Abstract
It is unknown how pelagic marine protists undergo diversification and speciation. Superficially, the open ocean appears homogeneous, with few clear barriers to gene flow, allowing extensive, even global, dispersal. Yet, despite the apparent lack of opportunity for genetic isolation, diversity is prevalent within marine taxa. A lack of candidate isolating mechanisms would seem to favor sympatric over allopatric speciation models to explain the diversity and biogeographic patterns observed in the oceans today. However, the ocean is a dynamic system, and both current and past circulation patterns must be considered in concert to gain a true perspective of gene flow through time. We have derived a comprehensive picture of the mechanisms potentially at play in the high latitudes by combining molecular, biogeographic, fossil, and paleoceanographic data to reconstruct the evolutionary history of the polar planktonic foraminifer Neogloboquadrina pachyderma sinistral. We have discovered extensive genetic diversity within this morphospecies and that its current “extreme” polar affinity did not appear until late in its evolutionary history. The molecular data demonstrate a stepwise progression of diversification starting with the allopatric isolation of Atlantic Arctic and Antarctic populations after the onset of the Northern Hemisphere glaciation. Further diversification occurred only in the Southern Hemisphere and seems to have been linked to glacial-interglacial climate dynamics. Our findings demonstrate the role of Quaternary climate instability in shaping the modern high-latitude plankton. The divergent evolutionary history of N. pachyderma sinistral genotypes implies that paleoceanographic proxies based on this taxon should be calibrated independently.
The lack of apparent barriers to gene flow in the open ocean coupled with the enormous population sizes and high inter-oceanic dispersal potential of pelagic taxa (1, 2) should greatly limit their ability to diversify and speciate through allopatric processes (3, 4). Yet, recent molecular data have revealed a previously unrecognized high degree of diversity in many pelagic groups (2-9). A variety of evolutionary processes, both allopatric and sympatric, have been proposed to explain the presence of such genetic diversity under open-ocean conditions (3, 4). To investigate which processes may be responsible, it is vital to have a clear perspective of the factors controlling reproductive isolation in the pelagic ecosystem. The pelagic planktonic foraminifera provide an ideal tool for addressing these important questions. The evolutionary history of individual morphospecies of planktonic foraminifera can be traced back in time with high resolution by using the enormous archive of well dated deep-sea sediment cores (10). In combination with paleoceanographic evidence, this makes it possible to interpret the findings of foraminiferal molecular genetic studies within a robust historical context and hence unravel mechanisms of diversification within the group and their links with global climate change.
Individual morphospecies of planktonic foraminifera are distributed within latitudinal provinces that follow surface temperature gradients, which means that subpolar and polar species effectively have bipolar distributions. Yet, it has been shown that transtropical gene flow occurs between populations of planktonic foraminiferal morphospecies predominant in Atlantic subpolar waters (2). The tropical/subtropical province therefore does not act as an allopatrically isolating barrier to gene flow between subpolar populations adapted to similar environmental conditions in opposite hemispheres. Here we investigate the history of gene flow between the most isolated climatic provinces: the Atlantic Arctic and Antarctic polar waters. These waters are environmentally highly distinct, yet both are dominated by one morphospecies of planktonic foraminifera, the left-coiling Neogloboquadrina pachyderma [sinistral (sin)].
Our study focuses on genetic variation in the small subunit (SSU) rRNA gene of N. pachyderma from the Atlantic Arctic and Antarctic subpolar/polar waters and from the Benguela upwelling system. We have discovered several distinct genetic types within the left-coiling polar variety of this morphospecies, lending additional support to the suggestion that biodiversity among planktonic foraminifera has been vastly underestimated when using shell morphology alone to define species (2, 6, 7). We have constructed a phylogeny of the extant representatives of the neogloboquadrinid group and used the molecular data to estimate the time of cryptic divergences within the N. pachyderma (sin) cluster. The effect of global climate change on the evolution of N. pachyderma (sin) and the implications for paleoceano-graphic proxies are discussed.
Materials and Methods
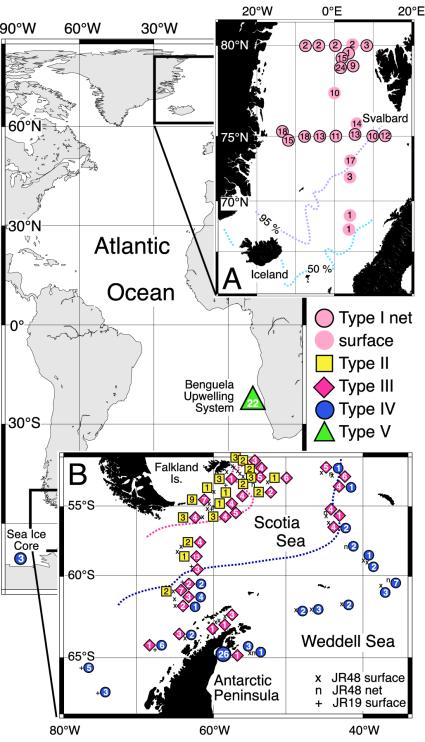
Sampling Localities. In the Arctic Atlantic (Fig. 1 Inset A), foraminifers were collected on board RV Polarstern (ARKXV/1+2, 1999), and specimens were obtained by using multinets (upper 500 m, 63-μm mesh) at 75°N (from 13°W to 13°E) and from a mixture of multinet and surface-pumped water samples (6-m depth, 63-μm mesh) in the Nordic seas between 80°N and 60°N. Twenty-four right-coiling N. pachyderma specimens were also collected from the Denmark Strait (13). Samples from the Benguela system off Namibia were collected from the RV Welwitschia (November 2001) by vertical plankton tow (50 m, 63-μm mesh) along a cruise transect between 2 and 30 nautical miles from the coast at 23°S (Fig. 1). South Atlantic samples were collected in 1997 and 2000 from the RRS James Clark Ross (cruises JR19 and JR48) along transects between the Falkland Islands and the Antarctic Peninsula in the northern Weddell Sea and Scotia Sea (Fig. 1 Inset B). Specimens were obtained either from vertical plankton tows (100 m, 63-μm mesh) or by pumping from 6-m depth through 63- or 125-μm screens. Ice cores were taken in the Bellingshausen Sea during RV Polarstern cruise ANTXVIII, leg 5b (2001; Fig. 1). Ice-core specimens were extracted from between 20- and 40-cm depth (-5.3°Cto - 2.8°C and ≈9.2%thou salinity). Subtropical specimens of Neogloboquadrina dutertrei and Pulleniatina obliquiloculata were collected off the Great Barrier Reef, Australia (14).
Fig. 1.
Sampling localities and distribution of the high-latitude and Benguela left-coiling (sin) N. pachyderma SSU genotypes (n = 566). (Inset A) Distribution pattern of N. pachyderma (sin) type I (n = 216) in the Fram Strait and Norwegian Sea. The contours denote the sediment “core-top” coiling ratio (% sin coiling), delineating the modern N. pachyderma (sin) province (11). (Inset B) Distribution pattern of N. pachyderma (sin) types II (n = 41), III (n = 117), and IV (n = 79) in the subpolar/polar Antarctic. The pink contour delineates the approximate position in austral summer of 2000 of the Sub-antarctic Front and the blue contour of the Polar Front as deduced from its average position (12) and from sea-surface temperature profiles generated during cruise JR48. The main map shows the location of the N. pachyderma (sin) type IV samples (n = 3) collected from the Bellingshausen Sea ice core and the location of the type V samples (n = 22) collected from the Benguela upwelling system.
Isolation and Sequencing of SSU Genes. DNA extraction, amplification by PCR, and automated sequencing of an ≈1,000-bp region of the terminal 3′ end of the foraminiferal SSU rRNA gene were as described (2). The gene fragments for N. pachyderma (dextral) were directly sequenced. Some limited degree of ambiguity was detected in the gene repeats for N. pachyderma (sin), and therefore it was sometimes necessary to clone the PCR products before sequencing. PCR products were cloned by using a pCR 2.1 TOPO TA cloning kit (Invitrogen) and sequenced by using universal primers.
Phylogenetic Analysis. Partial SSU rRNA-encoding DNA (rDNA) sequences were aligned manually within the genetic data environment 2.2 package (15). Six hundred eighty-five unambiguously aligned nucleotide sites were used in analyses incorporating all neogloboquadrinid taxa, with 835 sites used in analyses that excluded the highly divergent right-coiling N. pachyderma SSU genotype. Trees were rooted on the globorotaliid Globorotalia inflata. Phylogenetic trees were constructed by using the neighbor-joining (NJ) (16), Fitch-Margoliash (17), maximum-likelihood (ML) (18), and maximum-parsimony (19) methods within paup* 4.0d64 (20). For the NJ, Fitch-Margoliash, and ML methods a general time-reversible (GTR) model was used (21), with between-site rate variation accounted for by incorporating a proportion of invariant sites (I), with the remaining sites following a gamma distribution (Γ) (22). Parameters were estimated by using likelihood by iteration from an initial NJ tree. Genetic distances were estimated by using the GTR+I+Γ model. Bootstrap resampling (2,000 replicates) was used to assign support to branches in the trees (23).
Molecular Clock Analysis. To test the validity of a molecular clock, a likelihood ratio test was used to test deviations from a clock (24). Divergence times were estimated by using five different approaches: (i) GTR+I+Γ corrected pairwise distances using the equation T = K/2R (T = divergence time, K = pairwise distance, R = rate of substitution expressed as substitutions per site per year); (ii) an ultrametric (linearized clock) ML tree; (iii) Langley-Fitch (LF); (iv) LF local (LFL); and (v) penalized likelihood (PL). The LF, LFL, and PL methods were undertaken in the r8s package (25).
Results
Genetic Variation in N. pachyderma. Five hundred sixty-six specimens of N. pachyderma from the Atlantic Arctic and Antarctic waters and the Benguela upwelling system were examined. One right-coiling SSU genotype (n = 88) and five distinct left-coiling SSU genotypes (differing in their DNA sequences in the amplified SSU fragment) were identified (n = 478). After an extensive investigation of the northern Atlantic subpolar and polar waters (Fig. 1), only a single Northern Hemisphere left-coiling SSU genotype, type I (n = 216), was found. In contrast, four distinct left-coiling SSU genotypes were found in the Southern Hemisphere. Types II-IV were found in the Southern Ocean (Fig. 1). Type II (n = 41) tends to be generally distributed within warmer waters of the Subantarctic Front (Fig. 1). It was less common in the cooler waters to the south of the Subantarctic Front and was not found in the cold waters south of the Polar Front. Type III (n = 117) has a more widespread distribution (Fig. 1). It was most common in waters to the north of the Polar Front. It was less common in the cold waters to the south of the Polar Front, although a few individuals are found in the Antarctic Peninsula margin. Type IV (n = 82) was found only in the cold water, south of the Polar Front, and in the Bellingshausen Sea ice core (Fig. 1). A different N. pachyderma (sin) SSU genotype, type V (n = 22), was found in the Benguela upwelling system off Namibia (Fig. 1). The substantial diversity observed in the polar left-coiling variant N. pachyderma is in stark contrast to the presence of only one SSU genotype of the subpolar right-coiling variety found throughout the high-latitude Atlantic (2, 13, 26).
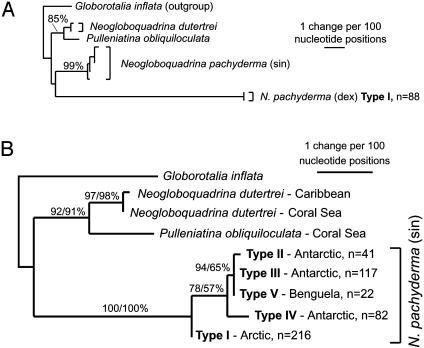
Phylogenetic Trees. The evolutionary relationships in the Neogloboquadrinidae (based on 685 unambiguously aligned nucleotide sites) are shown in Fig. 2A. In agreement with the fossil record, the N. dutertrei and P. obliquiloculata SSU genotypes cluster with 85% NJ bootstrap support. The Atlantic N. pachyderma SSU genotypes are principally divided into two distinct lineages. The first comprises a group of predominantly left-coiling SSU genotypes that cluster with 99% NJ bootstrap support. The second is represented by a single, predominantly right-coiling SSU genotype, N. pachyderma (dextral) type I. The right-coiling SSU genotype is highly divergent from the left-coiling SSU genotypes and falls on an exceptionally long branch in the tree (Fig. 2 A).
Fig. 2.
SSU rDNA phylogenetic trees highlighting the evolutionary relationships among the Neogloboquadrinidae. Trees were constructed both including (A) (685 sites, NJ) and excluding (B) (835 sites, ML) the highly divergent right-coiling (dextral) N. pachyderma SSU genotype. G. inflata was used as an outgroup. Bootstrap values (NJ/ML; expressed as a percentage) indicate support for branches in the trees.
To better resolve the relationships among the N. pachyderma left-coiling SSU genotypes, the right-coiling SSU genotype was excluded from the analysis. This permitted an additional 150 nucleotide sites to be recruited, providing a total of 835 sites for phylogenetic analyses (Fig. 2B). The principal division among the left-coiling SSU genotypes is between the Northern (type I, Arctic) and Southern (types II-V, Antarctic and Benguela) Hemispheres. Among the Southern Hemisphere SSU genotypes, type IV shows the greater divergence. Types II, III, and V are very closely related. They cluster together in the tree with 94%/65% NJ/ML bootstrap support (Fig. 2) and are defined mainly by differences in the variable regions of the SSU rDNA sequence (Fig. 3).
Fig. 3.
Sequence alignment showing the extensive variation in the first variable region of the SSU rRNA gene fragment. The variable region is shown in normal text. Conserved regions, alignable across all neogloboquadrinid and outgroup taxa and used in phylogenetic tree construction (Fig. 2), are shown in bold type (∼ indicates sequences not displayed; -indicates gaps introduced in aligning sequences). Additional differences are observed between SSU genotypes in other variable regions of the SSU rDNA fragment.
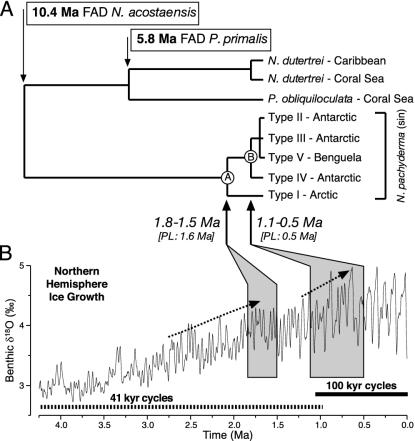
Molecular Dating. We have used the molecular data to estimate approximate dates for the cryptic divergences within the left-coiling N. pachyderma SSU genotype cluster. There are two well defined dates that can be used to calibrate the neoglobo-quadrinid tree. The divergence between N. dutertrei and N. pachyderma is one of the best constrained dates in the foraminiferal fossil record, with the earliest recorded appearance of Neogloboquadrina acostaensis dated at 10.4 million years ago (Ma) (27). The divergence between the N. dutertrei and P. obliquiloculata lineages occurs with the first appearance of Pulleniatina primalis, which is dated at 5.8 Ma (27).
There is significant between-lineage rate variation in the SSU rRNA gene of the foraminifera (28) that leads to difficulties in applying a molecular clock. Even within the Neogloboquadrinidae, the right-coiling N. pachyderma genotype has a significantly faster rate of evolution than the other members of the clade (Fig. 2 A). However, if the right-coiling N. pachyderma genotype is excluded, the remaining taxa are observed to evolve at relatively similar rates, and likelihood ratio tests indicate that the 835-nt data set (excluding the right-coiling genotype) does not deviate significantly from a molecular clock [log likelihood clock tree: -1,580.98669; log likelihood nonclock tree: -1,575.51264; χ2 (-2log Λ) = 10.948; df = 7; P > 0.1]. We have used five different approaches to estimate divergence times. The first three methods (pairwise distances, ultrametric tree, and LF; see Materials and Methods) reconstruct divergence times under the strict assumption of a molecular clock. The fourth, LFL, enables the molecular clock to be relaxed, permitting the rate for the N. dutertrei/P. obliquiloculata clade (which seems to have a slower rate of evolution from a simple comparison of branch lengths in the ML tree) to differ from the rest. The fifth method, PL, is clock-independent and estimates divergence times by allowing different branches to have different rates of evolution (29). Using these methods, we obtain time estimates of 1.8-1.5 Ma for the divergence of the Northern Hemisphere left-coiling N. pachyderma SSU genotype from the Southern Hemisphere left-coiling SSU genotypes (Fig. 4, node A, and Table 1) and 1.1-0.5 Ma for the divergence of the left-coiling N. pachyderma type IV from the other Southern Hemisphere SSU genotypes (Fig. 4, node B, and Table 1).
Fig. 4.
(A) PL chronogram (29) with branches proportional to time. The boxed divergence times are based on the foraminiferal fossil record (27) (FAD indicates first appearance datum). Estimates of divergence times of the cryptic divergences within N. pachyderma (sin) are indicated in italic type. (B) These estimates can be compared with a proxy record for global ice volume (30). Major intervals of ice-sheet growth in the Northern Hemisphere are indicated with dashed arrows (31).
Table 1. Time estimates of cryptic divergences within N. pachyderma (sin).
| Method | Node A | Node B |
|---|---|---|
| Pairwise distances (corrected using GTR+I+Γ model) [10.4 and 5.8 Ma datum] | 1.8 | 1.1 |
| Ultrametric Tree [10.4 datum] | 1.8 | 0.8 |
| LF [10.4 and 5.8 Ma datum] | 1.7 | 0.7 |
| LFL [10.4 and 5.8 Ma datum] | 1.5 | 0.5 |
| PL [10.4 and 5.8 Ma datum] | 1.6 | 0.5 |
Node A corresponds to the divergence of Northern Hemisphere type I from the Southern Hemisphere SSU genotypes (Fig. 4). Node B corresponds to the divergence of type IV from the other Southern Hemisphere SSU genotypes (Fig. 4). Divergence times (Ma) were estimated based on GTR+I+Γ corrected pairwise distances, an ultrametric ML tree, and by using the LF, LFL, and PL methods as implemented in the r8s package (25).
Discussion
Our findings provide perspective on the evolutionary history of a genetically divergent bipolar species. Through time, N. pachyderma (sin) has been transformed from a bipolar cosmopolitan (ref. 2 and this study) to a high-latitude specialist, leading to its isolation between the Northern and Southern Hemispheres. Cosmopolitanism is not the habit of N. pachyderma (sin) in the present day and this questions the suggestion of “ubiquitous” dispersal for all free-living marine protists (1). Recently, genetic divergence has also been reported between bipolar planktonic dinoflagellate protists from the high polar water mass and Antarctic Sea ice (32). However, without additional genetic, biogeographic, and fossil data, a detailed reconstruction of their evolutionary history was not possible. Fortunately, the comprehensive biogeographic and paleontological data available for the planktonic foraminifera, coupled with the much higher resolution in the planktonic foraminiferal SSU rRNA gene, enables us to build a detailed picture of their evolutionary history.
The global climate underwent a major cooling between 3.5 and 2.5 Ma, leading to a major expansion of the Northern Hemisphere ice sheets (Fig. 4B). This had a profound influence on Atlantic oceanography (31) and undoubtedly on the habitat of the bipolar common ancestor of the now genetically isolated N. pachyderma (sin) populations. The timing of the divergence between the Southern and Northern Hemisphere N. pachyderma (sin) (dated at ≈1.8-1.5 Ma from the molecular data; Fig. 4) suggests that their isolation occurred during the early Quaternary after ice-sheet growth. Our extensive genetic data set shows that the northern and southern SSU genotypes are mutually exclusive and that after their initial isolation, neither population was able to maintain a genetic exchange across the tropics, thus remaining genetically and geographically isolated until today. Subtle differences in shell morphology and ultrastructure between the Arctic and Antarctic N. pachyderma (sin) were observed earlier (33), suggesting a link between genetic and morphological traits associated with this divergence.
Although only one SSU genotype of N. pachyderma (sin) occurs in the North Atlantic today, we have found evidence of further diversification in the Southern Ocean. Of the four SSU genotypes identified, type IV is the earliest diverging (dated at ≈1.1-0.5 Ma from the molecular data; Fig. 4). This SSU genotype is confined to waters south of the Polar Front, and its identification in the ice-core sample (Fig. 1) suggests that the unique Antarctic adaptation to overwintering in sea ice (34) is also associated with this type. The estimated timing of the “extreme” polar adaptation in the Antarctic (1.1-0.5 Ma) is coincident with fossil-record estimates for true polar adaptation of N. pachyderma (sin) in the Northern Hemisphere [1.1 Ma (35 and 36)]. Our molecular data indicate that the Arctic and Antarctic populations of N. pachyderma (sin) were already genetically isolated at this time, implicating a globally acting environmental process as a likely mechanism leading to the evolution of extreme polar adaptations in both hemispheres. Indeed, the timing of the adaptations coincide with the mid-Pleistocene climate transition, a period when ice sheets expanded to their maximum proportions for the first time and the 100-thousand-year pacing of ice ages began (Fig. 4). The intensification of frontal systems around Antarctica and the onset of very cool glacials at this time [1.2-0.8 Ma (37-39)] are the most likely drivers of the divergence of type IV and its specific adaptation to life in the ice.
In addition to the mid-Pleistocene divergence of type IV, there is genetic evidence for a more recent diversification along the Subantarctic Front. Type II tends to be distributed within the warmer waters of the Subantarctic Front, whereas type III has a more widespread distribution (Fig. 1). It is possible that the three distinct SSU genotypes found in the Antarctic polar/subpolar waters may account for the morphological variability of N. pachyderma (sin) observed in sediments from the Southern Ocean (40), in which three broad morphological provinces of N. pachyderma (sin) have been recognized, their boundaries following the main Antarctic frontal systems.
An additional relict population of N. pachyderma (sin) was identified in the Benguela upwelling system off Namibia as a closely related but distinct type V. Unlike type IV, which shows greater divergence, the other three SSU genotypes are defined mainly by differences in the variable regions of the SSU rDNA sequence (Fig. 3). This suggests that their divergences occurred more recently. The close relationship between type V and types II and III clearly indicates that the Benguela system relict population has been seeded from the Southern Ocean. Indeed, it has been suggested (41) that during glacial periods, colder subpolar waters from the Southern Ocean could be advected into the Benguela current system. N. pachyderma (sin) has been present in the Benguela system for at least the last 420 thousand years (42), and it is likely that incursions into the Benguela system may have occurred repeatedly.
Paleoceanographers have long assumed that the presence of N. pachyderma (sin) in Pleistocene and Pliocene sediments is analogous to its current adaptation (43). Our data support previous suggestions (35, 36) that its present-day relationship cannot be extended to the entire time range of this morphospecies. Although N. pachyderma (sin) has been associated with high-latitude, cold waters ever since its first occurrence in the late Miocene (44), its current specific polar affinity did not evolve until much later in the mid-Pleistocene [1.1 Ma (refs. 35 and 36); 1.1-0.5 Ma (this study)].
N. pachyderma (sin) is the most important provider of paleoproxies on the state and variation of high-latitude oceans in the Quaternary. Darling et al. (2) confirmed that coiling direction in N. pachyderma is not a phenotypic response to temperature. The right-coiling genotype is genetically highly divergent from the left-coiling genotype, and the possibility for resolving the confusion of using the same species name for such genetically and ecologically distinct planktonic foraminifers should be investigated. We have confirmed that there is only one left-coiling N. pachyderma genotype (type I) in the Northern Atlantic, in which N. pachyderma dominates the polar assemblages (ref. 26 and this study). This is excellent news for paleoceanographers in light of the diversity we have discovered in the Southern Ocean. Morphological discrimination in the sediment, therefore, will not be a requirement for proxies in the Northern Hemisphere.
However, the genetic isolation and subsequent distinct evolutionary histories between the Arctic and Antarctic populations of N. pachyderma (sin) clearly imply that paleoproxies must be calibrated and applied separately in each hemisphere. Our study indicates that the genotypes of left-coiling N. pachyderma in the Northern and Southern Hemispheres have not mixed during the Quaternary, and therefore their adaptations would have diverged and remained distinct since their initial isolation in the early Pleistocene. The presence of several genetic types in the Southern Hemisphere implies that morphological recognition of these genotypes may be required for successful application of paleoproxies based on N. pachyderma (sin) in this region. If it proves possible to distinguish the individual genetic types, it may become possible to devise new proxies for the position of the Polar Front, extent of sea ice, and monitoring of advection of subpolar water masses into the Benguela upwelling system.
In common with many other pelagic organisms, planktonic foraminifera are cosmopolitan, inhabiting a large portion of the world's oceans with sustained genetic exchange (2, 4). Although they are clearly capable of long-distance dispersal and have the potential for genetic exchange on a global scale (2), we show that there are opportunities for isolation to occur in foraminiferal populations. Allopatric processes are clearly the most plausible explanation for the divergence between Arctic and Antarctic N. pachyderma (sin) populations. Allopatric isolation is also likely to be central to the diversification of type V, which is effectively peripherally isolated in the Benguela upwelling system, with only sporadic seeding from the Southern Ocean during glacial periods. The divergence of type IV can be best explained by a combination of ecological and geographical processes. This SSU genotype is restricted in distribution to the area south of the Polar Front but does show some degree of overlap with type III in its present-day distribution (Fig. 1). The area south of the Polar Front is largely covered by ice during the southern winter, and type IV is the only SSU genotype that is adapted to live in the sea ice. Individuals lacking this specific adaptation would not be able to persist there throughout the year, perhaps leading to the evolution of reproductive isolation as a consequence of divergent selection (45) between the seasonally ice-covered region and the rest of the Southern Ocean. Ecological processes may also account for the developing divergence between types II and III across the Subantarctic frontal system. The scenarios for the divergences among the Southern Ocean types are quite different from the bipolar and Benguela divergences, in which reproductive isolation would have evolved primarily as a byproduct (45) of geographical isolation.
Thus, it seems that allopatric and ecological processes can have an important role in the diversification of high-dispersal marine plankton and that paleoclimatic and paleoceanographic history are crucial to the understanding of present-day marine biogeographic patterns. We have demonstrated that barriers to gene flow do occur in the pelagic planktonic realm. If such barriers to gene flow are maintained for a sufficient period, speciation will ensue. The waxing and waning of continental ice sheets has had a profound effect on the dynamics of diversification among terrestrial organisms (46). Our study shows that the ups and downs of the Quaternary climate promoted the disruption of gene flow and facilitated diversification in the high-latitude oceanic realm as well.
Acknowledgments
We thank F. Kippert for molecular advice and assistance; R. Brinkmeyer for ice-core samples; D. Kroon, E. Stangeew, and J. Netzer for assistance on the FS Polarstern cruise Arktis XV/1-2; and R. Dingle and T. Paramor for sampling on the RRS James Clark Ross cruise JR19. We gratefully appreciate the support of the Research Institute of the Ministry of Fisheries and Resources (Namibia) staff for sampling from RV Welwitschia. This work was supported by the Natural Environment Research Council, the Leverhulme Trust, the Swedish Foundation for International Cooperation in Research and Higher Education, and the Swedish Natural Science Research Council.
Abbreviations: sin, sinistral; SSU, small subunit; rDNA, rRNA-encoding DNA; NJ, neighbor joining; ML, maximum likelihood; GTR, general time-reversible; LF, Langley-Fitch; LFL, LF local; PL, penalized likelihood; Ma, millions of years ago.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AY305329-AY305332).
References
- 1.Finlay, B. J. (2002) Science 296, 1061-1063. [DOI] [PubMed] [Google Scholar]
- 2.Darling, K. F., Wade, C. M., Steward, I. A., Kroon, D., Dingle, R. & Leigh Brown, A. J. (2000) Nature 405, 43-47. [DOI] [PubMed] [Google Scholar]
- 3.Palumbi, S. R. (1994) Annu. Rev. Ecol. Syst. 25, 547-572. [Google Scholar]
- 4.Norris, R. D. (2000) Paleobiology 26, Suppl., 236-258. [Google Scholar]
- 5.Knowlton, N. (1993) Annu. Rev. Ecol. Syst. 24, 189-216. [Google Scholar]
- 6.de Vargas, C., Norris, R., Zaninetti, L., Gibb, S. W. & Pawlowski, J. (1999) Proc. Natl. Acad. Sci. USA 96, 2864-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kucera, M. & Darling, K. F. (2002) Philos. Trans. R. Soc. London A 360, 695-719. [DOI] [PubMed] [Google Scholar]
- 8.Saez, A. G., Probert, I., Geisen, M., Quinn, P., Young, J. R. & Medlin, L. K. (2003) Proc. Natl. Acad. Sci. USA 100, 7163-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Garcia, P., Rodriguez-Valera, F., Pedros-Alio, C. & Moreira, D. (2001) Nature 409, 603-607. [DOI] [PubMed] [Google Scholar]
- 10.Kennett, J. P. & Srinivasan, M. S. (1983) Neogene Planktonic Foraminifera: A Phylogenetic Atlas (Hutchinson and Ross, Stroudsburg, PA).
- 11.Pflaumann, U., Duprat, J., Pujol, C. & Labeyrie, L. (1996) Paleoceanography 11, 15-35. [Google Scholar]
- 12.Orsi, A. H., Whitworth, T., III, & Nowlin, W. D., Jr. (1995) Deep Sea Res. Part II 42, 641-673. [Google Scholar]
- 13.Stewart, I. A., Darling, K. F., Kroon, D., Wade, C. M. & Troelstra, S. R. (2001) Mar. Micropaleontol. 43, 143-153. [Google Scholar]
- 14.Darling, K. F., Kucera, M., Wade, C., von Langen, P. & Pak, D. (2003) Paleoceanography 18, 10.1029/2001PA000723.
- 15.Smith, S. W., Overbeek, R., Woese, C. R., Gilbert, W. & Gillevet, P. M. (1994) Comput. Appl. Biosci. 10, 671-675. [DOI] [PubMed] [Google Scholar]
- 16.Saitou, N. & Nei, M. (1987) Mol. Biol. Evol. 4, 406-425. [DOI] [PubMed] [Google Scholar]
- 17.Fitch, W. M. & Margoliash, E. (1967) Science 155, 279-284. [DOI] [PubMed] [Google Scholar]
- 18.Felsenstein, J. (1981) J. Mol. Evol. 17, 368-376. [DOI] [PubMed] [Google Scholar]
- 19.Fitch, W. M. (1971) Syst. Zool. 20, 406-416. [Google Scholar]
- 20.Swofford, D. L. (2003) PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods) (Sinauer, Sunderland, MA), Version 4.
- 21.Lanave, C., Preparata, G., Saccone, C. & Serio, G. (1984) J. Mol. Evol. 20, 86-93. [DOI] [PubMed] [Google Scholar]
- 22.Gu, X., Fu, Y.-X. & Li, W.-H. (1995) Mol. Biol. Evol. 12, 546-557. [DOI] [PubMed] [Google Scholar]
- 23.Felsenstein, J. (1985) Evolution (Lawrence, Kans.) 39, 783-791. [DOI] [PubMed] [Google Scholar]
- 24.Huelsenbeck, J. P. & Rannala, B. (1997) Science 276, 227-232. [DOI] [PubMed] [Google Scholar]
- 25.Sanderson, M. J. (2003) Bioinformatics 19, 301-302. [DOI] [PubMed] [Google Scholar]
- 26.Bauch, D., Darling, K., Simstich, J., Bauch, H. A., Erlenkeuser, H. & Kroon, D. (2003) Nature 424, 299-302. [DOI] [PubMed] [Google Scholar]
- 27.Spencer-Cervato, C., Thierstein, H. R., Lazarus, D. B. & Beckmann, J.-P. (1994) Paleoceanography 9, 739-763. [Google Scholar]
- 28.Pawlowski, J., Bolivar, I., Fahrni, J. F., De Vargas, C., Gouy, M. & Zaninetti, L. (1997) Mol. Biol. Evol. 14, 498-505. [DOI] [PubMed] [Google Scholar]
- 29.Sanderson, M. J. (2002) Mol. Biol. Evol. 19, 101-109. [DOI] [PubMed] [Google Scholar]
- 30.Shackleton, N. J. (1995) in Paleoclimate and Evolution with Emphasis on Human Origins, eds. Vrba, E. S., Denton, G. H., Partridge, T. C. & Burckle, L. H. (Yale Univ. Press, New Haven, CT), pp. 242-248.
- 31.Driscoll, N. W. & Haug, G. H. (1998) Science 282, 436-438. [DOI] [PubMed] [Google Scholar]
- 32.Montresor, M., Lovejoy, C., Orsini, L., Procaccini, G. & Roy, S. (2003) Polar Biol. 26, 186-194. [Google Scholar]
- 33.Kennett, J. P. (1970) Contrrib. Cushman Found. Foram. Res. 21, 47-49. [Google Scholar]
- 34.Dieckmann, G. S., Spindler, M., Lange, M. A., Ackley, S. F. & Eicken, H. (1991) J. Foram. Res. 21, 182-189. [Google Scholar]
- 35.Huber, R., Meggers, H., Baumann, K.-H., Raymo, M. E. & Henrich, R. (2000) Palaeogeogr. Palaeoclimatol. Palaeoecol. 160, 193-212. [Google Scholar]
- 36.Kucera, M. & Kennett, J. P. (2002) Geology 30, 539-542. [Google Scholar]
- 37.Dieckmann, B. & Kuhn, G. (2002) Palaeogeogr. Palaeoclimatol. Palaeoecol. 182, 241-258. [Google Scholar]
- 38.Joseph, L. H., Rea, D. K., van der Pluijm, B. A. & Gleason, J. D. (2002) Earth Planet Sci. Lett. 201, 127-142. [Google Scholar]
- 39.Becquey, S. & Gersonde, R. (2002) Palaeogeogr. Palaeoclimatol. Palaeoecol. 182, 221-239. [Google Scholar]
- 40.Kennett, J. P. (1968) Micropaleontology 14, 305-318. [Google Scholar]
- 41.McIntyre, A., Ruddiman, W. F., Karlin, K. & Mix, A. C. (1989) Paleoceanography 4, 19-55. [Google Scholar]
- 42.Ufkes, E., Jansen, J. H. F. & Schneider, R. R. (2000) Mar. Micropaleontol. 40, 23-42. [Google Scholar]
- 43.Keller, G. (1979) Mar. Micropaleontol. 4, 159-172. [Google Scholar]
- 44.Bandy, O. L. (1972) Micropaleontology 18, 294-318. [Google Scholar]
- 45.Schluter, D. (2001) Trends Ecol. Evol. 16, 372-380. [DOI] [PubMed] [Google Scholar]
- 46.Hewitt, G. (2000) Nature 405, 907-913. [DOI] [PubMed] [Google Scholar]