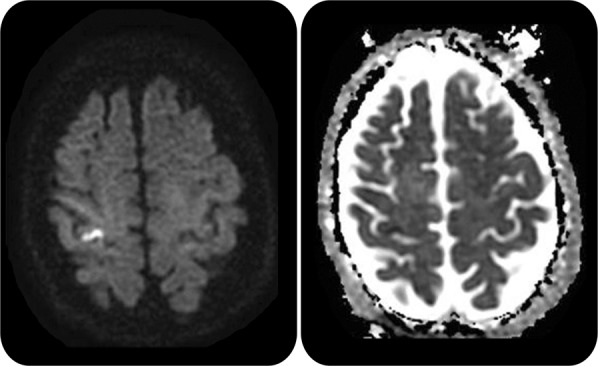
Diffusion-weighted imaging (b1000) (left) shows a hyperintense lesion along the right motor cortex (hand knob) that is hypointense on apparent diffusion coefficient map sequence (right) consistent with an acute infarct.
BACKGROUND
Identifying the stroke mechanism is a crucial aspect of secondary prevention of ischemic stroke. However, no stroke etiology is identified in 30%–40% of patients.1,2 Cryptogenic stroke is the term used to refer to strokes for which no definite cause can be identified.
The evaluation of a patient with ischemic stroke should include a careful history regarding symptom onset, progression, associated symptoms, and medical history. A history of neck injury and headache at the time of onset can suggest dissection as a cause, for example; associated palpitations or chest pain might suggest a cardiac embolic source. Careful general and neurologic examination may also help identify the possible stroke etiology (stigmata of peripheral emboli, for instance). Brain imaging (MRI or CT), vascular imaging, electrocardiography, laboratory testing including drug screen, telemetry, and echocardiography are also helpful.3
Cryptogenic stroke has been defined in several ways. A cryptogenic stroke, or stroke of undetermined etiology, as defined by the Trial of Org 10172 in Acute Stroke Treatment criteria, is a brain infarct not attributed to a definite source of large-vessel atherosclerosis, cardioembolism, or small-vessel disease, in the presence of (1) extensive cardiac, vascular, hematologic, and serologic evaluation; (2) incomplete evaluation; or (3) evidence of more than one competing cause.4 The Causative Classification System (CCS) requires brain imaging, imaging of cerebral vessels, and evaluation of heart function. According to the CCS, a cryptogenic stroke may be divided into “cryptogenic embolism” and “other cryptogenic.” Cryptogenic embolism refers to a stroke in which there is “angiographic evidence of abrupt cut-off consistent with a blood clot within otherwise angiographically normal looking intracranial arteries, imaging evidence of complete recanalization of previously occluded artery, or the presence of multiple acute infarctions that have occurred closely related in time without detectable abnormality in the relevant vessels.” Other cryptogenic is reserved for those not fulfilling the criteria of cryptogenic embolism.5
As is evident from the above diversity of criteria, the term cryptogenic is only as good as the testing that is done, i.e., how hard one looks for a cause; strokes may be considered cryptogenic because no cause is found after exhaustive testing, routine testing, or an incomplete evaluation. More recently, investigators have suggested use of the term embolic stroke of undetermined source to refer to patients with nonlacunar stroke in whom there is definitive absence of evidence of intracranial or extracranial stenosis of ≥50%, major risk source of cardiac embolism (such as atrial fibrillation), or other specific mechanism of stroke.6
A characteristic neuroimaging feature in cryptogenic stroke is evidence of one or more superficial hemispheric infarcts, suggestive of embolism, and present in up to 60% of cryptogenic stroke patients.7 About 65% of patients from the Stroke Databank who had infarcts of undetermined cause were considered to be due to less well-documented sources of embolism on further evaluation.1
Diagnostic approach
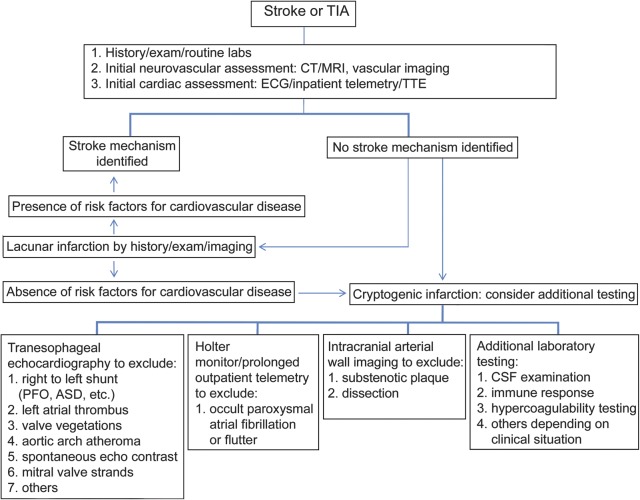
Vascular and general neurologists are often challenged to balance cost and thoroughness in the diagnostic evaluation of the patient with cryptogenic stroke. According to current guidelines, the initial stroke evaluation should include brain imaging (CT or MRI), noninvasive extracranial and intracranial vessel imaging, and cardiac monitoring for at least 24 hours.3 When those tests do not reveal an etiology, additional tests may be considered, such as conventional angiography, further cardiac evaluation (transesophageal echocardiography, Holter monitor, and long-term outpatient cardiac telemetry), and blood and CSF studies (e.g., Venereal Disease Research Laboratory, viral serologies, hypercoagulability evaluation). A suggested approach to the evaluation of patients with cryptogenic stroke is outlined in figure 2.
Figure 2. Suggested approach to the identification and further evaluation of cryptogenic stroke.
ASD = atrial septal defect; PFO = patent foramen ovale; TTE = transthoracic echocardiography.
Neuroimaging
A noncontrast head CT is cost-effective in the initial evaluation of stroke and has a very high sensitivity for excluding intracranial hemorrhage.8 However, it may not be a good tool for characterizing small infarcts, the identification of which may be important in establishing stroke mechanism. MRI is superior to CT in detecting acute ischemic lesions.9 Certain lesion patterns on diffusion-weighted MRI can help identify the stroke mechanism. For instance, multiple lesions in different vascular territories are suggestive of cardioembolism, whereas multiple small scattered lesions limited to one vascular distribution are suggestive of large-artery atherosclerosis.10 Thus cryptogenic stroke patients who have clinical and CT evidence of one lesion may benefit from an MRI, which may show small asymptomatic lesions in other vascular territories, potentially suggesting a cardioembolic source (figure 2). The presence of multiple infarcts, however, does not guarantee a cardiac mechanism: patients can have multiple simultaneous infarcts on neuroimaging from noncardioembolic mechanisms including vasculitis or atherosclerosis.
Vascular imaging
It is important to identify patients with large-vessel disease because their early risk of recurrence is higher than that of other stroke subtypes.11 In addition, aggressive medical therapy may reduce the risk of recurrent stroke. Various modalities are used to detect extracranial and intracranial large-vessel atherosclerotic stenosis, including ultrasound, magnetic resonance angiography, and CT angiography (CTA). In addition, CTA may be used to assess plaque morphology and transcranial Doppler ultrasound may be used to detect stenosis. Catheter angiography remains the gold standard in diagnosing intracranial atherosclerotic disease and evaluating its severity, but it is not used routinely because it is an invasive procedure carrying up to approximately a 2.5% risk of neurologic complications and a 0.1% risk of disabling stroke.12
Even when standard arterial luminal imaging is unrevealing, there is the possibility that the stroke is due to vessel wall abnormalities such as intraluminal plaque without significant stenosis and ulcerated substenotic plaque.13 While substenotic plaque is a well-recognized cause of myocardial infarction, the importance of substenotic plaques in causing stroke needs to be confirmed in larger prospective studies. These abnormalities may be detected by MRI sequences focused on the vessel wall (vessel wall imaging) rather than the lumen (figure 3). Similarly, an MRI of the neck with fat-suppressed sequences may be considered to diagnose cervical artery dissection, particularly in younger patients.
Figure 3. MRI.
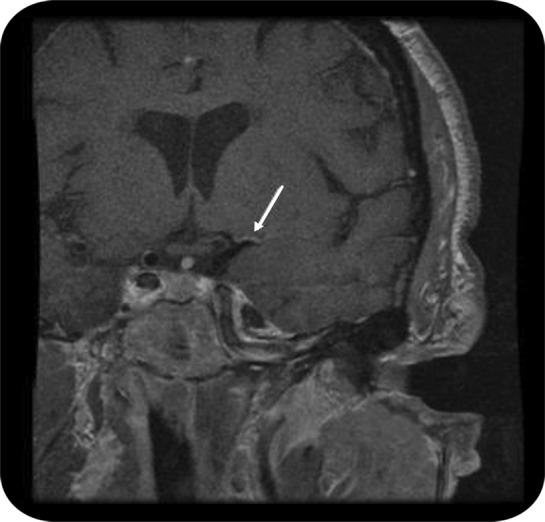
Arterial wall imaging study demonstrates enhancement consistent with atherosclerosis (arrow) in the proximal left middle cerebral artery territory in a patient with recurrent infarction in the territory of the deep white matter and basal ganglia of the left hemisphere.
Cardiac testing
The yield of advanced cardiac testing in patients with stroke depends on the pretest probability of finding a cardiac source. For instance, transesophageal echocardiography may be most useful in the patient with a potentially cardioembolic infarct pattern.
Transthoracic vs transesophageal echocardiography
In one study, ischemic stroke patients with an unknown etiology (before obtaining an echocardiogram) underwent both transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE). A potential cardiac source was detected in 55% of patients: 17% were identified on both TTE and TEE, and 39% were identified only on TEE.14 Thus TEE may be superior to TTE in identifying a cardiac etiology due to its higher sensitivity for aortic arch atheroma ≥4 mm and left atrial appendage thrombus.15 In another study, TEE identified a cardiac source in about 50% of patients when the basic evaluation including TTE failed to identify a source. In this study, a PFO was the most often detected potential cardiac source, followed by valvular abnormalities and aortic plaques.16 Both PFO and aortic arch atheroma are considered less well-documented cardiac sources, however, and thus strokes in patients with these abnormalities are still considered cryptogenic since the notion that they are the cause of the stroke is based primarily on epidemiologic associations. In addition, although the most recent PFO trials did not show a significant benefit of PFO closure over medical treatment,17 a concern with these trials is that they were not restricted to patients whose PFO is the likely stroke etiology. Therefore, whether PFO closure benefits such patients remains unanswered. Furthermore, in patients with cryptogenic stroke who were found to have a thick aortic arch atheroma (more than 4 mm), recent evidence suggests that antiplatelet is superior to anticoagulation therapy.18
Left atrial or left ventricular thrombus is identified in 1%–16% of patients with cryptogenic stroke, with TEE being more sensitive than TTE.14,15 When found, this should prompt consideration of anticoagulation. Furthermore, when endocarditis is clinically suspected, TEE is more sensitive than TTE in identifying valvular vegetations, suggesting the need for IV antibiotics. In addition, certain abnormalities seen on echocardiography have been associated with atrial fibrillation, such as left atrial enlargement16 and spontaneous echo contrast,19 indicating a potential value of more extensive cardiac evaluation, such as long-term outpatient cardiac monitoring to detect atrial fibrillation, in patients with these findings.
Thus, when a stroke etiology has not been identified (i.e., in patients with cryptogenic stroke and negative TTE, ECG, and inpatient telemetry), a TEE may be considered regardless of patient age to help identify the stroke etiology and guide stroke prevention strategies (figure 2).
Prolonged cardiac monitoring and other indicators of occult atrial fibrillation
Identification of atrial fibrillation (AF) is important because anticoagulation provides further benefit in stroke risk reduction compared to antiplatelet therapy. While standard 12-lead ECG and inpatient telemetry are useful in detecting chronic or frequent paroxysmal AF, they may not be sufficient to detect infrequent paroxysmal AF unassociated with cardiac symptoms.
While the rate of detection of AF in patients with cryptogenic stroke on routine cardiac testing (ECG, 24- to 48-hour telemetry) is up to 7%,20 the detection rate increases to approximately 25% when up to a 28-day cardiac monitor is used21 (table). The use of outpatient cardiac monitoring to detect paroxysmal AF appears cost-effective,22 though the optimal method and duration of monitoring remain uncertain.
Table.
Type of monitoring and detection of paroxysmal atrial fibrillation in patients with cryptogenic stroke
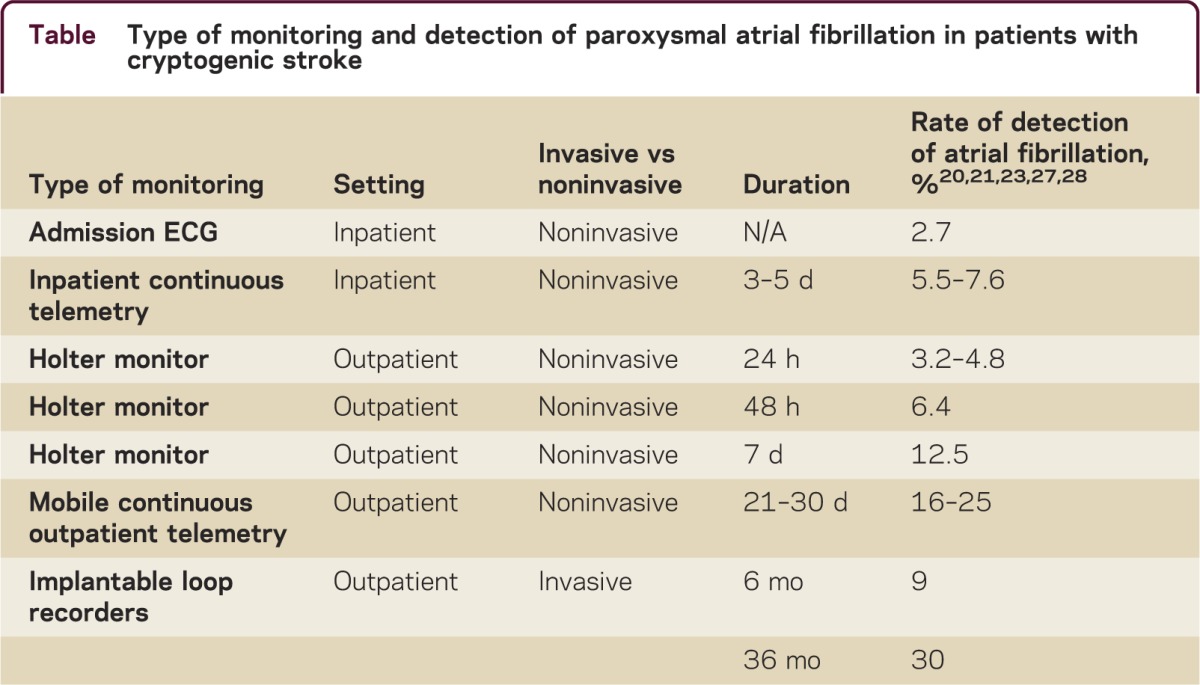
Invasive implantable devices such as loop recorders can provide an even longer duration of monitoring and may potentially increase the detection rate for paroxysmal AF. Data from the CRYSTAL-AF study showed that minimally invasive implantable cardiac monitors had higher yield in detecting AF than noninvasive short-term monitoring devices, with detection rates as high as 30% at 3 years.23 Thus minimally invasive prolonged cardiac monitoring may be considered in all patients with cryptogenic stroke to increase the yield of detecting atrial arrhythmias that would prompt anticoagulation therapy (figure 2). The value of these devices in preventing recurrent stroke remains under investigation.
In addition to TEE and cardiac monitoring, serum troponin and pro-brain natriuretic peptide (pro-BNP) are serum biomarkers associated with AF and stroke. Although cardioembolism is thought to be the mechanism of stroke in patients with elevated pro-BNP or troponin, it is difficult to establish a cause/effect relationship between stroke risk and elevated cardiac biomarkers because this is confounded by the fact that cardiac disease and elevated cardiac biomarkers are markers of systemic atherosclerosis. In a post hoc analysis of the Warfarin-Aspirin Recurrent Stroke Study, however, among those patients with stroke not known to have AF, treatment with warfarin was superior to aspirin in secondary stroke prevention among the 5% of patients with the most highly elevated N-terminal pro-BNP.24
Additional laboratory testing
Infectious, autoimmune, and inflammatory causes of vasculopathy constitute very rare mechanisms of stroke and may be considered when initial testing fails to identify an etiology. While paroxysmal atrial fibrillation is a potential culprit in older patients, in younger patients, genetic causes such as Fabry disease and mitochondrial encephalopathy with lactic acidosis and stroke are also rare causes of stroke and should be considered in the appropriate clinical setting. In certain clinical settings such as immunosuppressed patients or those with high exposure risk, and in patients with evidence of multifocal infarcts, infectious etiologies such as viruses (varicella-zoster virus, herpes simplex virus, and cytomegalovirus), syphilis, and tuberculosis should be considered, and confirmed by serum and CSF testing. Autoimmune causes of vasculitis, such as systemic lupus erythematosus and Wegener granulomatosis, are contemplated in patients with known systemic autoimmune disease or in those with signs, symptoms, or laboratory evidence of a systemic autoimmune disease. Serum testing for acquired antiphospholipid syndrome may also be considered when there is history of prior venous thromboembolism, second trimester abortion, or a rheumatologic disorder. When suspected by history and radiologic studies, CSF examination and meningeal biopsy should be considered to rule out primary CNS angiitis.
Inherited thrombophilia testing in young patients with cryptogenic stroke has a very high cost with extremely low diagnostic yield. Multiple case-control studies and a meta-analysis failed to show an association between inherited thrombophilia and stroke.25 The diagnostic utility of these tests is more robust in patients with sinus venous thrombosis or in patients with a positive family history of unprovoked venous thromboembolism or inherited thrombophilia.
Treatment
The mainstay of stroke prevention strategies in patients with cryptogenic stroke is the combination of antiplatelet therapy and stroke risk factor modification. Interestingly, there are studies showing a potential benefit from warfarin over aspirin in certain subgroups of cryptogenic stroke patients.26 However, this finding has not been replicated in appropriately designed double-blinded randomized trials and thus evidence for warfarin use in patients with cryptogenic stroke remains unclear.
DISCUSSION
Additional serum, imaging, and cardiac tests are available to help determine the stroke mechanism in patients with cryptogenic stroke. Clinicians need to be thorough yet cost-effective in choosing the diagnostic tests needed in each patient. The treatment of most patients with cryptogenic stroke consists of antiplatelet use and risk factor modification, though future trials may modify this approach.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
S. Yaghi reports no disclosures. M.S.V. Elkind serves/has served on scientific advisory boards for Jarvik Heart, Biogen Idec, and Boehringer-Ingelheim; serves as Resident & Fellow Section Editor for Neurology®; serves as a consultant for BMS-Pfizer Partnership, Janssen Pharmaceutical, and Daiichi Sankyo; receives research support from diaDexus, BMS-Sanofi Pharmaceutical Partnership, NIH (National Institute of Neurological Disorders and Stroke, NHLBI), American Heart Association National Board of Directors, American Heart Association Founders Affiliate Board of Directors, and American Heart Association NY City Board of Directors; and has participated in medicolegal proceedings. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Sacco RL, Ellenberg JH, Mohr JP, et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol 1989;25:382. [DOI] [PubMed] [Google Scholar]
- 2.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 2001;32:2735. [DOI] [PubMed] [Google Scholar]
- 3.Jauch EC, Saver JL, Adams HP, Jr, et al. ; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947 [DOI] [PubMed] [Google Scholar]
- 4.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41 [DOI] [PubMed] [Google Scholar]
- 5.Ay H, Furie KL, Singhal A, Smith WS, Sorensen AG, Koroshetz WJ. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol 2005;58:688–697 [DOI] [PubMed] [Google Scholar]
- 6.Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014;13:429–438 [DOI] [PubMed] [Google Scholar]
- 7.Lamy C, Giannesini C, Zuber M, et al. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale: the PFO-ASA Study: Atrial Septal Aneurysm. Stroke 2002;33:706. [DOI] [PubMed] [Google Scholar]
- 8.Sames TA, Storrow AB, Finkelstein JA, Magoon MR. Sensitivity of new-generation computed tomography in subarachnoid hemorrhage. Acad Emerg Med 1996;3:16–20 [DOI] [PubMed] [Google Scholar]
- 9.Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet 2007;369:293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang DW, Chalela JA, Ezzeddine MA, Warach S. Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Arch Neurol 2003;60:1730–1734 [DOI] [PubMed] [Google Scholar]
- 11.Purroy F, Jiménez Caballero PE, Gorospe A, et al. ; Stroke Project of the Spanish Cerebrovascular Diseases Study Group. Recurrent transient ischaemic attack and early risk of stroke: data from the PROMAPA study. J Neurol Neurosurg Psychiatry 2013;84:596–603 [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann TJ, Huston J, III, Mandrekar JN, Schleck CD, Thielen KR, Kallmes DF. Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology 2007;243:812–819 [DOI] [PubMed] [Google Scholar]
- 13.Freilinger TM, Schindler A, Schmidt C, et al. Prevalence of nonstenosing, complicated atherosclerotic plaques in cryptogenic stroke. JACC Cardiovasc Imaging 2012;5:397–405 [DOI] [PubMed] [Google Scholar]
- 14.De Bruijn SF, Agema WR, Lammers GJ, et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke 2006;37:2531–2534 [DOI] [PubMed] [Google Scholar]
- 15.Knebel F, Masuhr F, von Hausen W, et al. Transesophageal echocardiography in patients with cryptogenic cerebral ischemia. Cardiovasc Ultrasound 2009;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wozakowska-Kapłon B. Changes in left atrial size in patients with persistent atrial fibrillation: a prospective echocardiographic study with a 5-year follow-up period. Int J Cardiol 2005;101:47–52 [DOI] [PubMed] [Google Scholar]
- 17.Spencer FA, Lopes LC, Kennedy SA, Guyatt G. Systematic review of percutaneous closure versus medical therapy in patients with cryptogenic stroke and patent foramen ovale. BMJ Open 2014;4:e004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amarenco P, Davis S, Jones EF, et al. Clopidogrel plus aspirin versus warfarin in patients with stroke and aortic arch plaques. Stroke 2014;45:1248–1257 [DOI] [PubMed] [Google Scholar]
- 19.Black IW, Hopkins AP, Lee LC, Walsh WF. Left atrial spontaneous echo contrast: a clinical and echocardiographic analysis. J Am Coll Cardiol 1991;18:398–404 [DOI] [PubMed] [Google Scholar]
- 20.Rizos T, Güntner J, Jenetzky E, et al. Continuous stroke unit electrocardiographic monitoring versus 24-hour Holter electrocardiography for detection of paroxysmal atrial fibrillation after stroke. Stroke 2012;43:2689–2694 [DOI] [PubMed] [Google Scholar]
- 21.Bhatt A, Majid A, Razak A, Kassab M, Hussain S, Safdar A. Predictors of occult paroxysmal atrial fibrillation in cryptogenic strokes detected by long-term noninvasive cardiac monitoring. Stroke Res Treat 2011;172074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamel H, Hegde M, Johnson DR, Gage BF, Johnston SC. Cost-effectiveness of outpatient cardiac monitoring to detect atrial fibrillation after ischemic stroke. Stroke 2010;41:1514–1520 [DOI] [PubMed] [Google Scholar]
- 23.Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–2486 [DOI] [PubMed] [Google Scholar]
- 24.Longstreth WT, Jr, Kronmal RA, Thompson JL, et al. Amino terminal pro-B-type natriuretic peptide, secondary stroke prevention, and choice of antithrombotic therapy. Stroke 2013;44:714–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris JG, Singh S, Fisher M. Testing for inherited thrombophilias in arterial stroke: can it cause more harm than good? Stroke 2010;41:2985–2990 [DOI] [PubMed] [Google Scholar]
- 26.Sacco RL, Prabhakaran S, Thompson JL, et al. ; WARSS Investigators. Comparison of warfarin versus aspirin for the prevention of recurrent stroke or death: subgroup analyses from the Warfarin-Aspirin Recurrent Stroke Study. Cerebrovasc Dis 2006;22:4–12 [DOI] [PubMed] [Google Scholar]
- 27.Rojo-Martinez E, Sandín-Fuentes M, Calleja-Sanz AI, et al. High performance of an implantable Holter monitor in the detection of concealed paroxysmal atrial fibrillation in patients with cryptogenic stroke and a suspected embolic mechanism [article in Spanish]. Rev Neurol 2013;57:251–257 [PubMed] [Google Scholar]
- 28.Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467–2477 [DOI] [PubMed] [Google Scholar]