Summary
Perinatal arterial ischemic stroke (PAIS) can be an unrecognized cause of short- and long-term neurologic disability. Focal clonic seizure in the newborn period is the most common clinical presentation of PAIS. MRI is optimal in diagnosing PAIS; negative cranial ultrasound or CT does not rule out PAIS. Given the low rate of recurrence in combination with risk factors thought to be isolated to the maternal-fetal unit, anticoagulation or antiplatelet treatment is usually not recommended. The majority of newborns with PAIS do not go on to develop epilepsy, although further research is warranted in this area. Long-term morbidity, including motor, cognitive, and behavioral disabilities, can follow PAIS, necessitating early recognition, diagnosis, and therapy initiation.
With an estimated incidence of 1 per 4,000 live births,1 perinatal arterial ischemic stroke (PAIS) is defined as infarction in an arterial distribution between 28 weeks gestation and 28 days after birth. Most newborns with PAIS present with a focal clonic seizure (∼70%), typically within the first week after birth.2 Neurologic examination results are usually normal; however, some newborns may be systemically ill and have encephalopathy (39%), abnormalities of muscle tone (38%), or respiratory (26%) or feeding (24%) difficulties.2 While birth trauma and delivery-related anoxia were previously thought to be the primary causes of perinatal stroke, new studies suggest other pathogeneses, including inflammation, infection, and placental abnormalities,2 though the exact mechanisms remain unclear (figure 1).
Figure 1. Mechanisms of hypoxia-ischemia in the term newborn.
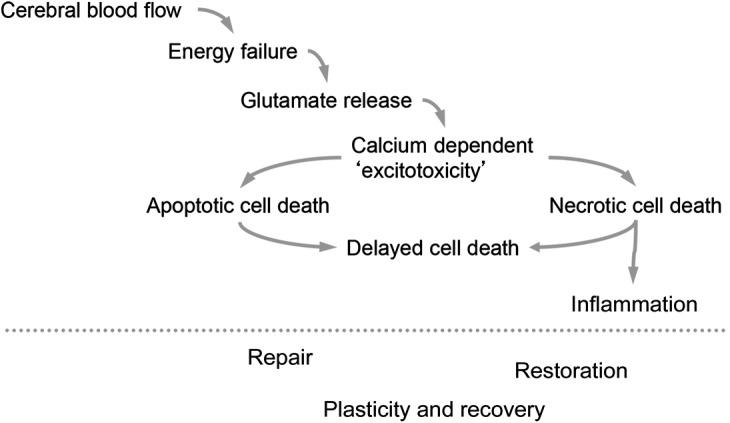
Following perinatal arterial ischemic stroke, there is a cascade of events from the resultant hypoxia-ischemia that evolve over time. Importantly, repair can occur long after the initial event in the term newborn.
PAIS is easy to miss in the newborn nursery if clinical suspicion and MRI are lacking. In this review, we emphasize the importance of early clinical recognition, allowing for appropriate diagnostic testing combined with early outpatient treatment interventions to aid in maximizing favorable neurologic outcomes.
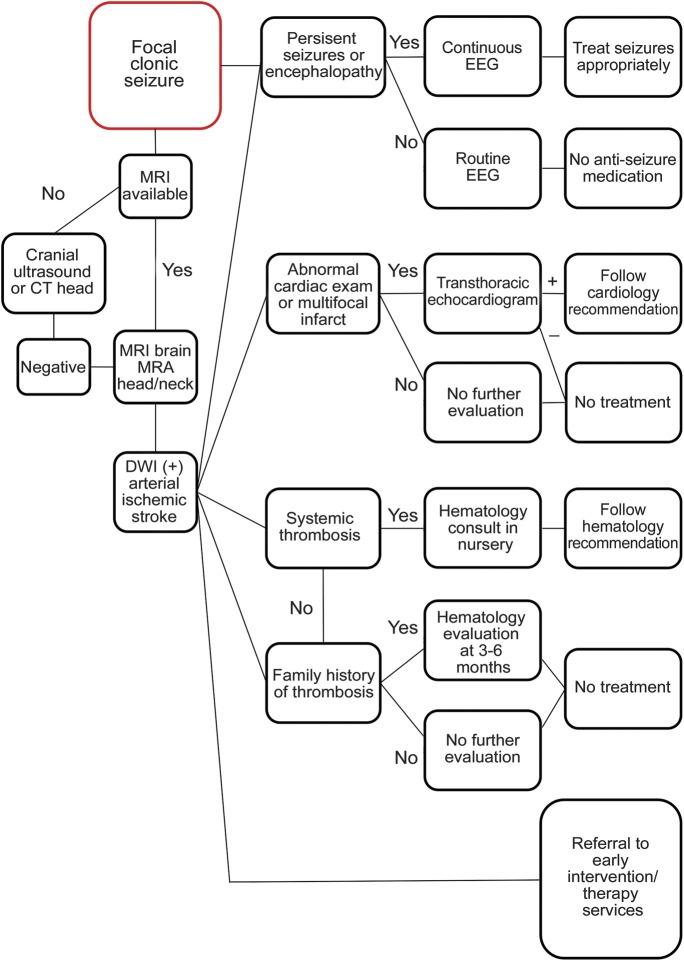
Organized approach to diagnosis and acute treatment
The goal of this article is to discuss the typical clinical presentation, differential diagnosis, testing, management, and acute treatment in newborns with PAIS (figure 2). This is not intended as a practice parameter, but rather suggestions to help guide the neurologist when caring for a newborn with PAIS. Other stroke etiologies (venous infarction and hemorrhagic stroke) and the evaluation of nonfocal neonatal seizures are beyond the scope of this review.
Figure 2. MRI/magnetic resonance angiography of a 1-day-old term newborn boy with right-sided clonic seizure.
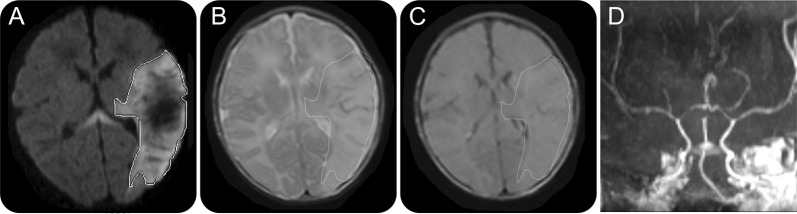
(A) Axial diffusion-weighted imaging (DWI) consistent with acute infarct on the left middle cerebral artery (MCA) distribution. (B) Axial T2 imaging shows hyperintensity (outlined) corresponding to area of restricted diffusion on DWI. (C) Axial fluid-attenuated inversion recovery imaging with subtle hyperintensity (outlined) corresponding to area of restricted diffusion on DWI. (D) Coronal magnetic resonance angiography demonstrates normal caliber and appearance of left MCA.
Case presentation
Patient 1 was a 1-day-old full-term boy (41 weeks gestation) with rhythmic movements of his right hand noted 12 hours after birth. He was born to a healthy gravida 1 para 0 woman with no history of smoking or illicit drug use. Prenatal care was consistent and initiated at 9 weeks postconception. There were no problems noted during the pregnancy. The newborn's mother noted decreased fetal movements the day before delivery, but stress testing had normal results. Labor was spontaneous; cesarean section was performed secondary to failure to progress. The newborn did not have spontaneous respirations at birth, and was given bag-mask ventilation for 2 minutes, after which respirations became spontaneous. Apgar scores were 5 at 1 minute and 9 at 5 minutes. Given his initial poor respiratory effort, the patient was taken to the newborn nursery for observation. Initial heel stick blood glucose was normal.
On examination, the patient was normothermic and his vital signs were normal. He was a healthy-appearing, appropriately sized term newborn boy. He breastfed well, without coughing or choking. He cried normally when taken off the breast but was easily consolable with a pacifier. His general physical examination results were normal, aside from a soft systolic murmur. He visually fixed for a few seconds, and optokinetic nystagmus was present. He blinked to bright light as well as loud sounds. There was no obvious facial asymmetry, and he had a symmetric cry. There was full range of motion of his neck. The patient had mild head lag, but normal tone of his trunk and extremities. He had no fisting of his hands. When supine, he was somewhat flexed, and had spontaneous, symmetric, antigravity movements of all his extremities. Neonatal reflexes were normal including suck, root, symmetric Moro, and stepping. The patient grimaced and withdrew to soft pinching of his extremities. His deep tendon reflexes were normal, and plantar responses were extensor. He had no abnormal movements.
Differential diagnosis
A well-appearing term newborn with isolated focal clonic seizures should be considered to have PAIS3; most newborns with PAIS have normal neurologic examination results. Intracranial hemorrhage (ICH), intraventricular hemorrhage (IVH), cerebral sinus venous thrombosis (CSVT), periventricular venous infarction (PVI), and hypoxic-ischemic injury can be clinically difficult to discern from PAIS, although encephalopathy is typically more frequent in these conditions.4,5 Infection is a common cause of nonfocal neonatal seizure and encephalopathy, and must be promptly addressed as with any ill newborn. Inborn errors of metabolism can be life-threatening and should be rapidly evaluated in the encephalopathic newborn with persistent seizures; additional metabolic defect screening may also present with seizure and encephalopathy. Cerebral structural lesions, including cortical malformations or tumor, present less commonly in the newborn period, but should be considered.
Evaluation
Although focal clonic seizure is the hallmark presentation of the term newborn with PAIS, the stability of the child must always be considered before any testing is performed. Blood culture, urinalysis, serum ammonia and glucose, and CSF analysis should be performed as soon as possible in the clinically ill-appearing newborn, as guided by the primary medical team.
Imaging
MRI of the brain is considered optimal for the diagnosis of PAIS. In situations where MRI is not available (or the newborn is too unstable for transport out of the intensive care unit, such as with severe respiratory, cardiac, or systemic disease), cranial ultrasound (CUS) can be a useful bedside screening tool. CUS may identify ICH, IVH, or large CSVT. However, given the low sensitivity of CUS in detecting ischemia, a negative CUS does not rule out PAIS.6 Alternatively, noncontrast CT may be utilized as a primary screen at institutions that do not have MRI capability, or if MRI cannot be acutely obtained. Similar to CUS, a noncontrast CT can readily identify ICH or IVH, and may detect CSVT. Nonetheless, PAIS or brain edema can be difficult to distinguish on CT scan due to the high water content of the neonatal brain.
MRI should be obtained for focal clonic seizure in a well-appearing newborn with normal neurologic examination results. Noncontrast MRI brain is recommended. Although contrast-enhanced magnetic resonance (MR) angiogram (MRA) of the head and neck is desirable to provide baseline arterial structure anatomy, repeating the MRI to complete vascular imaging is not generally recommended given the low rate of arterial abnormalities.2 If there is suspicion of a venous infarction (for instance, if the newborn is encephalopathic, dehydrated, or has signs of increased intracranial pressure), an MR venogram should be performed. Diffusion-weighted imaging (DWI) with apparent diffusion coefficient (ADC) is considered the most sensitive measure for identifying infarct in the neonatal brain6; T2-weighted sequences can also aid in the determination of ischemic injury (figure 3). Unlike in older children and adults, fluid-attenuated inversion recovery sequence is not helpful in determining ischemia with PAIS given the high water content of the neonatal brain.
Figure 3. Proposed evaluation of perinatal arterial ischemic stroke.
This is intended to be a suggested approach by the authors and is not the result of a consensus statement. DWI = diffusion-weighted imaging; MRA = magnetic resonance angiography.
EEG
Although neonatal seizures can have broad clinical manifestations that differ from those in older infants and children—including apneic events or “bicycling” limb movements—seizures associated with PAIS are typically focal and involve clonic movements of an arm or leg.3 In the well-appearing term neonate with isolated focal clonic seizure, routine EEG (30 minutes) is recommended as a baseline evaluation if available at the institution. Findings on EEG associated with PAIS may include slowing, isolated spike-wave discharges, or epileptiform discharges in the region corresponding to infarct.
Prolonged EEG is recommended in any term newborn who has repeated apneic or bradycardic episodes, or is persistently encephalopathic; although beyond the scope of this article, we refer the interested reader to recent guidelines by the American Clinical Neurophysiology Society.7 In nurseries that do not have this capability, transfer to an institution with neonatal EEG is strongly encouraged, and should be discussed with the primary medical team. Amplitude-integrated EEG may be a useful adjunctive tool for monitoring in the intensive care nursery, but should not take the place of multichannel EEG.
Laboratory tests
If there are any urgent hematologic concerns (i.e., systemic thrombosis or consumptive coagulopathy), pediatric hematology should guide acute testing and treatment. Genetic thrombophilias are uncommon in the otherwise healthy newborn with PAIS, although data are limited regarding their true importance.2,8–11 Therefore, genetic thrombophilia screening is generally recommended only if there is a strong family history noted, or the newborn has additional systemic arterial or venous thrombi. Referral to outpatient hematology (or a pediatric stroke center with hematology support) at 3–6 months is recommended in those situations, since immaturity of the newborn coagulation system may effect results in the newborn period, most notably antithrombin, protein C, and protein S levels.
Maternal antiphospholipid antibodies should be tested soon after delivery if possible. Acquired maternal antiphospholipid antibodies can cross the placenta, and have been associated with PAIS.11 Additionally, effort should be made to obtain the placenta for pathologic analysis.
Echocardiogram
Although rare, cardiac defects can have potentially detrimental outcomes if undiagnosed.2 If there is an abnormality on cardiac examination—or if the brain MRI is confirmatory of multiple emboli—transthoracic echocardiogram should be performed to evaluate for intracardiac thrombus or structural conditions that may predispose to thrombosis. The presence of a large patent foramen ovale (PFO) or atrial septal defect provides a route for thrombus from the right atrium or venous circulation to embolize to the brain. Many term newborns have a clinically insignificant PFO that ultimately closes on its own, and thus should not be treated as a true cardiac defect.
Management and treatment
As discussed earlier, acute treatment of suspected infection or other life-threatening processes should be addressed immediately by the primary medical team.
Stroke treatment
Unlike childhood arterial ischemic stroke, PAIS has a low 5-year recurrence rate (approximately 1%), except in those children with congenital heart disease or multiple genetic thrombophilias.10,12 Thus, initiation of anticoagulation agents, including heparin products or warfarin, or antiplatelet therapy such as aspirin, is not recommended in the newborn without identifiable risk factors.13 If congenital heart disease, multiple cerebral or systemic emboli, or severe thrombophilia is present, anticoagulation with low-molecular-weight heparin or unfractionated heparin,13 or aspirin 2–4 mg/kg/day, may be considered on a case-by-case basis with guidance from pediatric hematology and cardiology as appropriate.
Seizure medications
Seizure treatment of the otherwise well term newborn with PAIS and single focal seizure, or cluster of seizures, is controversial. These newborns commonly do not have seizures beyond the neonatal period; a recent study demonstrated that the rate of seizure recurrence was less than 25% at median follow-up of 18 months, with the majority of these events comprised of a single isolated seizure.14 Most perinatal stroke specialists do not recommend starting seizure medication in this group given the potential neuroinhibitory effects of these medications, although more research is warranted in this area.14
Acute management of persistent seizures should be treated as with any neonate, although the threshold for treatment varies among providers. Lorazepam 0.5 mg IV is recommended for convulsive seizures lasting longer than 5 minutes, or for brief seizure clusters. If seizures continue, or subclinical status epilepticus is seen on EEG, phenobarbital 20 mg/kg IV is the first-line agent for seizure control. Phenobarbital 2.5 mg/kg/day twice a day is a typical maintenance dose and can be given either IV or orally. Newborns can metabolize this medication quickly and may require higher doses; phenobarbital dosing is usually limited by side effects (sedation) rather than pharmacologic levels. Levetiracetam IV load of 20–30 mg/kg is a useful second-line agent for persistent seizures or status epilepticus; a maintenance starting dose is typically 15 mg/kg twice a day, and like phenobarbital can be given IV or orally.
Rehabilitation services
Approximately two-thirds of children with PAIS have poor long-term outcomes, ranging from mild to severe neurologic disability; deficits can be variable and recognized early in infancy (i.e., delayed motor milestone or early handedness), or later into childhood and adolescence (cognitive and behavioral disabilities).15–18 Newborns with infarct involving the posterior limb of the internal capsule or Wallerian degeneration of the cerebral peduncle are at particularly increased risk for hemiplegia.19 Prothrombotic disorders may also confer risk for worse motor and neurocognitive outcome.20 Most newborns may appear otherwise normal in the nursery, but eventually “grow into” their deficits with age. Therefore, we recommend that all newborns with PAIS should be discharged with early intervention referral, regardless of their examination results and functional capabilities. At the minimum, newborns should have physical therapy evaluation and ongoing outpatient follow-up with neurology if available. Given the risk of later-onset cognitive and behavioral disabilities (which can sometimes be difficult to recognize), neuropsychological testing in preschool, school age, and adolescence is highly recommended.
Case presentation (continued)
As phone consultation by a neurologist was available, the decision was made not to transfer the patient given his otherwise normal examination results. A brain MRI/MRA was performed at 3 days of age. The MRI revealed a large infarct in the left middle cerebral artery distribution with normal arterial caliber (figure 1). As the patient had one isolated focal clonic seizure at presentation, seizure medications were not given. There was no capability for routine EEG at the nursery and thus it was not performed. Echocardiogram was ordered to evaluate the murmur, and a small PFO was noted and considered clinically insignificant. Thrombophilia workup was not ordered. The patient was not started on any medication. He continued to do well in the nursery and was discharged home at 4 days old without early intervention services or neurology outpatient follow-up.
The patient was referred to the Stroke Clinic at 6 months of age, and was noted to have right homonymous hemianopia, right facial asymmetry, and decreased mobility of his right arm and leg. Notably, he had pronounced fisting of his right hand and decreased range of motion in his left ankle. Early intervention services were initiated after this evaluation. The patient is now 23 months old. He is socially engaging and playful. He receives speech therapy for mild language delay and has essentially caught up to his peers. He has a small right homonymous hemianopia that is clinically subtle with ongoing vision therapy. He participates in weekly physical and occupational therapy. His limited mobility and fisting of his right hand has improved considerably with therapy, and he now has almost full dexterity. He wears an ankle-foot orthosis, taking his first unassisted steps at 22 months of age. He has not had any spells concerning for seizures.
Early referral and initiation of rehabilitation therapies may have the greatest potential to affect long-term outcomes after PAIS, underscoring the importance of timely recognition.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
J. Armstrong-Wells receives research support from the NIH, University of Colorado Denver School of Medicine, Colorado Clinical and Translational Sciences Institute, and the American Heart Association. D. Ferriero serves on scientific advisory boards for NIH/National Institute of Neurological Disorders and Stroke; serves as an Associate Editor for Pediatric Research; receives publishing royalties for Principles and Practices of Pediatric Neurology (Elsevier, 2005); and receives research support from the NIH and Fondation LeDucq. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.

REFERENCES
- 1.Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol 2004;3:150–158 [DOI] [PubMed] [Google Scholar]
- 2.Kirton A, Armstrong-Wells J, Chang T, et al. Symptomatic neonatal arterial ischemic stroke: the International Pediatric Stroke Study. Pediatrics 2011;128:e1402–e1410 [DOI] [PubMed] [Google Scholar]
- 3.Gelfand AA, Glass HC, Elysa J, Ferriero DM. Focal clonic seizures suggest stroke in a newborn. Neurol Bull 2010;2:7–11 [Google Scholar]
- 4.Armstrong-Wells J, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Prevalence and predictors of perinatal hemorrhagic stroke: results from the Kaiser Pediatric Stroke Study. Pediatrics 2009;123:823–828 [DOI] [PubMed] [Google Scholar]
- 5.Moharir MD, Shroff M, Pontigon AM, et al. A prospective outcome study of neonatal cerebral sinovenous thrombosis. J Child Neurol 2011;26:1137–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowan F, Mercuri E, Groenendaal F, et al. Does cranial ultrasound imaging identify arterial cerebral infarction in term neonates? Arch Dis Child Fetal Neonatal Ed 2005;90:F252–F256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society's Guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol 2011;28:611–617 [DOI] [PubMed] [Google Scholar]
- 8.Miller SP, Wu YW, Lee J, et al. Candidate gene polymorphisms do not differ between newborns with stroke and normal controls. Stroke 2006;37:2678–2683 [DOI] [PubMed] [Google Scholar]
- 9.Profita THW, Bernard TJ, Goldenberg NA, Manco-Johnson MJ, Armstrong-Wells J. Biomarkers of hypercoagulability in perinatal arterial ischemic stroke. Ann Neurol 2010;68:S139 [Google Scholar]
- 10.Kurnik K, Kosch A, Strater R, et al. Recurrent thromboembolism in infants and children suffering from symptomatic neonatal arterial stroke: a prospective follow-up study. Stroke 2003;34:2887–2892 [DOI] [PubMed] [Google Scholar]
- 11.Simchen MJ, Goldstein G, Lubetsky A, Strauss T, Schiff E, Kenet G. Factor V Leiden and antiphospholipid antibodies in either mothers or infants increase the risk for perinatal arterial ischemic stroke. Stroke 2009;40:65–70 [DOI] [PubMed] [Google Scholar]
- 12.Rodan L, McCrindle BW, Manlhiot C, et al. Stroke recurrence in children with congenital heart disease. Ann Neurol 2012;72:103–111 [DOI] [PubMed] [Google Scholar]
- 13.Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke 2008;39:2644–2691 [DOI] [PubMed] [Google Scholar]
- 14.Wusthoff CJ, Kessler SK, Vossough A, et al. Risk of later seizure after perinatal arterial ischemic stroke: a prospective cohort study. Pediatrics 2011;127:e1550–e1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westmacott R, MacGregor D, Askalan R, deVeber G. Late emergence of cognitive deficits after unilateral neonatal stroke. Stroke 2009;40:2012–2019 [DOI] [PubMed] [Google Scholar]
- 16.Gold JJ, Trauner DA. Hippocampal volume and memory performance in children with perinatal stroke. Pediatr Neurol 2014;50:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trauner DA, Eshagh K, Ballantyne AO, Bates E. Early language development after perinatal stroke. Brain Lang 2013;127:399–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harbert MJ, Jett M, Appelbaum M, Nass R, Trauner DA. Perinatal risk factors and later social, thought, and attention problems after perinatal stroke. Stroke Res Treat 2012;2012:914546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirton A, Shroff M, Visvanathan T, deVeber G. Quantified corticospinal tract diffusion restriction predicts neonatal stroke outcome. Stroke 2007;38:974–980 [DOI] [PubMed] [Google Scholar]
- 20.Mercuri E, Cowan F, Gupte G, et al. Prothrombotic disorders and abnormal neurodevelopmental outcome in infants with neonatal cerebral infarction. Pediatrics 2001;107:1400–1404 [DOI] [PubMed] [Google Scholar]