Abstract
Asian patients with chronic myeloid leukemia (CML) tend to have different characteristics compared with patients from other regions, including younger age and smaller body size. The phase 3, open-label, randomized DASISION trial (NCT00481247), comparing dasatinib 100 mg once daily (QD) (n = 259) with imatinib 400 mg QD (n = 260) in newly diagnosed chronic phase CML (CML-CP), included a sizeable East Asian population (n = 60: dasatinib; n = 48: imatinib). In East Asian patients, dasatinib showed favorable 24-month rates of major molecular response (68% vs. 50% for imatinib) and complete cytogenetic response (92% vs. 88%), and more patients achieved BCR–ABL1 transcript levels ≤ 10% at 3 months with dasatinib (91% vs. 69%), similar to the overall population. Relative to non-East Asian patients, the incidence of rash, fluid-related events and grade 3/4 neutropenia and thrombocytopenia appeared to be higher in East Asians, regardless of treatment. Pharmacokinetic analysis revealed statistically non-significant increased dasatinib exposure among East Asian patients. Results support the use of dasatinib 100 mg QD as first-line CML treatment in both East Asian and non-East Asian patients.
Keywords: CML, dasatinib, imatinib, East Asian, first-line treatment
Introduction
Dasatinib is a BCR–ABL1 tyrosine kinase inhibitor, which is 325-fold more potent compared with imatinib, in vitro. Dasatinib has inhibitory activity against the majority of BCR–ABL1 mutations associated with clinical resistance to imatinib [1]. In the phase 3 DASISION trial (Dasatinib Versus Imatinib Study In Treatment-Naive Chronic Myeloid Leukemia), patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) were randomized to receive first-line treatment with either dasatinib 100 mg once daily (QD) or imatinib 400 mg QD. After a minimum 12-month follow-up, dasatinib showed superior efficacy compared with imatinib, including higher rates of complete cytogenetic response (CCyR) (83% vs. 72%; p = 0.001) and major molecular response (MMR) (46% vs. 28%; p < 0.0001), and an acceptable safety and tolerability profile [2]. Results after a minimum 24-month follow-up are consistent with the 12-month findings [3]. Dasatinib was approved as a first-line therapy for CML-CP based on DASISION trial results. Recent exploratory analyses of DASISION data showed that dasatinib provided faster, deeper responses compared with imatinib, and BCR–ABL1 transcript level reduction at 3 months was predictive of 36-month progression-free survival (PFS) and overall survival (OS) [4].
Asian patients with CML have different characteristics compared with patients from other regions. There is a lower incidence of CML in Asia (0.4–0.9 vs. 1.5 per 100 000 population) than in the United States, and Asian patients with CML tend to be younger (median age at diagnosis of 36–55 years vs. 65 years) and have smaller body size [5–7]. A recent report suggested that a proportion of East Asian patients with CML harbor a genetic polymorphism that confers resistance to imatinib [8]. This polymorphism, a common intronic deletion in the gene encoding BCL2-like 11 (BIM), has not been identified in non-East Asian patients with CML. Previous studies have suggested that the efficacy and toxicity profiles of imatinib in Asian patients are comparable to those observed in Western patients [6,9]. However, in clinical practice, imatinib doses lower than the recommended 400 mg QD dose are often prescribed to Asian patients. This is due, in part, to concerns relating to drug-induced toxicity and tolerability in Asian patients, who have a lower body surface area compared with their Western counterparts [6,10,11].
Studies of dasatinib in the second-line setting have shown that dasatinib safety and efficacy are similar in Asian and non-Asian patients. A pooled analysis of several single-arm clinical studies found that dasatinib efficacy and safety profiles were similar in Asian and non-Asian patients with CML (all phases), although among patients with CML-CP, Asian patients showed a trend toward higher response rates compared with non-Asians [5]. Pharmacokinetic (PK) parameters for dasatinib in Asian and non-Asian patients with prior imatinib therapy were also similar [5]. In a phase 1/2 trial of Japanese patients who had resistance or intolerance to imatinib, efficacy and safety findings for dasatinib were comparable to data from multinational trials [12].
East Asians represent a significant percentage of the world population, and there are increasing numbers of East Asians in American, European and Australian cities [13]. The phase 3 DASISION trial is one of few global multicenter CML trials with a sizeable East Asian population [5,6]. To determine the efficacy and safety of dasatinib 100 mg QD versus imatinib 400 mg QD in East Asian patients with newly diagnosed CML-CP, DASISION trial data were analyzed after a minimum 24-month follow-up. In addition, PK parameters for dasatinib were examined in East Asian and non-East Asian patients.
Methods
Study design and patients
DASISION (CA180-056; NCT00481247) is an ongoing open-label, multinational, randomized, phase 3 trial testing dasatinib versus imatinib in patients with CML-CP diagnosed within 3 months who had no previous treatment for CML (excluding anagrelide or hydroxyurea). Details of trial design, eligibility criteria and evaluations have been reported [2], and are briefly described in the Supplementary Methods section to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663.
Efficacy and safety assessments
Measurement of cytogenetic and molecular response, definitions of disease progression and BCR–ABL1 mutational analysis have been previously described [2] and are outlined in the Supplementary Methods section to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663.
Adverse events (AEs) were graded according to the Common Terminology Criteria for AEs version 3.0 of the US National Cancer Institute. To assess patients for pleural effusion, a chest radiograph was obtained in all patients at baseline and at 6 months after the start of treatment or more frequently if indicated clinically.
Statistical analyses
Efficacy was analyzed in all randomized patients based on the intention-to-treat principle. For response rates, 95% confidence intervals (CIs) were calculated using the Clopper and Pearson method. Times to MMR and CCyR were calculated in all patients, regardless of response, using competing risk analysis. Safety was analyzed in all patients who received ≥ 1 dose of study drug. No comparisons of geographic or other subpopulations were specified in the trial protocol, and the study was not powered to show a statistically significant difference between subgroups of the dasatinib and imatinib arms. Hence, any p-values provided are descriptive and not adjusted for multiple comparisons. Safety analyses only included events (AEs, dose modifications, deaths) occurring within the first 24 months of treatment.
PK analyses
Peripheral blood samples for PK analysis were collected from patients in the dasatinib arm only on day 1 (0.5, 6 and 8 h after dose administration), day 15 (pre-dose and 2 and 6 h after dose administration) and day 29 (pre-dose and 0.5 and 2 h after dose administration). Dasatinib plasma concentrations in DASISION were pooled with data from seven other clinical studies (Supplementary Table I to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663) for a population pharmacokinetic (PPK) analysis to characterize drug exposure and examine the potential effects of patient demographics and disease status [14,15]. Dasatinib steady-state exposure, including trough concentration (CminSS), peak concentration (CmaxSS) and area under the concentration curve (AUCSS), was derived from the PPK model for dasatinib-treated patients from DASISION with available concentration data. The analysis used a non-linear mixed effects model and individual PK parameters were estimated from the model.
Results
Patients and treatment
Of 519 patients randomized to receive either dasatinib (n = 259) or imatinib (n = 260) in the DASISION trial, 108 were enrolled at sites in East Asian countries: 49 in Japan, 37 in China, 11 in Korea and 11 in Singapore; 60 were randomized to receive dasatinib and 48 to receive imatinib.
Baseline characteristics of randomized patients were generally comparable between dasatinib- and imatinib-treated patients of the East Asian subpopulation (Supplementary Table II to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663). Among East Asian patients, median age was higher for patients receiving dasatinib compared with patients receiving imatinib (50 vs. 46 years). For non-East Asian patients, median age was lower for dasatinib compared with imatinib (45 vs. 49 years). East Asian patients had smaller median body weight (63 vs. 67 kg) and median body surface area (1.7 vs. 1.8 m2) compared with non-East Asian patients; values in the dasatinib and imatinib arms were similar for each subpopulation. Within the East Asian subpopulation, 6–8% of patients (3/48 imatinib-treated and 5/60 dasatinib-treated patients) had a high Hasford risk score compared with 22% (47/212 imatinib-treated and 44/199 dasatinib-treated patients) of non-East Asian patients.
Of 108 East Asian patients randomized (60 dasatinib, 48 imatinib), 59 received dasatinib treatment and all 48 received imatinib treatment (Table I). This present analysis was performed after all patients had a minimum 24-month follow-up. Mean treatment duration and median dose were similar for East Asian patients compared with non-East Asian patients (Table I and Supplementary Results to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663). At database lock, 82% of East Asian patients were receiving treatment, compared with 75% of non-East Asian patients, and for each subpopulation the fraction of patients remaining on treatment was similar for dasatinib and imatinib (Table I). Treatment is ongoing and a minimum 5-year follow-up is planned.
Table I.
Treatment status of study participants.
| Treated patients, n (%) |
||||
|---|---|---|---|---|
| East Asian patients |
Non-East Asian patients |
|||
| Dasatinib | Imatinib | Dasatinib | Imatinib | |
| Received treatment, n (% of randomized) | 59 (98) | 48 (100) | 199 (100) | 210 (99) |
| Still on treatment at last follow-up | 48 (81.4) | 40 (83.3) | 151 (75.9) | 154 (73.3) |
| Discontinued treatment | 11 (18.6) | 8 (16.7) | 48 (24.1) | 56 (26.7) |
| Reason for discontinuation | ||||
| Drug-related adverse event | 6 (10.2) | 2 (4.2) | 12 (6.0) | 10 (4.8) |
| Progression* | 3 (5.1) | 0 | 11 (5.5) | 17 (8.1) |
| Treatment failure | 1 (1.7) | 2 (4.2) | 7 (3.5) | 9 (4.3) |
| Poor adherence to therapy | 0 | 1 (2.1) | 0 | 1 (0.5) |
| Lost to follow-up | 0 | 1 (2.1) | 0 | 3 (1.4) |
| Subject request | 1 (1.7) | 2 (4.2) | 1 (0.5) | 1 (0.5) |
| Death | 0 | 0 | 4 (2.0) | 1 (0.5) |
| Other | 0 | 0 | 13 (6.5)† | 14 (6.7)‡ |
Progression was defined as doubling of white-cell count to more than 20 × 109 per liter in the absence of complete hematologic response (CHR), loss of CHR, increase in Philadelphia chromosome-positive bone marrow metaphases to > 35%, transformation to accelerated or blast phase, or death from any cause.
Other investigator-reported reasons included patients who discontinued due to adverse event (AE) unrelated to study drug (n = 5), withdrawal of consent (n = 4), pregnancy (n = 2), loss of complete cytogenetic response (CCyR) (n = 1) and insufficient molecular response (n = 1).
Other investigator-reported reasons included patients who discontinued due to AE unrelated to study drug (n = 1), withdrawal of consent (n = 3), pregnancy (n = 1), investigator request (n = 1), suboptimal response (n = 3), loss of molecular response (n = 2), no major molecular response (MMR) (n = 1), failure to achieve complete molecular response (n = 1) and confirmed occurrence of 8% pre-B-lymphoblasts in bone marrow (n = 1).
Efficacy
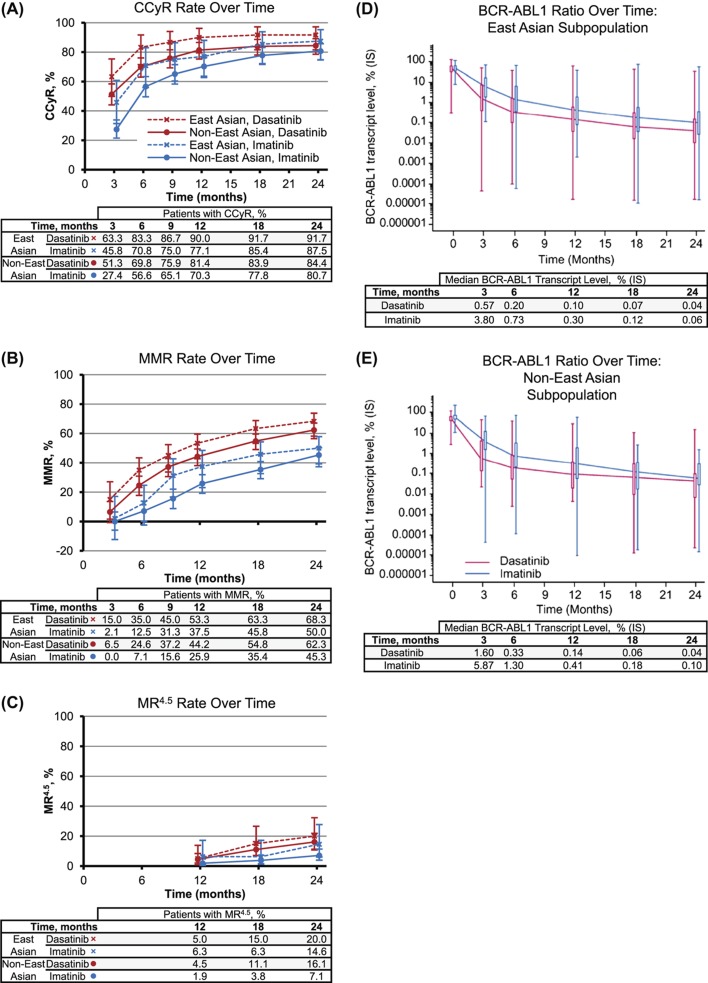
As in non-East Asian patients, East Asian patients had higher cytogenetic and molecular response rates and faster molecular responses with dasatinib compared with imatinib (Figure 1 and Supplementary Results to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663). East Asian patients showed a consistent trend for higher rates compared with non-East Asian patients at all time points and for all responses measured (CCyR, MMR and molecular response with 4.5 log reduction [MR4.5]) in both the dasatinib and imatinib arms (Figure 1).
Figure 1.
Molecular and cytogenetic responses. Responses to dasatinib (blue) and imatinib (red) for East Asian (diamonds) and non-East Asian (circles) populations (A–C) shown as percent of patients with CCyR (A), MMR (B) and MR4.5 (C), with error bars showing 95% confidence intervals. BCR–ABL1 kinetics for dasatinib (blue) and imatinib (red) in East Asian (D) and non-East Asian (E) subpopulations. Median BCR–ABL1 ratios, expressed as percent on the international scale, are indicated in the table below each time point.
Of evaluable East Asian patients with PCR assessments, 91% (51/56) receiving dasatinib had achieved BCR–ABL1 levels of ≤ 10% at 3 months, compared with 69% (33/48) receiving imatinib (p = 0.004). In evaluable non-East Asian patients, 82% (147/179) receiving dasatinib had achieved BCR–ABL1 levels of ≤ 10% at 3 months, compared with 63% (121/191) receiving imatinib (p < 0.0001). Median time to CCyR showed a trend toward being shorter among East Asian patients compared with non-East Asian patients for both dasatinib (3.1 vs. 3.7 months) and imatinib (5.6 vs. 6.1 months). Irrespective of response, median time to MMR was shorter for East Asian patients compared with non-East Asian patients (dasatinib: 11.8 vs. 16.7 months; imatinib: 28.2 months vs. not yet reached).
When patients were stratified by Hasford score, MMR rates were generally higher for patients with low risk compared with intermediate- or high-risk patients (Table II). MMR rates in imatinib-treated East Asian patients were an exception, showing an inverse trend. Additionally, East Asian patients with low or intermediate Hasford scores generally had higher MMR rates at 24 months compared with non-East Asian patients with similar Hasford scores, with the exception of imatinib-treated patients with low Hasford scores. However, the very small number of East Asian patients with high Hasford scores makes it difficult to compare response rates in these patients.
Table II.
Major molecular response (MMR) by 24 months according to Hasford risk groups.
| East Asian patients (n = 108) |
Non-East Asian patients (n = 411) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Dasatinib (n = 60) |
Imatinib (n = 48) |
Dasatinib (n = 199) |
Imatinib (n = 212) |
|||||
| Patients according to risk score, n | Responders, n (%) | Patients according to risk score, n | Responders, n (%) | Patients according to risk score, n | Responders, n (%) | Patients according to risk score, n | Responders, n (%) | |
| MMR | ||||||||
| Low Hasford score | 23 | 20 (87%) | 25 | 10 (40%) | 63 | 41 (65%) | 62 | 37 (60%) |
| Intermediate Hasford score | 32 | 20 (63%) | 20 | 12 (60%) | 92 | 56 (61%) | 103 | 46 (45%) |
| High Hasford score | 5 | 1 (20%) | 3 | 2 (67%) | 44 | 27 (61%) | 47 | 13 (28%) |
At 24 months, both East Asian and non-East Asian patients had high rates of PFS (dasatinib: 98% vs. 92% for East Asian vs. non-East Asian; imatinib: 98% vs. 91%) and OS (dasatinib: 98% vs. 94%; imatinib: 98% vs. 95%), as well as similar rates of transformation to accelerated phase/blast phase (AP/BP) CML (Supplementary Results to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663).
BCR–ABL1 mutational analyses
BCR–ABL1 mutation assessment of patients who discontinued identified 20 patients with mutations (10 dasatinib and 10 imatinib), including three East Asians (two dasatinib [both T315I], one imatinib [E355G]). Among non-East Asian patients, eight patients receiving dasatinib had mutations affecting three amino acids (T315I in five patients, F317L in two patients and F317I/ V299L in one patient), and nine patients receiving imatinib had mutations affecting nine different sites (E355G, M244V, G250E, E255K, F359V, L387M, H396P, E450G and D276G/F359C in one patient each). Best response and reasons for discontinuation were similar for East Asian and non-East Asian patients with mutations (Supplementary Results to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663).
Safety
Drug-related AEs with dasatinib and imatinib in the East Asian and non-East Asian subpopulations were primarily grade 1/2 (Supplementary Table III to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663). For either treatment arm, grade 3/4 neutropenia and thrombocytopenia showed trends for higher rates among East Asian patients compared with non-East Asian patients. In East Asian patients, grade 3/4 neutropenia and thrombocytopenia occurred in 21/59 (36%) and 17/59 (29%) receiving dasatinib and 19/48 (40%) and 7/48 (15%) receiving imatinib, respectively. In non-East Asian patients, rates of grade 3/4 neutropenia or thrombocytopenia were higher in patients treated with dasatinib versus imatinib. No grade 3/4 bleeding events were reported in East Asian patients receiving dasatinib, whereas 1/48 (2.1%) patients receiving imatinib reported such an event. In non-East Asian patients grade 3/4 bleeding events occurred in 1/199 (0.5%) receiving dasatinib and in 2/210 (1%) receiving imatinib.
As detailed in the Supplementary Results to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663, trends in non-hematologic AEs occurring in ≥ 10% of patients were similar for East Asian patients and non-East Asian patients in terms of comparisons between imatinib and dasatinib and time to onset (Figure 2 and Supplementary Table III to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663). When incidences of non-hematologic AEs occurring in ≥ 10% of patients were compared for East Asian versus non-East Asian patients, overall fluid retention, rash, nausea and fatigue showed a trend for higher rates in East Asian patients regardless of treatment (Figure 2 and Supplementary Table III to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663). Whereas headaches occurred in > 10% of non-East Asian patients across treatments, headaches in East Asian patients occurred with dasatinib but not imatinib. In the dasatinib arm, increased incidence of fluid retention among East Asian patients over non-East Asian patients (39% vs. 18%) was due to a higher incidence of superficial edema (17% vs. 9%) and pleural effusion (24% vs. 10%), as well as other types of fluid-related events (see footnote in Supplementary Table III to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663). In the imatinib arm, increased incidence of fluid retention AEs among East Asian compared with non-East Asian patients (65% vs. 38%) was primarily due to higher rates of generalized edema (31% vs. 2%). Increased rates of pleural effusion in East Asian patients compared with non-East Asian patients was evident for events of grade 1 (5% vs. 3%) and grade 2 (19% vs. 7%). Among East Asian patients who did and did not develop a pleural effusion, 24-month response rates were CCyR in 93% and 84%, and MMR in 64% and 71%, respectively. The same trend was seen in non-East Asian patients: CCyR in 89% and 78% of patients with versus without pleural effusion, and MMR in 63% and 62%, respectively.
Figure 2.
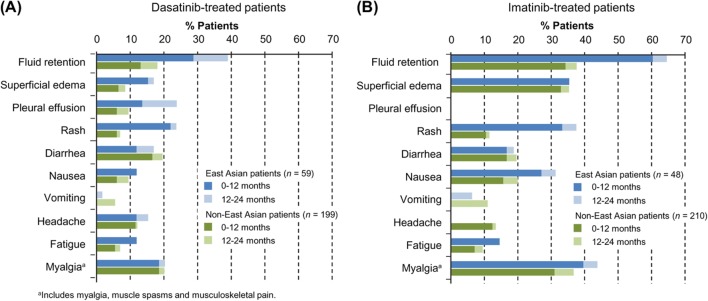
Rates of non-hematologic drug-related AEs of any grade (≥ 10%): change from 12 to 24 months. Rates of AEs at 12 and 24 months are shown for East Asian patients (blue) and non-East Asian patients (green). Bars representing 12-month data (dark) overlay those representing 24-month data (light) for visualization of AE rate increases between 12 and 24 months. Data from the dasatinib arm (A) are shown for comparison with the imatinib arm (B).
Grade 3/4 laboratory abnormalities (Supplementary Table III to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663) occurring in the East Asian subpopulation also occurred in non-East Asian patients, and included decreased phosphate, the most common grade 3/4 laboratory abnormality seen in non-East Asian patients (for both dasatinib and imatinib). Compared with non-East Asian patients, East Asian patients had higher rates of decreased calcium and decreased phosphate with dasatinib, and decreased potassium with imatinib.
Of East Asian patients in the dasatinib versus imatinib arms, 39% vs. 21% had a dose reduction, 68% vs. 54% had a dose interruption and 2% vs. 8% had a dose escalation. Of non-East Asian patients in the dasatinib versus imatinib arms, 21% vs. 12% had a dose reduction, 54% vs. 38% had a dose interruption and 7% vs. 18% had a dose escalation.
Among East Asian patients, 6/59 (10%) receiving dasatinib and 2/48 (4%) receiving imatinib discontinued treatment because of drug-related AEs, compared with 12/199 (6%) and 10/210 (5%), respectively, in the non-East Asian subpopulation (Table I). Drug-related AEs leading to discontinuation in East Asian patients included pleural effusion (n = 3), pericardial effusion (n = 1, this patient also had pleural effusion), elevated blood creatine phosphokinase (n = 1) and prolonged QT (n = 1) in the dasatinib arm; and thrombocytopenia (n = 2) and hypophosphatemia (n = 1) in the imatinib arm.
There were no deaths on study among East Asian patients, but two died more than 30 days after discontinuing study treatment; one in each treatment arm. The patient in the dasatinib arm died from infection 275 days after the last dose of dasatinib and 91 days after discontinuing study treatment due to disease progression; mutational analyses at the time of discontinuation indicated that this patient had the T315I mutation. The patient in the imatinib arm had discontinued due to loss of follow-up, but was subsequently reported to have progressed to CML-BP with thrombocytopenia, and died secondary to fatal gastrointestinal bleeding 57 days after the last recorded dose of imatinib. In the non-East Asian patient population there were 10 deaths on study (six in the dasatinib arm and four in the imatinib arm). A further 12 non-East Asian patients died during follow-up, more than 30 days after discontinuing study treatment (five in the dasatinib arm and seven in the imatinib arm).
Pharmacokinetics
The PPK analysis revealed that there were no significant differences in dasatinib pharmacokinetics between newly diagnosed patients with CML-CP (first-line) and treatment-experienced patients (second-line). The model was able to describe the observed concentration–time profiles equally well across the combined study population.
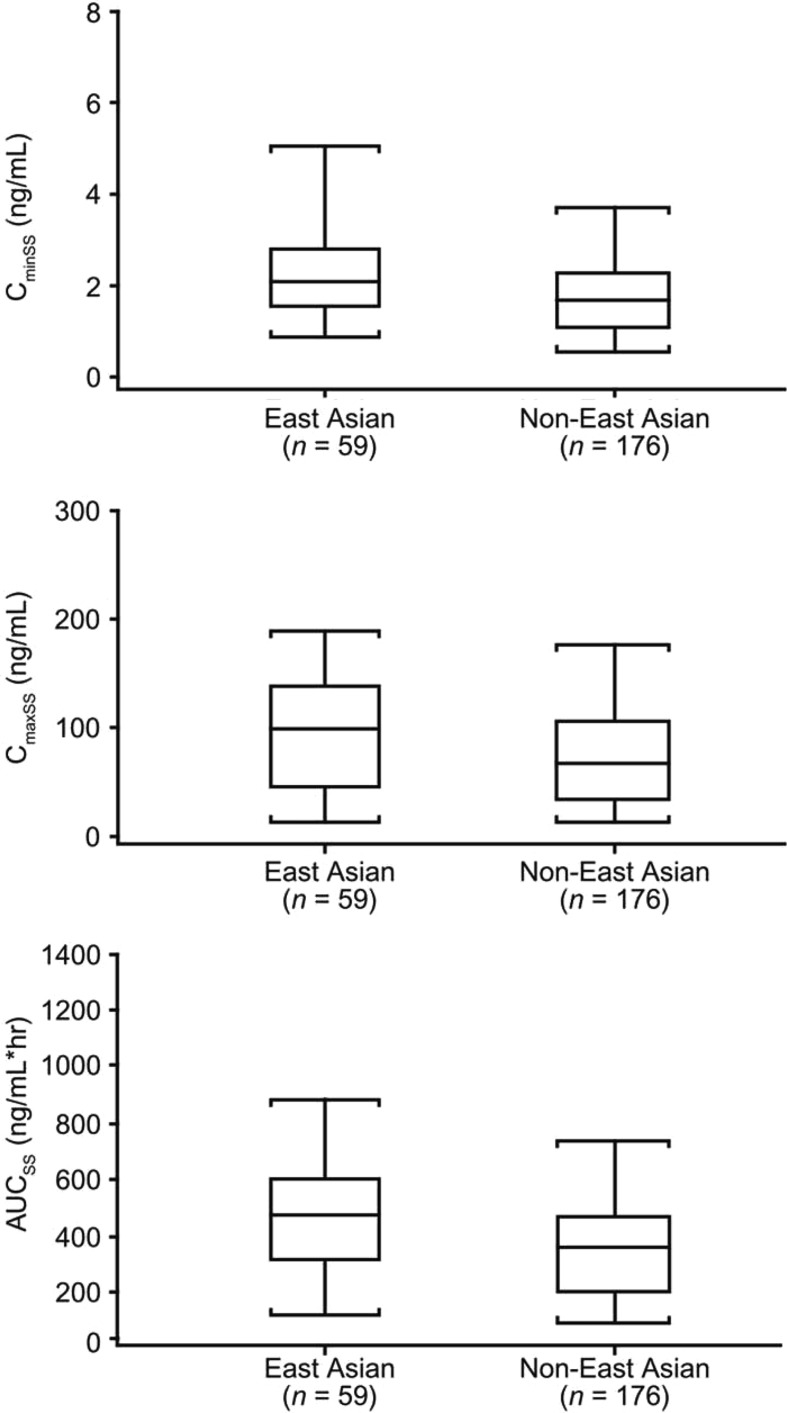
In DASISION, peripheral blood samples from 235 patients within the dasatinib arm were collected for PK analysis, of which 59 were from the East Asian subpopulation (Figure 3 and Supplementary Table IV to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663). In both the East Asian and non-East Asian subpopulations, mean terminal half-life of dasatinib was 6 h. Compared to non-East Asian patients, East Asian patients may have higher exposure to dasatinib based on steady-state AUC, Cmin and Cmax trends, but this did not reach statistical significance. When dasatinib PK levels were stratified by body weight, there was no obvious relationship between weight and CminSS, CmaxSS or CavgSS, both for the entire dasatinib-treated population (Supplementary Figure 1 to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663) and for the East Asian subpopulation (Supplementary Figure 2 to be found on at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663). The lower median body weight of the East Asian population does not appear to explain differences in PK parameters.
Figure 3.
Dasatinib steady-state exposure (CminSS, CmaxSS and AUCSS) of East Asian and non-East Asian patients. Horizontal line in interior of box represents median, box represents range between 25th and 75th percentiles of data (interquartile range) and whiskers represent 9th and 95th percentiles of data.
Discussion
Data from the East Asian subpopulation of the DASISION trial (24-month minimum follow-up) support the overall findings from the trial, including faster, deeper responses with dasatinib 100 mg QD compared with imatinib 400 mg QD [2,3]. In East Asian patients compared with non-East Asian patients, a similar efficacy advantage was seen for dasatinib over imatinib, including higher rates of CCyR and MMR at 12 and 24 months, MR4.5 by 24 months and BCR–ABL1 transcript levels ≤ 10% at 3 months. The advantage of achieving 3-month BCR–ABL1 levels ≤ 10% on long-term outcomes has been shown in retrospective exploratory analyses of DASISION [4] and imatinib studies [16–18]. CCyR and MMR rates in both treatment arms were 5–8% higher among East Asian patients compared with non-East Asian patients. Although fewer East Asian patients had a high Hasford risk score compared with non-East Asian patients (6–8% vs. 22%), this trend does not explain higher response rates seen in East Asian patients. East Asian patients with low or intermediate Hasford risk scores had higher MMR rates compared with non-East Asian patients with similar risk scores and receiving the same treatment. One exception appeared to be in imatinib-treated patients with low Hasford scores, which occurred at a higher rate in non-East Asian patients. During available follow-up, few East Asian patients have transformed to AP/BP CML, and rates of PFS and OS were similar for dasatinib compared with imatinib, and slightly higher than in non-East Asians. Since differences in response rates between East Asian and non-East Asian patients are small, further investigation is required to confirm these trends. Longer follow-up is needed to determine whether higher response rates for dasatinib versus imatinib will translate into improved long-term outcomes. A minimum 5-year follow-up is planned for the DASISION trial. For the potential differences in efficacy suggested by response rates, CI ranges overlapped in all cases, indicating that interpretations should be viewed with caution. Moreover, the study was not designed or powered to test differences between subpopulations.
Of the small number of patients for whom mutation data were available, three East Asian patients had BCR–ABL1 mutations, two receiving dasatinib and one receiving imatinib. These patients had similar reasons for discontinuation as observed in non-East Asian patients with mutations. Previous publications have reported that ˜30–60% of imatinib-resistant patients with CML-CP have BCR–ABL1 mutations: 54/198 (27%) Italian patients [19]; 44/134 (32.8%) US patients [20]; 28/111 (35.7%) Korean patients [21]; and 40/70 (57.1%) Chinese patients [22].
Safety profiles for East Asian and non-East Asian populations were largely consistent with previous findings [2,3], both in categories of AEs observed in > 10% of patients, and in specific AE rates showing differences between dasatinib and imatinib arms. For both populations, most AEs were low grade and most occurred during the first 12 months. Although the strength of the conclusions is limited by the East Asian sample size, this analysis suggests that compared with non-East Asians, East Asian patients may have a higher incidence of grade 3/4 neutropenia and thrombocytopenia, low-grade fluid-related AEs including pleural effusion, and rash. Potential higher rates of specific AEs in the Asian population may be associated with increased exposure to dasatinib and the higher median age in the dasatinib arm of the East Asian subpopulation. Similar AEs have been observed in Japanese patients receiving dasatinib in non-study settings with myelosuppression and pleural effusion indicated as the most common AEs associated with second-line dasatinib therapy for CML (all phases) or Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph + ALL) after imatinib failure [23]. Larger studies would be needed to fully explore whether certain AEs are truly more or less frequent in East Asian patients receiving a particular treatment.
Previous analyses have shown that cases of pleural effusion occurring during the DASISION trial were generally manageable by dose modification (interruption and/or reduction) with or without medical intervention (steroids and/or corticosteroids, and/or diuretics, with thoracentesis in occasional cases), and resulted in few discontinuations [24,25]. Despite the higher rate of pleural effusion observed among East Asian patients compared with non-East Asian patients (24% vs. 10%), occurrence of pleural effusion did not appear to impact the efficacy of dasatinib in either East Asian or non-East Asian patients, consistent with previous reports indicating no significant correlation between pleural effusion and outcome [26].
PK analysis was used to explore the hypothesis that higher rates of AEs in the East Asian population could be attributed to higher dasatinib exposure due to the mean body weight in East Asian patients being 5 kg lower than in non-East Asian patients. The PK analysis showed that although mean CminSS, CmaxSS and AUCSS were all > 30% higher in East Asian patients compared with non-East Asian patients, there was still considerable overlap in the distributions of exposure (as shown in Figure 3), and no statistical differences were seen. Furthermore, exposure parameters did not show a correlation with body weight (Supplementary Figures 1 and 2 to be found online at http://informahealthcare.com/doi/abs/10.3109/10428194.2013.866663). Given the high variability in the exposure results, however, larger populations need to be analyzed to confirm the relationship between body weight and exposure and the comparison of exposure parameters between East Asian and non-East Asian patients. A larger population would also be needed to explore whether higher exposure may contribute to a trend toward better response and higher incidence of certain AEs in East Asian patients.
Despite higher rates of certain AEs, overall rates of discontinuation for any reason, including drug-related AEs and treatment failure, were slightly lower in East Asian patients compared with non-East Asian patients (Table I), suggesting that AEs were manageable and did not impact efficacy. However, given the few East Asian patients who discontinued, the trend toward lower discontinuation rate needs confirmation, and could be influenced by the lower proportion of patients with high Hasford risk score.
In a previous comparison of Asian and non-Asian patients with imatinib resistance or intolerance treated with second-line dasatinib at a dose of 70 mg twice daily, no differences were seen in efficacy and safety results and PK profiles [5]. Patients in the present analysis received a different dasatinib dose (100 mg QD). Furthermore, the present analysis includes a larger population of dasatinib-treated Asian patients with CML-CP (60 vs. 13), a greater number of whom were available for PK analysis (59 vs. 4). Also, the patients had a more homogeneous disease profile in this study (all patients had newly diagnosed CML-CP, whereas in the previous analysis patients with several different disease phases were included), and were treated in a first-line setting (patients in the previous analysis were treated in a second-line setting). These factors help to explain any potential differences in findings. In this study, imatinib-related AE rates were also higher in the East Asian subpopulation, suggesting that PK levels of imatinib might be higher in the East Asian population compared with the non-East Asian population. Unfortunately, PK analysis was not performed for the imatinib arm, as the necessary samples were not collected.
Overall, findings from analysis of the DASISION 24-month data provide further support for use of dasatinib 100 mg QD as first-line treatment for patients with newly diagnosed CML-CP, in both East Asian and non-East Asian patients.
Acknowledgements
We thank the patients who participated in this Bristol-Myers Squibb-sponsored study and acknowledge the efforts of study staff at the investigational sites. We thank Dr. Tong Guo for providing statistical analysis and interpretation as well as reviewing for statistical accuracy. We also thank Dr. Xiaoning Wang for performing the population pharmacokinetic analyses and for providing technical expertise and review of this material. Professional medical writing and editorial assistance was provided by StemScientific, funded by BMS.
Potential conflict of interest
Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
Supplementary material available online
Supplementary Tables I–IV and Figures 1–2
References
- 1.O’Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119:1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saglio G, Kantarjian HM, Shah N, et al. Early response (molecular and cytogenetic) and long-term outcomes in newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): exploratory analysis of DASISION 3-year data. Blood. 2012;120((Suppl. 1)) [Google Scholar]
- 5.Kim DW, Goh YT, Hsiao HH, et al. Clinical profile of dasatinib in Asian and non-Asian patients with chronic myeloid leukemia. Int J Hematol. 2009;89:664–672. doi: 10.1007/s12185-009-0326-1. [DOI] [PubMed] [Google Scholar]
- 6.Au WY, Caguioa PB, Chuah C, et al. Chronic myeloid leukemia in Asia. Int J Hematol. 2009;89:14–23. doi: 10.1007/s12185-008-0230-0. [DOI] [PubMed] [Google Scholar]
- 7.Altekruse SF, Kosary CL, Krapcho M, et al. Surveillance Epidemiology and End Results (SEER) Cancer Statistics Review 1975–2007. http://seer.cancer.gov/csr/1975_2007/
- 8.Ng KP, Hillmer AM, Chuah CT, et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med. 2012;18:521–528. doi: 10.1038/nm.2713. [DOI] [PubMed] [Google Scholar]
- 9.Kim D, Goh HG, Kim SH, et al. Comprehensive therapeutic outcomes of frontline imatinib mesylate in newly diagnosed chronic phase chronic myeloid leukemia patients in Korea: feasibility assessment of current ELN recommendation. Int J Hematol. 2012;96:47–57. doi: 10.1007/s12185-012-1093-y. [DOI] [PubMed] [Google Scholar]
- 10.Park SJ, Choi IK, Seo HY, et al. Reduced dose of imatinib for patients with chronic myeloid leukemia and low body surface area. Acta Haematol. 2007;118:219–221. doi: 10.1159/000111777. [DOI] [PubMed] [Google Scholar]
- 11.Horikoshi A, Takei K, Sawada S. Effects of lower dose of imatinib to CML patients. Leuk Res. 2003;27:1167. doi: 10.1016/s0145-2126(03)00101-2. [DOI] [PubMed] [Google Scholar]
- 12.Sakamaki H, Ishizawa K, Taniwaki M, et al. Phase 1/2 clinical study of dasatinib in Japanese patients with chronic myeloid leukemia or Philadelphia chromosome-positive acute lymphoblastic leukemia. Int J Hematol. 2009;89:332–341. doi: 10.1007/s12185-009-0260-2. [DOI] [PubMed] [Google Scholar]
- 13.Castles CJ, Miller JF. Migration in the Asia-Pacific region. Migration Information Source. http://www.migrationinformation.org/feature/display.cfm?ID = 733
- 14.Sheiner LB. Learning versus confirming in clinical drug development. Clin Pharmacol Ther. 1997;61:275–291. doi: 10.1016/S0009-9236(97)90160-0. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Roy A, Hochhaus A, et al. Differential effects of dosing regimen on the safety and efficacy of dasatinib: retrospective exposure-response analysis of a phase III study. Clin Pharmacol Adv Appl. 2013;5:1–13. doi: 10.2147/CPAA.S42796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232–238. doi: 10.1200/JCO.2011.38.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanfstein B, Muller MC, Hehlmann R, et al. and the German CML Study Group. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML) Leukemia. 2012;26:2096–2102. doi: 10.1038/leu.2012.85. [DOI] [PubMed] [Google Scholar]
- 18.Hanfstein B, Müller M, Erben P, et al. the German CML-Study Group. Molecular response after 3 months of 1st line imatinib therapy is predictive for treatment failure and disease progression in patients with chronic phase chronic myeloid leukemia - a follow-up analysis of the German CML study IV. Blood. 2010;116((Suppl. 1)) [Google Scholar]
- 19.Soverini S,, Colarossi S, Gnani A,, et al. the GIMEMA Working Party on Chronic Myeloid Leukemia. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res. 2006;12:7374–7379. doi: 10.1158/1078-0432.CCR-06-1516. [DOI] [PubMed] [Google Scholar]
- 20.Jabbour E, Kantarjian H, Jones D, et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia. 2006;20:1767–1773. doi: 10.1038/sj.leu.2404318. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Kim D, Kim DW, et al. Analysis of Bcr-Abl kinase domain mutations in Korean chronic myeloid leukaemia patients: poor clinical outcome of P-loop and T315I mutation is disease phase dependent. Hematol Oncol. 2009;27:190–197. doi: 10.1002/hon.894. [DOI] [PubMed] [Google Scholar]
- 22.Qin Y, Chen S, Jiang B, et al. Characteristics of BCR-ABL kinase domain point mutations in Chinese imatinib-resistant chronic myeloid leukemia patients. Ann Hematol. 2011;90:47–52. doi: 10.1007/s00277-010-1039-5. [DOI] [PubMed] [Google Scholar]
- 23.Bristol-Myers Squibb. Post-marketing surveillance report of all cases in which dasatinib was administered in Japan: interim analysis. http://www.sprycel.jp/investigation.html#03
- 24.Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2011;119:1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laneuville P, Baccarani M, Cortes JE, et al. Analysis of patients (pts) with chronic phase chronic myeloid leukemia (CML-CP) who develop pleural effusion on first-line dasatinib: management and outcomes. J Clin Oncol. 2011;29((Suppl.)) [Google Scholar]
- 26.Kim D, Goh HG, Kim SH, et al. Long-term pattern of pleural effusion from chronic myeloid leukemia patients in second-line dasatinib therapy. Int J Hematol. 2011;94:361–371. doi: 10.1007/s12185-011-0921-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.