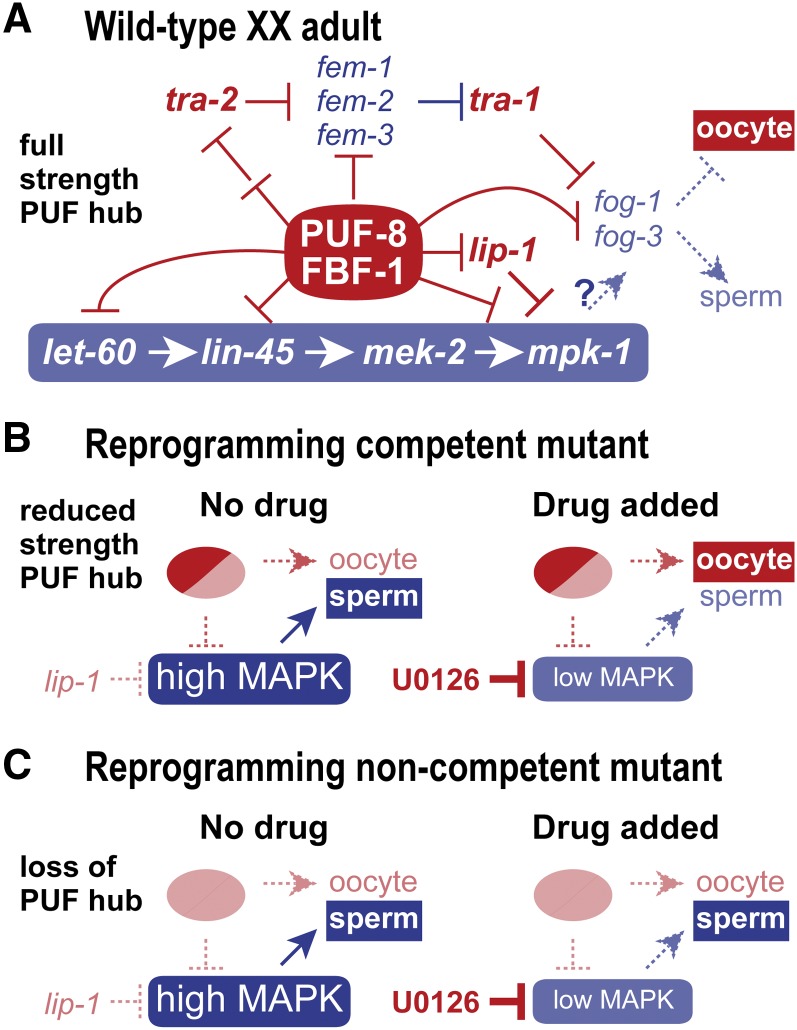
Figure 7.
Model for genotype-specific basis for reprogramming competence. (A) Simplified network for gamete fate specification in wild-type XX adults. The sex determination pathway is set to the female mode and oocyte fate specification; MAP kinase activity is low and therefore does not promote the sperm fate; PUF-8 and FBF-1 promote the oocyte fate via repression of both sex determination and MAP kinase pathways as well as repression of terminal gamete fate regulators fog-1 and fog-3. This model is based largely on established regulatory relationships, but also posits the existence of a PUF hub composed of PUF-8 and FBF-1 RNA-binding proteins. Relevant MPK-1 substrates are not known, but FOG-1 and FOG-3 proteins are candidates. See text for further explanation and references. (B) Model for sperm/oocyte specification in mutants with a reprogramming competent genotype (puf-8; lip-1 and fbf-1; lip-1). Without drug (left), germlines are set to the male mode of sperm fate specification. The PUF hub operates at reduced strength because one PUF protein is removed, and MAP kinase is hyperactivated because lip-1 is removed; the reduced-strength PUF hub is postulated to generate a sexually intermediate molecular signature. With drug (right), the U0126 MEK inhibitor reduces MAP kinase hyperactivation and permits the reduced-strength PUF hub to drive the oocyte fate. (C) Model for sperm/oocyte specification in mutants with a noncompetent genotype (puf-8 fbf-1; lip-1 depicted here). Without drug (left) or with drug (right), elimination of the PUF hub prevents oocyte fate specification regardless of MAP kinase activity. A similar explanation would hold for puf-8 fbf-1, which would not experience the hyperactivated ERK/MAPK depicted here for puf-8 fbf-1; lip-1.