Abstract
Background: Previous trials of binders in chronic kidney disease (CKD) stages 3–5 have shown only modest changes in serum phosphate but evaluated morning phosphate. It is unknown whether a circadian pattern of phosphate concentrations exists in CKD and is modifiable by dietary manipulation.
Objectives: We determined the circadian pattern of serum phosphate concentrations in CKD and whether it was modifiable by altering absorbable phosphate.
Design: This was a crossover feeding study in 11 CKD participants (estimated glomerular filtration rate: 30–45 mL · min−1 · 1.73 m−2) and 4 healthy control subjects. All subjects received high-phosphate (2500 mg/d), normal-phosphate (1500 mg/d), and low-phosphate (1000 mg/d plus 1000 mg lanthanum carbonate 3 times/d) diets for 5 d followed by a 10-d washout. After each 5-d feed, phosphate and other measurements were made every 4 h over 1 day.
Results: In CKD participants who consumed the high-phosphate diet, there were circadian changes in phosphate with lowest concentrations (±SDs) at 0800 (4.2 ± 0.5 mg/dL) and 2 peaks at 1600 and 0400 (4.5 ± 0.8 and 4.4 ± 0.6 mg/dL, respectively), which were similar to those in healthy controls. Results with the normal-phosphate diet were similar. The low-phosphate diet altered the circadian rhythm (P = 0.02) such that 0400 and 1600 peaks were absent. Differences in phosphate for lowest- compared with highest-phosphate diets were smallest at 0800 and largest at 1600 (0.5 compared with 1.0 mg/dL) in CKD. Circadian changes in phosphate were not explained by urine phosphate excretion, parathyroid hormone, or fibroblast growth factor-23.
Conclusions: A circadian pattern of serum phosphate is observed in CKD with lowest concentrations at 0800 and highest at 1600 and 0400. This circadian pattern is modifiable by phosphate intake and most evident at 1600. Future intervention studies targeting intestinal phosphate absorption should consider afternoon phosphate measurements.
INTRODUCTION
Higher serum phosphate concentrations have been linked to a number of adverse health outcomes in chronic kidney disease (CKD)5 including progression of kidney disease (1), cardiovascular disease (2), and all-cause mortality (3). Higher phosphate concentrations also stimulate higher concentrations of counter-regulatory hormones fibroblast growth factor-23 (FGF23) and parathyroid hormone (PTH), which may promote left ventricular hypertrophy and bone disease (4, 5). In 2009, Kidney Disease Improving Global Outcomes (KDIGO) clinical guidelines recommended the maintenance of serum phosphate concentrations within the normal laboratory range in CKD patients and recommended the use of dietary phosphate restriction or intestinal phosphate binders to accomplish this goal (6). However, since the KDIGO report, several studies have raised questions about the effectiveness of these approaches in patients with CKD stages 3–5 before the initiation of dialysis (7–12).
Existing studies that evaluated the effects of dietary phosphate restriction and binder use on serum phosphate concentrations in CKD patients have evaluated fasting morning serum phosphate concentrations (7–13). A study in healthy volunteers has shown considerable diurnal variation in serum phosphate concentrations with the lowest serum phosphate concentration in morning specimens and the least difference in serum phosphate concentrations on high- compared with low-phosphate diets at this time of day (14). Whether similar circadian patterns persist in CKD patients is uncertain (15, 16). In addition, whether the modification of dietary phosphate intake influences the circadian rhythm in CKD is unknown. If limiting phosphate intake has more dramatic effects on phosphate concentrations later in the day, the effect of binders on serum phosphate in CKD patients may have been underestimated in previous trials that evaluated morning specimens.
In the current article, we report a crossover dietary intervention study that evaluated circadian patterns of serum phosphate and effects of 3 diets with different phosphate contents in 11 patients with estimated glomerular filtration rates (eGFRs) from 30 to 45 mL · min−1 · 1.73 m−2 and 4 healthy control subjects. On the basis of data in healthy persons (14), we hypothesized a priori that the fasting 0800 serum phosphate concentration would show the least difference in serum phosphate concentrations across the 3 diets, and phosphate measurements at 1600 and 0400 would show both the highest concentrations and largest difference across diets.
SUBJECTS AND METHODS
Study population
From a large clinical practice in Denver, CO, we recruited 11 participants with eGFRs from 30 to 45 mL · min−1 · 1.73 m−2 and 4 healthy controls. Subjects were sequentially exposed to diets of known phosphate contents [high phosphate (2500 mg/d), normal phosphate (1500 mg/d), and low phosphate (1000 mg/d) combined with lanthanum carbonate (1000 mg 3 times/d) with meals]. Participants were provided meals and asked to consume nothing outside of provided meals during the study intervention. Each diet was provided for 5 d with a minimum of a 10-d washout period between diets. We designed each of the diets to have similar sodium, calcium, and total caloric contents but to differ in phosphate contents. Because of the varying absorption of phosphate shown in synthetic compared with natural sources, we chose to use natural food sources rather than phosphate supplementation, and therefore, the protein content increased in correlation with the phosphate content. Diets were designed to isolate phosphate content as much as possible within the limitations of the dietary protein:phosphate relation. Phosphate, calcium, and protein contents were determined by using an ash analysis of each diet at 2 time points both at the beginning and end of the trial. Total caloric and sodium contents were estimated with nutritional software. Diets were hand prepared by using fresh food and delivered to study participants. On the fifth day of each diet, participants were hospitalized for 24 h and underwent blood and urine assessments every 4 h. Serum phosphate was measured by using a standard clinical analyzer. PTH was measured by using a second generation immunoassay that measured intact PTH (Quest Diagnostics), and FGF23 was measured by using a second-generation C-terminal ELISA assay (Immutopics).
Ethics
Procedures followed in this study were approved by our local institutional review board and in accordance with the ethical standards with the Helsinki Declaration of 1975 as revised in 1983.
Statistics
Summary statistics were calculated for baseline characteristics including demographics, medical histories, and laboratory measures. Continuous measures were summarized by means (±SDs), and categorical variables were summarized by counts and percentages. To evaluate the presence of circadian rhythm, we used a double repeated-measures ANOVA. Repeated factors were diet (low, normal, and high phosphate) and hour of day (0800, 1200, 1600, 2000, 2400, 0400, 0800). Subjects were treated as random effects, and the covariance structure was set as unstructured for diet by autoregressive for hour of day. We tested diet × time interactions to determine whether the diurnal pattern of serum phosphate differed across diets. We developed summary figures showing mean (±SEs) of blood and urine concentrations of key analytes stratified by CKD status. All analyses were performed with SAS version 9.2 software (SAS Institute) and Stata SE version 11.0 software (Stata Corp).
RESULTS
Baseline characteristics of study participants are shown in Table 1. The mean age was 64 ± 14 y, 40% of subjects were men, and all subjects but one were white. The mean eGFR was 36 ± 7 mL · min−1 · 1.73 m−2 in the CKD group and 75 ± 14 mL · min−1 · 1.73 m−2 in healthy controls. Serum phosphate was within the normal laboratory range in both CKD and healthy participants (3.56 ± 0.60 and 3.65 ± 0.340 mg/dL, respectively). As expected, those with CKD had slightly lower serum calcium and 1,25-dihydroxyvitamin D concentrations and markedly higher intact PTH and FGF23 concentrations relative to those of healthy controls.
TABLE 1.
Baseline characteristics of study participants1
| All (n = 15) | CKD (n = 11) | Controls (n = 4) | 2 | |
| Age (y) | 64 ± 143 | 67 ± 14 | 56 ± 12 | 0.18 |
| M [n (%)] | 6 (40) | 4 (36) | 2 (50) | 0.63 |
| White [n (%)] | 14 (93) | 10 (91) | 4 (100) | 0.53 |
| Weight (lb) | 175 ± 40 | 179 ± 43 | 164 ± 27 | 0.52 |
| Height (in) | 66.4 ± 3.3 | 65.9 ± 3.0 | 67.8 ± 4.1 | 0.37 |
| eGFR (mL · min−1 · 1.73 m−2) | 46 ± 20 | 36 ± 7 | 75 ± 14 | <0.001 |
| Phosphate (mg/dL) | 3.59 ± 0.55 | 3.56 ± 0.60 | 3.65 ± 0.40 | 0.80 |
| Calcium (mg/dL) | 9.32 ± 0.40 | 9.20 ± 0.37 | 9.68 ± 0.22 | 0.34 |
| FGF23 (RU/mL) | 218 ± 120 | 271 ± 102 | 88 ± 17 | <0.01 |
| Intact PTH (pg/mL) | 62 ± 44 | 76 ± 44 | 27 ± 18 | 0.02 |
| 1,25(OH)2 (pg/mL) | 38.3 ± 12.1 | 36.5 ± 13.3 | 43.3 ± 6.8 | 0.36 |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; FGF23, fibroblast growth factor-23; PTH, parathyroid hormone; RU, relative units; 1,25(OH)2, 1,25-dihydroxyvitamin D.
For CKD patients compared with controls.
Mean ± SD (all such values).
The analysis of the diet composition revealed similar sodium and calcium contents. Caloric intake was also similar in low- and normal-phosphate diets and slightly higher in the high-phosphate diet. Phosphate contents were ∼2500, 1500, and 1000 mg in high-, normal-, and low-phosphate diets, respectively. The protein content was correlated with the phosphate content as expected (Table 2).
TABLE 2.
Diet composition
| Diet | Phosphorus | Calories | Protein | Sodium | Calcium |
| mg | kcal | g | mg | mg | |
| High phosphate | |||||
| Day 1 | 2470 | 2600 | 131 | 3350 | 1662 |
| Day 2 | 2467 | 2590 | 100 | 3300 | 1877 |
| Average | 2469 | 2595 | 115 | 3325 | 1770 |
| Normal phosphate | |||||
| Day 1 | 1498 | 1961 | 89 | 3184 | 1897 |
| Day 2 | 1481 | 2144 | 92 | 3252 | 1825 |
| Average | 1489 | 2053 | 91 | 3218 | 1861 |
| Low phosphate plus lanthanum | |||||
| Day 1 | 1031 | 2094 | 77 | 3498 | 1706 |
| Day 2 | 1179 | 2082 | 79 | 2699 | 1765 |
| Average | 1105 | 2088 | 78 | 3099 | 1735 |
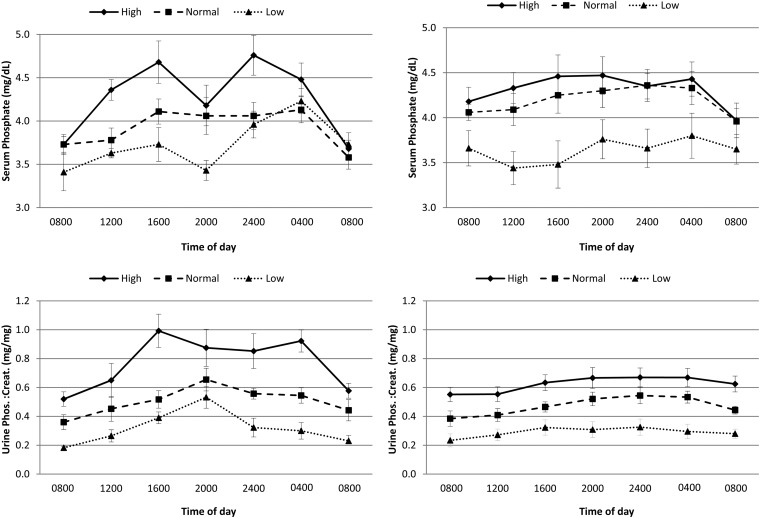
We examined serum phosphate data in healthy controls to provide a framework for comparisons. Serum phosphate concentrations differed significantly by diet in healthy controls with a 24-h average serum phosphate concentrations of 4.3 ± 0.3, 3.9 ± 0.3, and 3.7 ± 0.3 mg/dL in high, normal, and low-phosphate diets, respectively (P = 0.01). However, we observed a diet × time interaction (P = 0.02) that indicated that the circadian pattern of serum phosphate differed by diet (Figure 1). Serum phosphate was lowest at 0800 with all 3 diets and also showed the least difference across the 3 diets at 0800. With each diet, there was a peak in serum phosphate at 1600. There was also a second peak that occurred at 0400 with low- and normal-phosphate diets and occurred slightly earlier at 2400 with the high-phosphate diet. Across the 3 diets, the largest magnitude of difference in serum phosphate was observed at the 1600 time point.
FIGURE 1.
Serum phosphate concentration and urine phosphate:creatinine ratio in healthy controls (n = 4) and CKD patients (n = 11) throughout the day and across high-, normal-, and low-phosphate diets. Point estimates reflect mean concentrations, and error bars reflect SEs. The top panels show serum phosphate concentrations in healthy control and CKD participants. P values for diet × time interactions were 0.02 in the healthy control group and 0.02 in CKD participants. The bottom panels show urine phosphate:creatinine ratios in healthy control and CKD participants. P values for the diet × time interactions were 0.11 and 0.48 in healthy control and CKD participants, respectively. CKD, chronic kidney disease; Creat., creatine; Phos., phosphate.
A similar pattern was observed in participants with CKD. Here, we also observed that the mean serum phosphate was highest on the high-phosphate diet with time-averaged serum phosphate concentrations of 4.3 ± 0.6, 4.2 ± 0.6, and 3.6 ± 0.7 mg/dL with high-, normal-, and low-phosphate diets, respectively (P < 0.001). Again, we also observed a diet × time interaction (P = 0.02) that indicated that the circadian pattern of serum phosphate differed by diet. The lowest serum phosphate was observed at 0800 in high- and normal-phosphate diets (Figure 1). With the high-phosphate diet, we observed 2 peaks in serum phosphate, one at 1600–2000 and another at 0400, that were generally similar to data in healthy controls, albeit the valley between these peaks was less dramatic. With the normal-phosphate diet, serum phosphate increased from 0800 to 2400, remained relatively constant until 0400, and declined to its lowest concentration at 0800 once more. The pattern of increase in serum phosphate after 0800 was absent with the low-phosphate diet. Overall, the magnitude of change in serum phosphate across the 24-h period was less dramatic than that observed in healthy controls. For example with the high-phosphate diet, the difference between the highest and lowest serum phosphate concentration over the 24-h period was 0.3 mg/dL in CKD participants compared with 1.1 mg/dL in the healthy controls.
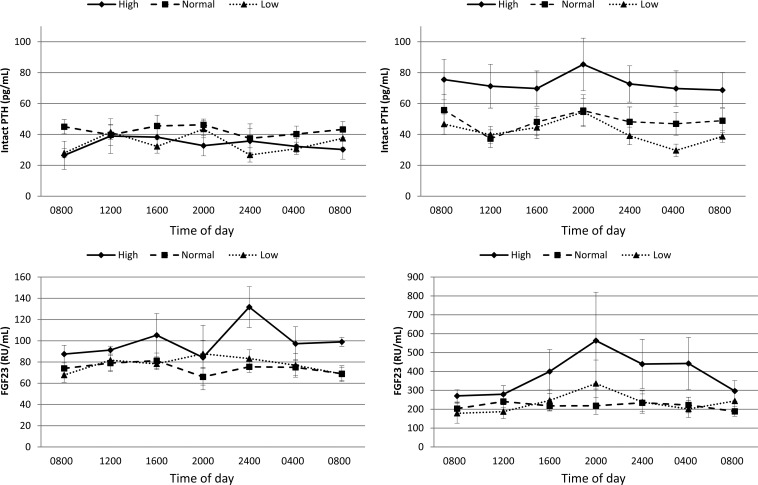
Because of the use of the morning serum phosphate sample in previous clinical trials and the consistent finding of a decline in serum phosphate between 0400 and 0800 on all 3 diets and both healthy controls and CKD participants in our study, we evaluated this period further. We compared urine phosphate–to–urine creatinine ratios in spot urine aliquots taken concurrent with blood measurements every 4 h. In the time period between 0400 and 0800, the urine phosphate–to–urine creatinine ratio decreased in healthy controls and CKD patients (Figure 1). We also examined the circadian pattern in serum intact PTH and FGF23 concentrations. Serum PTH concentrations were similar across diets in healthy controls (P = 0.54; Figure 2). In contrast, PTH concentrations differed significantly by diet in CKD participants (P = 0.03) with highest concentrations observed while consuming a high-phosphate diet. There was no consistent change in PTH concentrations between 0400 and 0800. Serum FGF23 concentrations were similar across diets in healthy controls (P = 0.08) and CKD participants (P = 0.15; Figure 2). There was no increase in FGF23 concentrations between 0400 and 0800 commensurate with the period of rapid decline in serum phosphate concentrations.
FIGURE 2.
Serum intact PTH and FGF23 concentrations in healthy controls (n = 4) and CKD patients (n = 11) throughout the day and across high-, normal-, and low-phosphate diets. Point estimates reflect mean concentrations, and error bars reflect SEs. The top panels show serum intact PTH concentrations in healthy control and CKD participants. P values for diet × time interactions were 0.48 and 0.98 in the healthy control group and CKD participants, respectively. The bottom panels show C-terminal FGF23 concentrations in healthy control and CKD participants. P values for diet × time interactions were 0.67 and 0.90 in healthy control and CKD participants, respectively. CKD, chronic kidney disease; FGF23, fibroblast growth factor-23; PTH, parathyroid hormone; RU, relative units.
DISCUSSION
We evaluated effects of modifying dietary phosphate intake on the circadian rhythm of serum phosphate in CKD patients and healthy controls. We showed that serum phosphate concentrations were consistently lowest at 0800 and had peaks at 1600 and 0400. This pattern was similar in CKD and healthy controls, albeit the magnitude of the difference in serum phosphate for comparison of lowest and highest concentrations across the day was less in CKD patients. A rapid decline in serum phosphate concentrations was observed between 0400 to 0800 with all 3 diets in both healthy controls and CKD patients. This decline was not accompanied by increases in urine phosphate excretion or PTH or FGF23 concentrations. A diet low in phosphate induced a lower time-averaged serum phosphate concentration and diminished peaks in serum phosphate in CKD patients and healthy controls. Effects of low- compared with high-phosphate diet were least evident at 0800 and, conversely, most evident at 1600 in healthy controls and CKD patients. These findings provide new insights to mechanisms of phosphate homeostasis and have implications for the design of future studies targeting dietary phosphate absorption in CKD patients.
Higher serum phosphate concentrations are associated with cardiovascular disease and mortality in CKD patients (2, 3, 17), and the KDIGO has recommended maintaining serum phosphate concentrations within the normal laboratory range in CKD stages 3–5 (6). Recently, several randomized clinical trials that evaluated intestinal phosphate binders compared with a placebo in CKD patients have either failed to detect significant differences in serum phosphate concentrations (9–12, 18) or observed quite-modest reductions despite the use of high doses of binders (7). These studies have evaluated fasting morning serum phosphate concentrations. Our study showed that serum phosphate concentrations were at their nadir at 0800 and had the least difference in response to dietary phosphate manipulation at this time of day. Serum phosphate concentrations were higher at both 1600 and 0400 and showed differences across phosphate diets at these time points. These findings suggest that previous studies may have underestimated the effect of binders on serum phosphate homeostasis by examining fasting morning specimens. Future studies investigating medications that target dietary phosphate absorption may be more likely to detect differences in serum phosphate concentrations if afternoon blood measurements are evaluated.
Although the difference in serum phosphate concentrations across diets were most modest at 0800 relative to other time points, we nonetheless observed that the low-phosphate diet still had lower phosphate concentrations than those of either normal- or high-phosphate diets in CKD patients at this time of day. This result was surprising because several previous randomized trials of binders in CKD patients have failed to detect differences in phosphate in morning specimens (9–12, 18). The disparate findings in our study may have reflected the concurrent use of a binder with the low-phosphate diet intervention. In an effort to further diminish intestinal absorption with the low-phosphate diet, participants took 1000 mg lanthanum carbonate 3 times/d concurrent with the low-phosphate diet. Recent studies in experimental animals have shown that intestinal phosphate binders upregulate the sodium phosphate co-transporter 2b, which is the major phosphate transporter in the small bowel (19, 20). Thus, it is possible that, when sodium phosphate co-transporter 2b is upregulated in response to binders, the intestine may hyperabsorb phosphate at periods when the binder is not present in the intestinal lumen, which may diminish effects of binders on serum phosphate concentrations. We hypothesized that the combination of low phosphate intake and lanthanum carbonate may have led to the larger difference in morning phosphate concentrations in our study relative to previous clinical trials. Thus, combination therapy may produce more-potent phosphate-lowering effects in CKD patients, which is a hypothesis that requires testing in future trials.
To our knowledge, only 2 previous feeding studies have evaluated circadian changes in phosphate concentrations in CKD patients (15, 16). Consistent with these studies, we confirmed a circadian pattern with lowest concentrations in the morning and higher concentrations in the afternoon. To our knowledge, our study made 2 additional novel contributions. First, the previous studies evaluated CKD patients on fixed phosphate intakes (15, 16). The evaluation of 3 diets that differed in phosphate contents allowed us to show that the circadian pattern of serum phosphate was altered by low phosphate intake in CKD patients, and the largest effect of phosphate diets on serum phosphate concentrations were observed in afternoon rather than morning. Second, in addition to serum phosphate, we measured urine phosphate, PTH, and FGF23. These data allowed us to show that the consistent decline in serum phosphate from 0400 to 0800 was not accompanied by an increase in urine phosphate excretion, PTH, or FGF23. The absence of an increase in urine phosphate excretion suggested that the decline in serum phosphate in the early morning hours may have been due to intracellular shifts, buffering with bone, or an acute decline in intestinal absorption. The later possibility seems less likely because the 0400 time point was ≥8 h after the last meal. Moreover, the lack of an increase in PTH or FGF23 and absence of an increase in urine phosphate excretion between 0400 and 0800 suggested that these hormones were unlikely to be responsible for the acute decline in serum phosphate. Other as yet unidentified mechanisms may be responsible and require future investigation (21).
Strengths of this study included the availability of measurements at multiple time points across the day, concurrent availability of urine phosphate and known phosphaturic hormones, use of controlled dietary feeding to evaluate 3 concentrations of phosphate intake, and evaluation of both CKD and healthy control participants. The study also had important limitations. First, we included only 4 healthy controls, and therefore, estimates may have lacked precision. However, observed circadian patterns in serum phosphate concentrations and responses to different phosphate intakes in these participants were consistent with those in previous reports (14). The low-phosphate diet altered the circadian rhythm of serum phosphate and rendered even the 0800 phosphate concentration lower than anticipated. Because the low-phosphate diet consisted of concurrent low phosphate intake and the use of lanthanum carbonate, we could not discern whether the effect of this diet on lowering 0800 phosphate would have been similar with either a low-phosphate diet or lanthanum carbonate alone. Previous studies in healthy individuals showed peak phosphate concentrations at 1400. We obtained specimens every 4 h at 1200 and 1600 and thus, we could not determine the exact timing of the true peak phosphate in CKD patients (14). All examinations occurred on day 5 of the respective diets. Longer-term effects of diets on the circadian rhythm on phosphate homeostasis remain uncertain. The limited sample size precluded an evaluation by strata of CKD severity.
In conclusion, serum phosphate concentrations have a circadian pattern in CKD patients with lowest concentrations in morning specimens and highest concentrations at 1600 and 0400. This pattern is similar to that of healthy controls but the magnitude of change in phosphate is blunted in CKD relative to healthy controls. Second, a low-phosphate diet in combination with lanthanum alters the circadian rhythm of phosphate concentrations in CKD patients such that 1600 and 0400 peaks are largely abrogated with this diet. Third, differences in serum phosphate concentrations comparing low- and high-phosphate diets in CKD patients had smallest differences at 0800 and the largest difference at 1600. Previous studies evaluating intestinal binders in CKD may have underestimated effects on serum phosphate by relying on morning specimens. Last, there is a consistent decline in serum phosphate concentrations from 0400 to 0800 without a commensurate increase in urine phosphate excretion, PTH, or FGF23. The mechanism or mechanisms responsible for this finding remain elusive and require additional study.
Acknowledgments
We thank the physicians of Denver Nephrology who have contributed patients to this clinical trial.
The authors’ responsibilities were as follows—GAB: designed the research; MSP and GAB: conducted the research; GS, MSP, and GAB: provided essential reagents or essential materials; JHI, CAMA, and GS: analyzed data; JHI: wrote the manuscript; JHI and GAB: had primary responsibility for the final content of the manuscript; CAMA: provided the preliminary interpretation of findings and presented the abstract at the American Society of Nephrology national meeting; and JHI, CAMA, GS, MSP, and GAB: critically reviewed and revised the manuscript. JHI has received honoraria from Shire Pharmaceuticals and Keryx Biopharmaceuticals and has consulted for Astra Zenica. GAB is a consultant for Nestlé Health Sciences. CAMA, GS, and MSP had no conflicts of interest.
Footnotes
Abbreviations used: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; FGF23, fibroblast growth factor-23; KDIGO, Kidney Disease Improving Global Outcomes; PTH, parathyroid hormone.
REFERENCES
- 1.Scialla JJ, Astor BC, Isakova T, Xie H, Appel LJ, Wolf M. Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol 2013;24:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 2009;20:381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 2005;16:520–8. [DOI] [PubMed] [Google Scholar]
- 4.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab 2006;91:3144–9. [DOI] [PubMed] [Google Scholar]
- 5.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011;121(11):4393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009;113:S1–130. [DOI] [PubMed] [Google Scholar]
- 7.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN, et al. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 2012;23:1407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chue CD, Townend JN, Moody WE, Zehnder D, Wall NA, Harper L, Edwards NC, Steeds RP, Ferro CJ. Cardiovascular effects of sevelamer in stage 3 CKD. J Am Soc Nephrol 2013;24:842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isakova T, Gutierrez OM, Smith K, Epstein M, Keating LK, Juppner H, Wolf M. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant 2011;26:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira RB, Cancela AL, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L, Carvalho AB, Jorgetti V, Canziani ME, Moyses RM. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol 2010;5:286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Parra E, Gonzalez-Casaus ML, Galan A, Martinez-Calero A, Navas V, Rodriguez M, Ortiz A. Lanthanum carbonate reduces FGF23 in chronic kidney disease stage 3 patients. Nephrol Dial Transplant 2011;26:2567–71. [DOI] [PubMed] [Google Scholar]
- 12.Seifert ME, de las Fuentes L, Rothstein M, Dietzen DJ, Bierhals AJ, Cheng SC, Ross W, Windus D, Davila-Roman VG, Hruska KA. Effects of phosphate binder therapy on vascular stiffness in early-stage chronic kidney disease. Am J Nephrol 2013;38:158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newsome B, Ix JH, Tighiouart H, Sarnak MJ, Levey AS, Beck GJ, Block G. Effect of protein restriction on serum and urine phosphate in the modification of diet in renal disease (MDRD) study. Am J Kidney Dis 2013;61:1045–6. [DOI] [PubMed] [Google Scholar]
- 14.Portale AA, Halloran BP, Morris RC., Jr Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for the renal production of 1,25-dihydroxyvitamin D. J Clin Invest 1987;80:1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moe SM, Zidehsarai MP, Chambers MA, Jackman LA, Radcliffe JS, Trevino LL, Donahue SE, Asplin JR. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011;6:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isakova T, Xie H, Barchi-Chung A, Smith K, Sowden N, Epstein M, Collerone G, Keating L, Juppner H, Wolf M. Daily variability in mineral metabolites in CKD and effects of dietary calcium and calcitriol. Clin J Am Soc Nephrol 2012;7:820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ix JH, De Boer IH, Peralta CA, Adeney KL, Duprez DA, Jenny NS, Siscovick DS, Kestenbaum BR. Serum phosphorus concentrations and arterial stiffness among individuals with normal kidney function to moderate kidney disease in MESA. Clin J Am Soc Nephrol 2009;4:609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chue CD, Edwards NC, Moody WE, Steeds RP, Townend JN, Ferro CJ. Serum phosphate is associated with left ventricular mass in patients with chronic kidney disease: a cardiac magnetic resonance study. Heart 2012;98:219–24. [DOI] [PubMed] [Google Scholar]
- 19.Radanovic T, Wagner CA, Murer H, Biber J. Regulation of intestinal phosphate transport. I. Segmental expression and adaptation to low-P(i) diet of the type IIb Na(+)-P(i) cotransporter in mouse small intestine. Am J Physiol Gastrointest Liver Physiol 2005;288:G496–500. [DOI] [PubMed] [Google Scholar]
- 20.Schiavi SC, Tang W, Bracken C, O'Brien SP, Song W, Boulanger J, Ryan S, Phillips L, Liu S, Arbeeny C, et al. Npt2b deletion attenuates hyperphosphatemia associated with CKD. J Am Soc Nephrol 2012;23:1691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berndt T, Thomas LF, Craig TA, Sommer S, Li X, Bergstralh EJ, Kumar R. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc Natl Acad Sci USA 2007;104:11085–90. [DOI] [PMC free article] [PubMed] [Google Scholar]