Abstract
Amphibian population declines and extinctions are occurring even in the world's least impacted areas. The introduction and spread of nonnative predators is one of many proposed causes of amphibian declines. Correlational studies have shown a negative relationship between introduced fishes and declining amphibians, but little direct experimental evidence is available. This study experimentally manipulated the presence and absence of widely introduced salmonids rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelinus fontinalis) to test the hypothesis that their introduction has contributed to the decline of the mountain yellow-legged frog (Rana muscosa). From 1996 to 2003, the introduced trout were removed from 5 lakes in a remote protected area of the Sierra Nevada, and 16 nearby lakes were used as controls, 8 with introduced trout and 8 without. To determine the vulnerable life stage, rainbow trout were placed in cages in three lakes containing amphibians. Removal of introduced trout resulted in rapid recovery of frog populations, and, in the caging experiment, tadpoles were found to be vulnerable to trout predation. Together, these experiments illustrate that introduced trout are effective predators on R. muscosa tadpoles and suggest (i) that the introduction of trout is the most likely mechanism responsible for the decline of this mountain frog and (ii) that these negative effects can be reversed.
Amphibian population declines are occurring worldwide, many in habitats regarded to be impacted little by human activities (1, 3). In theory, because amphibians have small home ranges, their populations should be secure in large parks and other protected habitats (4). Thus, the rapid decline and extinction of amphibian species from such areas is of great concern. Hypothesized mechanisms for declines in protected areas include emerging diseases (5, 6), UV radiation and climate change (7-9), increased levels of air pollution and pesticide use (10), introductions and spread of nonnative predators (11-15), and synergistic interactions (7, 16, 17). The direct effects of introduced predators have received less attention than the other factors, perhaps because the scale of the effect is usually thought to be localized (13). Many amphibian species that have declined or gone extinct are associated with montane aquatic habitats (18). Nonnative predators such as salmonid fishes are commonly introduced into aquatic ecosystems by humans (19), even in “protected” areas (20), and therefore may be an important factor in worldwide amphibian declines.
Predatory fishes are a major force structuring amphibian assemblages, particularly in permanent bodies of water because they can alter the distribution and abundance patterns of amphibians by extirpating local populations (21-23). Nonindigenous fishes have been extensively introduced into many naturally fishless areas on every continent except Antarctica (19), and permanent bodies of water in mountainous areas are often targets for introductions (20, 24). Although some salmonids such as Atlantic and Coho salmon (Salmo salar and Oncorhynchus kisutch) were introduced to establish commercial fisheries (25), others such as rainbow trout (Oncorhynchus mykiss), brown trout (Salmo trutta), and brook trout (Salvelinus fontinalis) were intended for recreational fishing (20, 24, 26). In the western U.S., an area experiencing severe amphibian declines, trout are reared in fish hatcheries and subsequently delivered by airplanes to remote regions, many designated as “wilderness” (19, 20). Thousands of lakes (>7,000) are stocked with trout on a regular basis (20), and trout now occupy up to 95% of larger, deeper mountain lakes in the western U.S. (20). Salmonids, especially trout, are highly effective predators (27), readily establish self-sustaining populations, and successfully colonize new habitats (28). These predatory fish exert strong effects on aquatic food webs (26, 29, 30). Surveys have shown that, where introduced trout are present, amphibians are often absent, thus possibly implicating introduced trout in amphibian declines (12, 31, 32). Introduction of nonnative trout continues on a large geographic scale, yet few studies have attempted to directly assess the effect introduced trout predation may be having on threatened amphibian populations (11). In addition, with the exception of a single study (33), no evidence exists to suggest whether impacts of nonnative trout are reversible.
The mountain yellow-legged frog, Rana muscosa, exists almost entirely on protected land in mountainous areas of California (Fig. 1) and yet has declined dramatically (34-36). The disjunct southern populations are federally listed as endangered, and the listing of the remaining Sierra Nevada populations as endangered was recently found to be “warranted” (37). This frog is an apparently ancient species comprising four genetically distant phylogeographic units, three of which are on the brink of extinction (38). Previous studies on amphibian decline have been criticized for lacking historical data against which to judge declines (39, 40). However, extensive museum records exist for this species (41), and these data have been used to confirm the decline of this once abundant montane frog (36). The mountain yellow-legged frog occurs mostly at high elevation (up to 3,700 m) and has a larval stage lasting up to 4 yr, making the species dependent on permanent bodies of water for successful reproduction (42). This frog apparently evolved without intense fish predation because nearly its entire range was historically fishless (41, 43). Trout introductions into Sierra Nevada fishless ecosystems began in the late 1800s (44). By the 1950s and 1960s, fish hatcheries provided hundreds of thousands of juvenile trout, and airplanes were used to add trout to even the most remote lakes (44). Wilderness areas and national parks in the U.S. are some of the most protected habitats on earth, yet today introduced trout occupy up to 90% of the habitat of R. muscosa in these areas in the Sierra Nevada (12). However, because some R. muscosa individuals overlap with introduced trout (12) and because the most severe declines were documented nearly 100 yr after trout introductions began, other hypotheses have been favored to explain the decline of the mountain yellow-legged frog (36).
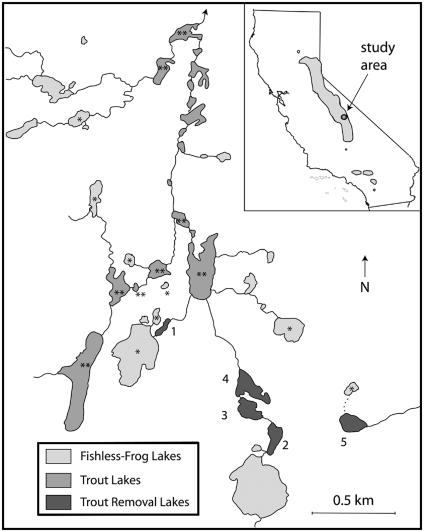
Fig. 1.
Distribution of R. muscosa and introduced trout in the Sixty Lake Basin, Kings Canyon National Park, CA. This 32-km2 basin contains 81 water bodies, of which only the largest are shown. Lakes labeled 1-5 are the trout removal lakes; fishless control lakes (*) and fish control lakes (**) are also shown. (Inset) The entire range of R. muscosa, including the Transverse Ranges in Southern California, and the Sierra Nevada, where this study took place.
To test whether introduced trout have a direct negative effect on R. muscosa populations, I conducted an 8-yr study in a remote, historically fishless area of the Sierra Nevada (Fig. 1; elevation 3,300 m). The site contains one of the largest remaining R. muscosa population complexes and therefore provides an opportunity to directly examine the interactions between introduced trout and R. muscosa. First, before manipulation, distribution and abundance patterns of R. muscosa and introduced trout were assessed. Introduced trout were then removed from five natural lakes, and the responses of frog populations at those lakes were compared with that at unmanipulated lakes. To test whether predation was the mechanism responsible for the pattern, introduced trout were placed within enclosures in three fishless lakes containing R. muscosa populations.
Methods
Premanipulation Frog and Fish Distribution. The Sixty Lake Basin (36.8186° north, 118.4251° west; 3,000- to 3,500-m elevation) Kings Canyon National Park, CA was selected as the study area because large populations of frogs were suspected to be in the area (unpublished data). In 1996, 1 yr before experiments began, 50 lakes and ponds in the study area (Fig. 1) were surveyed for R. muscosa and introduced trout. Standard visual encounter surveys (45) along shorelines were conducted for postmetamorphic R. muscosa (adults plus juveniles) and tadpoles (all size classes combined) during the warmest time of day, between 1000 and 1500 hours. The distribution of introduced trout was determined by using visual surveys for ephemeral water bodies and by using sinking monofilament gill nets in all permanent lakes (>1.5-m depth) (12). A single hand-deployed gill net was set for 8-24 h in each lake and was set perpendicular to shore. I compared the mean number of postmetamorphic frogs and tadpoles per 10 m of shoreline in lakes with introduced trout vs. in fishless lakes using a one-way ANOVA (46).
Trout Removal Experiment. To test whether introduced trout limit the size and distribution of R. muscosa populations, trout were removed from five lakes by using 35 hand-deployed gill nets (47). Removals began July 20, 1997, July 15, 1998, and August 15, 1999, in lakes 1, 2, and 3, respectively (Fig. 1). In August 2001, the National Park Service began removing trout from lakes 4 and 5 (Fig. 1). For this experiment, it was not possible to choose removal lakes at random. To prevent trout from recolonizing removal lakes during the course of the experiment, lakes selected had (i) no upstream populations of trout and (ii) downstream barriers (i.e., a waterfall with no jump pool) to prevent trout reascension. The sheer effort required to eradicate fish from entire lakes did not allow for removal to begin on all experimental lakes in the same year. To assess the consequences of fish removal on frog populations, I conducted counts of R. muscosa in the trout removal lakes (n = 5) and in a subset of fish-containing lakes (n = 8; “fish controls”) and fishless lakes (n = 8; “fishless controls”) from 1997 to 2003 (Fig. 1). Counts were conducted in the 21 lakes approximately every 2 wk from 1997 to 2001, twice per summer in 2002, and three times in 2003 (see Fig. 3). For the statistical analyses, in fish removal lakes, multiple counts from each lake were averaged, and one value was used for each year for each lake whereas, in the fish control and fishless control lakes, an average of all years was used for each lake in the comparison. Shoreline snorkeling surveys were conducted in experimental lakes and control lakes to search for young of year trout and frog egg masses. Lake perimeters were calculated by using a geographic information system and were used to standardize frog counts (number per 10 m of shoreline). I compared the mean density of postmetamorphic frogs and tadpoles in experimental trout-removal lakes (n = 5) vs. in fish controls lakes (n = 8) and fishless controls (n = 8) by using three statistical tests. First, to ensure that the five lakes I nonrandomly selected for removal were not different from fish control lakes in their premanipulation condition, I used a t test to compare the density of postmetamorphic frogs and tadpoles between these two lake types 1 yr before fish removal. Second, to determine whether fish removal led to an increase in frog populations, I compared the density of postmetamorphic frogs and tadpoles in the fish-removal lakes vs. in fish control lakes 1 yr after fish eradication began (t test). Finally, to determine whether frog populations in fish removal lakes reached the levels seen in fishless control lakes, I compared the density of postmetamorphic frogs and tadpoles in the fish-removal lakes vs. fishless control lakes (t test) 3 yr after eradication began. The last test included only three of the five trout-removal lakes because fish eradication did not begin until 2001 in lakes 4 and 5.
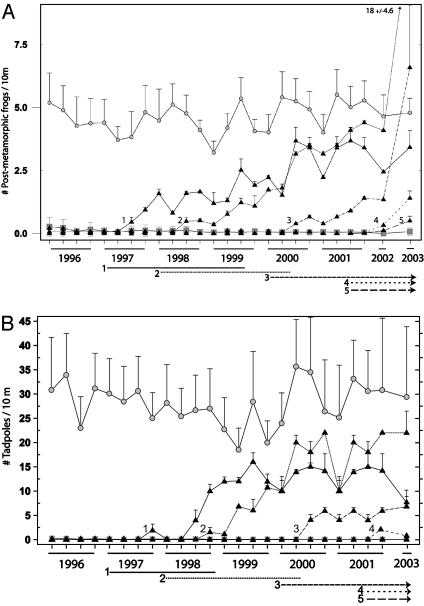
Fig. 3.
Density (mean ± SE) of postmetamorphic R. muscosa (A) and larval R. muscosa (B) in 21 lakes from 1996 to 2003. Filled triangles designate fish removal lakes (n = 5); numbers correspond to lake numbers in Fig. 1. Shaded circles are fishless control lakes (n = 8), and shaded squares are fish control lakes (n = 8). Horizontal lines at the bottom of each figure indicate the trout removal period for each of the removal lakes, and numbers correspond to individual lake numbers. No tadpole counts were conducted in 2002.
Predation on Tadpoles. To test whether trout predation on tadpoles was potentially responsible for the observed pattern of nonoverlap between trout and R. muscosa, in 1998 I constructed mesh enclosures (one per lake) along shorelines of three fishless lakes containing large R. muscosa populations. Enclosures were rectangular in shape (5 m × 3 m), with shoreline serving as one edge. Each enclosure consisted of a mesh fence buried in the substrate and extending 30 cm above waterline (max water depth = 75 cm). Bamboo poles secured the mesh fence. Enclosures were placed at known R. muscosa breeding sites before breeding began. Because some frogs laid egg masses in the cages, I did not move any egg masses or manipulate them in any way. Rainbow trout were captured with hand nets in nearby lakes and added to each enclosure (two trout per enclosure; mean total length = 33.5 cm). When tadpoles hatched from the egg masses, I recorded the number of tadpoles attacked by trout during daily 20-min observation periods at each enclosure. R. muscosa tadpoles take several years to metamorphose (42), and some that hatched in previous years (Gosner stage > 36) were confined inside the enclosures during cage construction. Attacks on these tadpoles were recorded separately from attacks on the newly hatched tadpoles. Enclosures were checked daily for tadpole carcasses. The experiment ran from June 15, 1998 to June 29, 1998, after which trout and cages were removed. One of the six trout died 12 h after it was placed in the enclosure. The remaining five trout survived throughout the entire 14-day experiment.
Results
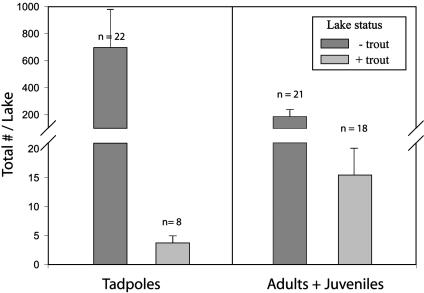
Premanipulation Comparison. In 1996, of the 50 lakes surveyed, 20 contained introduced trout (19 with rainbow trout, O. mykiss, and one, lake 5, with brook trout, S. fontinalis; Fig. 1). Mountain yellow-legged frog tadpoles were found in 30 lakes (22 fishless lakes and 8 trout lakes); postmetamorphic frogs (adults and juveniles) occurred in 39 lakes (21 fishless lakes and 18 trout-occupied lakes). Densities of postmetamorphic frogs and tadpoles were significantly higher in fishless lakes than in lakes containing introduced trout (tadpoles, F = 25.78; df = 1,49; P < 0.0001; adults plus juveniles, F = 19.55; df = 1,49; P < 0.0001; Fig. 2). A total of 30 large tadpoles (above Gosner stage 36; total length = 9-11 cm) were found in 8 fish lakes. In every case, tadpoles were found in lakes directly connected to fishless, frog-containing lakes located immediately upstream (<10 m). Although only data from 1996 are presented in Fig. 2, the analysis was repeated each year (excluding manipulated lakes) with the same result: R. muscosa overlapped with trout but only in small numbers and in close proximity to source populations.
Fig. 2.
Density (mean ± SE) of postmetamorphic and larval R. muscosa (no. per 10 m of shoreline) in trout-containing lakes vs. fishless lakes in the Sixty Lake Basin before experimental manipulations (1996). The number of lakes in each category is shown above each bar.
Trout Removal Experiment. The deployment of gill nets quickly depleted populations of introduced trout in the five removal lakes. By the end of the experiment, trout were extirpated from three of five lakes (lakes 1-3) and greatly reduced in the remaining two lakes. Brook trout in lake 5 were the most problematic to remove. Small numbers of young of year trout continued to be caught in lake 4 and especially in lake 5 in 2003; however, repeated gill net sets in lakes 1-3 failed to capture any trout by 2003. In the premanipulation comparison, the number of postmetamorphic frogs in lakes 1-5 did not differ from fish controls [0.048 ± 0.037 (n = 5) and 0.09 ± 0.03 (n = 8) (mean ± SE) postmetamorphic frogs per 10 m in removal lakes and fish control lakes, respectively; P = 0.39, t test]. The number of tadpoles also did not differ between removal and fish control lakes before manipulation [0.025 ± 0.04 (n = 5) and 0.097 ± 0.03 (n = 8) (mean ± SE) tadpoles per 10 m in removal lakes and fish control lakes, respectively; P = 0.174, t test]. The number of postmetamorphic R. muscosa in removal lakes 1 yr after fish removal began was significantly greater than in fish control lakes [Fig. 3A; 0.974 ± 0.12 (n = 5) and 0.0811 ± 0.09 (n = 8) (mean ± SE) postmetamorphic frogs per 10 m, in removal lakes and fishless control lakes, respectively; P < 0.0001, t test]. The same comparison for the number of tadpoles also showed a significant difference between the removal lakes and fish control lakes [Fig. 3B; 8.113 ± 2.35 (n = 5) and 0.102 ± 1.75 (n = 8) (mean ± SE) tadpoles per 10 m in removal lakes and fishless control lakes, respectively; P = 0.0183, t test]. Three years after removals began, there was no significant difference in postmetamorphic frog counts when comparing removal lakes and fishless control lakes [Fig. 3A; 6.85 ± 1.46 (n = 3) and 4.73 ± 0.89 (n = 8) (mean ± SE) postmetamorphic frogs in removal lakes and fishless control lakes, respectively; P = 0.24, t test]. Additionally, 3 yr after fish removals began, counts of tadpoles were not significantly different between removal lakes and fishless control lakes [Fig. 3B; 10.1 ± 10 (n = 3) and 29.62 ± 6.1 (n = 8) (mean ± SE) tadpoles per 10 m in removal lakes and fishless control lakes, respectively; P = 0.14, t test].
Evidence of successful frog breeding (egg masses and newly hatched tadpoles) was found in three of five trout removal lakes soon after initiation of trout removal. One year after trout removal began, frog eggs were found in lakes 1 and 2. In lake 3, ≈500 newly hatched tadpoles (total length <15 mm) were found 2 yr after removal began. From 1997 to 2003, frog egg masses were found yearly in all eight fishless control lakes, but none was found in any of the fish control lakes.
Predation on Tadpoles. In 1998, rainbow trout were observed eating R. muscosa tadpoles in all three enclosures placed in previously known frog-breeding sites. Seven egg masses (mean diameter = 4.5 cm; SE 1.2 cm) containing between 150 and 400 eggs each were deposited by frogs inside the trout enclosures, and many more were laid immediately next to the enclosures but not accessible to trout (mean = 50.33; SE = 2.4). During observation periods, trout did not attack egg masses but struck at and consumed tadpoles as they hatched. None of the newly hatched tadpoles within trout enclosures were found alive at the end of the experiment. Egg masses laid immediately outside enclosures hatched successfully. Sixty-five strikes by trout on large tadpoles were recorded (mean = 21.6 strikes per cage; SD = 11.6). In 63 of the strikes, tadpoles did not survive whereas, in two others, they escaped by hiding in the mud substrate. In addition, carcasses of 20 large (>5 cm TL) tadpoles were recovered from inside enclosures during the experiment.
Discussion
Several studies conducted in the western U.S. have provided correlational evidence of a strong negative association between introduced trout and amphibians (12, 31, 48). A similar negative correlation between introduced trout and mountain yellow-legged frogs was evident in my Sierra Nevada study basin (Fig. 2). The impact of introduced trout on amphibians is not unique to the American West. For example, in Costa Rica, harlequin frogs (Atelopus sp) disappeared in areas after trout introductions (49), amphibian species richness in Spain was reduced in areas with introduced trout (32), and field experiments in Australia implicate nonnative trout in amphibian declines (11).
In this study, I observed direct predation on R. muscosa tadpoles by introduced trout, and experimental removal of trout from lakes resulted in rapid increases in R. muscosa populations (Fig. 3 A and B). Just 1 yr after removals began, significant differences were detected in the number of postmetamorphic and larval R. muscosa in removal lakes as compared with fish control lakes. After 3 yr of growth without fish predators, removal lake frog populations (lakes 1-3, Fig. 1) were not distinguishable statistically from frog control lakes (n = 8). Population recovery in fish removal lakes began quickly because large nearby source populations (fishless controls) provided ample colonists and because frogs began reproducing in the fish removal lakes. R. muscosa eggs and newly hatched tadpoles were found in the three fish removal lakes with the greatest response (lakes 1-3). During the same period, hundreds of egg masses were regularly found in R. muscosa source populations whereas no R. muscosa egg masses or newly hatched tadpoles were ever found in fish control lakes (50). Interestingly, the removal lake that responded most slowly (lake 5) was different from the other trout removal lakes in that it (i) contained introduced brook trout (which were much more difficult to eradicate), (ii) was not connected by stream to a frog source population, and (iii) did not contain any evidence of R. muscosa reproduction by the end of the study. This study supports the previous prediction that R. muscosa populations can recover after trout disappearance (33) and underscores the importance of well connected source populations.
Trout introductions into the Sierra Nevada began in the late 1800s (44), and the vulnerability of R. muscosa to trout predation was noted in the earliest biological surveys of the Sierra Nevada (41, 51), but the accounts of a regional collapse of R. muscosa occurred much later (36). This delay explains why trout predation alone is not favored as the major reason for R. muscosa decline (36). The delay between the first trout introductions and the collapse of R. muscosa populations in the Sierra Nevada probably occurred because the initial effects of trout introductions were localized. During roughly the first 75-80 yr of introductions, trout fingerlings were carried in milk jugs and ferried over mountain passes with mules, technology that limited introductions to a small portion of the fishless Sierran ecosystems (44). By the late 1950s and 1960s, however, government agencies began using airplanes to drop fingerling trout by the hundreds of thousands into the majority of Sierran lakes (44). Trout introductions could be responsible for the sudden collapse of the species (i) if there was a dramatic drop in tadpole survival due to new fish predators, (ii) if there was a subsequent lapse of recruitment in young frogs, and (iii) if long-lived adults persisted even in fish-infested areas for some time but eventually died without being replaced. Additionally, small frog populations in suboptimal habitats isolated from fish could persist for some time but are hypothesized to go extinct even without additional fish introductions (52, 53). The lifespan of R. muscosa is not known, but other Ranid frogs (family Ranidae) are known to live many years (54). Metapopulation models predict that habitat loss and fragmentation (in this case trout eliminating frog habitat) can reduce the metapopulation capacity of a landscape and thus increase chance of extinction (52). However, there is usually a time lag after habitat loss before extinction occurs (52). This scenario fits the R. muscosa decline example well and, along with the results of this study, suggests that the introduced trout hypothesis has been undervalued in the analysis of the overall collapse of this frog species.
The alarming decline and, in some cases, extinctions of amphibians in protected areas around the world probably do not have a single cause (55). Understanding mechanisms responsible for declines is important because negative impacts may go unnoticed even though they may be widespread in protected and seemingly pristine areas. Ecologists consider predation to be a significant force shaping amphibian assemblages (2, 56-59) and introduced predators a major threat to worldwide biodiversity. The results of this study show that introduced trout can have dramatic effects on populations of montane amphibians but also demonstrates that amphibian populations have the ability to quickly recolonize habitat and establish large populations in a short period. Many of the most perplexing frog declines have occurred in protected montane habitats (1, 18, 36), areas that also have been targets for salmonid introductions. Amphibian species that evolved without fish predators are especially at risk from introduced fishes; therefore, conservation efforts should pay careful attention to fish introductions into these historically fishless areas because preventing fish introductions is likely to be easier than subsequently removing nonnative fish populations.
Acknowledgments
D. B. Wake, M. E. Power, W. Sousa, R. Knapp, and C. Briggs made helpful comments on the manuscript. I thank D. Graber, H. Werner, D. Boiano, R. Sanger, and G. Durkee from the National Park Service and J. Romansic, L. Chan, and T. Tunstall for help with extensive field work. V.T.V. was supported by National Institutes of Health/National Science Foundation Ecology of Infectious Disease Program Grant R01ES012067 from the National Institute of Environmental Health Sciences and by U.S. Geological Survey/Biological Resource Division/Natural Resources Preservation Program Grant 99WRSA0365.
References
- 1.Houlahan, J. E., Findlay, C. S., Schmidt, B. R., Meyer, A. H. & Kuzmin, S. L. (2000) Nature 404, 752-755. [DOI] [PubMed] [Google Scholar]
- 2.Resetarits, W. J., Jr., & Wilbur, H. M. (1989) Ecology 70, 220-228. [Google Scholar]
- 3.Alford, R. A. & Richards, S. J. (1999) Annu. Rev. Ecol. Syst. 30, 133-165. [Google Scholar]
- 4.Blaustein, A. R. & Wake, D. B. (1990) Trends Ecol. Evol. 5, 203-204. [Google Scholar]
- 5.Berger, L., Speare, R., Daszak, P., Green, D. E., Cunningham, A. A., Goggin, C. L., Slocombe, R., Ragan, M. A., Hyatt, A. D., McDonald, K. R., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 9031-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daszak, P., Berger, L., Cunningham, A. A., Hyatt, A. D., Green, D. E. & Speare, R. (1999) Emerg. Infect. Dis. 5, 735-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiesecker, J. M., Blaustein, A. R. & Belden, L. K. (2001) Nature 410, 681-684. [DOI] [PubMed] [Google Scholar]
- 8.Blaustein, A. R., Hoffman, P. D., Hokit, D. G., Kiesecker, J. M., Walls, S. C. & Hays, J. B. (1994) Proc. Natl. Acad. Sci. USA 91, 1791-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pounds, J. A., Fogden, M. P. L. & Campbell, J. H. (1999) Nature 398, 611-615. [Google Scholar]
- 10.Davidson, C., Shaffer, H. B. & Jennings, M. R. (2001) Ecol. Appl. 11, 464-479. [Google Scholar]
- 11.Gillespie, G. R. (2001) Biol. Conserv. 100, 187-198. [Google Scholar]
- 12.Knapp, R. A. & Matthews, K. R. (2000) Conserv. Biol. 14, 428-438. [Google Scholar]
- 13.Adams, M. J. (2000) Ecol. Appl. 10, 559-568. [Google Scholar]
- 14.Lawler, S. P., Dritz, D., Strange, T. & Holyoak, M. (1999) Conserv. Biol. 13, 613-622. [Google Scholar]
- 15.Kiesecker, J. M. & Blaustein, A. R. (1997) Ecology 78, 1752-1760. [Google Scholar]
- 16.Relyea, R. A. & Mills, N. (2001) Proc. Natl. Acad. Sci. USA 98, 2491-2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiesecker, J. M. & Blaustein, A. R. (1995) Proc. Natl. Acad. Sci. USA 92, 11049-11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams, S. E. & Hero, J.-M. (1998) Proc. R. Soc. London Ser. B 265, 597-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moyle, P. B. (1986) in Ecology of Biological Invasions of North America and Hawaii, eds. Mooney, H. A. & Drake, J. A. (Springer, New York), pp. 27-43.
- 20.Bahls, P. (1992) Northwest Sci. 66, 183-193. [Google Scholar]
- 21.Petranka, J. W. (1983) Copeia 1983, 624-628. [Google Scholar]
- 22.Hero, J.-M., Magnusson, W. E., Rocha, C. F. D. & Catterall, C. P. (2001) Biotropica 33, 131-141. [Google Scholar]
- 23.Hecnar, S. J. & M'Closkey, R. T. (1997) Biol. Conserv. 79, 123-131. [Google Scholar]
- 24.Donald, D. B. (1987) North Am. J. Fish. Manage. 7, 545-553. [Google Scholar]
- 25.Soto, D., Jara, F. & Moreno, C. (2001) Ecol. Appl. 11, 1750-1762. [Google Scholar]
- 26.Townsend, C. R. (1996) Biol. Conserv. 78, 13-22. [Google Scholar]
- 27.Flecker, A. S. & Townsend, C. R. (1994) Ecol. Appl. 4, 798-807. [Google Scholar]
- 28.Fausch, K. D., Taniguchi, Y., Nakano, S., Grossman, G. D. & Townsend, C. R. (2001) Ecol. Appl. 11, 1438-1455. [Google Scholar]
- 29.Power, M. E. (1990) Science 250, 811-814. [DOI] [PubMed] [Google Scholar]
- 30.Power, M. E. (1992) Ecology 73, 1675-1688. [Google Scholar]
- 31.Bradford, D. F. (1989) Copeia 1989, 775-778. [Google Scholar]
- 32.Brana, F., Frenchilla, L. & Orizaola, G. (1996) Herpetol. J. 6, 145-148. [Google Scholar]
- 33.Knapp, R. A., Matthews, K. R. & Sarnelle, O. (2001) Ecol. Monogr. 71, 401-421. [Google Scholar]
- 34.Bradford, D. F. (1991) J. Herpetol. 25, 174-177. [Google Scholar]
- 35.Bradford, D. F., Graber, D. M. & Tabatabai, F. (1994) Southwest. Nat. 39, 323-327. [Google Scholar]
- 36.Drost, C. A. & Fellers, G. M. (1996) Conserv. Biol. 10, 414-425. [Google Scholar]
- 37.Federal Register 67 127 (2002), pp. 44382-44392.
- 38.Macey, J. R., Strasburg, J. L., Brisson, J. A., Vredenburg, V. T., Jennings, M. & Larson, A. (2001) Mol. Phylogenet. Evol. 19, 131-143. [DOI] [PubMed] [Google Scholar]
- 39.Pechmann, J. H. K., Scott, D. E., Semlitsch, R. D., Caldwell, J. P., Vitt, L. J. & Gibbons, J. W. (1991) Science 253, 892-895. [DOI] [PubMed] [Google Scholar]
- 40.Pechmann, J. & Wilbur, H. (1994) Herpetologica 50, 65-84. [Google Scholar]
- 41.Grinnell, J. & Storer, T. (1924) Animal Life in the Yosemite (Univ. of Calif. Press, Berkeley).
- 42.Bradford, D. F. (1983) Ecology 64, 1171-1183. [Google Scholar]
- 43.Camp, C. L. (1917) Univ. Calif. Publ. Zool. 17, 59-62. [Google Scholar]
- 44.Pister, E. P. (2001) Ecosystems 4, 279-286. [Google Scholar]
- 45.Crump, M. L. & Scott, N. J. (1994) in Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians, eds. Heyer, W. R., Donnelly, M. A., McDiarmid, R. W., Hayek, L.-A. C. & Foster, M. S. (Smithsonian Institution Press, Washington, DC), pp. 84-92.
- 46.Zar, J. H. (1999) Biostatistical Analysis (Prentice-Hall, Englewood Cliffs, NJ).
- 47.Knapp, R. A. & Matthews, K. R. (1998) Restor. Ecol. 6, 207-213. [Google Scholar]
- 48.Pilliod, D. S. & Peterson, C. R. (2001) Ecosystems 4, 322-333. [Google Scholar]
- 49.Pough, F. H., Adrews, R. M., Cadle, J. E., Crump, M. L., Savitsky, A. H. & Wells, K. D. (1998) Herpetology (Prentice-Hall, Englewood Cliffs, NJ).
- 50.Vredenburg, V. T. (2002) Ph.D. thesis (Univ. of California, Berkeley).
- 51.Needham, P. H. & Vestal, E. H. (1938) Calif. Dept. Fish Game 24, 273-279. [Google Scholar]
- 52.Hanski, I. & Ovaskainen, O. (2002) Conserv. Biol. 16, 666-673. [Google Scholar]
- 53.Bradford, D. F., Tabatabai, F. & Graber, D. M. (1993) Conserv. Biol. 7, 882-888. [Google Scholar]
- 54.Esteban, M., Garcia-Paris, M. & Castanet, J. (1996) Can. J. Zool. 74, 1914-1921. [Google Scholar]
- 55.Blaustein, A. R. & Kiesecker, J. M. (2002) Ecol. Lett. 5, 597-608. [Google Scholar]
- 56.Werner, E. E. & McPeek, M. A. (1994) Ecology 75, 1368-1382. [Google Scholar]
- 57.Werner, E. E. (1998) in Experimental Ecology: Issues and Perspectives, eds. Resetarits, W. J., Jr., & Bernardo, J. (Oxford Univ. Press, Oxford), pp. 3-26.
- 58.Wilbur, H. M. (1997) Ecology 78, 2279-2302. [Google Scholar]
- 59.Relyea, R. A. (2000) Ecology 81, 2278-2289. [Google Scholar]