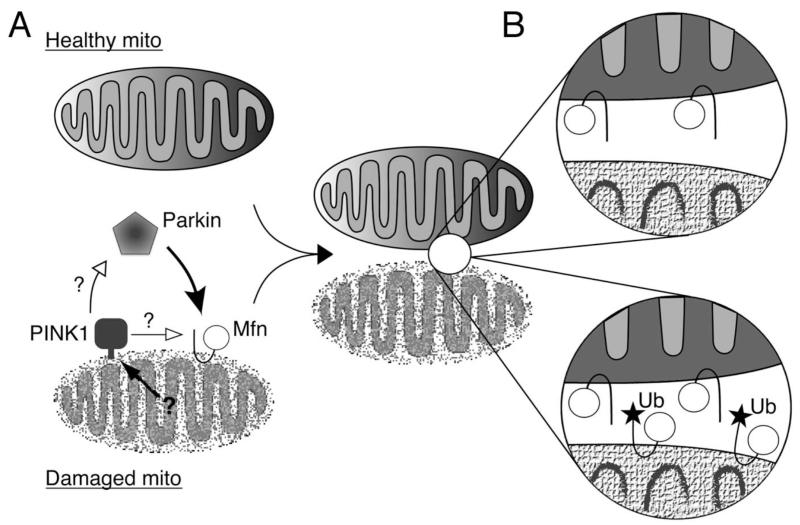
Much evidence links mitochondrial dysfunction to the death of neurons in Parkinson disease (PD), and is particularly emphasized by our growing understanding of the function of genes linked to recessively inherited PD such as PINK1, parkin and DJ-1. Recent work has revealed an exciting link between the PINK1-Parkin pathway and the autophagic turnover of dysfunctional mitochondrial (mitophagy). We have recently shown that mitofusin is ubiquitinated by Parkin when it is recruited to dysfunctional mitochondria. Recent work also shows that regulated fission and fusion events help segregate dysfunctional mitochondria prior to mitophagy. Here we hypothesize how Parkin-mediated ubiquitination of Mfn may play a role in this mechanism. In the past couple of years molecular and genetic studies have begun to uncover the way that the PINK1-Parkin pathway helps maintain mitochondrial homeostasis. A number of lines of evidence now indicate this pathway may regulate the autophagy of damaged or dysfunctional mitochondria. For instance, normally cytoplasmic Parkin translocates to mitochondria upon depolarization, and promotes their degradation by autophagy. A number of groups have shown that PINK1 is required to mediate Parkin translocation to damaged mitochondria and this is also necessary to promote mitophagy under these conditions (Fig. 1A). Currently, it is unclear exactly how PINK1 promotes or stimulates Parkin translocation, but this may require the sustained presence of PINK1 on the dsyfunctional mitochondria. Nevertheless, this mechanism provides a molecular framework that supports the previously established genetic relationship of PINK1 acting upstream of Parkin in a common pathway.
Figure 1.
Hypothetical mechanisms for how Mfn ubiquitination may affect mitochondrial segregation. (A) An unknown mechanism recognizes damaged mitochondria and stimulates PIN K1, by another unresolved mechanism that may involve stabilizing it on damaged mitochondria, to signal the recruitment of Parkin from the cytoplasm. Parkin then translocates and ubiquitinates Mfn on the outer surface. (B) Ubiquitination of Mfn may lead to its removal from the damaged mitochondria (top), or may interfere with Mfn-mediated tethering, a prerequisite for fusion (bottom). Either mechanism, or a combination of both, would reduce the capability of damaged mitochondria to re-fuse with the healthy network.
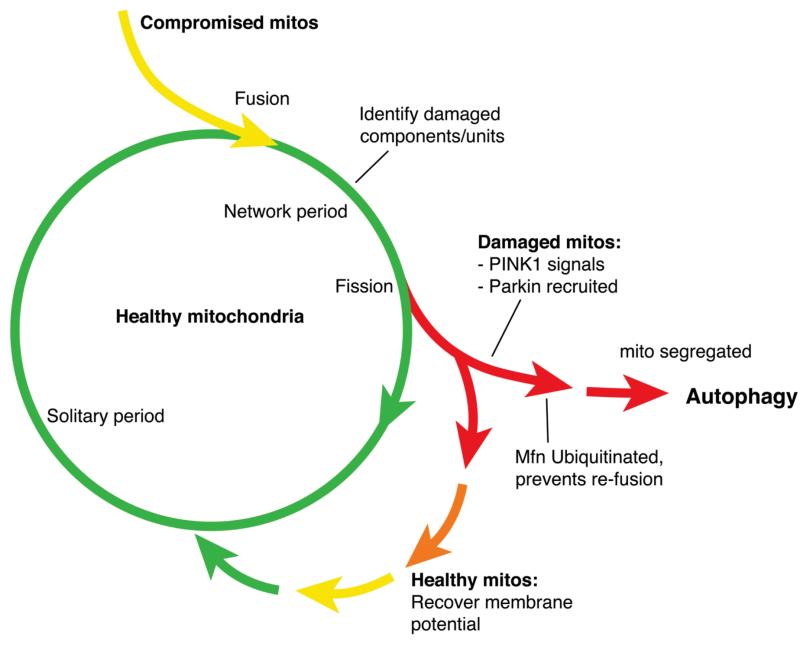
Another important insight came from the realization that PINK1 and parkin genetically interact with components of the mitochondrial fission and fusion machinery. Genetic studies in Drosophila suggest that the PINK1-Parkin pathway may act to promote mitochondrial fission or alternatively inhibit their fusion. This is particularly intriguing not only because aberrant mitochondrial fission/fusion has been linked to a growing list of neurodegenerative diseases, but also because emerging evidence suggests that mitochondrial dynamics may help segregate damaged mitochondria prior to autophagy (Fig. 2). This provides an attractive mechanism by which damaged mitochondrial components—proteins, lipids or DNA—can be ‘sorted out’ and sequestered for subsequent autophagy. The risk to cellular health here is that damaged mitochondria that are not cleared are in danger of rupturing, provoking apoptotic cell death; however, the mechanisms that regulate mitophagy of damaged mitochondria are poorly understood.
Figure 2.
Proposed function of PIN K1 and Parkin in regulating the segregation of damaged mitochondria. Mitochondrial fusion provides a mechanism for partially damaged (compromised) mitochondria to mix their contents with healthy mitochondria and allow complementation of dysfunctional components (proteins, lipids, mtDNA). Subsequent fission can generate a daughter mitochondrion that has accumulated a significant degree of damage such that complementation would be insufficient, and that must be degraded to avoid potentially catastrophic rupture. An unknown mechanism stimulates PIN K1 to signal to allow the recruitment of Parkin to that mitochondrion. Parkin ubiquitinates Mfn, which prevents re-fusion and segregates that mitochondrion for subsequent autophagy. (Fig. adapted withpermission from Twig et al. EMBO Journal 2008; 27:433-46).
Our recent work provides further insight into the molecular mechanism of how PINK1-Parkin mediate mitophagy. First, we showed in Drosophila cells that PINK1-mediated Parkin translocation and subsequent mitophagy is an important conserved mechanism. In addition, we identified the pro-fusion factor mitofusin (Mfn; also known as Marf in Drosophila) as a novel substrate of Parkin. These findings suggest a molecular mechanism that may explain the reported genetic interactions. Genetic perturbations that promote fragmentation suppress parkin and PINK1 mutant phenotypes. Consistent with this, we find that loss of parkin or PINK1 leads to an increased abundance of Mfn and hyperfused mitochondria.
However, a key unanswered question arising from these studies is how Parkin-mediated ubiquitination of Mfn may help segregate damaged mitochondria and stimulate mitophagy. Ubiquitination of the outer mitochondrial surface is thought to promote mitophagy by acting as a recruitment signal of the adaptor molecule p62/SQSTM1, but this could equally be mediated by ubiquitination of other abundant surface proteins such as VDAC1. Here, we hypothesize additional mechanisms by which the ubiquitination of Mfn specifically may promote the segregation of mitochondria to be degraded.
The modification of Mfn is a particularly attractive target since it plays a crucial role in the fusion of mitochondria.
We identified three isoforms of Mfn; unmodified Mfn (<92 kDa) and two higher molecular weight (100 kDa and <125 kDa) ubiquitinated isoforms that are Parkin dependent. These likely correspond to mono-ubiquitinated and multiubiquitinated Mfn, respectively. The first hypothesis proposes that ubiquitinated Mfn may be degraded by the proteasome, thus removing the pro-fusion factor from the outer surface. Cytoplasmic poly-ubiquitinated proteins are typically directed to the proteasome for degradation, however, membrane bound proteins are also extracted from the membrane and degraded by the proteasome. For example, misfolded proteins in the endoplasmic reticulum are degraded by a process known as ERAD. A similar mechanism could remove ubiquitinated Mfn specifically from damaged mitochondria and reduce their re-fusion capacity (Fig. 1B, top).
However, mono-ubiquitination does not typically lead to degradation. The ubiquitinated protein may develop new characteristics that affect its activity, subcellular localization or its interaction with other proteins. Indeed, mono-ubiquitination is becoming recognized as an important regulatory mechanism in controlling protein activity and signalling. A second hypothesis proposes that Mfn ubiquitination might physically interfere with the formation of trans Mfn dimers and prevent mitochondria tethering (Fig. 1B, bottom). This process is required for fusion, and its abrogation would thus preclude refusion of damaged mitochondria.
In summary, we propose the following mechanism by which regulated fusion and fission of mitochondria may contribute to a quality control process that recognizes and removes damaged mitochondria (Fig. 2). Following a fission event, a terminally damaged daughter mitochondrion is detected, perhaps by an internal mechanism, and stimulates PINK1 to promote the recruitment of Parkin. Parkin translocates to that mitochondrion where it ubiquitinates Mfn. The initial effect of Parkin mono-ubiquitination of Mfn may be to interfere with mitochondria tethering and exclude damaged mitochondria from rejoining the mitochondria network. Subsequently, p62/SQSTM1 may recognize multi-ubiquitinated Mfn on these mitochondria and recruit autophagosomes. In this way, mitochondria that have acquired an unsustainable amount of damage, for example through prolonged exposure to oxidative stress, are sequestered and safely destroyed, and a healthy mitochondrial network is maintained. Currently, it remains to be seen whether mammalian Parkin acts in an analogous fashion to regulate mitophagy. Furthermore, since Drosophila Mfn is the sole ubiquitously expressed mitofusin homologue and likely performs functions of both mammalian Mfn1 and Mfn2, it will be interesting to determine whether this mechanism may be mediated preferentially by Mfn1 or Mfn2. It will also be crucial to determine the mechanism that recognizes mitochondrial damage upstream of PINK1.