Abstract
Neurodevelopmental defects in humans represent a clinically heterogeneous group of disorders. Here, we report the genetic and functional dissection of a multigenerational pedigree with an X-linked syndromic disorder hallmarked by microcephaly, growth retardation, and seizures. Using an X-linked intellectual disability (XLID) next-generation sequencing diagnostic panel, we identified a novel missense mutation in the gene encoding 60S ribosomal protein L10 (RPL10), a locus associated previously with autism spectrum disorders (ASD); the p.K78E change segregated with disease under an X-linked recessive paradigm while, consistent with causality, carrier females exhibited skewed X inactivation. To examine the functional consequences of the p.K78E change, we modeled RPL10 dysfunction in zebrafish. We show that endogenous rpl10 expression is augmented in anterior structures, and that suppression decreases head size in developing morphant embryos, concomitant with reduced bulk translation and increased apoptosis in the brain. Subsequently, using in vivo complementation, we demonstrate that p.K78E is a loss-of-function variant. Together, our findings suggest that a mutation within the conserved N-terminal end of RPL10, a protein in close proximity to the peptidyl transferase active site of the 60S ribosomal subunit, causes severe defects in brain formation and function.
Keywords: X-linked, microcephaly, zebrafish, ribosome, translational elongation
NEURODEVELOPMENTAL defects in humans represent a diagnostic challenge. Displaying marked phenotypic overlap, examples include autism spectrum disorders (ASD), intellectual disability (ID), microcephaly, and seizures; in some instances, common genetic defects can underscore each of these clinical entities. For example, mutations in the voltage-gated sodium channel Nav1.2 encoded by SCN2A are associated with the manifestation of early infantile epilepsy (Sugawara et al. 2001). However, recent exome sequencing studies have also identified SCN2A mutations as rare contributors to disease in autism cohorts, thereby expanding the phenotypic spectrum underscored by Nav1.2 channel dysfunction (Sanders et al. 2012). A gender bias of ∼1.3–1.4 males to 1 female with a neurodevelopmental disorder has complicated further our mechanistic understanding of such defects (Leonard and Wen 2002; Ellison et al. 2013). One obvious explanation for an unbalanced representation of the sexes among individuals with a structural or functional brain defect is an abundance of developmentally important genes on the X chromosome. To date, >100 genes have been associated with ASD, ID, microcephaly, or seizures primarily in hemizygous males and, to some extent, their carrier mothers (De Brouwer et al. 2007; Tarpey et al. 2009; Lubs et al. 2012).
Here, we report the genetic dissection of a novel form of X-linked human genetic disease characterized by microcephaly, seizures, growth retardation, and hypotonia. Combined genetic, functional, and biochemical assays suggest that a missense mutation in RPL10, a component of the 60S large ribosomal subunit, can cause syndromic central nervous system defects, likely because of defects in bulk translation and increased apoptosis in the brain.
Materials and Methods
Clinical genetic screening and confirmatory testing
Nine members of the family consented for genetic testing. X chromosome inactivation status was established by analysis of DNA methylation at the human androgen receptor locus in DNA from the two mutation carrier mothers (individuals I-2 and II-4; Center for Genetic Testing, Saint Francis Health System). An X-linked intellectual disability (XLID) next-generation sequencing panel targeting 82 genes (supporting information, Table S1) was conducted at a commercial laboratory (Ambry Genetics), using a DNA sample from affected individual II-1. Segregation analysis of p.K78E was carried out by Sanger sequencing of RPL10 exon 5 in all seven additional available family members.
DNA constructs and in vitro transcription
We obtained a human wild-type (WT) RPL10 open reading frame (ORF) construct [pENTR221, Ultimate ORF Collection by Invitrogen (Carlsbad, CA); Life Technologies, clone IOH2895] and we generated constructs encoding missense variants p.K78E, p.L206M, p.H213Q, and p.S202N as described (Niederriter et al. 2013). Following sequence confirmation of the mutation and ORF integrity using Sanger sequencing, pENTR constructs were then cloned into the pCS2+ vector, using LR clonase II-mediated recombination (Life Technologies). Sequence-confirmed WT and mutant RPL10 constructs in the pCS2+ vector were linearized with NotI and transcribed in vitro, using the SP6 mMessage mMachine Kit (Ambion).
Zebrafish embryo manipulation and injections
We developed an in vivo complementation assay as described in Niederriter et al. (2013). Translation blocking (tb) (5′ TGCGATCTGTAACGTACACAATAAC 3′) and splice blocking (sb) (5′ AAAATACATGGCTTACCAGGAACAC 3′) morpholinos (MOs) (Gene Tools) were diluted to appropriate concentrations in nuclease-free water (0.5, 0.6, and 0.7 ng/nl for the tb-MO dose response; 1, 2, and 3 ng/nl for the sb-MO dose response; 0.6 ng/nl tb-MO for rescue experiments; and 0.7 ng/nl tb-MO or 3 ng/nl sb-MO for transferase-mediated dUTP nick end labeling (TUNEL) and phospho-histone H3 antibody staining) and injected into WT zebrafish embryos (Ekkwill × AB F1 outcross) at the one- to four-cell stage. To assess sb-MO efficiency, endogenous rpl10 expression was determined by extracting total RNA from 1 day postfertilization (dpf) embryos with Trizol (Invitrogen) according to manufacturer’s instructions. Oligo(dT)-primed total RNA was reverse transcribed using SuperScriptIII reverse transcriptase (Invitrogen) and the resulting complementary DNA (cDNA) was PCR amplified. To rescue morphant phenotypes, we injected tb-MO with 50 pg capped human messenger RNA (mRNA). Embryos were scored at 2 dpf and classified as normal or abnormal (microcephalic) when compared to age-matched controls from the same clutch. Embryos were then dechorionated, anesthetized with Tricaine solution, fixed in 4% paraformaldehyde solution overnight, and then transferred to 1× PBS prior to quantitative phenotypic analysis.
RNA in situ hybridization
We PCR amplified Danio rerio rpl10 transcript corresponding to cDNA clone MGC:56154 (GenBank: BC045950), using 1 dpf whole-embryo cDNA as template. We labeled sense and antisense RNA probes with digoxigenin and performed whole-mount RNA in situ hybridization on 2 dpf embryos as described in Thisse and Thisse (2008). Lateral images were acquired on a Nikon (Garden City, NY) AZ100 microscope, using Nikon NIS Elements Software.
Bright-field imaging and measurements
Lateral and dorsal images were acquired on a Nikon SMZ745 microscope, using Nikon NIS Elements Software (n = 30 larvae per injection batch; investigator masked to injection cocktail; repeated twice). We measured head size, body length, and somite angle with ImageJ software; for body length measurements (from lateral images), a polyline was drawn beginning at the anteriormost point of yolk attachment and terminating at the posteriormost point on the tail; for somite angle measurements (from lateral images), we measured the angle of the somite located at the midpoint between the yolk and the anus; for forebrain area measurements (from dorsal images), an outline was drawn beginning at the posteriormost point of eye and tracing around the head to terminate at the starting point. A Student’s t-test was used to determine the statistical significance of differences between injection batches.
Polysome gradients
Zebrafish larvae were anesthetized in tricaine solution at 5 dpf and decapitated with microsurgical scissors, and heads and bodies were lysed in separate pools in 200 mM KOAc, 15 mM MgCl2, 25 mM K-HEPES (pH 7.2), and 2% dodecylmaltoside (DDM) (n = 20 larvae per injection batch). For each sample, 250 A260 units of the tissue extracts were then layered over a 10–50% sucrose gradient and centrifuged for 3 hr at 35,000 rpm in a SW-41 rotor (Beckman-Coulter, Pasadena, CA). Gradients were collected using a Teledyne-Isco gradient fractionator with continuous absorbance monitoring at 254 nm.
Whole-mount TUNEL assay, phospho-histone H3 immunostaining, and fluorescence microscopy
We utilized terminal deoxynucleotidyl TUNEL to assay apoptosis, using the ApopTag rhodamine in situ Apoptosis Detection kit (Chemicon) as described in Golzio et al. (2012). For whole-mount anti-histone H3 immunostaining, we used anti-phospho-histone H3 (ser10)-R antibody (diluted 1:750; sc-8656-R, Santa Cruz) as described in Golzio et al. (2012). For each of TUNEL and phospho-histone H3 immunostaining, fluorescent signals were imaged on laterally positioned larvae and z-stacked on a Nikon AZ100 microscope, using NIS Elements AR software. We quantified staining by counting positive cells (histone H3) or pixels (TUNEL) in defined regions of the head by using ImageJ software; positive cells in the eyes were removed from cell counts for histone H3 (n = 20 embryos imaged per injection batch; masked scoring; repeated twice).
Results and Discussion
A novel missense variant in RPL10 segregates with X-linked syndromic microcephaly
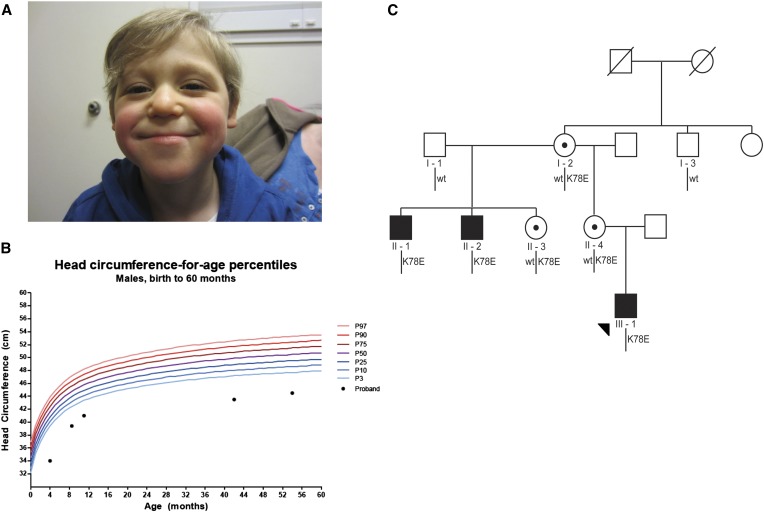
We consulted for an 11-month-old male of European-American origin who presented with a syndromic neurodevelopmental disorder of unknown etiology. Following a pregnancy that was complicated with polyhydramnios at 27 weeks gestation, the index case (III-1) was born at 35 weeks. He failed his newborn hearing screen and displayed multiple congenital abnormalities that included digit malformations, right cryptorchidism, sacral dimple, and dysmorphic craniofacial features (Table 1; Figure 1A). He underwent surgical procedures to correct some of his congenital abnormalities: pneumo-eustachian tube placement at 4 months, sacral lipoma removal at 9 months, and a right orchiopexy at 11 months. He had chronic reflux and growth retardation, was generally hypotonic, and was hospitalized for several pneumonias. At birth, his head circumference was 28 cm (2.6 standard deviations below the mean); his head circumference velocity declined, and at his most recent clinical assessment at age 4.5 years measured 44.5 cm (5 standard deviations below the mean, Figure 1B). Laboratory studies including chromosomal microarray, plasma amino acid, acylcarnitine, urine organic acids, creatine, and guanidoacetic acid were normal. Although both of his parents were reported to be healthy, a review of his family history revealed two maternal uncles who had unexplained syndromic encephalopathy disorders (Figure 1C). Both of the maternal uncles (II-1 and II-2) were evaluated and medical records were reviewed at 19 and 25 years, respectively. They shared multiple phenotypic features with that of the index case, including a history of microcephaly, seizures, growth retardation, hypotonia, genitourinary abnormalities, and prognathism (Table 1). Additionally, both maternal uncles were born with cardiac valve defects, developed hearing loss, are essentially nonverbal, and are minimally or nonambulatory (Table 1).
Table 1. Phenotypes of affected males with RPL10 p.K78E.
| Patient identifier | Wk of gestation | Birth weight | Head circumference | Microcephaly | Seizures | Severe growth retardation | Hypotonia | Craniofacial defects | Genitourinary abnormalities | Hand and foot malformations | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 (III-1) | 35 | 5 lb 3 oz (2.353 kg; 25th percentile) | 40.9 cm, −5.5 SD (11 mo) | + | + | + | + | Prognathic, thin upper lip, asymmetric dilation of the right temporal horn | Right cryptorchidism | Mild camptodactyly (second finger, left hand); proximal partial syndactyly (toes 2 and 3, left foot; toes 2–4, right foot) | GERD, recurrent fever/pneumonia, negative immune work-up, sacral lipoma |
| Case 2 (II-2) | 38 | 7 lb 6 oz (3.346 kg; 50th percentile) | 47.2 cm, −9.6 SD (19 yr) | + | + | + | + | Prognathic, with dental crowding | Hypospadias, right cryptorchidism | Tapered fingers | ASD, pulmonary artery stenosis, laryngomalacia, self-abusive behaviors, recurrent fevers/infection in childhood, chronic GERD, bilateral hearing loss, contractures of knees and ankles |
| Case 3 (II-1) | 37 | 6 lb 5 oz (2.864 kg; 25th percentile) | 41.75 cm, −6 SD (22 mo) | + | + | + | + | Prognathic, Protuberant ears, branchial cleft cyst | Hypospadias, cryptorchidism | Unilateral simian crease | VSD, recurrent fevers/infection in childhood, GERD, mild sensorineural hearing loss, movement disorder, nonverbal |
Abbreviations: ASD, atrial septal defect; GERD, gastroesophageal reflux disease; SD, standard deviations; VSD, ventricular septal defect.
Figure 1.
Microcephaly in an X-linked pedigree harboring RPL10 p.K78E. (A) The proband (III-1) at age 41 months. He displays microcephaly, a thin upper lip, and mandibular prognathism (see Table 1 for full phenotypic description). (B) Head circumference chart for the proband. Microcephaly was of prenatal onset and growth continued to follow a normal curve at this reduced trajectory. (C) X-linked segregation of RPL10 c.232A > G; p.K78E in a three-generation pedigree. Subsequent to identification of p.K78E in individual II-1 by a next-generation sequencing X-linked intellectual disability diagnostic panel, Sanger sequencing confirmed segregation of the mutation in three affected males and three carrier females. A healthy maternal great uncle (I-3) of the proband was wild type at this locus, and both carrier mothers (I-2 and II-4) displayed fully skewed X inactivation.
Suspicious of an X-linked disorder, we tested both heterozygous carrier mothers (I-2 and II-4) for nonrandom X-inactivation patterns. Favorable skewing is a well-documented phenomenon in which cells containing an active mutation-bearing X chromosome are selected against during cell division, resulting in a predominance of cells with an active X chromosome containing the normal allele (Migeon 1998; Van Den Veyver 2001). Evaluation of DNA methylation at the human androgen receptor locus in each of I-2 and II-4 produced results consistent with our hypothesis; each female showed fully skewed inactivation of their mutation-bearing X chromosome.
With no clear diagnostic criteria to assign the family to a described syndrome, we ordered an X-linked sequencing panel covering the coding regions and intron–exon boundaries of 82 genes implicated previously in XLID (XLID panel, Ambry Genetics; Table S1). An estimated 42% of affected individuals with a family history of XLID are anticipated to have a deleterious mutation in a gene represented on the panel (De Brouwer et al. 2007), and analytic sensitivity of this test is reported to be 83%. This approach identified a novel single-nucleotide change (c.232A > G; p.K78E) within the gene encoding 60S ribosomal protein L10 (RPL10), a gene on Xq28 reported previously to be an ASD candidate (Klauck et al. 2006). Mutational screening of all the other 81 genes was negative; this variant was also absent from all publicly available control exomes and genomes [NHLBI Exome Variant Server (EVS), dbSNP, and 1000 Genomes] and had not been reported in cases of XLID.
To explain further the significance of this variant, we conducted segregation analysis in eight available family members representative of three generations with Sanger sequencing of RPL10 exon 5. This variant segregated as expected for an X-linked disorder: the proband and both affected maternal uncles were hemizygous carriers of p.K78E; the proband’s mother (II-4) and his maternal grandmother (I-2) were heterozygous for p.K78E; and importantly, an unaffected great uncle of the index case (I-3) harbored the WT allele (Figure 1C).
Together, our mutational findings from the XLID panel, segregation with disease in multiple generations, and fully skewed X inactivation in carrier mothers provided genetic evidence suggesting that RPL10 p.K78E might be the primary cause of syndromic microcephaly in this family.
rpl10 suppression in zebrafish results in microcephaly and p.K78E is pathogenic
Missense mutations in RPL10 encoding p.L206M and p.H213Q have been reported previously to confer susceptibility to ASD (Klauck et al. 2006; Chiocchetti et al. 2011). Even so, individuals bearing these nonsynonymous changes were not reported to display microcephaly or the constellation of syndromic features present in the three hemizygous males with RPL10 p.K78E. Thus, the rarity of RPL10 variation among cases (3/521 ASD pedigrees) (Klauck et al. 2006; Chiocchetti et al. 2011) in the absence of replication in other ASD cohorts (Gong et al. 2009) and control cohorts bereft of functional variation (1/2443 males and 3/4060 females harboring missense RPL10 changes; EVS) precluded us from implicating p.K78E in severe neurodevelopmental phenotypes. Moreover, in silico prediction programs were conflicting; p.K78E was predicted to be benign by PolyPhen-2 (Adzhubei et al. 2010), but damaging by Mutation Taster (Schwarz et al. 2010) and SIFT (Kumar et al. 2009). Therefore, we turned to the developing zebrafish as a model both to determine the physiological relevance of RPL10 to disease and to test the pathogenic potential of p.K78E on RPL10 function.
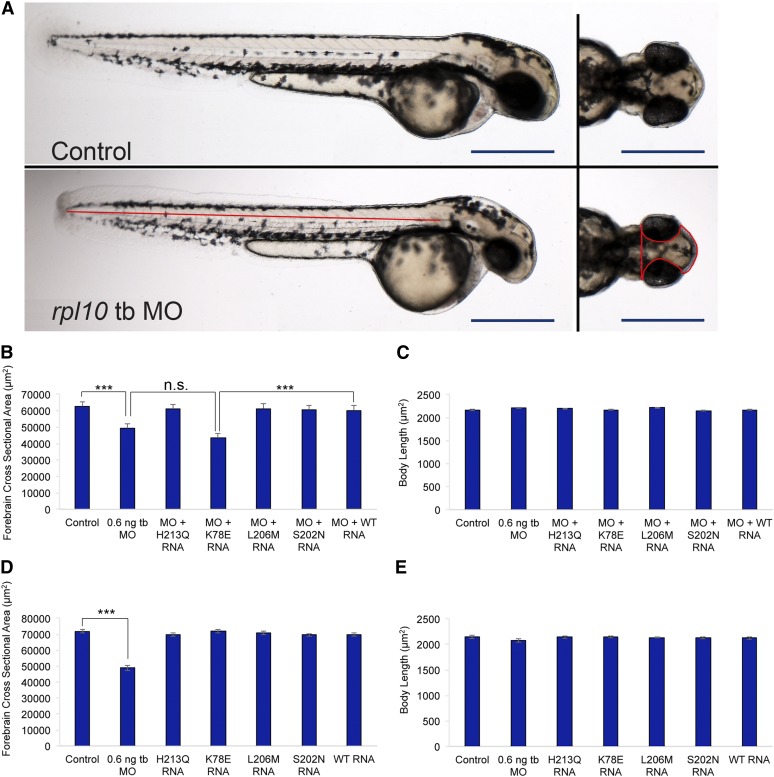
Previous studies have shown D. rerio to represent a useful surrogate to study neurodevelopmental defects in humans (Komoike et al. 2010; Tian et al. 2010; Bicknell et al. 2011; Golzio et al. 2012; Wan et al. 2012; Beunders et al. 2013; Dauber et al. 2013; Schaffer et al. 2014). Although reported previously to be expressed ubiquitously (Thisse and Thisse 2004), in situ hybridization of rpl10 riboprobes in 2 dpf embryos demonstrated enriched expression in the anterior vs. posterior structures, particularly at the midbrain–hindbrain boundary (Figure S1). Next, to determine the functional consequences of RPL10 suppression, we employed MO-induced knockdown of the single D. rerio ortholog (92% identity, 97% similarity). We designed a tb-MO targeting the translational start site of the rpl10 transcript. We injected one- to four-cell stage WT zebrafish embryos with increasing doses of tb-MO (0.5, 0.6, and 0.7 ng; n = 50 embryos per injection batch). We observed a microcephaly phenotype that was dose dependent at 2 dpf as determined by qualitative scoring masked to injection cocktails (Figure 2A; Figure S2, A and C). To assess this defect quantitatively, we measured the forebrain cross-sectional area of morphants and controls and observed a significant decrease in head size, but not body length, when evaluated at 2 dpf (P < 0.0001; n = 30; repeated twice; Figure 2, A–E; Table S2, Table S3), suggesting that rpl10 suppression did not cause a generalized developmental delay. Additionally, we recorded similar somite angle measurements in morphants vs. controls (mean somite angle of 99.8° vs. 99.1°, respectively; n = 30; repeated twice), and only a minor proportion of morphants displayed affected structures other than the head (tail extension defects; 3/60 evaluated), indicating that rpl10 suppression affects predominantly anterior structures. To replicate these observations, we designed a sb-MO targeting the donor site of rpl10 exon 2 (Figure S1, Figure S2, A and B); the sb-MO titration curve (1, 2, and 3 ng) resulted in a similar reduction of embryo head size at 2 dpf in a dose-dependent manner (Figure S2D). Next, we co-injected WT human RPL10 mRNA with tb-MO. This resulted in a significant improvement of the microcephaly phenotype according to both qualitative and quantitative measures (qualitative, 70% vs. 30% affected for MO alone vs. WT rescue, P < 0.0001; quantitative, mean cross-sectional area 49,337 µm2 vs. 60,203 µm2 for tb-MO alone vs. WT rescue; P < 0.0001; Figure 2, A–C; Table S2). Together, these data indicate that loss of rpl10 in developing zebrafish results in an anatomically similar neurodevelopmental phenotype to that of the hemizygous males harboring p.K78E.
Figure 2.
Suppression of rpl10 in zebrafish results in reduced head size and p.K78E is a loss-of-function variant. (A) Live larval images of control (top) and rpl10 tb-MO-injected embryos (bottom). Left panels show lateral views of whole larvae with similar body lengths; right panels show dorsal views showing a reduced head size in morphants. Red lines indicate head size measurements quantified in B and D and body length measurements quantified in C and E. Bars, 500 μm. (B) Quantification of head area for rpl10 MO and MO co-injected with human RPL10 mRNA. (C) Quantification of body length for rpl10 MO and MO co-injected with human RPL10 mRNA. (D) Quantification of head size for embryos injected with RPL10 mRNA alone. (E) Quantification of body length for embryos injected with RPL10 mRNA alone. Head area and body length measurements were carried out at 2 days postfertilization (dpf), using embryos injected with 0.6 ng tb-MO and/or 50 pg mRNA; n = 30 for each injection batch with masked scoring were repeated with similar results. Error bars indicate standard error of the mean (SEM). ***P < 0.0001 (two-tailed t-test comparisons between MO-injected and rescued embryos).
Both our group and others have shown that in vivo complementation assays in zebrafish embryos are a sensitive and specific approach to test the pathogenicity of missense mutations implicated in human genetic disease (Zaghloul et al. 2010; Wan et al. 2012; Niederriter et al. 2013; Davis et al. 2014). To test the effect of p.K78E on RPL10 function, co-injection of rpl10 tb-MO with mRNA harboring p.K78E failed to rescue the morphant phenotype and resulted in significantly decreased forebrain cross-sectional area (43,390 µm2) when compared to the WT rescue (60,203 µm2; P < 0.0001; n = 30; repeated twice); body length was not affected (P = 0.34; n = 30; Figure 2, B and C, Table S3). Comparison of the forebrain cross-sectional area for tb-MO plus p.K78E mRNA vs. MO-injected embryo batches was statistically indistinguishable (P = 0.066; n = 30; Figure 2, B and C). Next, we tested the two RPL10 variants associated previously with ASD and a putative benign variant (rs4909) also reported previously (Klauck et al. 2006). Co-injection of each of p.S202N, p.L206M, or p.H213Q encoding mRNAs with rpl10 tb-MO fully rescued the microcephaly phenotype, as indicated by similar forebrain cross-sectional areas and body lengths in comparison to batches co-injected with a cocktail of WT mRNA and rpl10 tb-MO (Figure 2, B and C). These data are not surprising, given that p.L206M and p.H213Q were reported as hypomorphic changes that do not disrupt the basic functions of translation, but do alter discrete cellular protein signatures that may result in the dysregulation of oxidative stress response (Klauck et al. 2006; Chiocchetti et al. 2014). Moreover, these C-terminally positioned mutations (a) can fully complement temperature-sensitive strains of RPL10 mutant yeast (Klauck et al. 2006) and (b) do not induce skewing of X inactivation in mutation carrier females, suggesting that they may be tolerated in some cellular contexts (Chiocchetti et al. 2011). Therefore, the functional capacity of RPL10 bearing each of these two variants probably exceeds the cellular threshold required to rescue the MO-induced microcephaly phenotypes in our zebrafish models, thereby resulting in a benign score.
Importantly, injection of each of the four missense RPL10 mRNAs alone resulted in relatively similar head sizes and body lengths in comparison to WT mRNA alone, arguing against mRNA toxicity or dominant negative effects (Figure 2, D and E, Table S2 and Table S3). Taken together, these data suggest that p.K78E is a pathogenic variant and is a functional null in this assay and support the genetic arguments from within our RPL10 pedigree to implicate RPL10 p.K78E as the driver of severe neurodevelopmental phenotypes.
Suppression of rpl10 results in decreased bulk translation in the zebrafish head
RPL10 is conserved among eukaryotic taxa and, in mammalian cells, is one of the 46 proteins that make up the 60S large ribosomal subunit in cooperation with three ribosomal (r)RNAs (Ben-Shem et al. 2011). The 60S subunit harbors the peptidyl transferase center (PTC) and the exit tunnel for newly synthesized polypeptides, whereas its functional partner, the 40S small ribosomal subunit, facilitates the interaction between transfer RNA (tRNA) and mRNA (Spahn et al. 2001; Klinge et al. 2011). Ribosomes are ubiquitous cellular components responsible for the translation of all mRNAs, and perturbed ribosome biogenesis and ribosome dysfunction can therefore give rise to numerous and varied downstream consequences (Scheper et al. 2007).
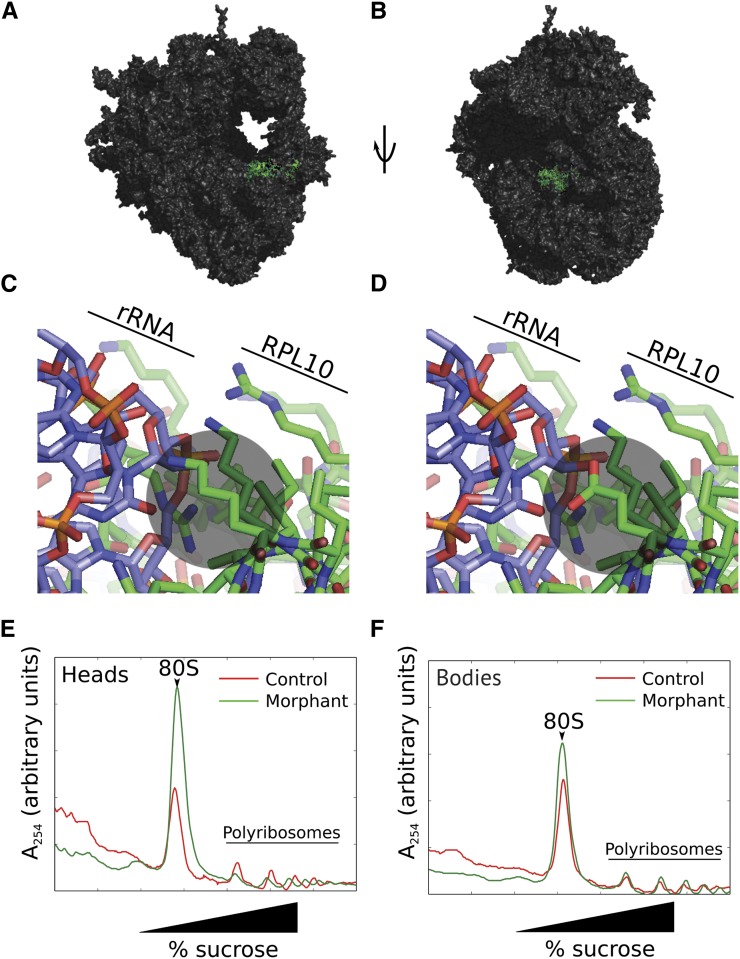
Advances in protein crystallography have enabled exquisite resolution of the structure of the 60S large ribosomal subunit (Klinge et al. 2011). RPL10 is one of six large subunit proteins with immediate proximity to the PTC (in addition to RPL3, RPL4, RPL8, RPL21, and RPL29; Figure 3, A and B); to date only RPL21 has been implicated in human genetic disease: a missense mutation at this locus has been associated with a nonsyndromic hair loss disorder, hereditary hypotrichosis simplex (HHS) (Zhou et al. 2011). The model suggests that mutation of K78, notably to an acidic residue, disrupts RPL10 protein–28S rRNA interactions (Figure 3, C and D), alters basic translational functions, and as a consequence alters significantly protein expression signatures to confer specific phenotypes to the central nervous system.
Figure 3.
rpl10 morphants display reduced bulk translation, especially in larval heads, as indicated by polyribosome structure. (A) Rendering of RPL10 in green on a eukaryotic ribosome in proximity to the peptidyl transferase active site. Protein Data Bank (PDB) entries 4a17, 4a19, and 2xzm were merged using a yeast ribosome (PDBs 2xzm and 3o58) as a guide. (B) As in A, rotated 90°. (C) Interaction of wild-type RPL10 K78 with the ribosomal (r)RNA. K78 interacts mostly with the negatively charged 28S rRNA and is indicated by a gray circle. (D) Simulation of the K78E mutation, indicated by a gray circle. (E) Sucrose gradient analysis of polyribosome structure in heads of 5 day postfertilization (dpf) rpl10 morphants and controls. Note the increase in 80S abundance with a corresponding decrease in polyribosomes for morphants vs. controls, indicative of a decrease in translational activity. (F) Sucrose gradient analysis of bodies of rpl10 morphants and controls. Polyribosome profiles are similar.
To gain preliminary insight into the biochemical underpinnings of the severe structural brain defects that result from altered RPL10, we asked whether reduction of RPL10 levels disrupted general protein synthesis. We injected WT zebrafish embryos with sb-MO and allowed them to grow to 5 dpf (Figure S2E). After separating heads from bodies, we analyzed polyribosome structure in each of the anterior and posterior portions of larvae. Morphant anterior structures displayed an increase in 80S ribosome abundance with a corresponding decrease in polyribosomes, consistent with a decrease in translation activity (Figure 3E). In contrast, polyribosome structure from bodies is relatively unchanged in morphants compared to controls (Figure 3F). These data indicate that RPL10 is important for translation specifically in zebrafish heads. At present, it is not known why loss of RPL10 expression alters polyribosome structure in the zebrafish head but not the posterior region. Such differences may reflect the apparently enriched expression of rpl10 in the anterior portion of the embryo or overall translation demands in specific spatiotemporal contexts.
rpl10 morphants display augmented apoptosis in the brain
Given the specific spatial reduction in bulk translation of rpl10 morphant brains, we wondered what cellular consequences were induced in the absence of RPL10. We modeled our hypothesis on reports from a clinically distinct ribosomopathy, Diamond–Blackfan anemia (DBA), in which mutations in ribosomal proteins can result in cell cycle arrest or induction of apoptosis (Aspesi et al. 2014). For example, studies using patient cells harboring mutations in the most common DBA gene, RPS19, can give rise to altered proliferation (Kuramitsu et al. 2008) and/or cell death (Gazda et al. 2006; Choesmel et al. 2007). Therefore, we tested these two possibilities by quantifying markers of cell cycle (M-phase marker phospho-histone H3) and cell death (TUNEL) in rpl10 morphants.
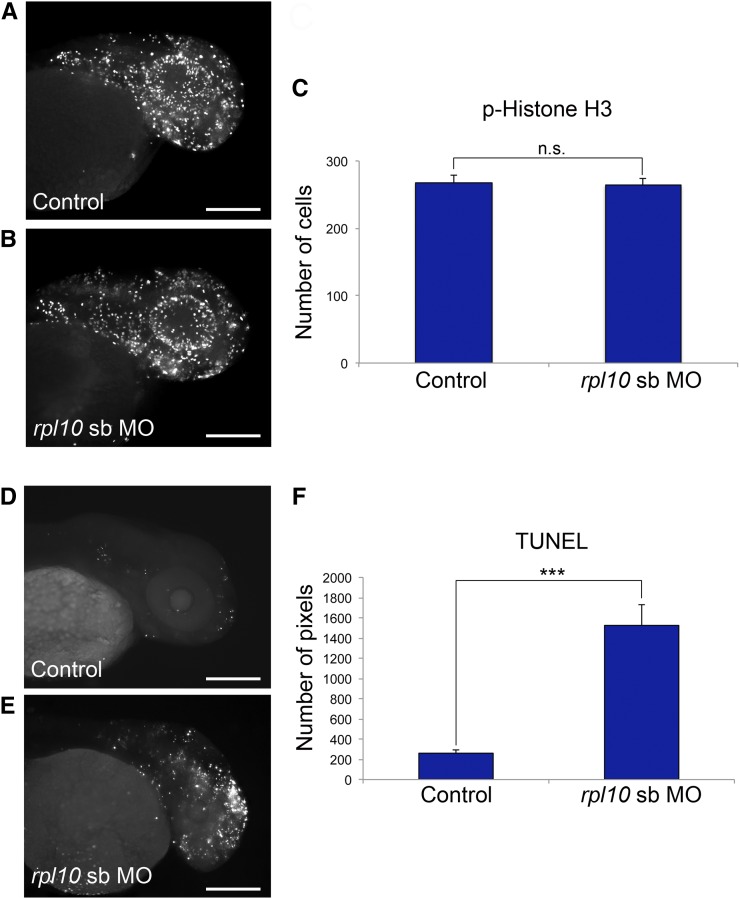
First, we injected WT embryos with rpl10 sb-MO (Figure 4, A and B), fixed them at 2 dpf, and stained them with anti-phospho-histone H3 antibody (n = 20 embryos per injection batch). We saw no difference in cell proliferation in morphants vs. controls upon quantification of stained cells in defined areas of the zebrafish head (Figure 4, A–C; repeated twice for the sb-MO, with similar results for the tb-MO, not shown). Moreover, we observed similar cell proliferation signatures at 1 dpf (Figure S3, A and B), a time point that typically precludes the observation of a head size defect in other zebrafish models of microcephaly (Golzio et al. 2012; Beunders et al. 2013).
Figure 4.
rpl10 morphants display normal cell proliferation and increased apoptosis in the brain. (A and B) Whole-mount phospho-histone H3 staining for proliferating cells (M-phase marker) in control and rpl10 morphants at 2 dpf (lateral views). (C) Quantification of phospho-histone H3-positive cells from 20 embryos each (control embryos or embryos injected with rpl10 MO). Data are represented as the mean ± SEM. n.s., nonsignificant (two-tailed t-test comparisons between sb-MO-injected and rescued embryos). (D and E) TUNEL staining for apoptotic cells in control and rpl10 morphants at 2 dpf (lateral views). (F) Quantification of TUNEL staining intensities from 20 embryos each (control embryos or embryos injected with rpl10 sb-MO). Data are represented as the mean ± SEM. ***P < 0.0001 (two-tailed t-test comparisons between MO-injected and rescued embryos); similar results were obtained with the rpl10 tb-MO. Bars, 250 μm.
Next, we monitored apoptosis in age-matched rpl10 sb-MO-injected embryos and controls, using whole-mount TUNEL staining. In contrast to the cell proliferation assay, we found a sixfold increase in cell death in the forebrains of rpl10 morphants in comparison to controls, determined by the number of stained pixels in laterally positioned images (Figure 4, D–F; P < 0.0001; n = 20 embryos quantified per injection batch; repeated twice for the sb-MO, with similar results for the tb-MO, not shown). While TUNEL staining in 1 dpf morphant embryos showed increased generalized apoptosis in the hindbrain and along the neural tube (Figure S3, C and D), it was temporally distinct from the localized cell death in the forebrain at 2 dpf, suggesting that apoptosis is likely to be a specific mechanistic driver of microcephaly in embryos with compromised RPL10 function.
Conclusion
Here, we report an X-linked pedigree with three hemizygous males who display severe central nervous system defects, including microcephaly and seizures in combination with growth retardation and a multitude of additional congenital defects. We propose that this novel syndrome is underscored by a missense p.K78E-encoding mutation in the 60S large ribosomal subunit component, RPL10. Our in vivo functional studies using zebrafish models support our human genetics data and highlight the power of a physiologically relevant vertebrate system to (a) establish relevance of a novel disease gene to human phenotypes; (b) determine variant pathogenic potential for private mutations; and (c) begin to elucidate the pathomechanism from biochemical evaluation of ribosomal output and cellular consequences, including cell death.
Although we are always cautious about elaborating findings from a single pedigree, our combined phenotypic, genetic, and functional data suggest that RPL10 dysfunction causes a novel ribosomopathy. To date, most reported mutations in ribosomal proteins have been associated with DBA. Frequently associated with loss-of-function mutations in at least 10 ribosomal components, DBA is a rare, clinically heterogeneous disorder hallmarked by red blood cell aplasia and incompletely penetrant defects in facio-skeletal development. Notably, affected individuals with DBA and our RPL10 family display overlapping features including syndactyly, mandibular and cleft defects, and genitourinary malformations (Vlachos et al. 2014). However, the defining features of each ribosomal disorder described here are distinctly different; DBA is characterized by fully penetrant anemia and the most prominent phenotypes in our RPL10 pedigree are in the central nervous system. Moreover, the affected males with a hemizygous RPL10 mutation are not anemic, suggesting that they do not have a variant form of DBA.
Finally, our findings add to the accumulating repertoire of ubiquitously expressed genes that give rise to tissue-specific phenotypes. Another such example includes cleavage and polyadenylation factor I subunit 1 (CLP1), a multifunctional kinase implicated in tRNA, mRNA, and small interfering RNA (siRNA) maturation that when mutated, gives rise to neurodegenerative disease (Schaffer et al. 2014). Also, the general pre-mRNA splicing factors, such as PRPF3, PRPF8, and PRPF31, are substantial contributors to isolated retinitis pigmentosa when rendered dysfunctional, yet mutation-bearing individuals do not display syndromic features (McKie et al. 2001; Vithana et al. 2001; Chakarova et al. 2002; Liu and Zack 2013). Although rpl10 is expressed widely in the zebrafish embryo (Thisse and Thisse 2004), but with augmented expression levels in the developing zebrafish head in comparison to the posterior region, our data indicate that central nervous system defects are likely due to altered quantitative, and potentially qualitative, translational activity restricted to the anterior structures. We do not know whether reduced bulk translation, altered translation of certain neuronal-specific transcripts, or both phenomena in concert result in microcephaly in hemizygous males with RPL10 mutations. Future systematic polysome profiling of cells derived from either affected individuals or model organisms corresponding to differing ribosomal components will be required to refine the precise mechanisms governing diverse and tissue-specific phenotypic outcomes.
Acknowledgments
We are grateful to the family in our study for their encouragement and support of our work. We acknowledge Dustin Dowless for technical assistance. This work was supported by funding from the Duke University Undergraduate Research Support Office (to A.L.W.), a National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Grant from the Brain and Behavior Research Foundation (to C.G.), National Institutes of Health (NIH) grant GM101533 (to C.V.N.), and the Simons Foundation Autism Research Initiative grant 239983 and NIH grant P50MH094268 (to N.K.). N.K. is a Distinguished George W. Brumley Professor.
Footnotes
Supporting information is available online at http://www.genetics.org/lookup/suppl/doi:10.1534/genetics.114.168211/-/DC1.
Communicating editor: L. B. Jorde
Literature Cited
- Adzhubei I. A., Schmidt S., Peshkin L., Ramensky V. E., Gerasimova A., et al. , 2010. A method and server for predicting damaging missense mutations. Nat. Methods 7: 248–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspesi A., Pavesi E., Robotti E., Crescitelli R., Boria I., et al. , 2014. Dissecting the transcriptional phenotype of ribosomal protein deficiency: implications for Diamond-Blackfan anemia. Gene 545: 282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shem A., Garreau de Loubresse N., Melnikov S., Jenner L., Yusupova G., et al. , 2011. The structure of the eukaryotic ribosome at 3.0 A resolution. Science 334: 1524–1529 [DOI] [PubMed] [Google Scholar]
- Beunders G., Voorhoeve E., Golzio C., Pardo L. M., Rosenfeld J. A., et al. , 2013. Exonic deletions in AUTS2 cause a syndromic form of intellectual disability and suggest a critical role for the C terminus. Am. J. Hum. Genet. 92: 210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell L. S., Walker S., Klingseisen A., Stiff T., Leitch A., et al. , 2011. Mutations in ORC1, encoding the largest subunit of the origin recognition complex, cause microcephalic primordial dwarfism resembling Meier-Gorlin syndrome. Nat. Genet. 43: 350–355 [DOI] [PubMed] [Google Scholar]
- Chakarova C. F., Hims M. M., Bolz H., Abu-Safieh L., Patel R. J., et al. , 2002. Mutations in HPRP3, a third member of pre-mRNA splicing factor genes, implicated in autosomal dominant retinitis pigmentosa. Hum. Mol. Genet. 11: 87–92 [DOI] [PubMed] [Google Scholar]
- Chiocchetti A., Pakalapati G., Duketis E., Wiemann S., Poustka A., et al. , 2011. Mutation and expression analyses of the ribosomal protein gene RPL10 in an extended German sample of patients with autism spectrum disorder. Am. J. Med. Genet. A. 155A: 1472–1475 [DOI] [PubMed] [Google Scholar]
- Chiocchetti A. G., Haslinger D., Boesch M., Karl T., Wiemann S., et al. , 2014. Protein signatures of oxidative stress response in a patient specific cell line model for autism. Mol Autism 5: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choesmel V., Bacqueville D., Rouquette J., Noaillac-Depeyre J., Fribourg S., et al. , 2007. Impaired ribosome biogenesis in Diamond-Blackfan anemia. Blood 109: 1275–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauber A., Golzio C., Guenot C., Jodelka F. M., Kibaek M., et al. , 2013. SCRIB and PUF60 are primary drivers of the multisystemic phenotypes of the 8q24.3 copy-number variant. Am. J. Hum. Genet. 93: 798–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E. E., Frangakis S., Katsanis N., 2014. Interpreting human genetic variation with in vivo zebrafish assays. Biochim. Biophys. Acta (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brouwer A. P., Yntema H. G., Kleefstra T., Lugtenberg D., Oudakker A. R., et al. , 2007. Mutation frequencies of X-linked mental retardation genes in families from the EuroMRX consortium. Hum. Mutat. 28: 207–208 [DOI] [PubMed] [Google Scholar]
- Ellison J. W., Rosenfeld J. A., Shaffer L. G., 2013. Genetic basis of intellectual disability. Annu. Rev. Med. 64: 441–450 [DOI] [PubMed] [Google Scholar]
- Gazda H. T., Kho A. T., Sanoudou D., Zaucha J. M., Kohane I. S., et al. , 2006. Defective ribosomal protein gene expression alters transcription, translation, apoptosis, and oncogenic pathways in Diamond-Blackfan anemia. Stem Cells 24: 2034–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzio C., Willer J., Talkowski M. E., Oh E. C., Taniguchi Y., et al. , 2012. KCTD13 is a major driver of mirrored neuroanatomical phenotypes of the 16p11.2 copy number variant. Nature 485: 363–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X., Delorme R., Fauchereau F., Durand C. M., Chaste P., et al. , 2009. An investigation of ribosomal protein L10 gene in autism spectrum disorders. BMC Med. Genet. 10: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauck S. M., Felder B., Kolb-Kokocinski A., Schuster C., Chiocchetti A., et al. , 2006. Mutations in the ribosomal protein gene RPL10 suggest a novel modulating disease mechanism for autism. Mol. Psychiatry 11: 1073–1084 [DOI] [PubMed] [Google Scholar]
- Klinge S., Voigts-Hoffmann F., Leibundgut M., Arpagaus S., Ban N., 2011. Crystal structure of the eukaryotic 60S ribosomal subunit in complex with initiation factor 6. Science 334: 941–948 [DOI] [PubMed] [Google Scholar]
- Komoike Y., Shimojima K., Liang J. S., Fujii H., Maegaki Y., et al. , 2010. A functional analysis of GABARAP on 17p13.1 by knockdown zebrafish. J. Hum. Genet. 55: 155–162 [DOI] [PubMed] [Google Scholar]
- Kumar P., Henikoff S., Ng P. C., 2009. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 4: 1073–1081 [DOI] [PubMed] [Google Scholar]
- Kuramitsu M., Hamaguchi I., Takuo M., Masumi A., Momose H., et al. , 2008. Deficient RPS19 protein production induces cell cycle arrest in erythroid progenitor cells. Br. J. Haematol. 140: 348–359 [DOI] [PubMed] [Google Scholar]
- Leonard H., Wen X., 2002. The epidemiology of mental retardation: challenges and opportunities in the new millennium. Ment. Retard. Dev. Disabil. Res. Rev. 8: 117–134 [DOI] [PubMed] [Google Scholar]
- Liu M. M., Zack D. J., 2013. Alternative splicing and retinal degeneration. Clin. Genet. 84: 142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubs H. A., Stevenson R. E., Schwartz C. E., 2012. Fragile X and X-linked intellectual disability: four decades of discovery. Am. J. Hum. Genet. 90: 579–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKie A. B., McHale J. C., Keen T. J., Tarttelin E. E., Goliath R., et al. , 2001. Mutations in the pre-mRNA splicing factor gene PRPC8 in autosomal dominant retinitis pigmentosa (RP13). Hum. Mol. Genet. 10: 1555–1562 [DOI] [PubMed] [Google Scholar]
- Migeon B. R., 1998. Non-random X chromosome inactivation in mammalian cells. Cytogenet. Cell Genet. 80: 142–148 [DOI] [PubMed] [Google Scholar]
- Niederriter A. R., Davis E. E., Golzio C., Oh E. C., Tsai I. C., et al. , 2013. In vivo modeling of the morbid human genome using Danio rerio. J. Vis. Exp. 78: e50338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders S. J., Murtha M. T., Gupta A. R., Murdoch J. D., Raubeson M. J., et al. , 2012. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485: 237–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer A. E., Eggens V. R., Caglayan A. O., Reuter M. S., Scott E., et al. , 2014. CLP1 founder mutation links tRNA splicing and maturation to cerebellar development and neurodegeneration. Cell 157: 651–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper G. C., van der Knaap M. S., Proud C. G., 2007. Translation matters: protein synthesis defects in inherited disease. Nat. Rev. Genet. 8: 711–723 [DOI] [PubMed] [Google Scholar]
- Schwarz J. M., Rodelsperger C., Schuelke M., Seelow D., 2010. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods 7: 575–576 [DOI] [PubMed] [Google Scholar]
- Spahn C. M., Beckmann R., Eswar N., Penczek P. A., Sali A., et al. , 2001. Structure of the 80S ribosome from Saccharomyces cerevisiae–tRNA-ribosome and subunit-subunit interactions. Cell 107: 373–386 [DOI] [PubMed] [Google Scholar]
- Sugawara T., Tsurubuchi Y., Agarwala K. L., Ito M., Fukuma G., et al. , 2001. A missense mutation of the Na+ channel alpha II subunit gene Na(v)1.2 in a patient with febrile and afebrile seizures causes channel dysfunction. Proc. Natl. Acad. Sci. USA 98: 6384–6389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpey P. S., Smith R., Pleasance E., Whibley A., Edkins S., et al. , 2009. A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation. Nat. Genet. 41: 535–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse, B., and C. Thisse, 2004 Fast release clones: a high throughput expression analysis. ZFIN Direct Data Submission. Available at: http://zfin.org
- Thisse C., Thisse B., 2008. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3: 59–69 [DOI] [PubMed] [Google Scholar]
- Tian J., Ling L., Shboul M., Lee H., O’Connor B., et al. , 2010. Loss of CHSY1, a secreted FRINGE enzyme, causes syndromic brachydactyly in humans via increased NOTCH signaling. Am. J. Hum. Genet. 87: 768–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Veyver I. B., 2001. Skewed X inactivation in X-linked disorders. Semin. Reprod. Med. 19: 183–191 [DOI] [PubMed] [Google Scholar]
- Vithana E. N., Abu-Safieh L., Allen M. J., Carey A., Papaioannou M., et al. , 2001. A human homolog of yeast pre-mRNA splicing gene, PRP31, underlies autosomal dominant retinitis pigmentosa on chromosome 19q13.4 (RP11). Mol. Cell 8: 375–381 [DOI] [PubMed] [Google Scholar]
- Vlachos A., Blanc L., Lipton J. M., 2014. Diamond Blackfan anemia: a model for the translational approach to understanding human disease. Expert Rev. Hematol. 7: 359–372 [DOI] [PubMed] [Google Scholar]
- Wan J., Yourshaw M., Mamsa H., Rudnik-Schoneborn S., Menezes M. P., et al. , 2012. Mutations in the RNA exosome component gene EXOSC3 cause pontocerebellar hypoplasia and spinal motor neuron degeneration. Nat. Genet. 44: 704–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul N. A., Liu Y., Gerdes J. M., Gascue C., Oh E. C., et al. , 2010. Functional analyses of variants reveal a significant role for dominant negative and common alleles in oligogenic Bardet-Biedl syndrome. Proc. Natl. Acad. Sci. USA 107: 10602–10607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Zang D., Jin Y., Wu H., Liu Z., et al. , 2011. Mutation in ribosomal protein L21 underlies hereditary hypotrichosis simplex. Hum. Mutat. 32: 710–714 [DOI] [PubMed] [Google Scholar]