Abstract
Aims
Islet amyloid is a hallmark in type 2 diabetic subjects, but its implication in clinical features and development of islet pathology is still unclear.
Methods
From 118 autopsy cases with type 2 diabetes, 26 cases with islet amyloid deposition (DA+) were selected. Twenty diabetic subjects without obvious amyloid deposition (DA−) matched for the age and diabetes duration and 20 non-diabetic subjects (ND) served for comparison. We examined the severity of amyloid deposition and its relationships with population of endocrine cells, expression of cell damage markers or macrophage infiltration. Correlation of clinical profile with islet pathology was also sought on the subset of the investigated patients.
Results
β-Cell volume density was nearly 40% less in DA+ and 20% less in DA− when compared to ND. Severity of amyloid deposition correlated with reduced volume densities of β-cell and α-cell, and increased body mass index (BMI), but not with duration of diabetes, age or HbA1c. Amyloid-rich islets contained an increased number of macrophages mixed with β-cells with oxidative stress-related DNA damage, characterized by γH2AX expression, and suppressed (pro)insulin mRNA expression.
Conclusions
In Japanese type 2 diabetic patients, islet amyloid was more common with severe β-cell loss and high BMI, associated with macrophage infiltration.
Keywords: β-cell loss, macrophage, morphometry, oxidative stress, pancreatic islet, type 2 diabetes
Introduction
Over a century ago, Opie first described hyalinization of islets in diabetes [1], which was later identified as amyloid, the most representative feature of the islet pathology in type 2 diabetes [2–4]. Westermark and Cooper found that the islet amyloid was composed of islet amyloid polypeptide (IAPP), or “amylin” peptide [5–8]. Experimental evidence has accumulated to support the toxic role of amyloid in β-cell death in diabetes. Substantial findings to underscore such consideration, however, are still lacking in human type 2 diabetes. There are only a few studies that explored correlation of amyloid deposition with β-cell loss in human type 2 diabetic patients, but the results are contradictory [2,9–11]. Westermark and his associates found a significant reduction of islet and β-cell area in diabetic patients with amyloid-rich islets [2,10], although others could not confirm such findings [11,12]. More recently, Jűrgens et al. [13] found robust β-cell loss in type 2 diabetic patients with amyloid-rich islets, which were accompanied by an increase in apoptotic β-cells.
There emerges growing evidence that suggests the involvement of proinflammatory process in the development of islet lesions in type 2 diabetes. In fact, macrophage infiltration in the islet with excessive production of cytokines and chemokines are encountered in animal and human type 2 diabetes [14,15]. Preliminary clinical trial with interleukin-1β receptor (IL-1βR) antagonist improved glucose intolerance in type 2 diabetic patients [16]. Information regarding a possible association between amyloid deposition, islet inflammation and β-cell loss in human type 2 diabetes is, however, still limited.
Prevalence of amyloid deposition was 80% or more in European and American diabetic patients [9,10], but modest in Asians, being nearly 40% in Chinese diabetic patients [17]. Among Chinese diabetic patients, islet amyloid deposition correlated with an increased pancreatic fat infiltration and fibrosis with higher levels in body mass index (BMI), blood pressure and HbA1c, compared to those without amyloid [17]. However, no information on the islet structure or the extent of β-cell loss was provided. Since islet amyloid was commonly detected in most diabetic Caucasians, the effects of amyloid deposition on the islet cells or clinical measures were not independently studied previously, but analyzed in the same diabetic cohort [2,13,18]. Thus, implication of amyloid deposition in the islet pathology is yet to be clarified.
We reported that amyloid deposition was less common in Japanese type 2 diabetic patients [19]. Our previous study consisted of younger diabetic subjects, 10 years in average when compared to those in Europeans or Americans reported in the literature [2,10,11,13]. We therefore consider that, with increasing subjects matched with age for investigation, it would be possible to find a significance of amyloid deposition in the development of islet pathology by comparison between amyloid-free diabetic patients and amyloid-rich diabetic patients.
Materials and methods
Subjects
From the archival autopsy files of Hirosaki University Hospital and affiliated related hospitals, 118 diabetic cases were procured from the year 2000. Diabetes was identified by their medical records and duration of diabetes was defined as the period after the clinical diagnosis or discovery of diabetes. Pancreatic tissues were obtained during full autopsies within 5 h after death and fixed in 10% buffered formalin. Multiple 4 μm thick sections of paraffin-embedded tissues of the body and tail of the pancreas were stained with hematoxylin and eosin (HE) and Congo-red for survey of the islet structure and presence of amyloid. Thirty-two cases (27.1%) were found to contain amyloid, but six cases were excluded for further analysis because of the lesions of pancreatic cancer (one case), exocrine pancreatitis (three cases) and scattered areas of autolysis (two cases). From the remaining 86 cases, subjects with pancreatic cancer (two cases), lesions of exocrine pancreatitis (four cases), autolysis (four cases), with end-stage liver disorders (two cases), potential causes of secondary diabetes such as myopathy (two cases) and adrenal tumor (one case) were excluded. Thereafter, we selected 20 cases without apparent amyloid deposition that were matched with age and duration of diabetes to those in DA+. Thus, 26 diabetic subjects with islet amyloid deposition (DA+) and 20 diabetic subjects without apparent amyloid deposition (DA−) were evaluated for the relationship between amyloid deposition and islet endocrine cell changes (Table 1).
Table 1.
Clinical summaries of investigated subjects.
| Non-diabetic | Diabetic without amyloid | Diabetic with amyloid | |
|---|---|---|---|
| Male/Female | 9;11 | 16;4 | 19;7 |
| Age (years) | 64.2 ± 2.9 | 65.7 ± 2.5 | 67.8 ± 2.5 |
| BMI (kg/m2) | 21.0 ± 0.7 (n = 16) | 22.4 ± 0.6 (n = 20) | 24.7 ± 0.7* (n = 17) |
| Duration of diabetes (years) | 11.4 ± 3.1 (n = 12) | 11.4 ± 2.2 (n = 19) | |
| HbA1c (%, NGSP) | 7.00 ± 0.3 (n = 17) | 7.46 ± 0.5 (n = 21) | |
| Treatment (%) | (n = 17) | (n = 23) | |
| Diet | 2 (10.0%) | 5 (19.2%) | |
| OHA | 7 (35.0%) | 9 (34.6%) | |
| Insulin | 5 (25.0%) | 9 (34.6%) | |
| Untreated | 3 (15.0%) | 0 (0%) | |
| Unknown | 3 (15.0%) | 3 (11.5%) | |
| Pancreatic weight (g) | 124.4 ± 7.9 (n = 19) | 120.1 ± 7.6 (n = 17) | 131.2 ± 11.7 (n = 18) |
Values are mean ± SE, *p < 0.05 versus non-diabetic, diabetic without amyloid.
BMI: Body mass index; OHA: Oral hypoglycemic agents.
For comparison with diabetic groups, we selected age- and sex-matched 20 non-diabetic cases without apparent amyloid deposition (ND). ND did not have an apparent history of diabetes themselves, or their family history, or previous evidence of hyperglycemia. Cases with a history of parenteral alimentation or continuous therapies with potential influences on glucose intolerance, such as steroid or cyclosporine, were not included in ND.
The study was approved by the institutional review board at the Hirosaki University School of Medicine and conformed the Declaration of Helsinki.
Pancreas specimens and detection of amyloid
From the paraffin blocks, several consecutive 4-μm thick sections were obtained. For the identification of amyloid and measurement of amyloid area, we conducted thioflavin-T staining (Wako Pure Chemicals, Osaka, Japan), which showed positive green fluorescence on fluorescent microscopy (Axio-Imager M1, Carl Zeiss, Tokyo). For the determination of islet area, the sections were incubated with monoclonal antibody to chromogranin A (1:1000) (Dako Cytomation, Glostrup, Denmark) overnight, followed by incubation with alkaline phosphatase-labeled rabbit anti-mouse immunoglobulin (1:1000, Dako). The reaction products were colorized with Vulcan Fast red chromogen (Biocare Medical, LLC, Concord, CA) and examined by fluorescent microscope. Since the pancreatic body area preserved most consistently the structural integrity, less confounded by fat infiltration or fibrosis, the data from the body represented the values in individuals in this study, as conducted by others [13]. Amyloid-positive areas were measured by point-counting method on at least 100 islets in each individual and expressed as percent area of pancreatic parenchyma, yielding the values of amyloid volume density relative to the area of pancreas parenchyma. Severity of amyloid was expressed as average percentage of amyloid area per islet area (sum of chromogranin and amyloid areas). Prevalence of amyloid-laden islets was obtained among 100 islets and expressed as a percentage in each case.
Immunostaining of islet endocrine cells, apoptosis, Ki67 and γH2AX
To characterize the composition of islet endocrine cells in each case, we conducted tetra-immunostaining of four endocrine hormones; insulin, glucagon, somatostatin and pancreatic polypeptide (PP). After deparaffinization and pretreatment with autoclave for antigen retrieval, the sections were first incubated with anti-PP antibody (1:3000) (Immuno-Biological Laboratories, Ltd., Gunma, Japan), followed by streptavidin-biotin-peroxidase (SAB) system (Nichirei Co., Tokyo, Japan). They were colorized with Ferange blue (Biocare Medical). Secondly, the sections were incubated with anti-glucagon antibody (1:3000) (Dako, Glostrup, Denmark) overnight, followed by incubation with SAB, and colorized with Vulcan Fast red. Thirdly, the sections were incubated with anti-insulin antibody (1:2000) (Santa Cruz Biotech. Inc., Santa Cruz, CA) followed by SAB and colorized with Vectastatin kit using diaminobenzidine as chromogen (Vector Lab. Inc., Burlingame, CA). Finally, δ-cells were labeled with anti-somatostatin antibody (1:1000) (Dako), reacted with SAB and colorized with Vina green chromogen kit (Biocare Medical). Thereafter, the sections were lightly counterstained with hematoxylin.
To explore cell death, proliferation rate and oxidative stress-related DNA damage, double immunostainings of anti-insulin with terminal deoxynucleotidyl transferase dUTP nick endlabeling (TUNEL) of an ApopTag® (Millipore, Bellerica, MA), Ki67 (MIB1) (1:2500) (Dako), and γH2AX (Ser139) (1:100) (Millipore) were performed, respectively, as previously described [19,20]. The sections were first incubated with ApopTag®, antibodies against Ki67, or γH2AX, and then antibody to insulin. Following incubation with the antibody conjugated with peroxidase or alkaline phosphatase, the reaction products were visualized with diaminobenzidine or with a fuchsin staining kit, respectively (Nichirei Co.).
Morphometric analysis of islet endocrine cells and proliferating β-cells
For determination of the fractional β-, α-, δ- and PP-cell area, the pancreatic sections were imaged at ×40 magnification. The β-cell area (β-cell volume density) and α-cell area (α-cell volume density), δ-cell area (δ-cell volume density) and PP-cell area (PP-cell volume density) to total parenchymal area were quantified at point count basis using Image J (Version 1.56, NIH, Bethesda, MD) [19–21]. At least, 100 islets were examined in each case. When the value of pancreas weight was available, the mass of islet, β-, α-, δ- and PP-cell was obtained by multiplication of β- and α-, δ- and PP-cell volume density by pancreas weight.
To determine β-cell growth, double-positive cells with insulin and Ki67 (among over 2000 β-cells) were examined. Since apoptotic cells were rarely found, we did not quantify the number because of unfeasibility to statistical analysis. Intensities of immunoreactions of γH2AX were evaluated by comparing with positive control specimens of lymph nodes showing many positive cells in the germinal center [20,22]. The reaction of the cells was defined as positive when the nuclei were clearly stained brown compared to negative background of the exocrine acinar cells. The percentage of positive cells was calculated by evaluating more than 500 β-cells in each case and comparison was made on the average values among ND, DA+ and DA−. The analysis was conducted in a random blinded manner.
Detection of islet macrophages
Infiltration of macrophages into the islets was examined by double immunohistochemistry of macrophage markers [23,24] and insulin. The sections were first incubated with monoclonal CD68 antibody (as a likely marker of M1 macrophage) (1:800) (PG-M1, DAKO) or CD163 antibody (as a likely marker of M2 macrophage) (1:100) (Cell Marque, Rocklin, CA), followed by incubation with anti-insulin antibody. The reaction products were colorized as described above. Infiltrated macrophages were quantified by counting the number of nuclei from at least 50 islets and expressed as the number per unit islet area.
To further characterize the marker expression of macrophage, double immunofluorescent staining using antibodies to inducible nitric oxide synthase (iNOS) (1:200) (Abcam, Cambridge, UK) [25] with CD68 antibody was conducted on deparaffinized sections from five subjects in each group. In brief, the sections were first incubated with antibody to iNOS followed by incubation with anti-rabbit antibody labeled with Alexa Fluor® 488 (1:250) (InVitrogen, Life Technol Inc., Carlsbad, CA). Then, the sections were reacted with CD68 antibody (1:250) followed by incubation with Alexa Fluor® 594-labeled anti-mouse antibody. The stained sections were observed under fluorescent microscope (Carl Zeiss). Double immunofluorescent staining using CD204 (macrophage scavenger receptor) (Clone SRA-E5) (TransGenic, Kumamoto, Japan) [26,27] and CD163 antibody was also conducted in a similar manner. First, CD204 antibody (1:200) was applied on the deparaffinized sections followed by incubation with AlexaFluor® 488-conjugated anti-mouse antibody (1:250) (InVitrogen). Then the sections were reacted with CD163 antibody followed by incubation with AlexaFluor® 594-conjugated anti-mouse antibody (1:250) (InVitrogen). The double positive cells for iNOS and CD68 and for CD204 and CD163 were counted on 50 islets in each case and expressed as the number per unit area of islets. The average value was obtained in each group and compared among groups.
In situ hybridization of (pro)insulin-mRNA
To detect the transcript expression of (pro)insulin, in situ hybridization (ISH) was performed on pancreatic tissues from ND and DA− and DA+ (n = 6 in each group) using a previously described method [19,28]. (Pro)insulin mRNA expression of β-cells was quantified on over 20 islets by densitometric analysis (Image-J) comparing with the background signals in the acinar area and average value was obtained in each subject.
Statistical analysis
Data in each group are presented as mean ± standard error (SE). Relationships between amyloid deposition and clinical profile or morphometric changes of islet endocrine cells were examined by correlation analysis. Since the data of BMI, pancreas weight, and HbA1c were not complete in the investigated subjects, the comparison or correlation was only sought among groups with available number of subjects. Statistical comparisons among ND, DA+ and DA− were carried out using analysis of variance with post-hoc Bonferroni’s corrections. The comparison of mean values between DA+ and DA− was made by non-parametric Mann–Whitney U-test. A simple regression was applied to the correlation analysis. p Values of <0.05 were taken as significant (StatView, Version 5.0.1, MountainView, CA).
Results
Clinical data and amyloid deposition
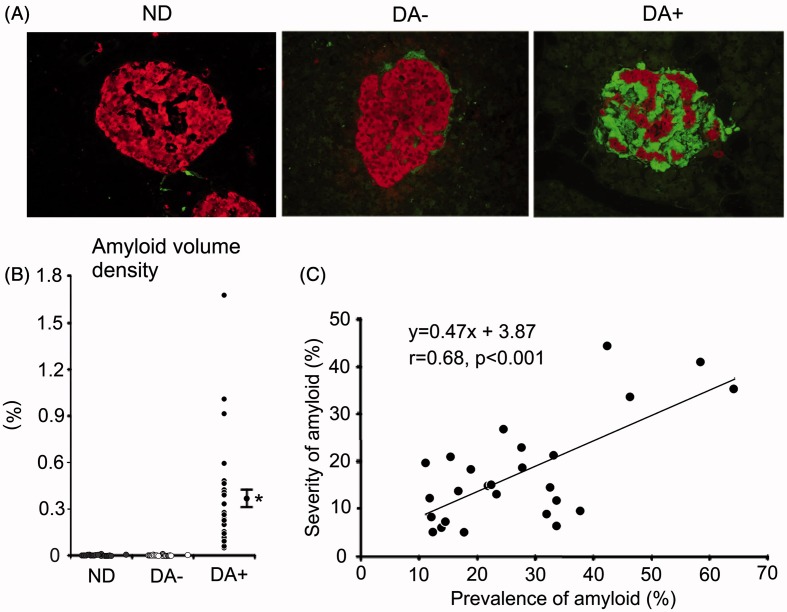
Clinical profiles of 20 cases of ND and 26 DA+ or 20 DA− are summarized in Table 1. More detailed information on the age, BMI, pancreas weight, diabetes duration, HbA1c values nearest to death and the causes of death are separately described in Supplemental Table 1. DA+ group showed marked deposition of amyloid occupying large area of islets (Figure 1). The extent of amyloid deposition on thioflavin-T staining was paralleled with that observed on the sections stained with HE and Congo-red, but the area of amyloid was possible to be much more critically evaluated on the thioflavin-stained slides. Amyloid volume density in DA+ all exceeded 0.05%, whereas that in DA− was less than 0.01% (0.0009–0.0094%) (Figure 1). Eight cases (40%) in DA− were completely free from amyloid. Among 20 cases of ND, five (20%) showed minimal amyloid deposition less than 0.0034% (0.0022–0.0033%) and other 15 cases were completely free from amyloid. Average prevalence of amyloid deposition was 27.1 ± 2.8% of the islets and area occupancy (severity of amyloid deposition) was 17.5 ± 2.2% of the islet area in DA+. The prevalence correlated well with the severity of amyloid deposition in DA+ (p < 0.001) (Figure 1).
Figure 1.
Amyloid deposition and endocrine cells in the islet of non-diabetic subject (ND), diabetic patient without obvious amyloid deposition (DA−) and with robust amyloid deposition (DA+), as depicted by thioflavin-T staining and chromogranin A immunoreactions on fluorescent microscopy (A). Red; chromogranin A reaction, and green for thioflavin-T staining. (B) Distribution of islet amyloid volume density in each group. DA− contained a trivial amount of amyloid (<0.005%), while amyloid volume density in DA+ exceeded 0.05%. Amyloid was negligible in ND. (C) Severity of amyloid deposition (amyloid occupancy in the islet) (%) well correlated with prevalence of amyloid-positive islets. *p < 0.001 versus non-diabetic and DA−. Bar stands for SE.
There was a significant increase in BMI and male preponderance in DA+ (n = 17) compared to DA− (n = 20), but no difference in duration of diabetes between DA+ (n = 19) and DA− (n = 12) or HbA1C between DA+ (n = 21) and DA− (n = 17) (Table 1). Pancreas weight was comparable among three groups (n = 18 in DA+, n = 17 in DA−, n = 19 in ND). There was no specific difference in diabetes treatment in two diabetic groups.
Islet morphometry
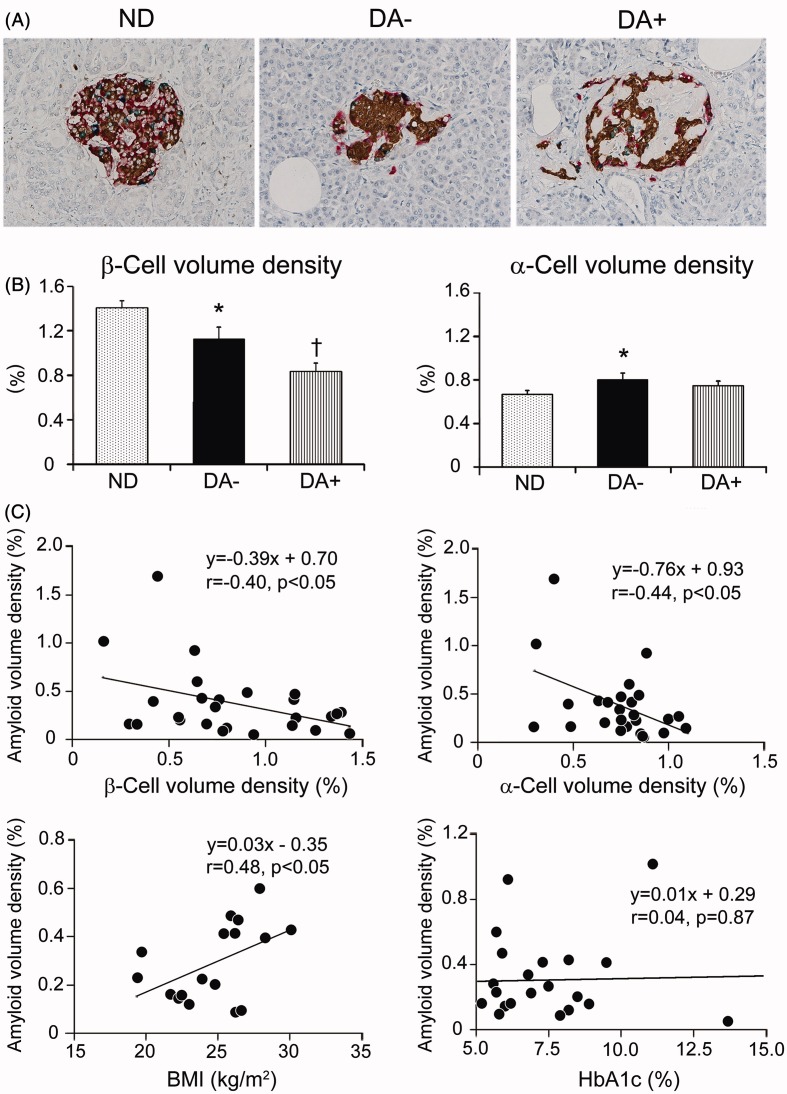
Tetra-immunostaining enabled precise population of four islet endocrine cells on the same sections in each case (Figure 2A). β-Cell volume density in DA− (n = 20) was reduced 20% compared to that in ND (n = 20) (p < 0.05), and that in DA+ (n = 26) was further reduced nearly 40% (Figure 2B). The difference between DA+ and DA− was significant (p < 0.01). Similarly, β-cell mass in DA+ (n = 18) was most severely depleted, followed by DA− (n = 17) compared to ND (n = 19) (Table 2). Contrariwise, α-cell volume density in DA− was increased compared to ND (p < 0.05), while it was comparable to that of ND. In contrast, there was no difference in α-cell mass or the mass of δ-cells or PP-cells among three groups (Table 2). The decline of β-cell volume density did not correlate with BMI, HbA1c or duration of diabetes in DA+ (Supplemental Figure 1).
Figure 2.
Islet endocrine cells tetra-immunostained in investigated subjects and correlation of amyloid deposition with β- and α-cell volume density or clinical parameters. (A) Tetra-immunostaining revealed distribution of four types of endocrine cells in the islets of non-diabetic subject (ND), diabetic subject without obvious amyloid deposition (DA−) and with amyloid deposition (DA+) (brown; β-cells, red; α-cells, green; δ-cells, blue; PP-cells). (B) There was a significant reduction of β-cell volume density in amyloid-free diabetic group (DA−) compared to non-diabetic group (ND) (*p < 0.05), and amyloid-rich diabetic group (DA+) showed further reduction (†p < 0.01 versus ND and DA−). α-Cell volume density was greater in DA− compared to ND (*p < 0.05) but that of DA+ was comparable to ND. (C) There was an inverse correlation between β-cell and α-cell volume density with amyloid volume density (p < 0.05). Clinicopathological analysis revealed a correlation between amyloid volume density and BMI (n = 17) (p < 0.05), but not HbA1c (n = 21).
Table 2.
Morphometric data on the islet and endocrine cells in non-diabetic and diabetic groups with or without amyloid.
| Islet mass | β-cell mass | α-cell mass | δ-cell mass | PP-cell mass | ||
|---|---|---|---|---|---|---|
| (g) | (g) | (g) | (g) | (g) | ||
| Non-diabetic | n = 19 | 2.73 ± 0.14 | 1.69 ± 0.09 | 0.81 ± 0.04 | 0.15 ± 0.02 | 0.03 ± 0.01 |
| Diabetic without amyloid | n = 17 | 2.50 ± 0.34 | 1.31 ± 0.15* | 1.03 ± 0.13 | 0.15 ± 0.02 | 0.03 ± 0.01 |
| Diabetic with amyloid | n = 18 | 2.38 ± 0.23 | 1.16 ± 0.12† | 1.06 ± 0.10 | 0.14 ± 0.02 | 0.25 ± 0.01 |
Values are mean ± SE.
*p < 0.05 versus non-diabetic; †p < 0.01 versus non-diabetic.
Correlation of amyloid deposition with morphometric data and clinical profile
There was an inverse correlation between amyloid volume density and β-cell volume density, indicating that the greater the amyloid area was, the smaller the β-cell volume density was (Figure 2C). In a similar manner, α-cell volume density inversely correlated with amyloid volume density. The amyloid deposition did not influence the data of δ-cells and PP-cells. There was no significant impact of age, HbA1c and duration of diabetes on the extent of amyloid deposition in DA+, while BMI significantly correlated with amyloid volume density although the subjects were limited to be partial (n = 17).
β-Cell proliferation
Double immunostainings of Ki67 and insulin disclosed low positive reactions in β-cells, yielding only 0.02–0.05% in diabetic group and 0.03–0.06% in ND. There was no difference in the average Ki67 positivity of β-cell and α-cell among three groups. Other δ-cells and PP-cells were infrequently positive for Ki67 and there was no difference in the appearance among three groups.
Macrophage infiltration
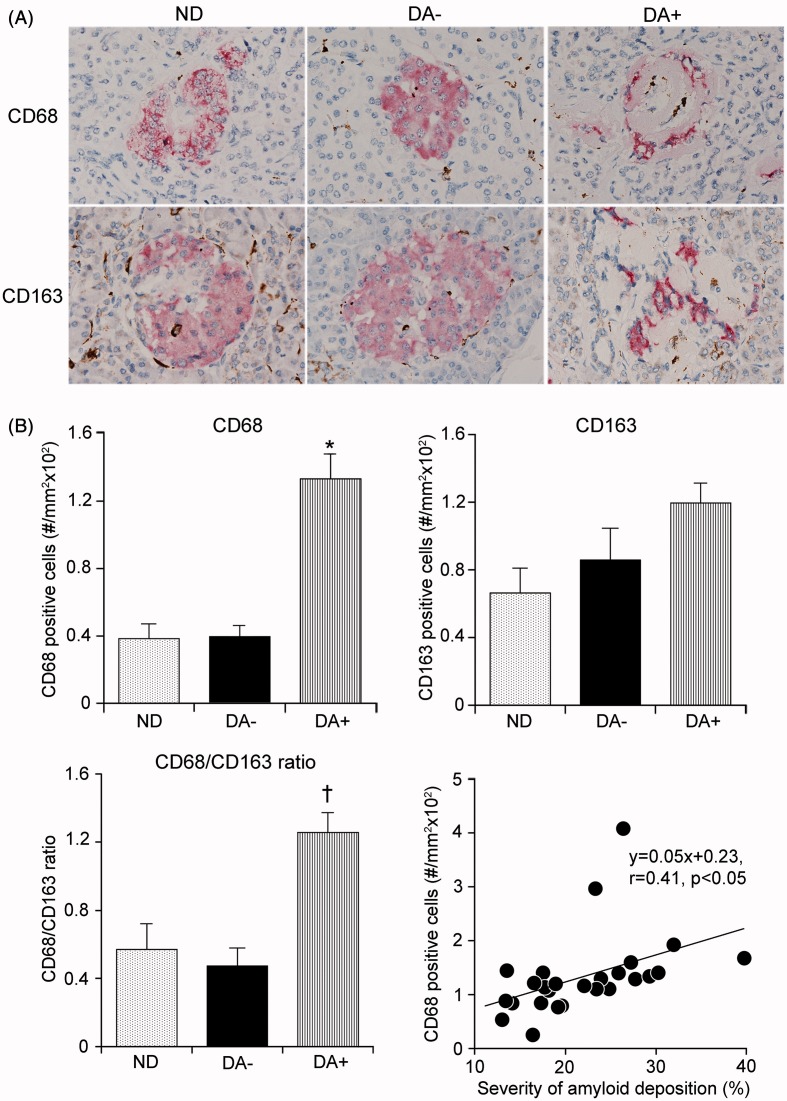
Within the amyloid-laden islets, there were frequently irregular-shaped cells buried in the amyloid or around the microvessels, positive for CD68 or CD163 (Figure 3A). There were only a few macrophages in amyloid-free islets in ND or DA−. Quantification of macrophages demonstrated a significant increase in the appearance of CD68- but not of CD163-cells in DA+ compared to ND and DA− (both p < 0.001 versus ND and DA+) (Figure 3B). Population of CD163 cells was slightly increased in DA− compared to ND but it was not significant. The increased population of CD68 cell well correlated with severity of amyloid volume density (p < 0.05) (Figure 3B), but not with β-cell volume density in DA+ (Supplemental Figure 2). CD68 cell infiltration was independent of an increase in BMI because comparison of BMI-matched DA+ (1.83 ± 0.75/mm2; n = 4) and DA− groups (0.46 ± 0.10/mm2; n = 10) in subjects with BMI less than 23 still showed a higher population of CD68 cells in DA+.
Figure 3.
Macrophage infiltration in the islet of diabetic pancreas. (A) Only sparse infiltrates of CD68 and CD163 macrophages were found in islets in non-diabetic group (ND) or amyloid-free diabetic group (DA−). In contrast, there were increased infiltrates of CD68 cells in the islets in amyloid-rich diabetic group (DA+). (B) Quantification demonstrated a significant increase in the population of CD68 cells in DA+ (*p < 0.001 versus ND and DA−), while the infiltrates of CD163 cells were not significant. The ratio of CD68 to CD163 cells was significantly greater in DA+ compared to those in ND and DA− (†p < 0.001). Infiltration of CD68 cells paralleled with amyloid volume density (p < 0.05).
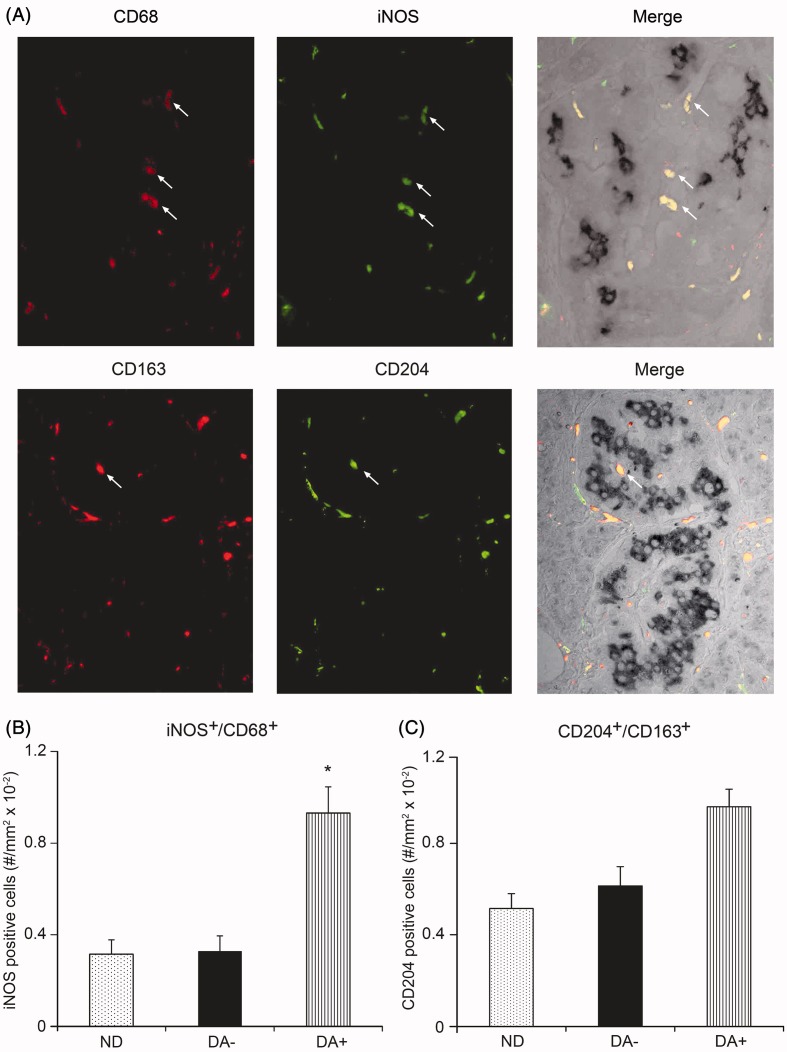
iNOS-positive cells were mostly consistent with CD68-positive cells, whereas CD163-positive cells were also positive for CD204. Quantification disclosed a significant increase in iNOS-positive CD68 cells in the islet of DA+ compared to those in ND and DA− (Figure 4A). In contrast to iNOS, there was no significant difference in the population of CD204-positive CD163 cells among groups (Figure 4B).
Figure 4.
Characterization of islet macrophages by double immunostainings with CD68 and inducible nitric oxide synthase (iNOS) (upper figures) or CD163 and CD204 (lower figures) in the islet of diabetic subject with amyloid deposition (A). Double immunostaining on subsets of each group (n = 5) demonstrated a significant increase in the appearance of iNOS-positive CD68 cells in a group of amyloid-rich diabetic group (DA+) (*p < 0.001 versus ND and DA−) (B). In contrast, the population of CD204-positive CD163 cells was comparable among three groups although there was a trend to increase in DA+ (C).
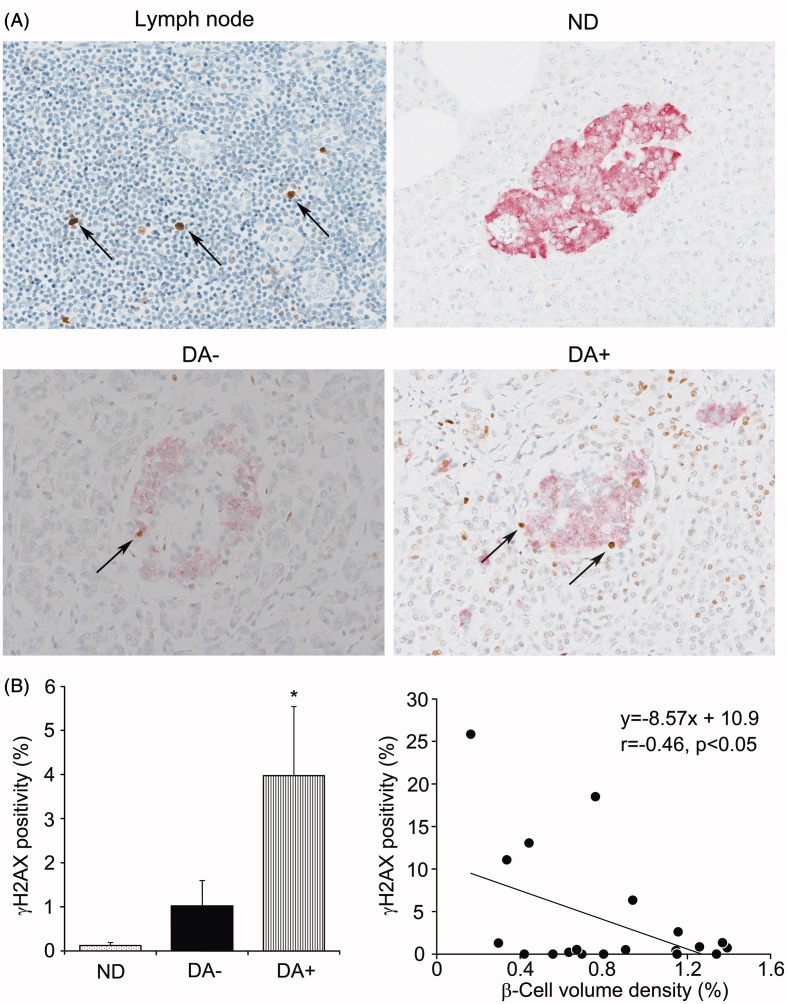
Identification of γH2AX-positive β-cells
Immunostainings of γH2AX revealed clearly positive reactions in the nuclei of β-cells in contrast to negative background of acinar cells or other stromal cells (Figure 5A). Population of positive cells for γH2AX was 0.47 ± 0.3% in average in ND, whereas it was 3.91 ± 1.3% in DA+, significantly greater in the latter (p < 0.01). In contrast, despite a trend toward an increase, there was no significant increase in γH2AX-positive cells in DA− compared to ND. Other endocrine cells were infrequently positive for γH2AX. There was an inverse correlation between the population of γH2AX-positive cells and β-cell volume density (Figure 5B). We could not find a significant correlation, however, between γH2AX-positivity and amyloid volume density or CD68 cell population (Supplemental Figure 3).
Figure 5.
γH2AX staining of the islets in non-diabetic and diabetic subjects with or without amyloid deposition. (A) Immunoreactions for γH2AX were identified as positive control of nuclear reactions in apoptotic cells in the lymphnode (LN). The population of γH2AX positive cells was the largest in amyloid-rich diabetic group (DA + ) but not so marked in amyloid-free diabetic group (DA−). (B) Comparison of the frequency showed a significant increase in γH2AX-positive cells in DA+ compared to ND and DA− (*p < 0.01 versus ND and DA−). Population of γH2AX-positive cells was inversely correlated with β-cell volume density (p < 0.05) but not with amyloid volume density in DA+.
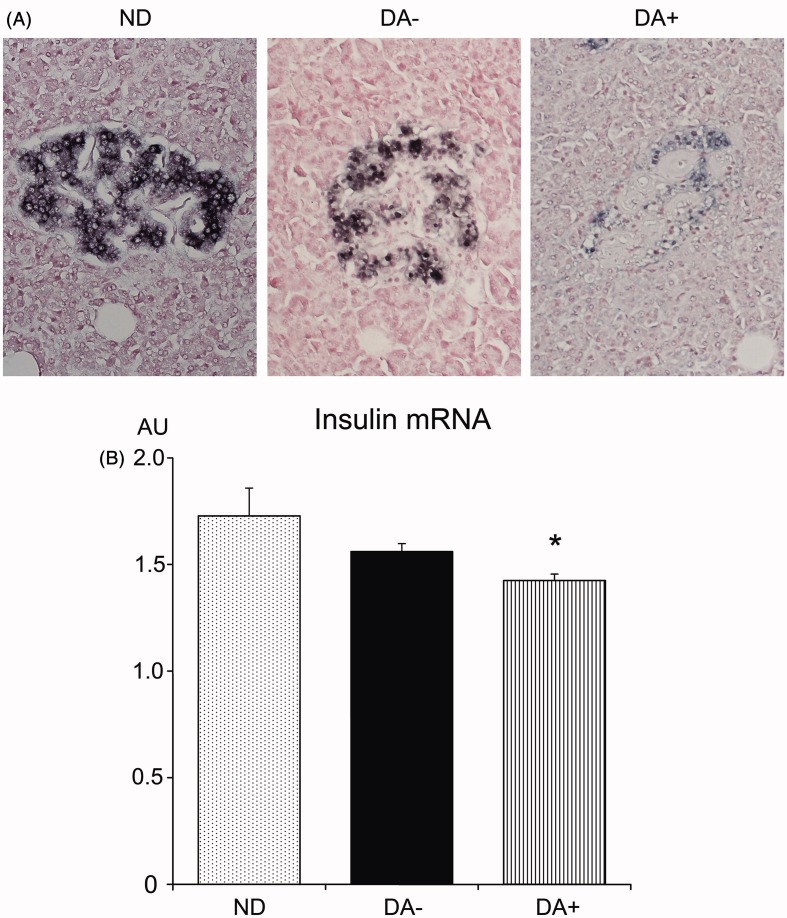
(Pro)insulin mRNA expressions
In situ hybridization of (pro)insulin-mRNA revealed positive reactions in the cytoplasm of β-cells in all three groups (Figure 6). While expressions in ND were intense, those in DA− and DA+ were suppressed. Semiquantitative estimations confirmed a significant reduction of (pro)insulin mRNA expressions in DA+ compared to those in ND, whereas the difference was not significant between ND and DA−.
Figure 6.
In situ hybridization of (pro)insulin mRNA in investigated subjects (n = 6 in each group). (A) There were strong positive signals in the cytoplasm of β-cells in the islet of non-diabetic subjects (ND). The reaction was slightly but not significantly reduced in amyloid-free diabetic group (DA−). There was marked reduction of (pro)insulin mRNA expression in amyloid-rich diabetic group (DA+). (B) Semi-quantitative evaluation confirmed significant reduction of insulin mRNA expression in DA+ compared to those in ND and DA− (*p < 0.05).
Discussion
This study demonstrated the augmented β-cell loss in Japanese type 2 diabetic patients with islet amyloid deposition. These findings are consistent with the results obtained from Caucasian diabetic patients [2,13]. Our findings also confirmed the association of amyloid deposition with increased infiltrates of macrophages [14,29]. We further demonstrated an increased population of γH2AX-positive β-cells and suppressed expression of (pro)insulin mRNA in amyloid-laden islets. Since these findings were less common in amyloid-free diabetic subjects, we suggest that amyloid deposition itself promotes β-cell damage that results in severe β-cell deficit.
Proinflammatory activation in the islet has recently been implicated in the pathogenesis of type 2 diabetes [30,31]. Consistent with the previous findings in Caucasian diabetic subjects [15], we found increased infiltration of macrophages in the islets, notably in islets with amyloid deposition (Figure 3). Population of macrophage in the islet intimately correlated with amyloid volume density. It is intriguing that the CD68 cells with iNOS expression were predominant over CD163 cells with CD204 expression. Hence, proinflammatory activation within the islet that associates accelerated β-cell loss may be comparable to other proinflammatory conditions in adipose tissues [32,33] or vascular walls with atherosclerotic changes encountered in insulin-resistant type 2 diabetes [34,35]. Under these circumstances, polarization of M1 over M2 macrophage population is recently proposed to be crucial for the progression of the lesions [30,31].
There is a possibility that macrophage infiltration may be secondary to the obesity since average BMI was significantly greater in DA+ than DA−. However, comparison of macrophage infiltration between DA+ and DA− matched with BMI (<23) confirmed higher population of CD68 cells in DA+. Thus, it may be speculated that amyloid has a causal role in the induction of proinflammatory activation in the islets in type 2 diabetes. In keeping with this contention, IAPP impairs the survival of β-cells in isolated islets by releasing cytokines such as IL-1β and IL-6 [36,37]. Activation of proinflammatory macrophages elicited by amyloid via inflammasome may release IL-1β, which in turn causes severe β-cell injury [38]. The fact that treatment with IL-1βR blockade ameliorated glucose control in type 2 diabetic patients may be supportive for the involvement of amyloid-related inflammation in the progression of this disease [16]. Nevertheless, other pathogenetic mechanisms of accelerated β-cell loss by amyloid fibrils or IAPP oligomers should also be taken into account. In fact, direct toxicity of amyloid fibrils to β-cells was demonstrated in in vitro studies [39,40]. Alternatively, IAPP suppresses the protective role of ER stress or autophagic processes, leading augmentation of β-cell injury [41,42].
Consistent with the results from Chinese diabetic patients [17], average BMI was greater in amyloid-rich diabetic group than that in amyloid-free diabetic group. We also found a correlation between amyloid volume density and BMI (Figure 2). However, we could not find a correlation between amyloid deposition and HbA1c, or duration of diabetes. The lower prevalence and severity of amyloid deposition in Japanese type 2 diabetic subjects may be attributed to much smaller average BMI (22–24) compared to American subjects (30–32). Despite the failure to find any other clinically relevant factors, we consider that insulin-resistance associated with increased BMI is possibly implicated in the trigger of amyloid deposition. Future investigations with increasing number of subjects may confirm the implication of such variables in the genesis of amyloid.
Compared with Caucasian type 2 diabetic patients, amyloid deposition was less common in Japanese diabetic patients. The relative infrequency of amyloid in our series may be due to the preparation of the slides from the body of the pancreas. Although the frequencies of amyloid-laden islets are described to be more common in the tail [10,43], the values of the body well reflected the amyloid areas in previous studies [10,13,43]. Consequently, our data are close to those in Chinese type 2 diabetic patients, but the prevalence of amyloid deposition appears to be still lower, perhaps due to the subjects with 10 years age difference. Despite the difference in the frequency and severity of amyloid deposition between Caucasians and Japanese subjects, our study indicated that its impact on β-cells was extremely similar, possibly promoting β-cell decline in type 2 diabetic patients. Both ethnic and environmental factors including life style and dietary components may also be involved in the above process.
It is clear that our study suffers from a number of limitations. Our clinical information on the investigated subjects is limited due to the insufficiency of the record at the terminal stage. It may also be suspected that the data may be confounded by the complicated diseases in their subjects such as severe arteriosclerosis or kidney failure [17]. Nevertheless, we believe that the relationships between BMI or HbA1c and islet morphometric data indicate the factors for the development of islet pathology. There might be a criticism on our method to evaluate only on the pancreas body. However, the tail is difficult for morphometry because of the frequent presence of fat infiltration. To increase the reproducibility of the results, we made an endeavor to investigate as many as the islets in a blinded way incorporating at least 15–20 frames including more than 100 islets. From our previous studies, approximately 100 islets are sufficient to obtain standardized data for islet morphometry [19,20]. Obviously, further investigations should be warranted to explore the precise role of amyloid deposition in the development of islet lesions in type 2 diabetes.
Acknowledgements
Technical assistances from Ms. Saori Ogasawara, Hiroko Mori, Misato Sakamoto, will be greatly appreciated. The authors acknowledge the editorial assistance of Drummond Bowden, Emeritus Professor, University of Manitoba, Canada.
Glossary
Abbreviations
- BMI
body mass index
- IL-1βR
interleukin-1β receptor
- iNOS
inducible nitric oxide synthase
Supplementary material available online
Declaration of interest
The authors report no conflicts of interest. This work was supported by a grant-in-aid to S.Y. from the Japan Promotion of Science and to H.M. from the Japan Diabetes Foundation and the Japan Promotion of Science.
Supporting information
Additional Supporting Information may be found in the online version of this article (supporting Table 1 and Figures 1, 2 and 3).
References
- 1.Opie EL. On the relation of diabetes mellitus to lesions of the pancreas. Hyaline degeneration of the islands of Langerhans. J Exp Med. 1901;5:527–40. doi: 10.1084/jem.5.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westermark P, Grimelius L. The pancreatic islet cells in insular amyloidosis in human diabetic and non-diabetic adults. Acta pathol Microbiol Scand Sect A. 1973;81:291–300. doi: 10.1111/j.1699-0463.1973.tb03538.x. [DOI] [PubMed] [Google Scholar]
- 3.Maloy AL, Longnecker DS, Greenberg ER. The relation of islet amyloid to the clinical type of diabetes. Hum Pathol. 1981;12:917–22. doi: 10.1016/s0046-8177(81)80197-9. [DOI] [PubMed] [Google Scholar]
- 4.Clark A, Cooper GJS, Lewis CE, Morris JF, Willis AC, Reid KBM, Turner RC. Islet amyloid formed from diabetes-associated-peptide may be pathogenic in type 2 diabetes. Lancet. 1987;ii:231–4. doi: 10.1016/s0140-6736(87)90825-7. [DOI] [PubMed] [Google Scholar]
- 5.Westermark P, Wernstedt C, Wilander E, Hayden DW, O’Brien TD, Johnson KH. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci USA. 1987;84:3881–5. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci USA. 1987;84:8628–32. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts AN, Leighton B, Todd JA, Cockburn D, Schofield PN, Sutton R, Holt S, et al. Molecular and functional characterization of amylin, a peptide associated with type 2 diabetes mellitus. Proc Natl Acad Sci USA. 1989;86:9662–6. doi: 10.1073/pnas.86.24.9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westermark P, Engström U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci USA. 1990;87:5036–40. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hull RL, Westermark GT, Westermark P, Kahn SE. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3629–43. doi: 10.1210/jc.2004-0405. [DOI] [PubMed] [Google Scholar]
- 10.Westermark P, Wilander E. The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia. 1978;15:417–21. doi: 10.1007/BF01219652. [DOI] [PubMed] [Google Scholar]
- 11.Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, Cooper GJS, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9:1591–9. [PubMed] [Google Scholar]
- 12.Steiner DF, Ohagi S, Nagamatsu S, Bell GI, Nishi M. Is islet amyloid polypeptide a significant factor in pathogenesis or pathophysiology of diabetes? Diabetes. 1991;40:305–9. doi: 10.2337/diab.40.3.305. [DOI] [PubMed] [Google Scholar]
- 13.Jűrgens CA, Toukatly MN, Fligner CL, Udayasankar J, Subramanian SL, Zraika S, Aston-Mourney K, et al. β-Cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol. 2011;178:2632–40. doi: 10.1016/j.ajpath.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homo-Delarche F, Calderari S, Irminger JC, Gangnerau MN, Coulaud J, Rickenbach K, Dolz M, et al. Islet inflammation and fibrosis in a spontaneous model of type 2 diabetes, the GK rat. Diabetes. 2006;55:1625–33. doi: 10.2337/db05-1526. [DOI] [PubMed] [Google Scholar]
- 15.Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–70. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 16.Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–26. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 17.Zao HL, Lai FMM, Tong PCY, Zhong DR, Yang D, Tomlinson B. Prevalence and clinicopathological characteristics of islet amyloid in Chinese patients with type 2 diabetes. Diabetes. 2003;52:2759–66. doi: 10.2337/diabetes.52.11.2759. [DOI] [PubMed] [Google Scholar]
- 18.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–10. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 19.Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45:85–96. doi: 10.1007/s125-002-8248-z. [DOI] [PubMed] [Google Scholar]
- 20.Mizukami H, Takahashi K, Inaba W, Tsuboi K, Kamata K, Yagihashi S. Age-associated changes of islet endocrine cells and the effect of body mass index in Japanese. J Diabetes Invest. 2014;5:38–47. doi: 10.1111/jdi.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inaba W, Mizukami H, Kamata K, Takahashi K, Tsuboi K, Yagihashi S. Effects of long-term treatment with the dipeptidyl peptidase-4 inhibitor vildagliptin on islet endocrine cells in non-obese type 2 diabetic Goto-Kakizaki rats. Eur J Pharmacol. 2012;691:297–306. doi: 10.1016/j.ejphar.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Mah LJ, El-Osta A, Karagiannis TC. γH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–86. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- 23.Kunisch E, Fuhrmann R, Roth A, Winter R, Lungershausen W, Kinne RW. Macrophage specificity of three anti-CD68 monoclonal antibodies (KP1, EBM11, and PGM1) widely used for immunohistochemistry and flow cytometry. Ann Rheum Dis. 2004;63:774–84. doi: 10.1136/ard.2003.013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadini GP, de Kreutzenberg SV, Boscaro E, Albiero M, Cappellari R, Kränkel N, Landmesser U, et al. An unbalanced monocyte polarisation in peripheral blood and bone marrow of patients with type 2 diabetes has an impact on microangiopathy. Diabetologia. 2013;56:1856–66. doi: 10.1007/s00125-013-2918-9. [DOI] [PubMed] [Google Scholar]
- 25.Ciccia F, Alessandro R, Rizzo A, Accardo-Palumbo A, Raimondo S, Raiata F, Guggino G, et al. Macrophage phenotype in the subclinical gut inflammation of patients with ankylosing spondylitis. Rheumatology. 2014;53:104–13. doi: 10.1093/rheumatology/ket323. [DOI] [PubMed] [Google Scholar]
- 26.Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216:15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- 27.Kawamura K, Komohara Y, Takaishi K, Katabuchi H, Takeya M. Detection of M2 macrophages and colony-stimulating factor 1, expression in serous and mucinous ovarian epithelial tumors. Pathol Int. 2009;59:300–5. doi: 10.1111/j.1440-1827.2009.02369.x. [DOI] [PubMed] [Google Scholar]
- 28.McKenzie KJ, Hind C, Farquaharson MA, McGill M, Foulis AK. Demonstration of insulin production and storage in insulinomas by in situ hybridization and immunocytochemistry. J Pathol. 1997;181:218–22. doi: 10.1002/(SICI)1096-9896(199702)181:2<218::AID-PATH732>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 29.de Koning EJ, van den Brand JJ, Mott VL, Charge SB, Hansen BC, Bodkin NL, Morris JF, et al. Macrophages and pancreatic islet amyloidosis. Amyloid. 1988;5:247–54. doi: 10.3109/13506129809007297. [DOI] [PubMed] [Google Scholar]
- 30.Donath MY. Targeting inflammation in the treatment of type 2 diabetes. Diabetes Obes Metab. 2013;15:193–6. doi: 10.1111/dom.12172. [DOI] [PubMed] [Google Scholar]
- 31.Eguchi K, Manabe I. Macrophages and islet inflammation in type 2 diabetes. Diabetes Obes Metab. 2013;15:152–8. doi: 10.1111/dom.12168. [DOI] [PubMed] [Google Scholar]
- 32.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 33.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 35.Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscl Thromb Vasc Biol. 2009;29:1419–23. doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- 36.Ehses JA, Lacraz G, Giroix MH, Schmidlin F, Coulaud J, Kassis N, Irminger JC, et al. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc Natl Acad Sci USA. 2009;106:13998–4003. doi: 10.1073/pnas.0810087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westwell-Roper C, Dai DL, Soukhatcheva G, Potter KJ, van Rooijen N, Ehses JA, Verchere CB. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol. 2011;187:2755–65. doi: 10.4049/jimmunol.1002854. [DOI] [PubMed] [Google Scholar]
- 38.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, et al. Activation of the Nlrp3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368:756–60. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 40.Sparr E, Engel MF, Sakharov DV, Sprong M, Jacobs J, de Kruijff B, Höppener JW, et al. Islet amyloid polypeptide-induced membrane leakage involves uptake of lipids by forming amyloid fibers. FEBS Lett. 2004;577:117–20. doi: 10.1016/j.febslet.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 41.Matveyenko AV, Gurlo T, Daval M, Butler AE, Butler PC. Successful versus failed adaptation to high-fat diet-induced insulin resistance: the role of IAPP-induced beta-cell endoplasmic reticulum stress. Diabetes. 2009;58:906–16. doi: 10.2337/db08-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rivera JF, Gurlo T, Daval M, Huang CJ, Matveyenko AV, Butler PC, Costes S. Human-IAPP disrupts the autophagy/lysosomal pathway in pancreatic β-cells: protective role of p62-positive cytoplasmic inclusions. Cell Death Differ. 2011;18:415–26. doi: 10.1038/cdd.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark A, Holman RR, Mattews DR, McGill M, Foulis AK. Non-uniform distribution of islet amyloid in the pancreas of ‘maturity-onset’ diabetic patients. Diabetologia. 1984;27:527–8. doi: 10.1007/BF00290389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.