Abstract
Dexmedetomidine, an imidazoline compound, is a highly selective α2-adrenoceptor agonist with sympatholytic, sedative, amnestic, and analgesic properties. In order to minimize the patients' pain and anxiety during minimally invasive spine surgery (MISS) when compared to conventional surgery under general anesthesia, an adequate conscious sedation (CS) or monitored anesthetic care (MAC) should be provided. Commonly used intravenous sedatives and hypnotics, such as midazolam and propofol, are not suitable for operations in a prone position due to undesired respiratory depression. Dexmedetomidine converges on an endogenous non-rapid eye movement (NREM) sleep-promoting pathway to exert its sedative effects. The great merit of dexmedetomidine for CS or MAC is the ability of the operator to recognize nerve damage during percutaneous endoscopic lumbar discectomy, a representative MISS. However, there are 2 shortcomings for dexmedetomidine in MISS: hypotension/bradycardia and delayed emergence. Its hypotension/bradycardiac effects can be prevented by ketamine intraoperatively. Using atipamezole (an α2-adrenoceptor antagonist) might allow doctors to control the rate of recovery from procedural sedation in the future. MAC, with other analgesics such as ketorolac and opioids, creates ideal conditions for MISS. In conclusion, dexmedetomidine provides a favorable surgical condition in patients receiving MISS in a prone position due to its unique properties of conscious sedation followed by unconscious hypnosis with analgesia. However, no respiratory depression occurs based on the dexmedetomidine-related endogenous sleep pathways involves the inhibition of the locus coeruleus in the pons, which facilitates VLPO firing in the anterior hypothalamus.
Keywords: adrenergic alpha-2 receptor agonists, conscious sedation, dexmedetomidine, minimally invasive surgical procedures, percutaneous discectomy
INTRODUCTION
Operators sometimes perform minimally invasive spine surgery (MISS) while ignoring the primary concerns of their patients including anxiety and pain during the perioperative period. There is considerable difference of opinion between the two groups. From the operators' perspective, the MISS is considered to be less invasive and less painful compared with conventional surgery. Therefore, they confidently choose local anesthesia along with conscious sedation (CS) or monitored anesthetic care (MAC) for the MISS. In contrast, patients have suffered through a considerable period of intractable spinal pain before the operation and expect the procedure to completely alleviate this pain. Patients believe the procedure of MISS will provoke no pain or endurable pain due to their doctors' explanation of the procedure and its inherent minimal damage to the normal structures.
It is difficult to find a combination of intravenous drugs for CS or MAC to guarantee no respiratory depression without intubation during MISS in a prone position. Due to delaying the procedure and an increased risk of infection while performing MISS, it is difficult to halt the procedure and rotate the patient from the prone position to the supine position in order to secure the airway. In addition, recognition of nerve damage during the MISS must be ensured. Therefore, patients are given insufficient analgesia and sedation while enduring intolerable anxiety and pain. The doctor-patient relationship may be broken even after a successful MISS due to the patient's painful experience. Patients who have suffered from intolerable anxiety and pain are unlikely to recommend the MISS to their colleagues or other potential patients.
Dexmedetomidine, an imidazoline compound, is a highly selective α2-adrenoceptor agonist with sedative, amnestic, sympatholytic, and analgesic properties. Its great merit is providing a passage from a cooperative conscious sedation to an uncooperative, unconscious hypnosis while securing the ability of self-reporting the risk of upcoming potential nerve damage and insuring spontaneous respiration. These merits of dexmedetomidine will provide an ideal anesthetic condition regardless of adverse effects such as hypotension/bradycardia intraoperatively and delayed emergence postoperatively.
The brief history of the drug, the mechanisms of amnesia/sedation, analgesia, and sympatholysis, the pharmacokinetics and pharmacodynamics, and the clinical application, especially for a procedural sedation during percutaneous endoscopic lumbar discectomy (PELD) - a representative MISS - will be focused on in this paper.
BRIEF HISTORY
Medetomidine (Domitor®, Orion Corporation, Espoo, Finland) was launched as an animal sedative in 1987. Dexmedetomidine (Precedex®, Abbott Laboratories, North Chicago, IL), the S-enantiomer of medetomidine, received approval from the Food and Drug Administration (FDA) in 1999, and was indicated for sedation of initially intubated and mechanically ventilated adult patients during treatment in an intensive care setting for up to 24 hours.
An additional FDA-approved indication of this drug in 2008 was sedation of non-intubated patients prior to and/or during surgical and other procedures. This 200 µg of 2 ml dexmedetomidine must be diluted in 0.9% sodium chloride solution prior to administration.
The premixed ready-to-use preparation (Precedex® Premix, Hospira Inc, Lake Forest, IL) is available in 50 and 100 ml sizes since 2012.
Various α2 adrenoreceptor ligands are now available. In the chemical class of imidazoline: oxymetazoline, brimonidine for ocular hypertension and clonidine for hypertension and pain, as well as dexmedetomidine. In the chemical class of guanidine: guanfacine and guanabenz for hypertension have been developed [1].
MECHANISMS OF ACTION
Dexmedetomidine, an imidazoline compound, is a highly selective α2-adrenoceptor agonist with sedative, amnestic, sympatholytic, and analgesic properties. It has 8 times more selectivity for α2 receptors than clonidine (clonidine selectivity: α2 : α1 = 200 : 1; dexmedetomidine selectivity: α2 : α1 = 1,620 : 1), especially for the 2A subtype. On the other hand, it also has twice as much selectivity for imidazoline receptors than clonidine (clonidine selectivity: α2 : imidazoline = 15 : 1; dexmedetomidine selectivity: α2 : imidazoline = 30 : 1), especially for type 1 [2,3].
Adrenoceptors are found in nearly all peripheral tissues and in many neuronal populations within the central nervous system. Both norepinephrine and epinephrine play important roles in the control of blood pressure, myocardial contractile rate and force, airway reactivity, and a variety of metabolic functions. Adrenoceptor activation in the locus coeruleus (LC) has direct and indirect effects on the activity of many other neuronal nuclei within the brain [4].
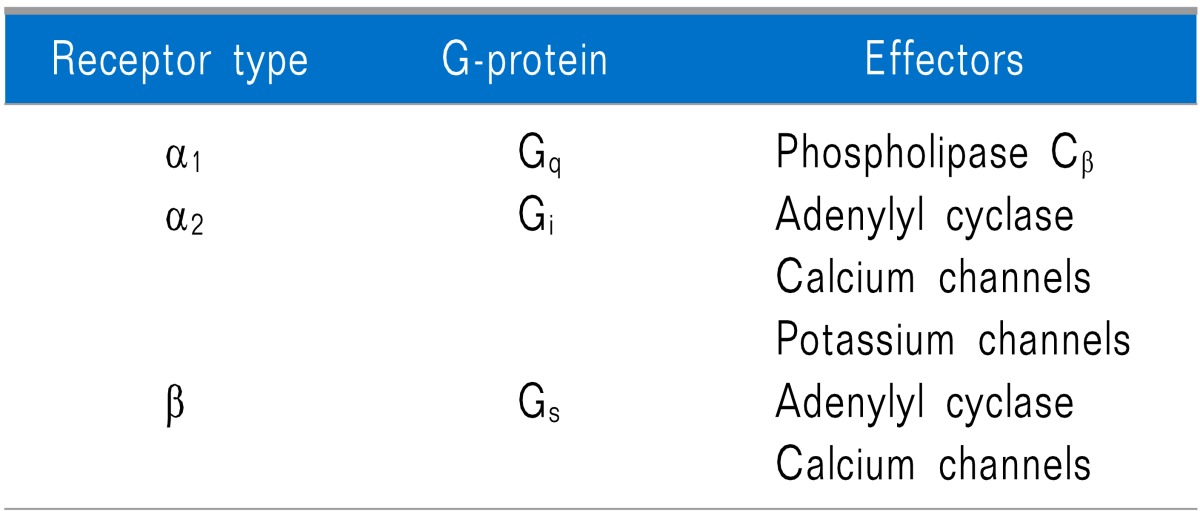
Adrenoceptors can be classified into α1 (α1A, α1B, and α1D), α2 (α2A, α2B, and α2C), β1, β2, and β3 subtypes. Multiple subtypes often coexist in a particular tissue or even on an individual cell. They can mediate opposing (i.e., α2 versus β), redundant (i.e., β1 and β2), or synergistic responses (i.e., α1 and β2). According to adrenergic receptors, they have their own preferred linkages to G-protein families and effectors (Table 1) [5]. Dexmedetomidine blocks presynaptic α2 receptors as a negative feedback system resulting in suppression of norepinephrine (NE) release [6].
Table 1.
Preferred Linkages of Adrenergic Receptors to G-protein Families and Effectors
1. Action mechanisms of sedation and hypnosis
The sedative/hypnotic response to dexmedetomidine is mediated in the LC in the dorsal wall of the rostral pons, located in the lateral floor of the fourth ventricle. The LC is known as the site of the largest noradrenergic cell body aggregate of the brain, including a high density of α2-adrenergic receptors. Wakefulness or vigilance is associated with an increase in the firing rate of the LC [7]. Agonist activation of α2-adrenergic receptors in the LC suppresses the firing of the noradrenergic neurons, resulting in hypotension, bradycardia, and sedation/hypnosis [8]. The sedation/hypnosis induced by dexmedetomidine mimics normal physiologic sleep in its cardiovascular and respiratory effects, and involves an endogenous sleep-promoting pathway [8,9].
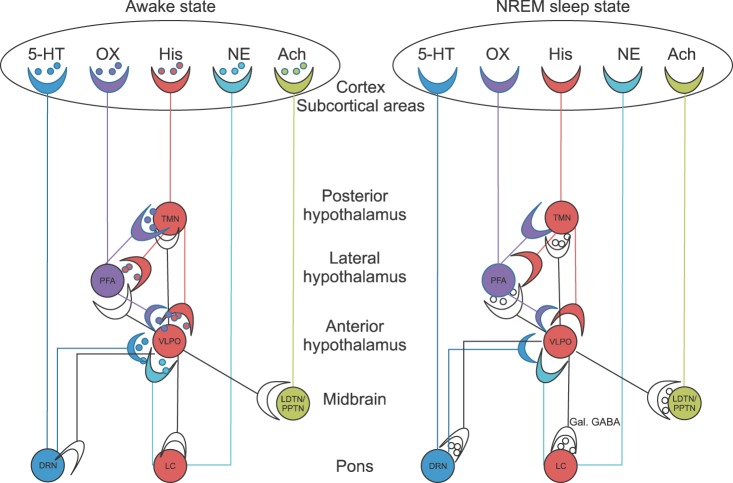
The normal endogenous sleep-promoting pathway, which converts from a wakeful state to a non-rapid eye movement (NREM) state, is found in the pons, midbrain, hypothalamus, and cortex. The main arousal-sleep pathway begins with an inhibition of noradrenergic neurons in the LC in the pons releases a tonic noradrenergic inhibition of the ventrolateral preoptic nucleus (VLPO) in the anterior hypothalamus. The activated VLPO releases γ-aminobutyric acid (GABA) and galanin (GAL) into the tuberomammillary nucleus (TMN) in the posterior hypothalamus. The TMN, as representative of arousal centers, inhibits its release of arousal-promoting histamine into the cortex (PFA, and VLPO), and thus induces loss of consciousness.
Other arousal-promoting neurotransmitters and their pathways to the cortex are: 1) serotonin (5-hydroxytryptamine, 5-HT) from the dorsal raphe nucleus (DRN) in the pons; 2) orexin (ORX, hypocretins) from the perifornical area (PFA) in the lateral hypothalamus; 3) NE from the LC in the pons, and 4) acetylcholine (ACh) from the laterodorsal tegmental nuclei (LDTN) and pedunculopontine tegmental nuclei (PPTN).
Therefore, if the arousal-promoting neurotransmitters - including histamine, as well as 5-HT, ORX, NE, and Ach - are released from TMN, DRN, PFA, LC, and LDTN/PPTN, respectively, into the cortex, wakefulness is promoted.
When the wakeful state is converted to the NREM sleep state, the most characteristic feature of neurotransmitters in the brain is an increased release of GAL and GABA into the TMN, LC, DRN, PFA, and LDTN/PPTN from the VLPO. And other notable neurotransmitter changes are a decreased release of ORX from the PFA into the TMN and the VLPO and a decreased release of histamine from the TMN into the VLPO and PFA. The release of all 5 arousal-promoting neurotransmitters decreases in the cortex as well.
The answer for why dexmedetomidine shows relatively rapid and complete awake-sleep transitions (one falls asleep and suddenly wakens) might be explained from the flip-flop switch model. The perifornical area in the lateral hypothalamus has ORX neurons which connect TMN and VLPO. Since the VLPO neurons do not have ORX receptors, the ORX neurons serve primarily to reinforce the monoaminergic tone (histamine, NE, and 5-HT), rather than directly inhibiting the VLPO on their own. During wakefulness, the monoaminergic nuclei inhibit the VLPO, thereby relieving the inhibition of the monoaminergic cells, ORX neurons, and Ach neurons. On the other hand, during sleep, the firing of the VLPO neurons inhibits the monoaminergic cell groups. This also allows it to inhibit the ORX neurons, further preventing monoaminergic activation that might interrupt sleep. The direct mutual inhibition between the VLPO and the monoaminergic cell groups forms a classic flip-flop switch, which produces sharp transitions in state, but is relatively unstable. The addition of the orexin neurons stabilizes the switch [10].
Sleep spindles are one of the most important sleep electroencephalogram (EEG) micro-events, originating in the thalamus and then propagating to the cortex. They are considered sleep maintaining events that block the transfer of sensory information to the cerebral cortex during sleep, thus preventing sleep-interrupting arousals. Spindles are short-lasting, oscillatory EEG waveforms with a frequency typically in the high α and low β bands (11-14 Hz) occurring with highest density during S2 sleep and with lower density in deeper S3 and S4 slow-wave sleep. Dexmedetomidine produces a state closely resembling physiological S2 sleep in humans and activates normal non-rapid eye movement sleep (NREM)-promoting pathways [11].
In conclusion, the sedative/hypnotic mechanism of dexmedetomidine-related endogenous sleep pathways involves the inhibition of the LC in the pons, which facilitates VLPO firing in the anterior hypothalamus. The increased release of GABA and GAL at the terminals of the VLPO inhibits TMN firing, which is required for the sedative response in the cortex (Fig. 1) [8,9].
Fig. 1.
Sedative and hypnotic mechanism of dexmedetomidine-related endogenous sleep pathways. If the arousal-promoting neurotransmitters - including histamine, as well as 5-hydroxytryptamine (5-HT), orexin (ORX, hypocretin), norepinephrine (NE), and acetylcholine (Ach) - are released from the tuberomammillary nucleus (TMN), dorsal raphe nucleus (DRN), perifornical area (PFA), locus coeruleus (LC), and laterodorsal tegmental nuclei (LDTN) and pedunculopontine tegmental nuclei (PPTN), respectively, into the cortex, wakefulness is promoted. When the wakeful state is converted to the NREM sleep state, the most characteristic feature of neurotransmitters in the brain is an increased release of galanin (GAL) and gamma-aminobutyric acid (GABA) into the TMN, LC, DRN, PFA, and LDTN/PPTN from the ventrolateral preoptic nucleus (VLPO). And other notable neurotransmitter changes are a decreased release of ORX from the PFA into the TMN and VLPO and a decreased release of histamine from the TMN into the VLPO and PFA. The release of all 5 arousal-promoting neurotransmitters decreases in the cortex as well.
2. Action mechanisms of lack of respiratory depression
Dexmedetomidine has a biphasic effect on respiratory drive, with low doses decreasing and higher doses increasing resting ventilation in dogs. The locus coeruleus is an important site for the action of α2 adrenoceptor agonists. The locus coeruleus is involved in arousal reactions; suppression of its activity by alpha-2 adrenoceptor agonists can result in a state similar to sleep with mild respiratory depression. There is no significant effect on hypercapnic or hypoxic ventilatory drive with α-2 adrenoceptor stimulation [12].
Dexmedetomidine did have some respiratory effects, as would be expected because (1) one of its sites of action is the LC, which is known to play a role in both respiratory control and sleep modulation, and (2) dexmedetomidine converges on the natural sleep pathway to exert its sedative effects, whereas natural sleep results in ventilation modulation. However, the respiratory effects of dexmedetomidine were markedly different from those of opioids or intravenous/inhalative anesthetics. Compared with baseline, during dexmedetomidine infusions, the hypercapnic ventilatory response was unchanged, the respiration rate was significantly increased, and the overall apnea/hypopnea index was significantly decreased. The distribution of ventilatory cycle time/inspiratory time showed an increased peak. More importantly, dexmedetomidine exhibited a hypercapnic arousal phenomenon similar to what has been described during natural sleep [13].
In conclusion, dexmedetomidine shows limited respiratory depression with sedation or hypnosis similar to normal physiologic sleep.
3. Action mechanisms of analgesic property
Part of the mechanism by which dexmedetomidine produces an antinociceptive effect is based on action made directly on the LC. Furthermore, the action of dexmedetomidine in the LC in turn may result in an increase in the activation of α2 adrenoceptors in the spinal cord [14].
Intrathecal administration of dexmedetomidine alleviates mechanical allodynia induced by chronic constriction injury. Western blotting reveals that dexmedetomidine reduces the activation of microglia and the up-regulation of interleukin-18 protein expression in the ipsilateral lumbar spinal dorsal horn [15].
Systemic α2-adrenoceptor stimulation may facilitate inhibitory synaptic responses in the superficial dorsal horn to produce analgesia mediated by activation of the pontospinal noradrenergic inhibitory system [16]. The α2 adrenoceptor is highly enriched in spinal dorsal horn and involved in descending noradrenergic pain modification. Action of α2-adrenoceptors inhibits N-methyl-D-aspartate (NMDA) receptor-mediated nociceptive transmission in the spinal dorsal horn of mice with inflammatory pain [17].
In conclusion, dexmedetomidine shows an antinociceptive effect by action directly on the LC. It also potentiates descending noradrenergic pain modification and inhibits NMDA receptor-mediated nociceptive transmission in the spinal dorsal horn.
4. Action mechanisms of sympatholysis
The activation of presynaptic α2-adrenoceptors on sympathetic nerves and in the central nervous system induces sympatholysis, whereas the activation of vascular postsynaptic receptors causes both vasoconstriction (through the activation of α2-adrenoceptors on vascular smooth muscle cells) and vasodilatation (through the activation of α2-adrenoceptors on endothelial cells).
Alpha2-agonists induce sympatholytic effects through α2-adrenoceptors in the central nervous system. Intracerebroventricular administration of dexmedetomidine induces cardiovascular responses mediated by sympathetic inhibition and parasympathetic activation. It also has the potential to decrease blood pressure in a dose-dependent manner because of its α2-agonist activity on the sympathetic ganglia and the resulting sympatholytic effects [18].
THE PHARMACOKINETICS AND PHARMACODYNAMICS
1. Pharmacokinetics
Dexmedetomidine undergoes almost complete biotransformation through direct glucuronidation and cytochrome P450 metabolism (hydroxylation, mediated by CYP2A6), with very little excretion of unchanged molecules in the urine (95%) or feces (4%). It may be necessary to decrease the typical dose in patients with hepatic failure. The elimination half-life is approximately 2 hours. Dexmedetomidine exhibits linear kinetics when infused in the recommended dose range from 0.2 to 0.7 µg/kg/h for no more than 24 hours.
The steady-state volume of distribution is 118 L, and the distribution phase is rapid, with a half-life of distribution of approximately 6 minutes. The average protein binding of dexmedetomidine is 94%, with negligible protein binding displacement by fentanyl, ketorolac, theophylline, digoxin, and lidocaine, all drugs commonly used during anesthesia [2].
There have been no significant sex or age-based differences in the pharmacokinetic profile, even in elderly patients, and pharmacokinetics of the active dexmedetomidine molecule do not change in patients with renal failure. There is, however, a theoretical possibility of accumulation of metabolites of biotransformation because of the high degree of renal clearance of these metabolites [2].
2. Pharmacodynamics
Dexmedetomidine is an α-adrenoceptor agonist with dose dependent α2-adrenoceptor selectivity. At higher doses (>1 mg/kg) or in rapid infusions of lower doses, both α1- and α2-adrenoceptor activities are observed. Patients treated with dexmedetomidine required either no additional sedative medication or only small doses of add-on medications [2].
A biphasic cardiovascular response is observed after application of dexmedetomidine. Initial transient increase of blood pressure and a reflex decrease in heart rate can be explained by the peripheral α2B-adrenoceptor stimulation of vascular smooth muscle and can be attenuated by a slow infusion. Both of these effects are caused by the inhibition of the central sympathetic outflow overriding the direct stimulating effects. Another possible explanation for the subsequent decrease of heart rate is the stimulation of the presynaptic α2-adrenoceptor, leading to a decreased NE release [19,20].
The application of a single high dose of dexmedetomidine reduces NE and epinephrine releases by as much as 92% in young healthy volunteers. This seems to be more important than either central α2-adrenoceptor agonism or non-α adrenaline imidazole-preferring receptors in effecting the change [21].
CLINICAL APPLICATION FOR MISS
After an additional FDA-approved indication of dexmedetomidine in 2008 for sedation of non-intubated patients prior to and/or during surgical and other procedures, various clinical applications have been introduced [22,23,24].
Most spinal procedures including MISS should be performed in the prone position. Also, most sedative/hypnotic drugs, including midazolam and propofol, never fail to cause respiratory depression. It is difficult to use these drugs on the non-intubated patients who receive MISS procedure in the prone position due to the fatal defect of respiratory depression.
Dexmedetomidine can be used for procedural sedation in the prone position without concern of respiratory depression occurring. Its common adverse effects, bradycardia and hypotension, can be prevented using ketamine. When both dexmedetomidine and ketamine are used together, dexmedetomidine may prevent the tachycardia, hypertension, salivation, and emergence phenomena from ketamine, whereas ketamine may prevent the bradycardia and hypotension, which has been reported with dexmedetomidine. An additional benefit of the added ketamine is an increased speed of sedation, compared with the slow onset time when dexmedetomidine is the sole agent [25]. However, the dose of ketamine over 1 mg/kg in adults may show a range of perceptual distortions, including referential ideas, affective flattening, and alogia, but not hallucination [26].
In case of patients with severe anxiety, a small dose (3-5 mg) of intramuscular injection of midazolam 1 hour before arriving at the operating room can cause antegrade amnesia to rid the patient of unpleasant preoperative memories and anxiety. For the incisional pain, a 5% lidocaine patch is applied to the anticipated working channel site [27]. Intramuscular administration of midazolam compared to intravenous administration has a lower probability of provoking respiratory depression during the operation. A preventive intravenous antibiotic is administrated 30 minutes before operation.
While draping the operation field, continuous intravenous administration of dexmedetomidine mixed with less than 1 mg/kg of ketamine is started. After infusion of 0.4 mg (2 vials, 7 µg/kg) of dexmedetomidine, a favorable skin incisional condition with unconscious sedation is usually accomplished. Fifty µg of fentanyl and 30 mg of ketorolac for analgesia are given in a single dose. It is not recommended to use the more potent ultra-short-acting opioid analgesic, remifentanil, instead of fentanyl, in opioid-naïve patients. It may cause more severe nausea and vomiting during the operation. These adverse effects can delay the progression of the operation by 10 minutes to 1 hour as well as make it necessary to change the position of the patient to maintain an open airway.
Before the anulotome, 50 µg of fentanyl is administered intravenously to relieve the severe intractable pain caused by the anulotome. After the removal of the targeted herniated nucleus pulposus, the infusion of dexmedetomidine should be discontinued to reduce the delayed emergence of the patient.
This delayed emergence from the dexmedetomidine, lasting from 3 to 8 hours, is a major fault of the sedative/hypnotic. This results in an extended fasting period for the patient, similar to that of general anesthesia with a muscle relaxant. It is necessary to prepare an intravenous fluid and electrolyte balance until the patient can eat and drink regularly. Guardians of the patient should be briefed by the operator on the delayed emergence period to reduce anxiety from such a long period without contact between patient and guardian.
Bispectral index (BIS; Aspect Medical Systems Inc., Natick, MA), a monitor of cortical electrical activity, has been used to access the level of sedation/hypnosis for various intravenous or inhalative agents. However, the BIS cannot tell us anything about arousability, and its predictive value is not proven. In physiologic sleep, BIS levels less than 30 were detected, and the volunteers were arousable. This is not the case if propofol and inhalative agents like isoflurane or desflurane are used and these deep levels are achieved. One characteristic of dexmedetomidine seems to be its easy arousability without clinical signs of stress [28]. BIS is not useful for some individual hypnotic agents (ketamine, dexmedetomidine, nitrous oxide, xenon, and opioids) to predict arousability [29].
BIS values at Observer's Assessment of Alertness and Sedation (OAA/S) scores of 1, 2, 3, 4, and 5 during dexmedetomidine sedation were noted as 95, 62, 45.5, 39.5, and 24.5, respectively in a previous study. These BIS values showed a different decreasing pattern during the infusion of propofol: 95.5, 78, 67, 57, and 34 from OAA/S score 1 to 5, respectively. The calculated cutoff BIS values for OAA/S scores of ≤ 2 (patient responds only after mild prodding or shaking) were 46 for dexmedetomidine and 67 for propofol [30]. If the BIS value is over 46 after infusion of dexmedetomidine, the patient can respond and report their potential nerve injury during the MISS.
CONCLUSIONS
Dexmedetomidine provides an ideal sedative and hypnotic surgical condition during MISS in a prone position. Additional administration of a small dose of ketamine reassures operators from the worries about respiratory depression with hypotension and bradycardia. It also allows patients to report a potential nerve injury even in a deep sedative and hypnotic state. The greatest drawback is delayed emergence after infusion of high dose of dexmedetomidine.
Footnotes
This study was partially supported by a grant from Pusan National University for a 2-year study between May 2014 and Feb 2016.
References
- 1.Newman-Tancredi A, Nicolas JP, Audinot V, Gavaudan S, Verrièle L, Touzard M, et al. Actions of alpha2 adrenoceptor ligands at alpha2A and 5-HT1A receptors: the antagonist, atipamezole, and the agonist, dexmedetomidine, are highly selective for alpha2A adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:197–206. doi: 10.1007/pl00005243. [DOI] [PubMed] [Google Scholar]
- 2.Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001;14:13–21. doi: 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chrysostomou C, Schmitt CG. Dexmedetomidine: sedation, analgesia and beyond. Expert Opin Drug Metab Toxicol. 2008;4:619–627. doi: 10.1517/17425255.4.5.619. [DOI] [PubMed] [Google Scholar]
- 4.Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, et al. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- 5.Insel PA. Seminars in medicine of the Beth Israel Hospital, Boston. Adrenergic receptors--evolving concepts and clinical implications. N Engl J Med. 1996;334:580–585. doi: 10.1056/NEJM199602293340907. [DOI] [PubMed] [Google Scholar]
- 6.Haselman MA. Dexmedetomidine: a useful adjunct to consider in some high-risk situations. AANA J. 2008;76:335–339. [PubMed] [Google Scholar]
- 7.Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76:948–952. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:979–984. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 10.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 11.Huupponen E, Maksimow A, Lapinlampi P, Särkelä M, Saastamoinen A, Snapir A, et al. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol Scand. 2008;52:289–294. doi: 10.1111/j.1399-6576.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 12.Khan ZP, Ferguson CN, Jones RM. alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146–165. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- 13.Hsu YW, Cortinez LI, Robertson KM, Keifer JC, Sum-Ping ST, Moretti EW, et al. Dexmedetomidine pharmacodynamics: part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101:1066–1076. doi: 10.1097/00000542-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology. 1996;84:873–881. doi: 10.1097/00000542-199604000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Li SS, Zhang WS, Ji D, Zhou YL, Li H, Yang JL, et al. Involvement of spinal microglia and interleukin-18 in the anti-nociceptive effect of dexmedetomidine in rats subjected to CCI. Neurosci Lett. 2014;560:21–25. doi: 10.1016/j.neulet.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Funai Y, Pickering AE, Uta D, Nishikawa K, Mori T, Asada A, et al. Systemic dexmedetomidine augments inhibitory synaptic transmission in the superficial dorsal horn through activation of descending noradrenergic control: an in vivo patch-clamp analysis of analgesic mechanisms. Pain. 2014;155:617–628. doi: 10.1016/j.pain.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan QQ, Li L, Wang WT, Yang X, Suo ZW, Hu XD. Activation of α2 adrenoceptors inhibited NMDA receptor-mediated nociceptive transmission in spinal dorsal horn of mice with inflammatory pain. Neuropharmacology. 2014;77:185–192. doi: 10.1016/j.neuropharm.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Seyrek M, Halici Z, Yildiz O, Ulusoy HB. Interaction between dexmedetomidine and α-adrenergic receptors: emphasis on vascular actions. J Cardiothorac Vasc Anesth. 2011;25:856–862. doi: 10.1053/j.jvca.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–1142. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Sulaiman S, Karthekeyan RB, Vakamudi M, Sundar AS, Ravullapalli H, Gandham R. The effects of dexmedetomidine on attenuation of stress response to endotracheal intubation in patients undergoing elective off-pump coronary artery bypass grafting. Ann Card Anaesth. 2012;15:39–43. doi: 10.4103/0971-9784.91480. [DOI] [PubMed] [Google Scholar]
- 21.Kallio A, Scheinin M, Koulu M, Ponkilainen R, Ruskoaho H, Viinamäki O, et al. Effects of dexmedetomidine, a selective alpha 2-adrenoceptor agonist, on hemodynamic control mechanisms. Clin Pharmacol Ther. 1989;46:33–42. doi: 10.1038/clpt.1989.103. [DOI] [PubMed] [Google Scholar]
- 22.Shukry M, Miller JA. Update on dexmedetomidine: use in nonintubated patients requiring sedation for surgical procedures. Ther Clin Risk Manag. 2010;6:111–121. doi: 10.2147/tcrm.s5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: a review of clinical applications. Curr Opin Anaesthesiol. 2008;21:457–461. doi: 10.1097/ACO.0b013e328305e3ef. [DOI] [PubMed] [Google Scholar]
- 24.Gerlach AT, Dasta JF. Dexmedetomidine: an updated review. Ann Pharmacother. 2007;41:245–252. doi: 10.1345/aph.1H314. [DOI] [PubMed] [Google Scholar]
- 25.Tobias JD. Dexmedetomidine and ketamine: an effective alternative for procedural sedation? Pediatr Crit Care Med. 2012;13:423–427. doi: 10.1097/PCC.0b013e318238b81c. [DOI] [PubMed] [Google Scholar]
- 26.Pomarol-Clotet E, Honey GD, Murray GK, Corlett PR, Absalom AR, Lee M, et al. Psychological effects of ketamine in healthy volunteers. Phenomenological study. Br J Psychiatry. 2006;189:173–179. doi: 10.1192/bjp.bp.105.015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim KH. Use of lidocaine patch for percutaneous endoscopic lumbar discectomy. Korean J Pain. 2011;24:74–80. doi: 10.3344/kjp.2011.24.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Triltsch AE, Welte M, von Homeyer P, Grosse J, Genähr A, Moshirzadeh M, et al. Bispectral index-guided sedation with dexmedetomidine in intensive care: a prospective, randomized, double blind, placebo-controlled phase II study. Crit Care Med. 2002;30:1007–1014. doi: 10.1097/00003246-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Johansen JW. Update on bispectral index monitoring. Best Pract Res Clin Anaesthesiol. 2006;20:81–99. doi: 10.1016/j.bpa.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Kasuya Y, Govinda R, Rauch S, Mascha EJ, Sessler DI, Turan A. The correlation between bispectral index and observational sedation scale in volunteers sedated with dexmedetomidine and propofol. Anesth Analg. 2009;109:1811–1815. doi: 10.1213/ANE.0b013e3181c04e58. [DOI] [PubMed] [Google Scholar]