Mitochondrial dysfunction has long been implicated in the pathogenesis of Parkinson disease (PD). Recent research has highlighted that two proteins encoded by genes linked to familial PD, PINK1 and parkin, play a role in the autophagic degradation of dysfunctional mitochondria (mitophagy).
We have recently shown that mitochondrial dysfunction in PINK1-deficient human dopaminergic cells correlates with decreased autophagic flux and can be rescued by parkin expression. Further dissection of PINK1-parkin-dependent mitophagy indicates that the ubiquitination of mitofusins 1 and 2 is an early event. Here, we discuss how ubiquitination of the mitofusins might facilitate mitochondria degradation and the potential for activating mitophagy as a treatment for diseases affecting brain and muscle.
It has recently been established that PINK1 and parkin are involved in the clearance of depolarized mitochondria (mitochondria with decreased mitochondrial membrane potential required to drive ATP synthesis). Several groups have shown that following depolarization, PINK1 promotes the translocation of parkin from the cytosol to the mitochondria. The exact mechanism by which PINK1 senses depolarization is unclear, but increasing evidence suggests that full-length PINK1 protein levels are increased in depolarized mitochondria, perhaps as a result of increased protein stability/decreased processing. PINK1 is a putative serine/threonine kinase, thus phosphorylation of parkin via a kinase cascade might be the means of recruitment of this E3 ubiquitin ligase to mitochondria.
A number of groups have reported an increase in the ubiquitination of mitochondrial proteins following parkin translocation to mitochondria. Upon induction of mitophagy we found that mitofusin 1 (MFN-1) and mitofusin 2 (MFN-2) are rapidly ubiquitinated in a PINK1- and parkin-dependent manner. The MFNs are transmembrane GTPases located in the outer membrane of mitochondria and participate in the fusion of this organelle. Drosophila express a sole mitofusin homologue (also known as Marf) and this protein is a substrate of parkin, and is ubiquitinated following induction of mitophagy. A key question is how does ubiquitination of MFN-1/2 facilitate mitophagy?
Ubiquitination of both MFNs could simply be a signal to recruit ubiquitinbinding proteins such as p62/SQSTM1, which could mediate the aggregation and loading of damaged mitochondria into autophagosomes. Induction of mitophagy results in the ubiquitination of several additional mitochondrial proteins, most of which are still unidentified. We speculate that certain of these other ubiquitinated proteins, rather than MFN-1/2, are acting as tags to recruit p62/SQSTM1. A more attractive hypothesis is that ubiquitination of MFN-1/2 regulates mitochondrial dynamics, which would help to segregate damaged mitochondria destined for mitophagy. Mitochondria continuously undergo fusion and fission events and form a dynamic network throughout the cell (Fig. 1). This process has been proposed as an important mechanism to modulate mitochondrial metabolism and mitochondrial DNA (mtDNA) integrity. Elegant experiments have shown that depolarized/damaged mitochondria undergo fission from the mitochondria network and it is to these mitochondria only that parkin translocates and ubiquitinates its substrates.
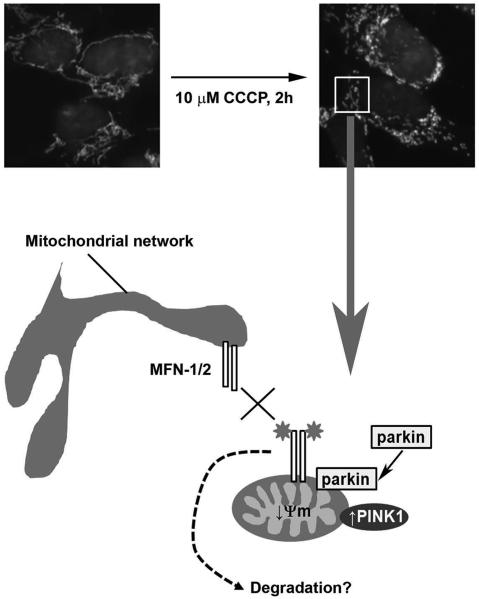
Figure 1.
Immunofluorescent staining of mitochondria with an antibody against the mitochondrial protein MTCO 1 (white) in dopaminergic SH-SY5Y neuroblastoma cells indicates that the mitochondrial network undergoes fission within two hours of depolarization of mitochondria by the mitochondrial toxin CCCP. Depolarized mitochondria (↓ Ψm) display an increase in PIN K1 protein levels, which recruits parkin to mitochondria. Parkin then ubiquitinates (stars) several mitochondrial proteins including MFN-1 and MFN-2. We postulate that ubiquitination prevents the refusion of depolarized/damaged mitochondria with the mitochondrial network (cross) allowing them to be segregated for degradation by mitophagy. Ubiquitination could prevent refusion by degrading the pro-fusion proteins via the proteasome or autophagy. Alternatively, ubiquitination might inhibit MFN GTP ase activity or sterically block inter-mitochondrial and mitochondria-endoplasmic reticulum tethering by the MFNs.
Ubiquitination of MFN-1/2 could prevent the refusion of these damaged mitochondria with the mitochondrial network (Fig. 1). Ubiquitination might be a post-translational modification that directly inhibits MFN GTPase activity or physically interferes with the MFN homo- and heterodimers between mitochondria.
Alternatively, ubiquitination could prevent refusion by promoting the degradation of the mitofusins by the proteasome or autophagy. The parkin-dependent ubiquitination of MFN-1 and MFN-2 is different. MFN-1 exhibits multiple ubiquitin moieties, while only three isoforms of MFN-2 are detected: unmodified MFN-2, and two higher molecular weight species indicative of di- and triubiquitination. Parkin has been reported to catalyze both K48- and K63-linked ubiquitin chains. Typically, degradation of proteins by the proteasome requires K48-linkage and a chain of more than four ubiquitin moieties, whereas K63-linked mono-ubiquitinated or poly-ubiquitinated proteins are degraded by autophagy. The type of linkage for each MFN needs to be determined. The ubiquitination pattern of MFN-2 would suggest that it is not a proteasome substrate. Drosophila MFN is more homologous to MFN-2, and it is interesting to note that both the fly and human proteins exhibit a similar ubiquitination pattern.
Little is known about MFN-1 other than its involvement in mitochondrial fusion. However, MFN-2 has been implicated in several other processes that might be relevant to mitophagy including mitochondria-ER connections and the transport of mitochondria along microtubules. Determining whether MFN-1 and MFN-2 have differing roles in mitophagy also needs to be addressed.
Mitochondrial and lysosomal function decrease with age and both have been implicated in age-related disorders such as PD. Dysfunctional mitochondria produce less ATP and generate increased levels of reactive oxygen species, which can further damage proteins, lipids and DNA throughout the cell. Therefore clearance of damaged mitochondria is required to maintain cellular homeostasis and prevent cell death. This is particularly relevant to post-mitotic cells such as neurons. Damage to mitochondria, and in particular mtDNA, by reactive oxygen species will accumulate over time, affecting the expression of key subunits of the electron transport chain, which will further impair oxidative phosphorylation.
Indeed, an increased frequency of mitochondrial deletions has been reported in the Substantia nigra of both aged and parkinsonian brains. Impaired mitophagy over several years could well be a contributing factor.
In our studies we found that long-term silencing of PINK1 expression results in inhibition of ATP synthesis and decreased autophagy. Since parkin is downstream of PINK1, we reasoned that overexpression of parkin could increase autophagic/mitophagic flux. This is the case, and crucially mitochondrial ATP synthesis is no longer inhibited, implying that damaged mitochondria are being removed.
Other studies have noted that activation of autophagy/mitophagy can have beneficial effects. Expression of parkin reduces the mutant load of cell lines harboring a particular hereditary mtDNA mutation, whereas rapamycin treatment can reduce cell death induced by the mitochondria toxin rotenone. These findings raise the possibility that maintenance of mitophagy in tissues such as neurons and muscle, which are particularly dependent on mitochondria and exhibit declining mitochondrial function with age, could be a useful tool in ameliorating the effects of inherited and age-related disorders that afflict these tissues.