Abstract
Introduction
There is increasingly a double burden of under-nutrition and obesity in women of reproductive age. Preconception underweight or overweight, short stature and micronutrient deficiencies all contribute to excess maternal and fetal complications during pregnancy.
Methods
A systematic review and meta-analysis of the evidence was conducted to ascertain the possible impact of preconception care for adolescents, women and couples of reproductive age on maternal, newborn and child health (MNCH) outcomes. A comprehensive strategy was used to search electronic reference libraries, and both observational and clinical controlled trials were included. Cross-referencing and a separate search strategy for each preconception risk and intervention ensured wider study capture.
Results
Maternal pre-pregnancy weight is a significant factor in the preconception period with underweight contributing to a 32% higher risk of preterm birth, and obesity more than doubling the risk for preeclampsia, gestational diabetes. Overweight women are more likely to undergo a Cesarean delivery, and their newborns have higher chances of being born with a neural tube or congenital heart defect. Among nutrition-specific interventions, preconception folic acid supplementation has the strongest evidence of effect, preventing 69% of recurrent neural tube defects. Multiple micronutrient supplementation shows promise to reduce the rates of congenital anomalies and risk of preeclampsia. Although over 40% of women worldwide are anemic in the preconception period, only one study has shown a risk for low birth weight.
Conclusion
All women, but especially those who become pregnant in adolescence or have closely-spaced pregnancies (inter-pregnancy interval less than six months), require nutritional assessment and appropriate intervention in the preconception period with an emphasis on optimizing maternal body mass index and micronutrient reserves. Increasing coverage of nutrition-specific and nutrition-sensitive strategies (such as food fortification; integration of nutrition initiatives with other maternal and child health interventions; and community based platforms) is necessary among adolescent girls and women of reproductive age. The effectiveness of interventions will need to be simultaneously monitored, and form the basis for the development of improved delivery strategies and new nutritional interventions.
Keywords: preconception, overweight, folic acid, nutrition
Introduction
Nutritional status is an important aspect of health and wellness before and during pregnancy. Under nutrition in women contributes to 20% of maternal deaths, and is a significant risk factor for stillbirths, preterm births, small for gestational age and low birth weight babies [1-7], yet in most countries 10-20% of women are underweight [8]. Maternal short stature heightens the risk for obstructed labor, obstetric fistula and maternal mortality, as well as birth asphyxia leading to neonatal death, and is often the result of girls being stunted since childhood [9]. Pre-pregnancy overweight and obesity has been linked to two of the foremost causes of maternal mortality [10,11] - hypertensive disorders of pregnancy [12-15] and gestational diabetes mellitus (GDM) [16,17]- as well as an entire spectrum of adverse pregnancy outcomes [1-7], including poor lactation practices [18,19], obstetric anesthesia-related complications [20], prolonged gestation [21,22], maternal infectious morbidity [23] and decreased success with trial of labor [24-27]. Maternal obesity is a cause for stillbirths, fetal and neonatal death [3,28-31], and moreover, perpetuates the obesity epidemic since children of obese women are more likely to be obese themselves [17,32-36].
In addition to weight, micronutrient status is also linked to pregnancy outcomes. The recent Cochrane review [37] found a strong protective effect (RR 0.28, 95% CI 0.15-0.52) of folic acid on recurrent neural tube defects (NTDs). Other meta-analyses of randomized and observational studies showed a reduction in recurrence risk of 69 to 100% [38] and a reduction in occurrence risk of 42 [39] to 62% [40], yet less than half of all women regularly consume folic acid before conception [41]. Despite research evidence linking iron deficiency with maternal mortality, around 40% of women are anemic globally [9]. Other micronutrients such as zinc and calcium have been found to improve maternal and newborn outcomes when supplementation is provided during pregnancy- it seems likely that ensuring adequate intake of these micronutrients earlier, which is during the preconception period, would be of added benefit for undernourished girls and women and in the case of unplanned pregnancies. Folic acid, B vitamins and zinc have been shown to affect early fetal development, even before women realize they are pregnant. Micronutrient supplementation or fortification is currently being used as strategies to improve nutrition even in resource-poor settings since many girls and women are chronically undernourished [42].
There is a dearth of intervention trials to address under-nutrition or obesity in women of reproductive age. Weight and micronutrient status during pregnancy is influenced by a number of factors such as food insecurity and birth spacing that require broad interventions, hence the aim should to achieve and sustain optimal nutritional intake and weight before pregnancy. In addition, even for women who are overweight or obese, losing weight is not recommended during gestation and therefore weight and nutritional status should be reviewed between pregnancies. Nutritional risks and interventions are an important component of preconception care, defined for the purpose of this review as “any intervention provided to women and couples of childbearing age, regardless of pregnancy status or desire, before pregnancy, to improve health outcomes for women, newborns and children” (Detailed discussion of the importance and scope of preconception care is given elsewhere) [43].
This paper presents the findings of a systematic review that was undertaken to consolidate the evidence for nutritional risks before pregnancy, and ascertain the effectiveness of providing interventions during the preconception period (versus periconception or prenatal) on maternal, newborn and child health (MNCH) outcomes. The first section discusses pre-pregnancy weight, which is followed by diet and exercise as interventions to achieve and maintain optimal weight. This is followed by the sections on folic acid, multivitamin and iron supplementation in the preconception period. The review also looks beyond efficacy of an intervention to studies that examined impact of strategies used to increase uptake.
Methods
We systematically reviewed all literature published up to 2011 to identify studies describing the effectiveness of preconception (period before pregnancy and between pregnancies) nutritional risks and interventions and their impact on maternal, newborn and child health (MNCH) outcomes. Electronic databases such as PubMed, Cochrane Libraries, Embase, and WHO Regional Databases were searched to identify the studies. We included systematic reviews, experimental and observational studies. Papers were also identified by hand searching references from included studies. No language or date restrictions were applied in the search. The findings were presented at international meeting [44,45] and shared with professionals in the relevant fields of maternal and child health, following which results were updated based on current searches (through end of 2012) and expert opinion. Studies were included if they reported the nutritional risks and interventions for MNCH outcomes. Methodology is described in detail elsewhere [43].
For the studies that met the final inclusion criteria, two review authors abstracted data describing study identifiers and context, study design, intervention specifics and outcome effects into a standardized abstraction sheets. The quality of experimental studies were assessed using Cochrane criteria [46], whereas STROBE guidelines were used to assess the quality of observational studies [47]. We conducted meta-analyses for individual studies and pooled statistics was reported as the odds ratio (OR) and relative risk (RR) between the experimental and control groups with 95% confidence intervals (CI). Mantel–Haenszel pooled RR and corresponding 95% CI were reported or the Der Simonian–Laird pooled RR and corresponding 95% CI where there was an unexplained heterogeneity. All analyses were conducted using the software Review Manager 5.1 [48]. Heterogeneity was quantified by Chi2 and I2, in situations of high heterogeneity, causes were explored and random effect models were used.
Results
The review identified 2034 papers from search in all databases. After the initial title and abstract screening, 198 full texts were reviewed to identify papers which met the inclusion criteria and had the outcomes of our interest. One hundred and forty six studies were finally selected for abstraction and analysis (Figure 1). Information related to each included study can be found on the following link: https://globalmotherchildresearch.tghn.org/site_media/media/articles/Preconception_Report.pdf
Figure 1.
Search flow diagram
Nutritional risks
Maternal pre-pregnancy weight
In order to define the categories of weight that are not normal, the World Health Organization and the National Institutes of Health grouped weight into four categories according to individuals’ body mass index: underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0-29.9 kg/m2), and obese (30.0 kg/m2) [49]. The literature shows a BMI-dependent relationship between pre-pregnancy obesity and adverse pregnancy outcomes [50,51]. Further, excessive postpartum weight retention is a risk not only for subsequent pregnancies [52,53], but also for the development of maternal chronic diseases. Although guidelines exist for gestational weight gain according to maternal pre-pregnancy BMI, however gestational weight gain is not discussed further as it falls outside the scope of preconception care. Previous reviews have assessed maternal overweight and obesity using various cutoff points to define obesity, and have linked them to only one outcome of interest. This review extensively examines any MNCH outcomes that have been reported with all weight categories, grouping the data from individual studies into underweight or overweight and comparing these to women with normal BMI as defined above (please see table for data included here).
The review identified 34 studies that discussed maternal underweight [11,13,15,30,31,54-81]. This review found that pre-pregnancy underweight significantly increases the risk of preterm birth by 32% (RR 1.32, 95% CI 1.22-1.43) (Figure 2). Pre-pregnancy underweight was also found to significantly increase the risk of small-for-gestational age babies (RR 1.64, 95% CI 1.22-2.21)., Although previous work has found a significant effect of pre-pregnancy underweight on the risk of having low birth weight babies (RR 1.64 [82] and OR 1.82 [83], this review found a non-significant risk (RR 1.37, 95% CI 0.46-4.13) perhaps because of the low number of studies included since these were the only ones to assess maternal weight status before pregnancy. No effect was found for pre-pregnancy underweight on hypertensive disorders of pregnancy, GDM, large-for-gestational age or macrosomia, or any congenital birth defects.
Figure 2.
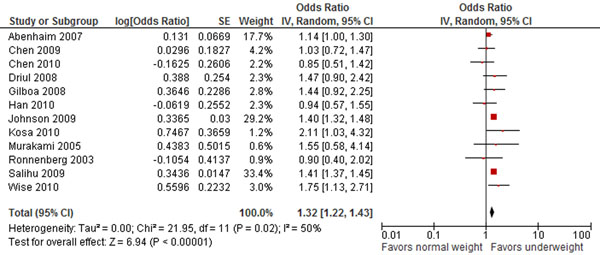
Pre pregnancy underweight and risk for preterm birth: evidence from observational studies Citations to the included studies: Abenhaim 2007 [55], Chen 2009 [56], Driul 2008 [57], Johnson 2009 [58], Kosa 2010 [59], Murakami 2005 [60], Ronnenberg 2003 [61], Salihu 2009 [62], Wise 2010[63], Chen 2010 [64], Gilboa 2008 [65], Han 2010 [66].
The review identified 41 studies that reported outcomes for overweight women [1,7,11,13,15,29,54-60,63-67,71-81,84-89]. The results presented in this review for the increased risk of hypertensive disorders of pregnancy with maternal overweight are not truly representative of the association since various studies categorized this outcome differently. The risk of preeclampsia (OR 2.28; 95% CI: 2.04-2.55) (Figure 3) and GDM (OR 1.91; 95% CI: 1.58-2.32), however, approximately doubles with pre-pregnancy overweight, and the effect was even greater for pre-pregnancy obesity. The data for impact of pre-pregnancy obesity is not presented since it is similar to the results for maternal overweight. The Pooled analysis of seven observational studies showed a clear increase of 42% in caesarean delivery among overweight women (OR 1.42, 95% CI 1.21-1.66). This review found inconclusive evidence for the effect of pre-pregnancy overweight on preterm births, instrumental delivery, or fetal distress. Perinatal outcomes that were significantly associated with maternal overweight before conception include macrosomia, large-for-gestational age babies (OR 1.63, 95% CI 1.51-1.76), and birth defects-notably neural tube defects and congenital heart defects (OR 1.15, 95% CI 1.07-1.24).
Figure 3.
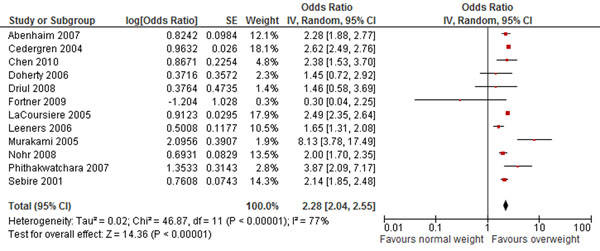
Pre pregnancy overweight and risk for preeclampsia: evidence from observational studies Citations to the included studies: Abenhaim 2007 [55], Driul 2008 [57], Fortner 2009 [75], Murakami 2005 [60], Nohr 2008 [67], Cedergren 2004 [29], Chen 2010 [64], Doherty 2006 [11], Leeners 2006 [13], LaCoursiere 2005 [74], Phithakwatchara 2007 [85], Sebire 2001[1]
Nutritional interventions
Diet, exercise and weight loss
Consumption of calorie-dense but nutritiously poor foods and physical inactivity are concerning for the health of all people, but disproportionately affect mothers and their young children. Fortunately, weight is a modifiable risk factor and evidence supports weight change as an intervention to improve MNCH outcomes [50,51,56,73,90]. Although this review demonstrated that maternal underweight increases the chances of preterm birth (25%), and small-for-gestational age babies (64%), the review found a scarcity of evidence for interventions to improve the macro-nutritional status of women before pregnancy.
As shown in the previous section, maternal overweight and obesity is a major risk factor for poor maternal and child outcomes. There is some evidence to support exercise as an intervention to decrease the risk of GDM, preeclampsia, and maternal weight gain, improve birth weight, and increase the chance of a normal delivery [91]. This review expands upon previous work [92,93] and examines whether diet and/or exercise are effective in reducing weight in women, and if this impacts MNCH outcomes.
The review 23 identified studies [94-116]. The trials found all used a control group; however they were carried out in women of different ages, and included different interventions. Women in the intervention group lost an average of up to 3.5kg. Interventions that combined calorie restriction and physical activity, involved a support system and monitoring, and were sustained over longer periods effected more weight change. A case control study [96] showed that women with perceived strenuous physical activity before pregnancy had a 78% reduced risk of preeclampsia.
Folic acid supplementation
Folic acid is a B-vitamin whose bioavailability from dietary sources lags behind that achieved through supplementation, and whose deficiency is associated with congenital abnormalities, especially neural tube defects [117]. Multiple case-control, cohort and quasi-randomised controlled trials have been carried out that provide a strong evidence base to support the effectiveness of folic acid supplementation in preventing birth defects and their consequent morbidity and mortality. Folic acid supplementation has thus become a primary periconceptional intervention.
The analyses showed that folic acid has a strong protective effect on preventing recurrent NTDs (RR 0.31, 95% CI 0.14-0.66) when it was restricted to randomized double-blind placebo-controlled studies, however this effect was no longer significant when two observational studies were included- (RR 0.43, 95% CI 0.13-1.40) (Figure 4). The review also found a significant effect of multivitamin supplementation in preventing NTDs, both occurrent and recurrent, although the only included study that did not show a beneficial effect actually separated folic acid from multivitamins, while the rest included folic-acid containing supplements. Hence, it is doubtful that multivitamins without folic acid have a protective effect against NTDs, which is confirmed by the MRC study (RR for multivitamins versus placebo 0.61, 95% CI 0.26-1.45) [118].
Figure 4.
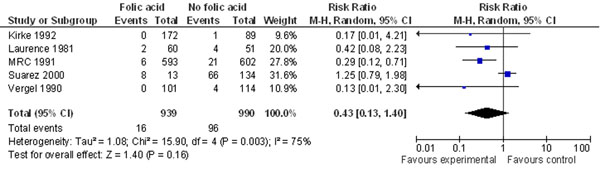
Folic acid supplementation and risk of recurrent neural tube defects: evidence from observational and experimental studies Citations to the included studies: Kirke 1992 [119], Laurence 1981 [120], MRC 1991 [118], Suarez 2000 [121], Vergel 1990 [122]
Previous reviews have not shown a benefit of folic acid/multivitamin supplementation on orofacial clefts, and although this review added three case-control studies and two prospective cohorts, the effect sizes adhered to unity. However, reviews that include all studies on folic acid/multivitamin supplementation simultaneously do show a modest protective effect [123-126], especially for cleft lip.
This review identified [118-120,122,127-198] 73 studies on the effect of folic acid supplementation and congenital heart defects. The result is mixed at best- pooling of two randomized trials, one cohort and one case-control trial showed a risk reduction of 42% in this analysis (Figure 5).
Figure 5.
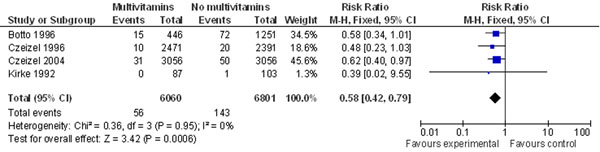
Folic acid supplementation and risk of congenital heart defects: evidence from observational and experimental studies Citations to the included studies: Botto 1996 [146], Czeizel 1996 [134], Czeizel 2004 [135], Kirke 1992 [119]
No review has shown a consistent effect of folic acid/multivitamin supplementation on maternal and pregnancy outcomes- including ectopic pregnancy, miscarriage, stillbirths, preterm births, low birth weight, and other birth defects. Further, the apprehension that widespread folic acid supplementation or fortification would lead to increased rates of multiple gestation was not shown to be significant in this review (RR 0.99, 95% CI 0.94-1.05) or previous work (OR 1.02 with supplementation and maximum annual increase in twinning rates of 4.6% with fortification) [199].
The results from three randomized double-blind placebo-controlled studies yield a 69% reduced risk (RR of 0.31, 95% CI 0.14-0.66) for recurrent NTDs with periconceptional folic acid supplementation. The MRC study probably provides the most accurate estimate for this intervention since it was a multicenter prospective randomized trial [118]. The remaining studies all suffer from low response rates, however, only Suarez et al. 2000 [121] has results inconsistent with the pooled analysis. This could be attributed to recall and selection bias in the study or to primary intake in this population being from dietary sources with lower bioavailability.
Multivitamins supplementation
There is incontrovertible data to support the routine use of multivitamins by women of reproductive age, to improve their own health as well as their potential mother and child outcomes. Although previous systematic reviews and meta-analyses have analysed the unique role of periconceptional folic acid (versus multivitamins) on MNCH outcomes, they have included only randomised and quasi-randomised trials. In addition, while periconceptional supplementation is in itself an intervention, it would have a greater impact if it were implemented for all women with the potential to become mothers. For limb reduction defects (RR ranges from 0.43-0.59 for all analyses) and congenital urinary tract anomalies (RR ranges from 0.17-0.68 for all analyses), the evidence shows a modest but persistent risk reduction with the use of multivitamins, rather than folic acid.
Pooling two cohort studies, the review also found a significant 27% reduction in the risk of preeclampsia (Figure 6) by maternal periconceptional multivitamin supplementation. This review also found a 43% risk reduction of multivitamins for multiple congenital abnormalities (Figure 7).
Figure 6.
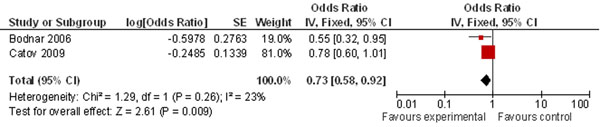
Multivitamin supplementation and risk of preeclampsia: evidence from observational studies Citations to the included studies: Bodnar 2006 [155], Catov 2009 [156]
Figure 7.
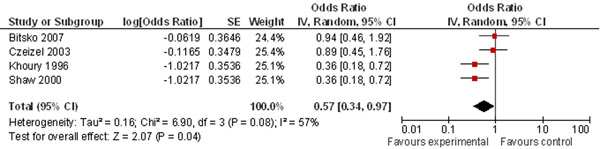
multivitamin supplementation and risk of multiple congenital anomalies: evidence from observational studies Citations to the included studies: Bitsko 2007 [149], Czeizel 2003 [150], Khoury 1996 [151], Shaw 2000 [152]
Pooling data from 5 case-control and 2 cohort-controlled trials conducted in relatively high-income countries (HICs), resulted in a significant 49% decrease in risk (RR 0.51, 95% CI 0.31-0.82) (Figure 8) of occurrent neural tube defects due to periconceptional multivitamin supplementation. The case-control study by Bower & Stanley 1992 was inconsistent with the other study results, possibly owing to the small total number of women using periconceptional multivitamins, or to recall bias among case mothers [129]. There was substantial heterogeneity between studies, however repeat analysis using random effects did not change the results.
Figure 8.
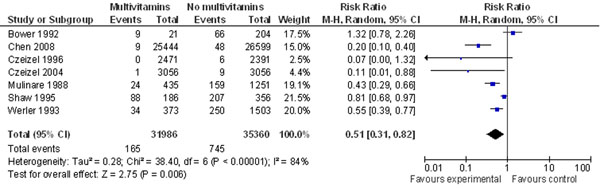
multivitamin supplementation and risk of concurrent neural tube defects: evidence from observational studies Citations to the included studies: Bower 1992 [129], Chen 2008[133], Czeizel 1996 [134], Czeizel 2004 [135], Mulinare 1988 [136], Shaw 1995 [200], Werler 1993 [138]
A recent community intervention in China [201] provided multivitamins to the intervention group from 3 months pre-pregnancy until the end of the first trimester. The intervention resulted in lower incidence of stillbirth (0.70% in intervention group vs. 1.55% in control group; p <0.001), malformation (0.23% in intervention group vs. 0.70% in control group; p <0.001) and low birth weight (0.39% in intervention group vs. 0.84% in control group; p <0.001) compared to the control group, and better growth indicators at birth.
Iron supplementation
Anemia is a common problem among women of reproductive age, especially in low and middle income countries (LMICs) where low dietary intake of bioavailable iron combined with endemic infectious diseases such as helminthiasis puts women at increased risk in the preconception period. Low preconception hemoglobin and ferritin levels increase the risk of poor fetal growth and low birth weight [202]. The literature shows that iron supplementation during pregnancy can be a protective factor against low birth weight [203], and given alone or with folic acid it is effective in increasing iron stores and preventing anemia during later gestation [204].
The review identified 6 studies [205-210]. Berger et al. [205] tested a weekly combined iron-folic acid intervention in the preconception period among Vietnamese women and similarly found the supplementation significantly improved iron status and reduced anemia when compared to baseline. In the Philippines ferritin levels improved, however hemoglobin lagged behind, possibly due to women being deficient in other micronutrients related to heme formation which were not supplemented during the study [206]. The results of these interventions on a national level were similar to a previous randomized-controlled trial in Bangladesh [207] where iron-folic acid given as a powdered supplement added to food decreased anemia among non-pregnant women; however this benefit did not extend to those women who became pregnant. Recent trials in Vietnam [208,209] combined iron-folic acid supplementation with intermittent deworming and demonstrated significant reduction in anemia, lower rates of helminthic infection, high compliance, and increase in birth weight in intervention districts versus control. In India, the country with the world’s highest proportion of maternal anemia, the same intervention was carried out in adolescent girls, resulting in a substantial drop in anemia prevalence [210].
Discussion
Maternal overweight and obesity is a growing problem across the world, but women in LMICs and lower socioeconomic strata continue to be at risk of undernourishment [211]. Both pre-pregnancy overweight and underweight are risk factors for poor maternal and child health outcomes, however overweight and obesity results in significantly greater health risks and associated costs. This review identified the association of maternal underweight with preterm birth and small for gestational age babies and the findings are comparable to previous meta-analysis which showed an increased risk of 29% [212] and another study which showed an increased risk of 37% [62]. Similarly the results on association of pre-pregnancy overweight on pre-eclampsia and GDM is consistent with previous reviews that show the risk of preeclampsia typically doubles for each 5 to 7 kg/m2 increase in BMI [213]; and the OR of developing GDM is 1.97-2.14 for overweight women, and 3.01-3.56 for obese women [16,214]. The pooled analysis on risk of caseation section and other outcomes are also consistent with previous reviews [2,6,16,215,216]. Given that weight is a modifiable risk factor, research must now focus on how healthcare interventions and public health campaigns can reduce these risks.
There is a strong need for evidence to demonstrate the effectiveness of interventions to achieve optimal pre-pregnancy weight, especially for those women who are underweight. This review confirms earlier evidence [217] that promoting improvement in diet and exercise through sustained, daily changes, with the help of a support system results in weight loss and higher levels of physical activity. Although preceding work illustrates examples of population-scale interventions, more research is needed to support how small-scale initiatives targeted at women with childbearing potential can be implemented on a wider scale. In HICs countries, obese women are increasingly opting to undergo weight loss surgeries, and a review of laparoscopic adjustable gastric banding (LAGB) shows lower gestational weight gain and better maternal and neonatal outcomes for these women compared to obese women not undergoing LAGB prior to pregnancy; however the outcomes were not improved compared to women of normal weight [218].
The role of nutrition in promoting health is well defined. What women eat determines more than just their own health, it is also vital to healthy pregnancies and newborns, and in fact research now shows that nutritional status in early childhood affects health throughout life. Pregnancy, or planning for pregnancy, provides an impetus for women to change non-healthful behaviors. Many women are still unaware of how much their nutritional status impacts their pregnancy outcomes, and improving women’s nutrition and weight-related behaviors should therefore begin during their earlier reproductive years.
Folic acid supplementation has been proven to reduce the risk of NTDs, both recurrent and occurrent and the results are confirmed by the meta-analyses undertaken by De-Regil et al. [37] However, further research is needed to show whether this benefit extends to prevention of orofacial clefts and congenital cardiovascular abnormalities. Although major health organizations promote the use of folic acid by women of reproductive age through clinical guidelines and recommendations [219], and the prevalence of folic acid use is reportedly high in the prenatal period, most women do not use folic acid in the periconceptional period, even if they are aware of its benefits. A recent systematic review [41] demonstrated that even in high income countries, only half of all women use folic acid before conception, therefore protective levels cannot be achieved before the critical period of neural tube closure. Reasons for low prevalence of use are confirmed by other studies [220-228] and include low maternal education and socioeconomic status; young maternal age; lack of a partner; and unplanned pregnancy. It is necessary therefore to improve awareness and use of folic acid supplements among all women of reproductive age so that even women with unplanned pregnancies are protected.
Multicomponent interventions increase use transiently and do not achieve universal coverage, although those with personal counseling in addition to mass campaigns have been shown to be more effective [229]. Fortification has thus been proposed as a means to prevent approximately half of all NTDs occurring annually and 13% of neonatal mortality attributed to NTDs [40], especially in areas with high prevalence of NTDs [230-232]. However, ongoing efforts must be made to supplement women at risk of a recurrent NTDs and women who are more folate-depleted [233,234]. A novel idea has been to incorporate folate into contraceptive pills, which also helps to bridge the gap between when a woman discovers she is pregnant and neural tube closure, even without periconceptional folate use [235]. In order to provide all women (including those at risk of recurrence) with an adequate dose of folic acid, public health policy in some countries now mandates that staple foods, such as flour, be fortified with folic acid.
The studies on iron demonstrate that large-scale nutritional intervention is feasible in LMICs contexts, and results in better biochemical indices. Given the global magnitude of maternal anemia, however, it is surprising that only one trial further assessed birth weight as a measure of improved maternal and newborn health. Iron fortification of foods such as flour, rice, sugar, juice, and fish or soy sauce in various countries has also been shown to improve iron status among women of reproductive age [236-240], but again the analyses of the fortification trials do not assess pregnancy outcomes in the long-term. As with folic acid, preventive iron supplementation may require greater community mobilization and social marketing for increased effectiveness [205] and to contribute to improved women’s and maternal health in developing regions.
Conclusion
Maternal malnutrition remains a serious global health issue, particularly in LMICs. The median prevalence of low body mass index among women in the preconception period is 10.9% among 24 countries with a recent Demographic and Health Survey, while 42% of women are anemic when they become pregnant [241]. Underweight and deficiencies of essential nutrients coupled with the increasing burden of obesity have consequences during pregnancy and for newborns. These negative effects are amplified in adolescents or women with closely-spaced pregnancies since they have depleted nutritional reserves, which results in stillbirths, neonatal deaths, low birth weight and preterm births [242]. Overweight and obesity further predispose to maternal hypertensive disorders and gestational diabetes.
Among nutrition-specific interventions periconceptional folic acid supplementation significantly reduces the risk of recurrent NTDs. There is growing interest in multiple micronutrient supplementation in at-risk populations in whom multiple deficiencies often coexist. Data for multiple micronutrient supplementation from a small number of controlled trials shows a persistent lowering of rates of congenital anomalies and preeclampsia. Other nutrition-specific interventions (iron, calcium, balanced protein energy supplementation) have only been studied in pregnant women, or if they have been studied during the preconception period the outcomes are limited to changes in biochemical markers while pregnancy and birth outcomes were not assessed.
Strategies for implementation of nutrition-specific interventions in the preconception period are needed especially to reach women in low- and middle-income countries. At present, food fortification with micronutrients is noted to be the most cost-effective large scale method. However, different approaches are needed to specifically increase uptake among women of reproductive age, noting the critical links between poor maternal nutritional status and its wide-ranging determinants and consequences. Nutritional-sensitive interventions improve population health, education and development and countries investing in such strategies have had greater gains in both nutrition and health outcomes. Integrating nutrition with maternal and child health initiatives and developing community-based platforms that are able to reach populations are especially promising.
Competing interests
We do not have any financial or non-financial competing interests for this review.
Peer review
Peer review reports are included in additional file 1.
Supplementary Material
Peer review reports.
Contributor Information
Sohni V Dean, Email: sohni.dean@gmail.com.
Zohra S Lassi, Email: zohra.lassi@aku.edu.
Ayesha M Imam, Email: ayeshaimam09@gmail.com.
Zulfiqar A Bhutta, Email: zulfiqar.bhutta@aku.edu.
Acknowledgment
The publication of these papers and supplement was supported by an unrestricted grant from The Partnership for Maternal, Newborn and Child Health.
Declarations
This article has been published as part of Reproductive Health Volume 11 Supplement 2, 2014: Preconception interventions. The full contents of the supplement are available online at http://www.reproductive-health-journal.com/supplements/11/S3. Publication charges for this collection were funded by the Partnership for Maternal, Newborn & Child Health (PMNCH).
References
- Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, Regan L, Robinson S. Maternal obesity and pregnancy outcome: a study of 287213 pregnancies in London. International journal of obesity. 2001;25(8):1175–1182. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- Arendas K, Qiu Q, Gruslin A. Obesity in pregnancy: pre-conceptional to postpartum consequences. J Obstet Gynaecol Can. 2008;30(6):477–488. doi: 10.1016/S1701-2163(16)32863-8. [DOI] [PubMed] [Google Scholar]
- Hall LF, Neubert AG. Obesity and pregnancy. Obstetrical & gynecological survey. 2005;60(4):253. doi: 10.1097/01.ogx.0000158509.04154.9e. [DOI] [PubMed] [Google Scholar]
- Rode L, Nilas L, Wøjdemann K, Tabor A. Obesity-related complications in Danish single cephalic term pregnancies. Obstetrics & Gynecology. 2005;105(3):537. doi: 10.1097/01.AOG.0000152304.39492.1c. [DOI] [PubMed] [Google Scholar]
- Rosenberg TJ, Garbers S, Chavkin W, Chiasson MA. Prepregnancy weight and adverse perinatal outcomes in an ethnically diverse population. Obstetrics & Gynecology. 2003;102(5 Part 1):1022. doi: 10.1016/j.obstetgynecol.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Yu CKH, Teoh TG, Robinson S. Obesity in pregnancy. BJOG: An International Journal of Obstetrics & Gynaecology. 2006;113(10):1117–1125. doi: 10.1111/j.1471-0528.2006.00991.x. [DOI] [PubMed] [Google Scholar]
- Callaway LK, Prins JB, Chang AM, McIntyre HD. The prevalence and impact of overweight and obesity in an Australian obstetric population. Medical Journal of Australia. 2006;184(2):56. doi: 10.5694/j.1326-5377.2006.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- Bhutta ZA, Chopra M, Axelson H, Berman P, Boerma T, Bryce J, Bustreo F, Cavagnero E, Cometto G, Daelmans B. Countdown to 2015 decade report (2000-10): taking stock of maternal, newborn, and child survival. Lancet. 2012;375(9730):2032–2044. doi: 10.1016/S0140-6736(10)60678-2. [DOI] [PubMed] [Google Scholar]
- Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. American Journal of Public Health. 2001;91(3):436. doi: 10.2105/ajph.91.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty DA, Magann EF, Francis J, Morrison JC, Newnham JP. Pre-pregnancy body mass index and pregnancy outcomes. International Journal of Gynecology & Obstetrics. 2006;95(3):242–247. doi: 10.1016/j.ijgo.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Bodnar LM, Wisner KL, Moses-Kolko E, Sit DKY, Hanusa BH. Prepregnancy body mass index, gestational weight gain and the likelihood of major depression during pregnancy. The Journal of clinical psychiatry. 2009;70(9):1290. doi: 10.4088/JCP.08m04651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeners B, Rath W, Kuse S, Irawan C, Imthurn B, Neumaier-Wagner P. BMI: new aspects of a classical risk factor for hypertensive disorders in pregnancy. Clinical Science. 2006;111:81–86. doi: 10.1042/CS20060015. [DOI] [PubMed] [Google Scholar]
- Robinson HE, O’Connell CM, Joseph KS, McLeod NL. Maternal outcomes in pregnancies complicated by obesity. Obstetrics & Gynecology. 2005;106(6):1357. doi: 10.1097/01.AOG.0000188387.88032.41. [DOI] [PubMed] [Google Scholar]
- Samuels-Kalow ME, Funai EF, Buhimschi C, Norwitz E, Perrin M, Calderon-Margalit R, Deutsch L, Paltiel O, Friedlander Y, Manor O. Prepregnancy body mass index, hypertensive disorders of pregnancy, and long-term maternal mortality. American journal of obstetrics and gynecology. 2007;197(5):490. doi: 10.1016/j.ajog.2007.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu SY, Kim SY, Schmid CH, Dietz PM, Callaghan WM, Lau J, Curtis KM. Maternal obesity and risk of cesarean delivery: a meta analysis. Obesity Reviews. 2007;8(5):385–394. doi: 10.1111/j.1467-789X.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- Lu GC, Rouse DJ, DuBard M, Cliver S, Kimberlin D, Hauth JC. The effect of the increasing prevalence of maternal obesity on perinatal morbidity* 1. American journal of obstetrics and gynecology. 2001;185(4):845–849. doi: 10.1067/mob.2001.117351. [DOI] [PubMed] [Google Scholar]
- Li R, Jewell S, Grummer-Strawn L. Maternal obesity and breast-feeding practices. American Journal of Clinical Nutrition. 2003;77(4):931. doi: 10.1093/ajcn/77.4.931. [DOI] [PubMed] [Google Scholar]
- Hilson JA, Rasmussen KM, Kjolhede CL. High prepregnant body mass index is associated with poor lactation outcomes among white, rural women independent of psychosocial and demographic correlates. Journal of Human Lactation. 2004;20(1):18. doi: 10.1177/0890334403261345. [DOI] [PubMed] [Google Scholar]
- Saravanakumar K, Rao SG, Cooper GM. Obesity and obstetric anaesthesia. Anaesthesia. 2006;61(1):36–48. doi: 10.1111/j.1365-2044.2005.04433.x. [DOI] [PubMed] [Google Scholar]
- Stotland NE, Washington AE, Caughey AB. Prepregnancy body mass index and the length of gestation at term. American journal of obstetrics and gynecology. 2007;197(4):378. doi: 10.1016/j.ajog.2007.05.048. [DOI] [PubMed] [Google Scholar]
- Rayco-Solon P, Fulford AJ, Prentice AM. Maternal preconceptional weight and gestational length. American journal of obstetrics and gynecology. 2005;192(4):1133–1136. doi: 10.1016/j.ajog.2004.10.636. [DOI] [PubMed] [Google Scholar]
- Myles TD, Gooch J, Santolaya J. Obesity as an independent risk factor for infectious morbidity in patients who undergo cesarean delivery. Obstetrics & Gynecology. 2002;100(5 Part 1):959. doi: 10.1016/s0029-7844(02)02323-2. [DOI] [PubMed] [Google Scholar]
- Bujold E, Hammoud A, Schild C, Krapp M, Baumann P. The role of maternal body mass index in outcomes of vaginal births after cesarean. American journal of obstetrics and gynecology. 2005;193(4):1517–1521. doi: 10.1016/j.ajog.2005.03.041. [DOI] [PubMed] [Google Scholar]
- Carroll CS. Vaginal birth after cesarean section versus elective repeat cesarean delivery: Weight-based outcomes* 1. American journal of obstetrics and gynecology. 2003;188(6):1516–1522. doi: 10.1067/mob.2003.472. [DOI] [PubMed] [Google Scholar]
- Durnwald CP, Ehrenberg HM, Merce BM. The impact of maternal obesity and weight gain on vaginal birth after cesarean section success. American journal of obstetrics and gynecology. 2004;191(3):954–957. doi: 10.1016/j.ajog.2004.05.051. [DOI] [PubMed] [Google Scholar]
- Goodall PT, Ahn JT, Chapa JB, Hibbard JU. Obesity as a risk factor for failed trial of labor in patients with previous cesarean delivery. American journal of obstetrics and gynecology. 2005;192(5):1423–1426. doi: 10.1016/j.ajog.2004.12.075. [DOI] [PubMed] [Google Scholar]
- Stephansson O, Dickman PW, Johansson A, Cnattingius S. Maternal weight, pregnancy weight gain, and the risk of antepartum stillbirth* 1. American journal of obstetrics and gynecology. 2001;184(3):463–469. doi: 10.1067/mob.2001.109591. [DOI] [PubMed] [Google Scholar]
- Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstetrics & Gynecology. 2004;103(2):219. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- Kristensen J, Vestergaard M, Wisborg K, Kesmodel U, Secher NJ. Pre pregnancy weight and the risk of stillbirth and neonatal death. BJOG: An International Journal of Obstetrics & Gynaecology. 2005;112(4):403–408. doi: 10.1111/j.1471-0528.2005.00437.x. [DOI] [PubMed] [Google Scholar]
- Nohr EA, Bech BH, Davies MJ, Frydenberg M, Henriksen TB, Olsen J. Prepregnancy obesity and fetal death: a study within the Danish National Birth Cohort. Obstetrical & gynecological survey. 2005;61(1):7. doi: 10.1097/01.AOG.0000172422.81496.57. [DOI] [PubMed] [Google Scholar]
- Kitsantas P, P L. Maternal obesity, health status during pregnancy, and breastfeeding initiation and duration. 2010. [DOI] [PubMed]
- Mesman I, R T, Bonsel GJ, Gemke RJ, van der Wal MF, Vrijkotte TG. Maternal pre-pregnancy body mass index explains infant's weight and BMI at 14 months: results from a multi-ethnic birth cohort study. 2009. [DOI] [PubMed]
- Olson CM, S M, Dennison BA. Maternal weight gain during pregnancy and child weight at age 3 years. 2009. [DOI] [PubMed]
- Ellen A Nohr1, M V, Jennifer L Baker1, Thorkild IA Sørensen1, Jorn Olsen1, Kathleen M Rasmussen1. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy1,2, 2008. [DOI] [PubMed]
- Lu GC, Rouse DJ, DuBard M, Cliver S, Kimberlin D, Hauth JC. The effect of the increasing prevalence of maternal obesity on perinatal morbidity. American Journal of Obstetrics and Gynecology. 2001;185(4):845–849. doi: 10.1067/mob.2001.117351. [DOI] [PubMed] [Google Scholar]
- De-Regil LM, Fernández-Gaxiola AC, Dowswell T, Peña-Rosas JP. Effects and safety of periconceptional folate supplementation for preventing birth defects. Cochrane Database of Systematic Reviews. 2010;10:CD00795. doi: 10.1002/14651858.CD007950.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse SD, Collins JS. Folic acid supplementation and neural tube defect recurrence prevention. Birth Defects Research Part A: Clinical and Molecular Teratology. 2007;79(11):737–742. doi: 10.1002/bdra.20394. [DOI] [PubMed] [Google Scholar]
- Blom HJ. Folic acid, methylation and neural tube closure in humans. Birth Defects Research Part A: Clinical and Molecular Teratology. 2009;85(4):295–302. doi: 10.1002/bdra.20581. [DOI] [PubMed] [Google Scholar]
- Blencowe H, Cousens S, Modell B, Lawn J. Folic acid to reduce neonatal mortality from neural tube disorders. International Journal of Epidemiology. 2010;39(Supplement 1):i110. doi: 10.1093/ije/dyq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JG, Vermeulen MJ, Meier C, Wyatt PR. Risk of congenital anomalies detected during antenatal serum screening in women with pregestational diabetes. Qjm. 2004;97(10):651. doi: 10.1093/qjmed/hch107. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan U, Lowe A, Vir S, Kumar S, Mohanraj R, Chaturvedi A, Noznesky EA, Martorell R, Mason JB. Public health interventions, barriers, and opportunities for improving maternal nutrition in India. Food Nutr Bull. 2012;33(2 Suppl):S71–92. doi: 10.1177/15648265120332S105. [DOI] [PubMed] [Google Scholar]
- Dean SV, Lassi ZS, Imam AM, Bhutta ZA. Preconception Care: closing the gap in the continuum of care to accelerate improvements in maternal, newborn and child health. Reproductive Health. 2014. [DOI] [PMC free article] [PubMed]
- Dean S, Rudan I, Althabe F, Girard AW, Howson C, Langer A, Lawn J, Reeve M-E, Teela KC, Toledano M. Setting research priorities for preconception care in low-and middle-income countries: aiming to reduce maternal and child mortality and morbidity. PLoS Med. 2013;10(9):e1001508. doi: 10.1371/journal.pmed.1001508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO WHO. Meeting to develop a global consensus on preconception care to reduce maternal and childhood mortality and morbidity. Geneva: World Health Organization Headquarters; 8211. [Google Scholar]
- Higgins JPT, Green S, editor. The Cochrane Collaboration. 2008. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.0 [updated February 2008] Available from http://www.cochranehandbook.org. [Google Scholar]
- Brand RA. Editorial: standards of reporting: the CONSORT, QUORAM, and STROBE guidelines. Clin Orthop Relat Res. 2009. [DOI] [PMC free article] [PubMed]
- Review Manager (RevMan). [computer program]. Version 5.0: Copenhagen: Nordic Cochrane Collaboration; 2008. [Google Scholar]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic. WHO Obesity Technical Report Series 894. Geneva, Switzerland: World Health Organization; 2000. [PubMed] [Google Scholar]
- Raatikainen K, Heiskanen N, Heinonen S. Transition from Overweight to Obesity Worsens Pregnancy Outcome in a BMI-dependent Manner&ast. Obesity. 2006;14(1):165–171. doi: 10.1038/oby.2006.20. [DOI] [PubMed] [Google Scholar]
- Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. The Lancet. 2006;368(9542):1164–1170. doi: 10.1016/S0140-6736(06)69473-7. [DOI] [PubMed] [Google Scholar]
- Hoff GL, Cai J, Okah FA, Dew PC. Pre-Pregnancy Overweight Status between Successive Pregnancies and Pregnancy Outcomes. Journal of Women's Health. 2009;18(9):1413–1417. doi: 10.1089/jwh.2008.1290. [DOI] [PubMed] [Google Scholar]
- Joseph NP, Hunkali KB, Wilson B, Morgan E, Cross M, Freund KM. Pre-pregnancy body mass index among pregnant adolescents: gestational weight gain and long-term post partum weight retention. Journal of Pediatric and Adolescent Gynecology. 2008;21(4):195–200. doi: 10.1016/j.jpag.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Ota E, Haruna M, Suzuki M, Anh DD, Tho LH, Tam NTT, Thiem VD, Anh NTH, Isozaki M, Shibuya K. Maternal body mass index and gestational weight gain and their association with perinatal outcomes in Viet Nam. Bulletin of the World Health Organization. 2011;89(2):127–136. doi: 10.2471/BLT.10.077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abenhaim HA, Kinch RA, Morin L, Benjamin A, Usher R. Effect of prepregnancy body mass index categories on obstetrical and neonatal outcomes. Archives of Gynecology and Obstetrics. 2007;275(1):39–43. doi: 10.1007/s00404-006-0219-y. [DOI] [PubMed] [Google Scholar]
- Chen A, Klebanoff MA, Basso O. Pre-pregnancy body mass index change between pregnancies and preterm birth in the following pregnancy. Paediatric and Perinatal Epidemiology. 2009;23(3):207–215. doi: 10.1111/j.1365-3016.2009.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driul L, Cacciaguerra G, Citossi A, Martina MD, Peressini L, Marchesoni D. Prepregnancy body mass index and adverse pregnancy outcomes. Archives of Gynecology and Obstetrics. 2008;278(1):23–26. doi: 10.1007/s00404-007-0524-0. [DOI] [PubMed] [Google Scholar]
- Johnson TS, Rottier KJ, Luellwitz A, Kirby RS. Maternal prepregnancy body mass index and delivery of a preterm infant in Missouri 1998–2000. Public Health Nursing. 2009;26(1):3–13. doi: 10.1111/j.1525-1446.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Kosa JL, Guendelman S, Pearl M, Graham S, Abrams B, Kharrazi M. The Association Between Pre-pregnancy BMI and Preterm Delivery in a Diverse Southern California Population of Working Women. Maternal and Child Health Journal. 2010. pp. 1–10. [DOI] [PMC free article] [PubMed]
- Murakami M, Ohmichi M, Takahashi T, Shibata A, Fukao A, Morisaki N, Kurachi H. Prepregnancy body mass index as an important predictor of perinatal outcomes in Japanese. Archives of Gynecology and Obstetrics. 2005;271(4):311–315. doi: 10.1007/s00404-004-0629-7. [DOI] [PubMed] [Google Scholar]
- Ronnenberg AG, Wang X, Xing H, Chen C, Chen D, Guang W, Guang A, Wang L, Ryan L, Xu X. Low preconception body mass index is associated with birth outcome in a prospective cohort of Chinese women. Journal of Nutrition. 2003;133(11):3449. doi: 10.1093/jn/133.11.3449. [DOI] [PubMed] [Google Scholar]
- Salihu HM, Mbah AK, Alio AP, Clayton HB, Lynch O. Low pre-pregnancy body mass index and risk of medically indicated versus spontaneous preterm singleton birth. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2009;144(2):119–123. doi: 10.1016/j.ejogrb.2009.02.047. [DOI] [PubMed] [Google Scholar]
- Wise LA, Palmer JR, Heffner LJ, Rosenberg L. Prepregnancy body size, gestational weight gain, and risk of preterm birth in African-American women. Epidemiology. 2010;21(2):243. doi: 10.1097/EDE.0b013e3181cb61a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CW, Tsai CY, Sung FC, Lee YY, Lu TH, Li CY, Ko MC. Adverse birth outcomes among pregnancies of teen mothers: age specific analysis of national data in Taiwan. Child: Care, Health and Development. 2010;36(2):232–240. doi: 10.1111/j.1365-2214.2009.01039.x. [DOI] [PubMed] [Google Scholar]
- Gilboa SM, Correa A, Alverson CJ. Use of spline regression in an analysis of maternal prepregnancy body mass index and adverse birth outcomes: does it tell us more than we already know? Annals of epidemiology. 2008;18(3):196–205. doi: 10.1016/j.annepidem.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Han YS, Ha EH, Park HS, Kim YJ, Lee SS. Relationships between pregnancy outcomes, biochemical markers and pre-pregnancy body mass index. International Journal of Obesity. 2010. [DOI] [PMC free article] [PubMed]
- Nohr EA, Vaeth M, Baker JL, Sorensen TIA, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. American Journal of Clinical Nutrition. 2008;87(6):1750. doi: 10.1093/ajcn/87.6.1750. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Inoue K, Doi M, Matsumoto M, Ogasawara K, Fukuoka H, Nagai Y. Risk factors for term small for gestational age infants in women with low prepregnancy body mass index. Journal of Obstetrics and Gynaecology Research. 2010;36(3):506–512. doi: 10.1111/j.1447-0756.2010.01170.x. [DOI] [PubMed] [Google Scholar]
- Frederick IO, Williams MA, Sales AE, Martin DP, Killien M. Pre-pregnancy body mass index, gestational weight gain, and other maternal characteristics in relation to infant birth weight. Maternal and Child Health Journal. 2008;12(5):557–567. doi: 10.1007/s10995-007-0276-2. [DOI] [PubMed] [Google Scholar]
- Yekta Z, Ayatollahi H, Porali R, Farzin A. The effect of pre-pregnancy body mass index and gestational weight gain on pregnancy outcomes in urban care settings in Urmia-Iran. BMC Pregnancy and Childbirth. 2006;6(1):15. doi: 10.1186/1471-2393-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu SY, Bachman DJ, Callaghan WM, Whitlock EP, Dietz PM, Berg CJ, O'Keeffe-Rosetti M, Bruce FC, Hornbrook MC. Association between obesity during pregnancy and increased use of health care. New England Journal of Medicine. 2008;358(14):1444. doi: 10.1056/NEJMoa0706786. [DOI] [PubMed] [Google Scholar]
- Dietz PM, Callaghan WM, Morrow B, Cogswell ME. Population-based assessment of the risk of primary cesarean delivery due to excess prepregnancy weight among nulliparous women delivering term infants. Maternal and Child Health Journal. 2005;9(3):237–244. doi: 10.1007/s10995-005-0003-9. [DOI] [PubMed] [Google Scholar]
- Getahun D, Ananth CV, Peltier MR, Salihu HM, Scorza WE. Changes in prepregnancy body mass index between the first and second pregnancies and risk of large-for-gestational-age birth. American journal of obstetrics and gynecology. 2007;196(6):530. doi: 10.1016/j.ajog.2006.12.036. [DOI] [PubMed] [Google Scholar]
- LaCoursiere D, Bloebaum L, Duncan JD, Varner MW. Population-based trends and correlates of maternal overweight and obesity, Utah 1991-2001. American journal of obstetrics and gynecology. 2005;192(3):832–839. doi: 10.1016/j.ajog.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Fortner RT, Pekow P, Solomon CG, Markenson G, Chasan-Taber L. Prepregnancy body mass index, gestational weight gain, and risk of hypertensive pregnancy among Latina women. American Journal of Obstetrics and Gynecology. 2009;200(2):167–167. doi: 10.1016/j.ajog.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Saftlas AF, Wang W, Risch H, Woolson R, Hsu CD, Bracken MB. Prepregnancy body mass index and gestational weight gain as risk factors for preeclampsia and transient hypertension. Annals of epidemiology. 2000;10(7):475–475. doi: 10.1016/s1047-2797(00)00167-8. [DOI] [PubMed] [Google Scholar]
- Gilboa SM, Correa A, Botto LD, Rasmussen SA, Waller DK, Hobbs CA, Cleves MA, Riehle-Colarusso TJ. Association between prepregnancy body mass index and congenital heart defects. American Journal of Obstetrics and Gynecology. 2010;202(1):51. doi: 10.1016/j.ajog.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Oddy WH, De Klerk NH, Miller M, Payne J, Bower C. Association of maternal pre pregnancy weight with birth defects: Evidence from a case–control study in Western Australia. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2009;49(1):11–15. doi: 10.1111/j.1479-828X.2008.00934.x. [DOI] [PubMed] [Google Scholar]
- Waller DK, Shaw GM, Rasmussen SA, Hobbs CA, Canfield MA, Siega-Riz AM, Gallaway MS, Correa A. Prepregnancy obesity as a risk factor for structural birth defects. Archives of Pediatrics and Adolescent Medicine. 2007;161(8):745. doi: 10.1001/archpedi.161.8.745. [DOI] [PubMed] [Google Scholar]
- Li Z, Liu J, Ye R, Zhang L, Pei L, Zheng X, Ren A. Maternal prepregnancy body mass index and risk of neural tube defects: A population-based case-control study in Shanxi province, China. Birth Defects Research Part A: Clinical and Molecular Teratology. 2010. [DOI] [PubMed]
- Waller DK, Tita ATN, Werler MM, Mitchell AA. Association between prepregnancy maternal body mass index and the risk of having an infant with a congenital diaphragmatic hernia. Birth Defects Research Part A: Clinical and Molecular Teratology. 2003;67(1):73–76. doi: 10.1002/bdra.10003. [DOI] [PubMed] [Google Scholar]
- Han Z, Mulla S, Beyene J, Liao G, McDonald SD. Maternal underweight and the risk of preterm birth and low birth weight: a systematic review and meta-analyses. International Journal of Epidemiology. 2011;40(1):65. doi: 10.1093/ije/dyq195. [DOI] [PubMed] [Google Scholar]
- Salihu HM, Lynch ON, Alio AP, Mbah AK, Kornosky JL, Marty PJ. Extreme maternal underweight and feto-infant morbidity outcomes: a population-based study. Journal of Maternal-Fetal and Neonatal Medicine. 2009;22(5):428–434. doi: 10.1080/14767050802385764. [DOI] [PubMed] [Google Scholar]
- Jensen DM, Damm P, Sørensen B, Mølsted-Pedersen L, Westergaard JG, Ovesen P, Beck-Nielsen H. Pregnancy outcome and prepregnancy body mass index in 2459 glucose-tolerant Danish women. American journal of obstetrics and gynecology. 2003;189(1):239–244. doi: 10.1067/mob.2003.441. [DOI] [PubMed] [Google Scholar]
- Phithakwatchara N, Titapant V. The effect of pre-pregnancy weight on delivery outcome and birth weight in potential diabetic patients with normal screening for gestational diabetes mellitus in Siriraj Hospital. JOURNAL-MEDICAL ASSOCIATION OF THAILAND. 2007;90(2):229. [PubMed] [Google Scholar]
- Barau G, Robillard P. Linear association between maternal pre pregnancy body mass index and risk of caesarean section in term deliveries. BJOG: An International Journal of Obstetrics & Gynaecology. 2006;113(10):1173–1177. doi: 10.1111/j.1471-0528.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- Kaiser PS, Kirby RS. Obesity as a risk factor for cesarean in a low-risk population. Obstetrics & Gynecology. 2001;97(1):39. doi: 10.1016/s0029-7844(00)01078-4. [DOI] [PubMed] [Google Scholar]
- Vahratian A, Siega-Riz AM, Savitz DA, Zhang J. Maternal pre-pregnancy overweight and obesity and the risk of cesarean delivery in nulliparous women. Annals of epidemiology. 2005;15(7):467–474. doi: 10.1016/j.annepidem.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Frederick IO, Rudra CB, Miller RS, Foster JC, Williams MA. Adult weight change, weight cycling, and prepregnancy obesity in relation to risk of preeclampsia. Epidemiology. 2006;17(4):428. doi: 10.1097/01.ede.0000221028.33245.0b. [DOI] [PubMed] [Google Scholar]
- Glazer NL, Hendrickson AF, Schellenbaum GD, Mueller BA. Weight change and the risk of gestational diabetes in obese women. Epidemiology. 2004;15(6):733. doi: 10.1097/01.ede.0000142151.16880.03. [DOI] [PubMed] [Google Scholar]
- Gavard JA, Artal R. Effect of exercise on pregnancy outcome. Clinical Obstetrics and Gynecology. 2008;51(2):467. doi: 10.1097/GRF.0b013e31816feb1d. [DOI] [PubMed] [Google Scholar]
- Birdsall KM, Vyas S, Khazaezadeh N, Oteng Ntim E. Maternal obesity: a review of interventions. International journal of clinical practice. 2009;63(3):494–507. doi: 10.1111/j.1742-1241.2008.01910.x. [DOI] [PubMed] [Google Scholar]
- Galtier F, Raingeard I, Renard E, Boulot P, Bringer J. Optimizing the outcome of pregnancy in obese women: from pregestational to long-term management. Diabetes & metabolism. 2008;34(1):19–25. doi: 10.1016/j.diabet.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Fortner RT, Pekow PS, Whitcomb BW, Sievert LL, Markenson G, Chasan-Taber L. Physical Activity and Hypertensive Disorders of Pregnancy among Hispanic Women. Medicine & Science in Sports & Exercise. 2011;43(4):639. doi: 10.1249/MSS.0b013e3181f58d3e. [DOI] [PubMed] [Google Scholar]
- Martin CL, Brunner Huber LR. Physical Activity and Hypertensive Complications During Pregnancy: Findings from 2004 to 2006 North Carolina Pregnancy Risk Assessment Monitoring System. Birth. 2010;37(3):202–210. doi: 10.1111/j.1523-536X.2010.00407.x. [DOI] [PubMed] [Google Scholar]
- Rudra CB, Williams MA, Lee I. Perceived exertion during prepregnancy physical activity and preeclampsia risk. Medicine & Science in Sports & Exercise. 2005;37(11):1836. doi: 10.1249/01.mss.0000175862.41620.41. [DOI] [PubMed] [Google Scholar]
- Rudra CB, Sorensen TK, Luthy DA, Williams MA. A prospective analysis of recreational physical activity and preeclampsia risk. Medicine & Science in Sports & Exercise. 2008;40(9):1581. doi: 10.1249/MSS.0b013e31817cab1. [DOI] [PubMed] [Google Scholar]
- Saftlas AF, Logsden-Sackett N, Wang W, Woolson R, Bracken MB. Work, leisure-time physical activity, and risk of preeclampsia and gestational hypertension. American journal of epidemiology. 2004;160(8):758. doi: 10.1093/aje/kwh277. [DOI] [PubMed] [Google Scholar]
- Sorensen TK, Williams MA, Lee I. Recreational physical activity during pregnancy and risk of preeclampsia. Hypertension. 2003;41(6):1273. doi: 10.1161/01.HYP.0000072270.82815.91. [DOI] [PubMed] [Google Scholar]
- Dempsey JC, Butler CL, Sorensen TK, Lee I. A case-control study of maternal recreational physical activity and risk of gestational diabetes mellitus* 1. Diabetes research and clinical practice. 2004;66(2):203–215. doi: 10.1016/j.diabres.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Dempsey JC, Sorensen TK, Williams MA, Lee I. Prospective study of gestational diabetes mellitus risk in relation to maternal recreational physical activity before and during pregnancy. American journal of epidemiology. 2004;159(7):663. doi: 10.1093/aje/kwh091. [DOI] [PubMed] [Google Scholar]
- Oken E, Ning Y, Rifas-Shiman SL, Radesky JS, Rich-Edwards JW, Gillman MW. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstetrics and gynecology. 2006;108(5):1200. doi: 10.1097/01.AOG.0000241088.60745.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudra CB, Williams MA, Lee I. Perceived exertion in physical activity and risk of gestational diabetes mellitus. Epidemiology. 2006;17(1):31. doi: 10.1097/01.ede.0000184474.33629.cd. [DOI] [PubMed] [Google Scholar]
- Tobias DK, Zhang C, van Dam RM, Bowers K, Hu FB. Physical Activity Before and During Pregnancy and Risk of Gestational Diabetes Mellitus. Diabetes Care. 2011;34(1):223. doi: 10.2337/dc10-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Solomon CG, Manson JAE, Hu FB. A prospective study of pregravid physical activity and sedentary behaviors in relation to the risk for gestational diabetes mellitus. Archives of internal medicine. 2006;166(5):543. doi: 10.1001/archinte.166.5.543. [DOI] [PubMed] [Google Scholar]
- Kinnunen TI, Pasanen M, Aittasalo M, Fogelholm M, Weiderpass E, Luoto R. Reducing postpartum weight retention – a pilot trial in primary health care. Nutrition Journal. 2007;6(1):21. doi: 10.1186/1475-2891-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim Adegboye AR, Linne YM, Lourenco PMC. Diet or exercise, or both, for weight reduction in women after childbirth. 2007. status and date: Edited (no change to conclusions) [DOI] [PubMed]
- Black MM, Hager ER, Le K, Anliker J, Arteaga SS, DiClemente C, Gittelsohn J, Magder L, Papas M, Snitker S. Challenge! Health Promotion/Obesity Prevention Mentorship Model Among Urban, Black Adolescents. Pediatrics. 2010;126(2):280. doi: 10.1542/peds.2009-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiben G, Lissner L. Health Hunters–an intervention to prevent overweight and obesity in young high-risk women. International journal of obesity. 2005;30(4):691–696. doi: 10.1038/sj.ijo.0803167. [DOI] [PubMed] [Google Scholar]
- Faucher MA, Mobley J. A Community Intervention on Portion Control Aimed at Weight Loss in Low-Income Mexican American Women. Journal of Midwifery & Women's Health. 2010;55(1):60–64. doi: 10.1016/j.jmwh.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Gokee LaRose J, Tate DF, Gorin AA, Wing RR. Preventing Weight Gain in Young Adults:: A Randomized Controlled Pilot Study. American journal of preventive medicine. 2010. [DOI] [PMC free article] [PubMed]
- Ostbye T, Krause KM, Lovelady CA, Morey MC, Bastian LA, Peterson BL, Swamy GK, Brouwer RJN, McBride CM. Active Mothers Postpartum:: A Randomized Controlled Weight-Loss Intervention Trial. American journal of preventive medicine. 2009;37(3):173–180. doi: 10.1016/j.amepre.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MW, Nitzke S, Brown R. Design and Outcomes of a Mothers In Motion Behavioral Intervention Pilot Study. Journal of Nutrition Education and Behavior. 2010;42(3S):11–21. doi: 10.1016/j.jneb.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey-Berino J, West D, Krukowski R, Prewitt E, VanBiervliet A, Ashikaga T, Skelly J. Internet delivered behavioral obesity treatment. Preventive medicine. 2010. [DOI] [PMC free article] [PubMed]
- Mediano MFF, Barbosa JSO, Moura AS, Willett WC, Sichieri R. A randomized clinical trial of home-based exercise combined with a slight caloric restriction on obesity prevention among women. Preventive medicine. 2010. [DOI] [PMC free article] [PubMed]
- Rock CL, Flatt SW, Sherwood NE, Karanja N, Pakiz B, Thomson CA. Effect of a Free Prepared Meal and Incentivized Weight Loss Program on Weight Loss and Weight Loss Maintenance in Obese and Overweight Women. JAMA: The Journal of the American Medical Association. 2010;304(16):1803. doi: 10.1001/jama.2010.1503. [DOI] [PubMed] [Google Scholar]
- Hall J, Solehdin F. Folic acid for the prevention of congenital anomalies. European journal of pediatrics. 1998;157(6):445–450. doi: 10.1007/s004310050850. [DOI] [PubMed] [Google Scholar]
- Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338(760):131–137. [PubMed] [Google Scholar]
- Kirke PN, Daly LE, Elwood JH. A randomised trial of low dose folic acid to prevent neural tube defects. The Irish Vitamin Study Group. British Medical Journal. 1992;67(12):1442. doi: 10.1136/adc.67.12.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence KM, James N, Miller MH, Tennant GB, Campbell H. Double-blind randomised controlled trial of folate treatment before conception to prevent recurrence of neural-tube defects. British medical journal (Clinical research ed) 1981;282(6275):1509. doi: 10.1136/bmj.282.6275.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez L, Hendricks KA, Cooper SP, Sweeney AM, Hardy RJ, Larsen RD. Neural tube defects among Mexican Americans living on the US-Mexico border: effects of folic acid and dietary folate. American journal of epidemiology. 2000;152(11):1017–1023. doi: 10.1093/aje/152.11.1017. [DOI] [PubMed] [Google Scholar]
- Vergel RG, Sanchez LR, Heredero BL, Rodriguez PL, Martinez AJ. Primary prevention of neural tube defects with folic acid supplementation: Cuban experience. Prenatal diagnosis. 1990;10(3):149–152. doi: 10.1002/pd.1970100303. [DOI] [PubMed] [Google Scholar]
- Botto LD, Olney RS, Erickson JD. Vitamin supplements and the risk for congenital anomalies other than neural tube defects. John Wiley & Sons; 2004. pp. 12–21. [DOI] [PubMed] [Google Scholar]
- Johnson KJ, Alexander BH, Doody MM, Sigurdson AJ, Linet MS, Spector LG, Hoffbeck RW, Simon SL, Weinstock RM, Ross JA. Childhood cancer in the offspring born in 1921–1984 to US radiologic technologists. British journal of cancer. 2008;99(3):545–550. doi: 10.1038/sj.bjc.6604516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh YI, Bollano E, Einarson TR, Koren G. Prenatal multivitamin supplementation and rates of congenital anomalies: a meta-analysis. J Obstet Gynaecol Can. 2006;28(8):680–689. doi: 10.1016/S1701-2163(16)32227-7. [DOI] [PubMed] [Google Scholar]
- Badovinac RL, Werler MM, Williams PL, Kelsey KT, Hayes C. Folic acid–containing supplement consumption during pregnancy and risk for oral clefts: A meta analysis. Birth Defects Research Part A: Clinical and Molecular Teratology. 2007;79(1):8–15. doi: 10.1002/bdra.20315. [DOI] [PubMed] [Google Scholar]
- Suarez L, Hendricks KA, Cooper SP, Sweeney AM, Hardy RJ, Larsen RD. Neural tube defects among Mexican Americans living on the US-Mexico border: effects of folic acid and dietary folate. American journal of epidemiology. 2000;152(11):1017. doi: 10.1093/aje/152.11.1017. [DOI] [PubMed] [Google Scholar]
- Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, Mulinare J, Zhao P, Wong LYC, Gindler J. Prevention of neural-tube defects with folic acid in China. New England Journal of Medicine. 1999;341(20):1485. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- Bower C, Stanley FJ. Periconceptional vitamin supplementation and neural tube defects; evidence from a case-control study in Western Australia and a review of recent publications. Journal of Epidemiology and Community Health. 1992;46(2):157. doi: 10.1136/jech.46.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich M, Kristoffersen K, Rolschau J, Grinsted P, Schaumburg E, Foged N. The influence of folic acid supplement on the outcome of pregnancies in the county of Funen in Denmark. Part II. Congenital anomalies. A randomised study. Eur J Obstet Gynecol Reprod Biol. 1999;87(2):111–113. discussion 103-114. [PubMed] [Google Scholar]
- Central Technical Co-ordinating Unit, ICMRCentral Technical Co-ordinating Unit I. Multicentric study of efficacy of periconceptional folic acid containing vitamin supplementation in prevention of open neural tube defects from India. Indian J Med Res. 2000;112:206–211. [PubMed] [Google Scholar]
- Smithells RW, Sheppard S, Schorah CJ, Seller MJ, Nevin NC, Harris R, Read AP, Fielding DW. Apparent prevention of neural tube defects by periconceptional vitamin supplementation. Archives of disease in childhood. 1981;56(12):911. doi: 10.1136/adc.56.12.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Song X, Ji Y, Zhang L, Pei L, Chen J, Liu J, Li C, Zheng X. Prevention of NTDs with periconceptional multivitamin supplementation containing folic acid in China. Birth Defects Research Part A Clinical and Molecular Teratology. 2008;82(8):592–596. doi: 10.1002/bdra.20471. [DOI] [PubMed] [Google Scholar]
- Czeizel AE. Reduction of urinary tract and cardiovascular defects by periconceptional multivitamin supplementation. American Journal of Medical Genetics Part A. 1996;62(2):179–183. doi: 10.1002/(SICI)1096-8628(19960315)62:2<179::AID-AJMG12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Dobó M, Vargha P. Hungarian cohort controlled trial of periconceptional multivitamin supplementation shows a reduction in certain congenital abnormalities. Birth Defects Research Part A Clinical and Molecular Teratology. 2004;70(11):853–861. doi: 10.1002/bdra.20086. [DOI] [PubMed] [Google Scholar]
- Mulinare J, Cordero JF, Erickson JD, Berry RJ. Periconceptional use of multivitamins and the occurrence of neural tube defects. JAMA. 1988;260(21):3141. [PubMed] [Google Scholar]
- Shaw GM, Schaffer D, Velie EM, Morland K, Harris JA. Periconceptional vitamin use, dietary folate, and the occurrence of neural tube defects. Epidemiology. 1995;6(3):219–226. doi: 10.1097/00001648-199505000-00005. [DOI] [PubMed] [Google Scholar]
- Werler MM, Shapiro S, Mitchell AA. Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA. 1993;269(10):1257. [PubMed] [Google Scholar]
- Bower C, Miller M, Payne J, Serna P. Folate intake and the primary prevention of non neural birth defects. Australian and New Zealand Journal of Public Health. 2006;30(3):258–261. doi: 10.1111/j.1467-842x.2006.tb00867.x. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Timar L, Sarkozi A. Dose-dependent effect of folic acid on the prevention of orofacial clefts. Pediatrics. 1999;104(6):e66. doi: 10.1542/peds.104.6.e66. [DOI] [PubMed] [Google Scholar]
- Hayes C, Werter MM, Willett WC, Mitchell AA. Case-control study of periconceptional folic acid supplementation and oral clefts. American journal of epidemiology. 1996;143(12):1229. doi: 10.1093/oxfordjournals.aje.a008710. [DOI] [PubMed] [Google Scholar]
- Johnson CY, Little J. Folate intake, markers of folate status and oral clefts: is the evidence converging? International journal of epidemiology. 2008;37(5):1041. doi: 10.1093/ije/dyn098. [DOI] [PubMed] [Google Scholar]
- Itikala PR, Watkins ML, Mulinare J, Moore CA, Liu Y. Maternal multivitamin use and orofacial clefts in offspring. Teratology. 2001;63(2):79–86. doi: 10.1002/1096-9926(200102)63:2<79::AID-TERA1013>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Van Beynum IM, Kapusta L, Bakker MK, Den Heijer M, Blom HJ, de Walle HEK. Protective effect of periconceptional folic acid supplements on the risk of congenital heart defects: a registry-based case-control study in the northern Netherlands. European heart journal. 2009. [DOI] [PubMed]
- Scanlon KS, Ferencz C, Loffredo CA, Wilson PD, Correa-Villaseñor A, Khoury MJ, Willett WC. Preconceptional folate intake and malformations of the cardiac outflow tract. Epidemiology. 1998;9(1):95. [PubMed] [Google Scholar]
- Botto LD, Khoury MJ, Mulinare J, Erickson JD. Periconceptional multivitamin use and the occurrence of conotruncal heart defects: results from a population-based, case-control study. Pediatrics. 1996;98(5):911. [PubMed] [Google Scholar]
- Li DK, Daling JR, Mueller BA, Hickok DE, Fantel AG, Weiss NS. Periconceptional multivitamin use in relation to the risk of congenital urinary tract anomalies. Epidemiology. 1995;6(3):212. doi: 10.1097/00001648-199505000-00004. [DOI] [PubMed] [Google Scholar]
- Yang Q, Khoury MJ, Olney RS, Mulinare J. Does periconceptional multivitamin use reduce the risk for limb deficiency in offspring? Epidemiology. 1997;8(2):157–161. doi: 10.1097/00001648-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Bitsko RH, Reefhuis J, Romitti PA, Moore CA, Honein MA. Periconceptional consumption of vitamins containing folic acid and risk for multiple congenital anomalies. American Journal of Medical Genetics Part A. 2007;143(20):2397–2405. doi: 10.1002/ajmg.a.31950. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Medveczky E. Periconceptional multivitamin supplementation and multimalformed offspring. Obstetrics & Gynecology. 2003;102(6):1255. doi: 10.1016/j.obstetgynecol.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Shaw GM, Moore CA, Lammer EJ, Mulinare J. Does periconceptional multivitamin use reduce the risk of neural tube defects associated with other birth defects? Data from two population-based case-control studies. American Journal of Medical Genetics Part A. 1996;61(1):30–36. doi: 10.1002/(SICI)1096-8628(19960102)61:1<30::AID-AJMG6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Croen LA, Todoroff K, Tolarova MM. Periconceptional intake of vitamin supplements and risk of multiple congenital anomalies. American journal of medical genetics. 2000;93(3):188–193. doi: 10.1002/1096-8628(20000731)93:3<188::aid-ajmg5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Catov JM, Bodnar LM, Ness RB, Markovic N, Roberts JM. Association of periconceptional multivitamin use and risk of preterm or small-for-gestational-age births. American journal of epidemiology. 2007;166(3):296. doi: 10.1093/aje/kwm071. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Dudas I, Metneki J. Pregnancy outcomes in a randomised controlled trial of periconceptional multivitamin supplementation. Archives of Gynecology and Obstetrics. 1994;255(3):131–139. doi: 10.1007/BF02390940. [DOI] [PubMed] [Google Scholar]
- Bodnar LM, Tang G, Ness RB, Harger G, Roberts JM. Periconceptional multivitamin use reduces the risk of preeclampsia. American journal of epidemiology. 2006;164(5):470. doi: 10.1093/aje/kwj218. [DOI] [PubMed] [Google Scholar]
- Catov JM, Nohr EA, Bodnar LM, Knudson VK, Olsen SF, Olsen J. Association of periconceptional multivitamin use with reduced risk of preeclampsia among normal-weight women in the Danish National Birth Cohort. American journal of epidemiology. 2009;169(11):1304. doi: 10.1093/aje/kwp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo EB, Biglieri A. Impact of folic acid fortification on women nutritional status and on the prevalence of neural tube defects. Archivos argentinos de pediatría. 2008;106:492–498. doi: 10.1590/S0325-00752008000600004. [DOI] [PubMed] [Google Scholar]
- Mersereau P, Kilker K, Carter H, Fassett E, Williams J, Flores A, Prue C, Williams L, Mai C, Mulinare J. Spina bifida and anencephaly before and after folic acid mandate—United States, 1995–1996 and 1999–2000. Morbidity and Mortality Weekly Report. 2004;53(17):362–365. [PubMed] [Google Scholar]
- De Wals P, Rusen ID, Lee NS, Morin P, Niyonsenga T. Trend in prevalence of neural tube defects in Quebec. Birth Defects Research Part A: Clinical and Molecular Teratology. 2003;67(11):919–923. doi: 10.1002/bdra.10124. [DOI] [PubMed] [Google Scholar]
- Gucciardi E, Pietrusiak MA, Reynolds DL, Rouleau J. Incidence of neural tube defects in Ontario, 1986-1999. Canadian Medical Association Journal. 2002;167(3):237. [PMC free article] [PubMed] [Google Scholar]
- Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LYC. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. Jama. 2001;285(23):2981. doi: 10.1001/jama.285.23.2981. [DOI] [PubMed] [Google Scholar]
- Liu S, West R, Randell E, Longerich L, O'Connor KS, Scott H, Crowley M, Lam A, Prabhakaran V, McCourt C. A comprehensive evaluation of food fortification with folic acid for the primary prevention of neural tube defects. BMC Pregnancy and Childbirth. 2004;4(1):20. doi: 10.1186/1471-2393-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López Camelo JS, Orioli IM, Dutra MG, Nazer Herrera J, Rivera N, Ojeda ME, Canessa A, Wettig E, Fontannaz AM, Mellado C. Reduction of birth prevalence rates of neural tube defects after folic acid fortification in Chile. American Journal of Medical Genetics Part A. 2005;135(2):120–125. doi: 10.1002/ajmg.a.30651. [DOI] [PubMed] [Google Scholar]
- Persad VL, Van den Hof MC, Dube JM, Zimmer P. Incidence of open neural tube defects in Nova Scotia after folic acid fortification. Canadian Medical Association Journal. 2002;167(3):241. [PMC free article] [PubMed] [Google Scholar]
- Ray JG, Meier C, Vermeulen MJ, Boss S, Wyatt PR, Cole DEC. Association of neural tube defects and folic acid food fortification in Canada. The Lancet. 2002;360(9350):2047–2048. doi: 10.1016/S0140-6736(02)11994-5. [DOI] [PubMed] [Google Scholar]
- Sayed AR, Bourne D, Pattinson R, Nixon J, Henderson B. Decline in the prevalence of neural tube defects following folic acid fortification and its cost benefit in South Africa. Birth Defects Research Part A: Clinical and Molecular Teratology. 2008;82(4):211–216. doi: 10.1002/bdra.20442. [DOI] [PubMed] [Google Scholar]
- Simmons CJ, Mosley BS, Fulton Bond CA, Hobbs CA. Birth defects in Arkansas: Is folic acid fortification making a difference? Birth Defects Research Part A: Clinical and Molecular Teratology. 2004;70(9):559–564. doi: 10.1002/bdra.20063. [DOI] [PubMed] [Google Scholar]
- Williams LJ, Mai CT, Edmonds LD, Shaw GM, Kirby RS, Hobbs CA, Sever LE, Miller LA, Meaney FJ, Levitt M. Prevalence of spina bifida and anencephaly during the transition to mandatory folic acid fortification in the United States. Teratology. 2002;66(1):33–39. doi: 10.1002/tera.10060. [DOI] [PubMed] [Google Scholar]
- Williams LJ, Rasmussen SA, Flores A, Kirby RS, Edmonds LD. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995-2002. Pediatrics. 2005;116(3):580. doi: 10.1542/peds.2005-0592. [DOI] [PubMed] [Google Scholar]
- Li Z, Gindler J, Wang H, Berry RJ, Li S, Correa A, Zheng J, Erickson JD, Wang Y. Folic acid supplements during early pregnancy and likelihood of multiple births: a population-based cohort study. The Lancet. 2003;361(9355):380–384. doi: 10.1016/s0140-6736(03)12390-2. [DOI] [PubMed] [Google Scholar]
- Vollset SE, Gjessing HK, Tandberg A, Rønning T, Irgens LM, Baste V, Nilsen RM, Daltveit AK. Folate supplementation and twin pregnancies. Epidemiology. 2005;16(2):201. doi: 10.1097/01.ede.0000152914.84962.13. [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Watkins ML, Chiu V, Erickson JD, Petitti DB. Food fortification with folic acid and rate of multiple births, 1994–2000. Birth Defects Research Part A: Clinical and Molecular Teratology. 2004;70(12):948–952. doi: 10.1002/bdra.20088. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Nelson V, Selvin S, Schaffer DM. Food fortification with folic acid and twinning among California infants. American Journal of Medical Genetics Part A. 2003;119(2):137–140. doi: 10.1002/ajmg.a.20145. [DOI] [PubMed] [Google Scholar]
- Signore C, Mills JL, Cox C, Trumble AC. Effects of folic acid fortification on twin gestation rates. Obstetrics & Gynecology. 2005;105(4):757. doi: 10.1097/01.AOG.0000154886.40318.5e. [DOI] [PubMed] [Google Scholar]
- Chevrier C, Perret C, Bahuau M, Zhu H, Nelva A, Herman C, Francannet C, Robert Gnansia E, Finnell RH, Cordier S. Fetal and maternal MTHFR C677T genotype, maternal folate intake and the risk of nonsyndromic oral clefts. American Journal of Medical Genetics Part A. 2007;143(3):248–257. doi: 10.1002/ajmg.a.31462. [DOI] [PubMed] [Google Scholar]
- Little J, Gilmour M, Mossey PA, FitzPatrick D, Cardy A, Clayton-Smith J, Fryer AE. Folate and Clefts of the Lip and Palate-A UK-Based Case-Control Study: Part I: Dietary and Supplemental Folate. The Cleft Palate-Craniofacial Journal. 2008;45(4):420–427. doi: 10.1597/06-150.1. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Laurent C, Rasmussen SA. Maternal nutrient intakes and risk of orofacial clefts. Epidemiology. 2006;17(3):285. doi: 10.1097/01.ede.0000208348.30012.35. [DOI] [PubMed] [Google Scholar]
- van Rooij I, Swinkels DW, Blom HJ, Merkus H, Steegers-Theunissen RPM. Vitamin and homocysteine status of mothers and infants and the risk of nonsyndromic orofacial clefts* 1. American Journal of Obstetrics and Gynecology. 2003;189(4):1155–1160. doi: 10.1067/s0002-9378(03)00592-1. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Lie RT, Solvoll K, Taylor J, McConnaughey DR, Åbyholm F, Vindenes H, Vollset SE, Drevon CA. Folic acid supplements and risk of facial clefts: national population based case-control study. BMJ. 2007;334(7591):464. doi: 10.1136/bmj.39079.618287.0B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Walle HEK, De Jong Van Den Berg LTW. Insufficient folic acid intake in the Netherlands: what about the future? Teratology. 2002;66(1):40–43. doi: 10.1002/tera.10078. [DOI] [PubMed] [Google Scholar]
- Kannan S, Menotti E, Scherer HK, Dickinson J, Larson K. Folic acid and the prevention of neural tube defects: A survey of awareness among Latina women of childbearing age residing in southeast Michigan. Health promotion practice. 2007;8(1):60. doi: 10.1177/1524839905278934. [DOI] [PubMed] [Google Scholar]
- Kari JA, Bardisi ES, Baitalmal RM, Ageely GA. Folic acid awareness among female college students: neural tube defects prevention. Saudi Med J. 2008;29(12):1749–1751. [PubMed] [Google Scholar]
- Sillender M, Pring DW. How effective was the Health Education Authority s folic acid campaign? Journal of Obstetrics and Gynaecology. 2000;20(3):271–276. doi: 10.1080/01443610050009593. [DOI] [PubMed] [Google Scholar]
- Der Pal de Bruin V. The Dutch ‘Folic Acid Campaign’–have the goals been achieved? Paediatric and Perinatal Epidemiology. 2000;14(2):111–117. doi: 10.1046/j.1365-3016.2000.00251.x. [DOI] [PubMed] [Google Scholar]
- Chan A, Pickering J, Haan EA, Netting M, Burford A, Johnson A, Keane RJ. " Folate before pregnancy": the impact on women and health professionals of a population-based health promotion campaign in South Australia. Obstetrical & Gynecological Survey. 2002;57(1):8. doi: 10.5694/j.1326-5377.2001.tb143471.x. [DOI] [PubMed] [Google Scholar]
- Pastuszak A, Bhatia D, Okotore B, Koren G. Preconception counseling and women's compliance with folic acid supplementation. Canadian Family Physician. 1999;45(2053) [PMC free article] [PubMed] [Google Scholar]
- Stevenson RE, Allen WP, Pai GS, Best R, Seaver LH, Dean J, Thompson S. Decline in prevalence of neural tube defects in a high-risk region of the United States. Pediatrics. 2000;106(4):677. doi: 10.1542/peds.106.4.677. [DOI] [PubMed] [Google Scholar]
- Robbins JM, Cleves MA, Collins HB, Andrews N, Smith LN, Hobbs CA. Randomized trial of a physician-based intervention to increase the use of folic acid supplements among women. American Journal of Obstetrics and Gynecology. 2005;192(4):1126–1132. doi: 10.1016/j.ajog.2004.10.620. [DOI] [PubMed] [Google Scholar]
- Watson MJ, Watson LF, Bell RJ, Halliday JL, Burford N, Brennecke SP. A randomized community intervention trial to increase awareness and knowledge of the role of periconceptional folate in women of child-bearing age. Health Expect. 1999;2(4):255–265. doi: 10.1046/j.1369-6513.1999.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M, Watson L, Bell R, Halliday J. The increasing knowledge of the role of periconceptional folate in Victorian women of child-bearing age: follow-up of a randomised community intervention trial. Aust N Z J Public Health. 2001;25(5):389–395. [PubMed] [Google Scholar]
- Chacko MR, Anding R, Kozinetz CA, Grover JL, Smith PB. Neural tube defects: knowledge and preconceptional prevention practices in minority young women. Pediatrics. 2003;112(3):536. doi: 10.1542/peds.112.3.536. [DOI] [PubMed] [Google Scholar]
- Hauser KW, Lilly CM, Frías JL. Florida health care providers' knowledge of folic acid for the prevention of neural tube defects. Southern medical journal. 2004;97(5):437. doi: 10.1097/00007611-200405000-00004. [DOI] [PubMed] [Google Scholar]
- Watkins ML, Brustrom J, Schulman J. Effectiveness of a free folic acid supplement program in family planning clinics. Birth Defects Research Clinical and Molecular Teratology. 2004;70(6):403–407. doi: 10.1002/bdra.20035. [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Watkins ML, Ershoff D, Petitti DB, Chiu V, Postlethwaite D, Erickson JD. Design and evaluation of interventions promoting periconceptional multivitamin use. American Journal of Preventive Medicine. 2003;25(1):17–24. doi: 10.1016/s0749-3797(03)00097-7. [DOI] [PubMed] [Google Scholar]
- Egen V, Hasford J. Prevention of neural tube defects: effect of an intervention aimed at implementing the official recommendations. Sozial-und Präventivmedizin/Social and Preventive Medicine. 2003;48(1):24–32. doi: 10.1007/s000380300003. [DOI] [PubMed] [Google Scholar]
- Mullenix A. Reaching Women and Health Care Providers with Women’s Wellness Messages. NC Med J. 2009;70(5) [PubMed] [Google Scholar]
- Baro L, Martinez-Ferez A, Rodriguez C, Valero A, Fonolla J, Lucena A, Jimenez J, Boza JJ, Lopez-Huertas E. The administration of a multivitamin/mineral fortified dairy product improves folate status and reduces plasma homocysteine concentration in women of reproductive age. International journal for vitamin and nutrition research. 2004;74(3):234–240. doi: 10.1024/0300-9831.74.3.234. [DOI] [PubMed] [Google Scholar]
- de Weerd S, Thomas CMG, Cikot RJLM, Steegers-Theunissen RPM, de Boo TM, Steegers EAP. Preconception counseling improves folate status of women planning pregnancy. Obstetrics & Gynecology. 2002;99(1):45. doi: 10.1016/s0029-7844(01)01573-3. [DOI] [PubMed] [Google Scholar]
- Muggli EE, Halliday JL. Folic acid and risk of twinning: a systematic review of the recent literature, July 1994 to July 2006. Medical Journal of Australia. 2007;186(5):243. doi: 10.5694/j.1326-5377.2007.tb00882.x. [DOI] [PubMed] [Google Scholar]
- Shaw GM, O'Malley CD, Wasserman CR, Tolarova MM, Lammer EJ. Maternal periconceptional use of multivitamins and reduced risk for conotruncal heart defects and limb deficiencies among offspring. American journal of medical genetics. 1995;59(4):536–545. doi: 10.1002/ajmg.1320590428. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jun PEIL, Ming SX, Gong C, Ying ZX. Impact of Periconceptional Multi-micronutrient Supplementation on Gestation: A Population-based Study. Biomed Environ Sci. 2013;26(1):23–31. doi: 10.3967/0895-3988.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Ronnenberg AG, Wood RJ, Wang X, Xing H, Chen C, Chen D, Guang W, Huang A, Wang L, Xu X. Preconception hemoglobin and ferritin concentrations are associated with pregnancy outcome in a prospective cohort of Chinese women. Journal of Nutrition. 2004;134(10):2586. doi: 10.1093/jn/134.10.2586. [DOI] [PubMed] [Google Scholar]
- Imdad A, Yakoob MY, Bhutta Z. Effect of breastfeeding promotion interventions on breastfeeding rates, with special focus on developing countries. BMC Public Health. 2011;11(Suppl 3):S24. doi: 10.1186/1471-2458-11-S3-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Rosas JP, V F. Effects and safety of preventive oral iron or iron+folic acid supplementation for women during pregnancy. 2009. [DOI] [PubMed]
- Berger J, T H, Cavalli-Sforza T, Smitasiri S, Khan NC, Milani S. Community mobilization and social marketing to promote weekly iron-folic acid supplementation in women of reproductive age in Vietnam: impact on anemia and iron status. 2005. [DOI] [PubMed]
- Angeles-Agdeppa I, P L, Ramos AC, Etorma UM, Cavalli-Sforza T, Milani S. Government-Industry Partnership in Weekly Iron-Folic Acid Supplementation for Women of Reproductive Age in the Philippines. 2005. [DOI] [PubMed]
- Khambalia AZ, O'Connor DL, Macarthur C, Dupuis A, Zlotkin SH. Periconceptional iron supplementation does not reduce anemia or improve iron status among pregnant women in rural Bangladesh. The American journal of clinical nutrition. 2009;90(5):1295–1302. doi: 10.3945/ajcn.2009.28350. [DOI] [PubMed] [Google Scholar]
- Casey GJ, J D, Phuc TQ, Tinh TT, Tho DH. Long-term weekly iron-folic acid and de-worming is associated with stabilised haemoglobin and increasing iron stores in non-pregnant women in Vietnam. 2010. [DOI] [PMC free article] [PubMed]
- Passerini L, C G, Biggs BA, Cong DT, Phu LB, Phuc TQ, Carone M, Montresor A. Increased birth weight associated with regular pre-pregnancy deworming and weekly iron-folic acid supplementation for Vietnamese women. 2012. [DOI] [PMC free article] [PubMed]
- Vir SC, S N, Nigam AK, Jain R. Weekly iron and folic acid supplementation with counseling reduces anemia in adolescent girls. 2008. [DOI] [PubMed]
- Mendez MA, Monteiro CA, Popkin BM. Overweight exceeds underweight among women in most developing countries. American Journal of Clinical Nutrition. 2005;81(3):714. doi: 10.1093/ajcn/81.3.714. [DOI] [PubMed] [Google Scholar]
- Han YW. Oral Health and Adverse Pregnancy Outcomes–What’s Next? Journal of Dental Research. 2011;90(3):289. doi: 10.1177/0022034510381905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14(3):368. doi: 10.1097/00001648-200305000-00020. [DOI] [PubMed] [Google Scholar]
- Torloni MR, Betrán AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, Valente O. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta analysis. Obesity Reviews. 2009;10(2):194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- Poobalan AS, Aucott LS, Gurung T, Smith WCS, Bhattacharya S. Obesity as an independent risk factor for elective and emergency caesarean delivery in nulliparous women–systematic review and meta analysis of cohort studies. Obesity Reviews. 2009;10(1):28–35. doi: 10.1111/j.1467-789X.2008.00537.x. [DOI] [PubMed] [Google Scholar]
- Nuthalapaty FS, Rouse DJ. The impact of obesity on obstetrical practice and outcome. Clinical obstetrics and gynecology. 2004;47(4):898. doi: 10.1097/01.grf.0000135358.34673.48. [DOI] [PubMed] [Google Scholar]
- WHO. Consultation on Antiretroviral Treatment for Prevention of HIV Transmission. Meeting Report. Geneva, Switzerland; 2009. [Google Scholar]
- Vrebosch L, B S, VansantMaternal and neonatal outcome after laparoscopic adjustable gastric banding: a systematic. 2012. [DOI] [PubMed]
- Cornel MC, Erickson JD. Comparison of national policies on periconceptional use of folic acid to prevent spina bifida and anencephaly (SBA) Teratology. 1997;55(2):134–137. doi: 10.1002/(SICI)1096-9926(199702)55:2<134::AID-TERA3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Bener A, Al Maadid MGA, Al-Bast DAE, Al-Marri S. Maternal knowledge, attitude and practice on folic acid intake among Arabian Qatari women. Reproductive Toxicology. 2005;21(1):21–25. doi: 10.1016/j.reprotox.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Brough L, Rees GA, Crawford MA, Dorman EK. Social and ethnic differences in folic acid use preconception and during early pregnancy in the UK: effect on maternal folate status. Journal of human nutrition and dietetics. 2009;22(2):100–107. doi: 10.1111/j.1365-277X.2008.00936.x. [DOI] [PubMed] [Google Scholar]
- Coll O, Pisa S, Palacio M, Quintó L, Cararach V. Awareness of the use of folic acid to prevent neural tube defects in a Mediterranean area. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2004;115(2):173–177. doi: 10.1016/j.ejogrb.2003.12.009. [DOI] [PubMed] [Google Scholar]
- CDC. The importance of preconception care in the continuum of women's health care. Obstet Gynecol Clin North Am. 2005;106(3):665–666. doi: 10.1097/00006250-200509000-00052. [DOI] [PubMed] [Google Scholar]
- De Walle HE, Van der Pal KM. Periconceptional folic acid in The Netherlands in 1995. Socioeconomic differences. Journal of epidemiology and community health. 1998;52(12):826. doi: 10.1136/jech.52.12.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell SR, Barnett AG, Underwood MR. The use of pre-conceptional folic acid as an indicator of uptake of a health message amongst white and Bangladeshi women in Tower Hamlets, east London. Family Practice. 2001;18(3):300. doi: 10.1093/fampra/18.3.300. [DOI] [PubMed] [Google Scholar]
- Nawapun K, Phupong V. Awareness of the benefits of folic acid and prevalence of the use of folic acid supplements to prevent neural tube defects among Thai women. Archives of Gynecology and Obstetrics. 2007;276(1):53–57. doi: 10.1007/s00404-006-0305-1. [DOI] [PubMed] [Google Scholar]
- Tamim H, Harrison G, Atoui M, Mumtaz G, El-Kak F, Seoud M, Yunis K. Preconceptional folic acid supplement use in Lebanon. Public health nutrition. 2009;12(05):687–692. doi: 10.1017/S136898000800298X. [DOI] [PubMed] [Google Scholar]
- Paudel P, W K, Silpakar SK. Awareness of periconceptional folic acid supplementation among Nepalese women of childbearing age: a cross-sectional study. 2012. [DOI] [PubMed]
- Stockley L, Lund V. Use of folic acid supplements, particularly by low-income and young women: a series of systematic reviews to inform public health policy in the UK. Public health nutrition. 2008;11(08):807–821. doi: 10.1017/S1368980008002346. [DOI] [PubMed] [Google Scholar]
- Botto LD, Lisi A, Bower C, Canfield MA, Dattani N, De Vigan C, De Walle H, Erickson DJ, Halliday J, Irgens LM. Trends of selected malformations in relation to folic acid recommendations and fortification: an international assessment. Birth Defects Research Part A: Clinical and Molecular Teratology. 2006;76(10):693–705. doi: 10.1002/bdra.20307. [DOI] [PubMed] [Google Scholar]
- Oakley GP. Folate deficiency is an“imminent health hazard” causing a worldwide birth defects epidemic. Birth Defects Research Part A Clinical and Molecular Teratology. 2003;67(11):903–904. doi: 10.1002/bdra.10141. [DOI] [PubMed] [Google Scholar]
- W NJ. Folic acid and the prevention of neural-tube defects. 2004.
- Locksmith GJ, Duff P. Preventing neural tube defects: the importance of periconceptional folic acid supplements. Obstetrics & Gynecology. 1998;91(6):1027. doi: 10.1016/s0029-7844(98)00060-x. [DOI] [PubMed] [Google Scholar]
- Kondo A, Kamihira O, Ozawa H. Neural tube defects: Prevalence, etiology and prevention. International Journal of Urology. 2009;16(1):49–57. doi: 10.1111/j.1442-2042.2008.02163.x. [DOI] [PubMed] [Google Scholar]
- Holzgreve W, P K, Koletzko B. Adding folate to the contraceptive pill: a new concept for the prevention of neural tube defects.J Matern Fetal Neonata. 2012. [DOI] [PubMed]
- Van Thuy P, Berger J, Nakanishi Y, Khan NC, Lynch S, Dixon P. The use of NaFeEDTA-fortified fish sauce is an effective tool for controlling iron deficiency in women of childbearing age in rural Vietnam. The Journal of nutrition. 2005;135(11):2596–2601. doi: 10.1093/jn/135.11.2596. [DOI] [PubMed] [Google Scholar]
- Sadighi J, Mohammad K, Sheikholeslam R, Amirkhani MA, Torabi P, Salehi F, Abdolahi Z. Anaemia control: lessons from the flour fortification programme. Public health. 2009;123(12):794–799. doi: 10.1016/j.puhe.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Hotz C, Porcayo M, Onofre Gn, GarcÃa-Guerra A, Elliott T, Jankowski S, Greiner T. Efficacy of iron-fortified Ultra Rice in improving the iron status of women in Mexico. Food & Nutrition Bulletin. 2008;29(2):140–149. doi: 10.1177/156482650802900208. [DOI] [PubMed] [Google Scholar]
- Blanco-Rojo R, Perez-Granados AM, Toxqui L, Gonzalez-Vizcayno C, Delgado MA, Vaquero MP. Efficacy of a microencapsulated iron pyrophosphate-fortified fruit juice: a randomised, double-blind, placebo-controlled study in Spanish iron-deficient women. British Journal of Nutrition. 2011;105(11):1652. doi: 10.1017/S0007114510005490. [DOI] [PubMed] [Google Scholar]
- Biebinger R, Zimmermann MB, Al-Hooti SN, Al-Hamed N, Al-Salem E, Zafar T, Kabir Y, Petry N, Hurrell RF. Efficacy of wheat-based biscuits fortified with microcapsules containing ferrous sulfate and potassium iodate or a new hydrogen-reduced elemental iron: a randomised, double-blind, controlled trial in Kuwaiti women. British Journal of Nutrition. 2009;102(09):1362–1369. doi: 10.1017/S0007114509990353. [DOI] [PubMed] [Google Scholar]
- Bhutta ZA, S R. Global nutrition epidemiology and trends. 2012. [DOI] [PubMed]
- Dean SV, Lassi ZS, Imam AM, Bhutta ZA. Preconception care: promoting reproductive planning. Reproductive Health. 2014. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peer review reports.


