Abstract
OBJECTIVES
Evidence suggests inflammation is associated with cognitive impairment, but previous epidemiological studies have reported conflicting results.
DESIGN
Prospective population-based cohort.
SETTING
Epidemiology of Hearing Loss Study participants.
PARTICIPANTS
Individuals without cognitive impairment in 1998–2000 (N = 2,422; 1,947 with necessary data).
MEASUREMENTS
Cognitive impairment (Mini-Mental State Examination score <24 or diagnosis of dementia) was ascertained in 1998–2000, 2003–2005, and 2009– 2010. Serum C-reactive protein (CRP) and interleukin-6 (IL-6) were measured in 1988–1990, 1998–2000, and 2009–2010; tumor necrosis factor-alpha was measured from 1998–2000.
RESULTS
Participants with high CRP in 1988–1990 and 1998–2000 had lower risk of cognitive impairment than those with low CRP at both time points (hazard ratio (HR) = 0.46, 95% confidence interval (CI) = 0.26–0.80). Risk did not differ according to 10-year IL-6 profile or baseline inflammation category in the whole cohort. In sensitivity analyses restricted to statin nonusers, those with high IL-6 at both times had greater risk of cognitive impairment than those with low IL-6 at both times (HR = 3.35, 95% CI = 1.09–10.30). In secondary analyses, each doubling of IL-6 change over 20 years was associated with greater odds of cognitive impairment in 2009–2010 in the whole cohort (odds ratio (OR) = 1.40, 95% CI = 1.04–1.89), whereas a doubling of CRP change over 20 years was associated with cognitive impairment only in statin nonusers (OR = 1.32, 95% CI = 1.06–1.65).
CONCLUSION
With data collected over 20 years, this study demonstrated greater likelihood of cognitive impairment in individuals with repeated high or increasing IL-6. The inconsistent CRP findings may reflect effects of statin medications, survival effects, or adverse effects associated with chronically low CRP. Further studies of long-term inflammation and cognitive impairment are needed.
Keywords: cognition, dementia, inflammation, epidemiology, population-based
Dementia is a significant public health burden. A recent study estimated that 35.6 million people worldwide had dementia in 2010, and this number is expected to double every 20 years, to 115.4 million in 2050.1 Many of the shared risk factors for cardiovascular disease (CVD) and other chronic conditions are also thought to be associated with dementia, including inflammation.2–4
Relatively slight elevations in levels of inflammatory markers, including C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), have been independently associated with CVD,5,6 diabetes mellitus, 7–9 cancer,10 frailty, and mortality.11–13 Inflammation is integrally involved in atherosclerosis,14 which is one mechanism by which it might also be involved in the development of cognitive impairment. Inflammatory molecules have been observed in the cerebrospinal fluid and amyloid plaques of individuals with Alzheimer’s disease (AD).15 Prior longitudinal epidemiological studies have reported mixed results from analyses of inflammatory marker levels with dementia or cognitive impairment,16–23 provoking a recent meta-analysis that reported that higher CRP and IL-6 levels are associated with modestly greater risk of all-cause dementia.24
One concern in previous studies was whether inflammatory markers measured at a single time point would reflect long-term inflammation exposure. In the current study, IL-6 and CRP levels measured from three examination phases were used to better characterize long-term inflammation exposure status. The aim of the study was to determine whether long-term inflammation was associated with incident cognitive impairment in a large population-based cohort.
METHODS
Study Population
The Epidemiology of Hearing Loss Study (EHLS) is a population- based longitudinal study of sensory loss and aging in Beaver Dam, Wisconsin (1993–present). In 1987–1988, a private census identified 43- to 84-year-old residents of the city or township of Beaver Dam, Wisconsin. There were 5,924 eligible participants, and in 1988–1990, 4,926 (83%) of these participated in the first examination phase of the Beaver Dam Eye Study (BDES).25 Those who participated in the baseline BDES and were alive on March 1, 1993 (n = 4,541) were eligible for the EHLS, and 3,753 (82.6%) participated in the first EHLS examination phase from 1993–1995.26 Follow-up examinations were conducted at 5-year intervals (1998–2000, 2003–2005, and 2009–2010), and all achieved participation rates >80%.27,28
Cognition was first measured for all participants at the EHLS examination in 1998–2000 (baseline in these analyses). Participants with prevalent cognitive impairment (as defined below) in 1998–2000 were excluded from the current study. Participants without cognitive impairment in 1998–2000 were eligible (n = 2,422). The Health Sciences institutional review board of the University of Wisconsin approved this study, and informed consent was obtained from each participant.
Outcome
The Mini-Mental State Examination (MMSE) was administered in 1998–2000, 2003–2005, and 2009–2010.29 The MMSE is a brief test of general cognitive function and is often used as a screening device for cognitive impairment. 30 Cognitive impairment was defined as a MMSE score <24 (out of 30) or a self- or proxy-reported diagnosis of AD or dementia.
In 2009–2010, additional cognitive tests were administered: Trail Making Test Parts A and B (TMT-A, TMT-B), Digit Symbol Substitution Test (DSST), Verbal Fluency Test (VFT), and Auditory Verbal Learning Test (AVLT).31 Two memory questions were added: “Have you, your family, or your physician ever expressed concerns about your memory?” and “Do (your) memory loss symptoms interfere with your ability to do your own day-to-day activities?” For each test, participants who performed worse than the third to fifth percentile based on published age-specific normative data were considered impaired in the corresponding cognitive domain: executive function (DSST,32 TMT-A,33 TMT-B33), memory (AVLT34), or language (VFT; for VFT, cut-points were set to 1.5 SDs below the mean and were also education-specific, based on available normative data35). Based on recommended criteria for mild cognitive impairment (MCI)36 and all-cause dementia,37 cognitive test performance and responses to the memory questions were used to categorize participants as having normal cognition or MCI or dementia. MCI was defined as a positive response to the first question (memory concerns), impairment in at least one cognitive domain, and not meeting the definition for dementia. Dementia was defined as a positive response to the latter question (memory affects functioning) and two or three impaired domains or a self- or proxy report of physician-diagnosed AD or dementia. For these analyses, MCI and dementia were subsequently collapsed into one category, MCI/dementia.
This study was limited to participants without cognitive impairment in 1998–2000. Incident cognitive impairment was ascertained in 2003–2005 and 2009–2010, and MCI/dementia was ascertained in 2009–2010.
Inflammatory Markers
Blood samples from 1998–2000 and 2009–2010 were assayed for inflammatory markers in 2011 at the University of Minnesota. Serum CRP was measured using a high-sensitivity latex particle-enhanced immunoturbidimetric assay kit (Roche Diagnostics, Indianapolis, IN). The interassay coefficient of variation (CV) for the laboratory was 4.5%. The reference range was 0 to 5 mg/L. Serum IL-6 was measured using the quantitative sandwich enzyme technique of enzyme-linked immunosorbent assay (Quanti-Kine High Sensitivity Kit, R & D Systems, Minneapolis, MN). For IL-6, the interassay CV was 11.7%. Samples from 1998–2000 were assayed for serum TNF-α using a human TNF-α enzyme-linked immunosorbent assay (QuantiGlo, R&D Systems). The interassay CV was 13.0%.
Data on inflammatory markers from 10 years before baseline (1988–1990) were available from the BDES.38 Serum CRP was measured using a high-sensitivity latex particle-enhanced immunoturbidimetric assay kit (Kamiya Biomedical Co., Seattle, WA). The reference range was 0 to 0.5 mg/dL. The CV was 4.5%. Although reagents from a different manufacturer were used for these earlier CRP measurements, the same laboratory performed the assays using the same method as the later CRP measurements (described above), and their comparison study found excellent correlation between levels measured using reagents from the two manufacturers (correlation coefficient = 0.998). Serum IL-6 was measured for a sample of participants as part of a substudy using the same methods for the IL-6 at the later time points (described above). The reported CV was 6.5% to 9.6%.
Covariates
Unless otherwise noted, covariate data were obtained from the baseline examination phase (1998–2000). Education was self-reported and categorized as <12, 12, 13–15, or 16 or more years. General mental health was measured using the Mental Component Score (MCS) of the Medical Outcomes Study 36-item Short-Form Health Survey (SF-36), in which higher scores indicate better mental health.39 Participants self-rated their overall health as excellent, good, fair, or poor; fair and poor were combined for analyses. Participants self-reported smoking behavior (never, former, current) and exercise (number of times per week engaged in regular activity long enough to work up a sweat). Ever heavy drinking was defined as regular consumption of four or more alcoholic drinks per day, self-reported at any examination phase from 1988–2000. Height and weight were measured, and body mass index (BMI) was calculated by dividing weight (kg) by height (meters) squared. A BMI of 30.0 kg/m2 or more was considered obese. Apolipoprotein E (APOE) genotype was measured (Children’s Hospital of Philadelphia, Center for Applied Genomics) in participants of the 5-year follow-up phase (2003–2005) and was available for 87% (n = 1,691) of the 1,947 participants in the analytical sample. Histories of transient ischemic attack, CVD (myocardial infarction, stroke, or angina pectoris), and arthritis were based on self-reported physician diagnosis. Diabetes mellitus was defined as a self-reported physician diagnosis, treatment for diabetes mellitus, or a glycosylated hemoglobin level of 6.5% or greater. Systolic and diastolic blood pressure were measured according to the Hypertension Detection and Follow- Up Program protocol.40 Hypertension was defined as systolic blood pressure of 140 mmHg or greater, diastolic pressure of 90 mmHg or greater, or a self-reported physician diagnosis of hypertension and current use of hypertension medications. Participants brought all medications used in the past month, and examiners recorded medication names. Statin use was defined as use of any statin medication in the past month. Participants were also asked whether they had used aspirin at least twice per week for 3 or more months. Nonsteroidal anti-inflammatory drug (NSAID) use was defined as an affirmative response to this question or presence of aspirin or nonaspirin NSAIDs among the medications used in the past month.
Statistical Analyses
Statistical analyses were conducted using SAS (SAS Institute, Inc., Cary, NC). Of the eligible participants (without cognitive impairment at baseline, n = 2,422), 475 were not included in any analyses because they were missing necessary exposure or outcome data (mostly losses to follow- up due to death). Of the remaining 1,947 participants (the analytical sample), baseline characteristics of those who developed incident cognitive impairment during follow- up (n = 178) were compared with characteristics of those who did not (n = 1,769). These comparisons used the Cochran-Mantel-Haenszel statistic for general association 41 and were age and sex adjusted. The Kaplan–Meier method was used to estimate the 10-year cumulative incidence of cognitive impairment in the analytical sample (n = 1,947).
Marker levels were modeled primarily as categorical variables to compare participants with high inflammation at two time points with those with low inflammation at two time points. Thus, 10-year CRP and IL-6 profiles were based on marker levels in 1988–1990 and 1998–2000. Ten-year CRP profiles were high-high (CRP ≥2.81 mg/L at both time points), low-low (CRP <1.15 mg/L at both points), and all other (remaining participants). Likewise, 10-year IL-6 profiles were high-high (IL-6 >2.64 pg/mL at both points), low-low (IL-6 ≤1.64 pg/mL at both points), or all other. These cut-points for the 10-year profiles were tertiles from the distributions of 1988–1990 levels in participants without cognitive impairment at baseline.
For separate analyses with baseline (1998–2000) inflammation categories only, cut-points were defined according to tertiles from the distribution of 1998–2000 levels in participants without cognitive impairment (for CRP: <1.44, 1.44–3.61, ≥3.62 mg/L; for IL-6: ≤1.187, 1.188–2.26, >2.26 pg/mL; for TNF-α: ≤1.01, 1.02–1.477, >1.477 pg/mL).
Discrete-time Cox regression models were used to estimate the associations between baseline categories and 10-year inflammation profiles, respectively, and incident cognitive impairment in 2003–2005 or 2009–2010. The proportional hazards assumption was tested with interaction terms for inflammation categories by time, and no significant violations were detected. MCI and dementia were ascertained only in 2009–2010, and all analyses with the MCI/dementia outcome used logistic regression.
Models were initially adjusted for age, sex, and education (Model 1). Other potential confounders (described in Covariates section) were considered for inclusion in Model 2 to determine whether inflammation categories were independently associated with cognitive impairment risk after further adjustment for risk factors and comorbid disease. Covariates were retained in Model 2 if they moderately changed inflammation estimates or were statistically significant. Some covariates were conceptualized not only as potential confounders, but also as potential mediators (e.g., stroke, myocardial infarction), yet were ultimately included in Model 2 because the associations between inflammation and cognitive impairment were stable upon adjustment, making a separate mediation model redundant. During the multivariable modeling process, within each outcome, similar sets of covariates were retained across the various inflammation exposures, with discordant variables making little difference. Thus, for each outcome, a standard set of covariates was used and noted in the respective table.
Potential effect modification according to APOE ε4 carrier status was tested by including an interaction term for APOE ε4 carrier status by categorical inflammation variable, along with a main effect term for APOE ε4 carrier status, in each respective Model 1. Because statin use may lower inflammation,42–45 sensitivity analyses excluded those using statins during a given exposure period. Additional sensitivity analyses excluded those with CRP levels >10 mg/L, which may indicate an acute inflammatory response.5
To compare results with those of a previous study,18 secondary analyses used change in CRP and IL-6 levels over the 20-year period as continuous variables with a log (base 2) transformation to accommodate the skewed data (e.g., log2(CRP2009–10/CRP1988–90)). For example, with the CRP transformation, each one-unit increase in log2(CRP2009–10/CRP1988–90) corresponds to a doubling in CRP 20-year change. Thus, odds ratios will quantify the effect of a doubling in 20-year change in CRP or IL-6. Logistic regression models were used to examine the associations between 20-year change in inflammatory marker levels and the odds of incident cognitive impairment in 2009–2010 and, separately, MCI/dementia in 2009–2010. Models included the same standard sets of covariates determined in the analyses with categorical inflammation. Because CRP trajectories have been shown to differ considerably in statin users, these 20-year change analyses were repeated in only participants reporting statin nonuse at all five phases.
RESULTS
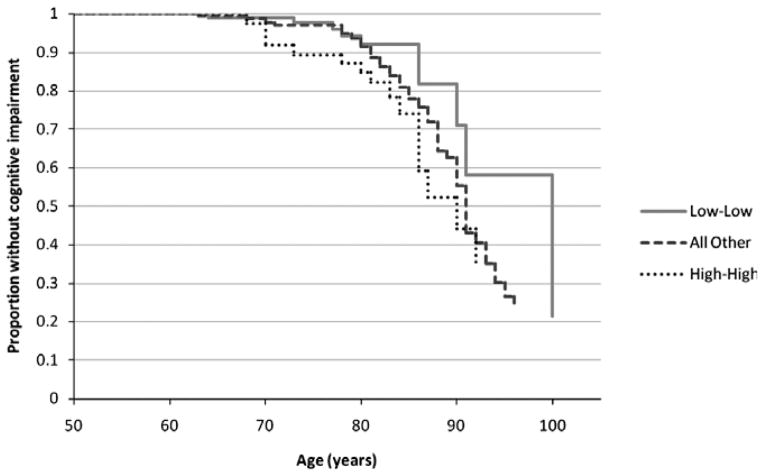
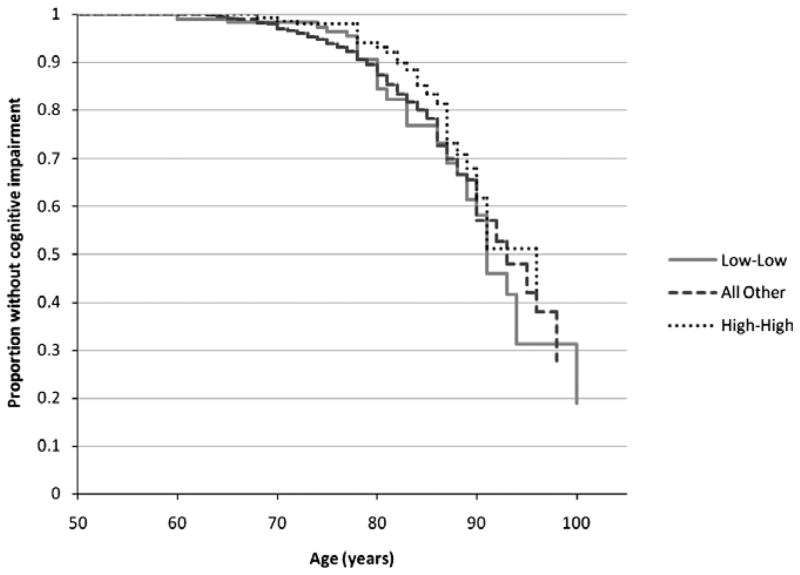
Participants who developed incident cognitive impairment at the 5- or 10-year follow-up were older and less educated and had worse mental health (lower SF-36 MCS) than those who did not develop cognitive impairment, and greater proportions reported ever heavy drinking, a history of stroke, and fair or poor self-rated health (Table 1). The 10-year cumulative incidence of cognitive impairment was 10.3% (n = 1,947). The plots of the crude proportions without cognitive impairment by age illustrated greater difference according to 10-year IL-6 profile (Figure 1) than CRP profile (Figure 2).
Table 1.
Baseline Characteristics of Analytical Samplea According to Development of Incident Cognitive Impairment in Participants without Cognitive Impairment at Baseline (1998–2000)
| Characteristic | Did Not Develop Cognitive Impairment, n = 1,769 | Developed Cognitive Impairment, n = 178 | Age- and Sex-Adjusted P-Valueb |
|---|---|---|---|
| Male, n (%) | 724 (40.9) | 72 (40.5) | .31 |
| Age, n (%) | |||
| 53–59 | 504 (28.5) | 7 (3.9) | <.001 |
| 60–69 | 692 (39.1) | 42 (23.6) | |
| 70–79 | 457 (25.8) | 84 (47.2) | |
| 80–97 | 116 (6.6) | 45 (25.3) | |
| Education, years, n (%) | |||
| <12 | 211 (11.9) | 58 (32.6) | <.001 |
| 12 | 887 (50.1) | 84 (47.2) | |
| 13–15 | 317 (17.9) | 22 (12.4) | |
| ≥ 16 | 354 (20.0) | 14 (7.9) | |
| Obese (body mass index ≥ 30.0 kg/m2), n (%) | 847 (48.7) | 75 (43.4) | .59 |
| Smoking status, n (%) | |||
| Never | 823 (47.0) | 86 (49.1) | .05 |
| Past | 746 (42.6) | 70 (40.0) | |
| Current | 182 (10.4) | 19 (10.9) | |
| Ever heavy drinking, n (%) | 404 (23.0) | 51 (29.1) | .006 |
| Exercise ≥ 1/week, n (%) | 785 (44.8) | 66 (37.7) | .75 |
| Diabetes mellitus, n (%) | 195 (11.1) | 25 (14.3) | .65 |
| Hypertension, n (%) | 984 (55.7) | 123 (69.5) | .13 |
| Cardiovascular disease, n (%) | 220 (12.6) | 31 (17.7) | .70 |
| History of stroke, n (%) | 23 (1.3) | 9 (5.1) | .04 |
| Self-rated health, n (%) | |||
| Excellent | 388 (22.2) | 24 (13.7) | .008 |
| Good | 1,172 (67.0) | 112 (64.0) | |
| Fair or poor | 190 (10.9) | 39 (22.3) | |
| Nonsteroidal anti-inflammatory drug use, n (%) | 1,269 (72.5) | 132 (75.4) | .60 |
| Aspirin use, n (%) | 923 (52.8) | 105 (60.0) | .44 |
| Statin use, n (%) | 360 (20.6) | 38 (21.7) | .95 |
| APOE ε4 carrier, n (%)c | 229 (14.8) | 16 (11.0) | .40 |
| Medical Outcomes Study 36-item Short-Form Health Survey Mental Component Subscale score, mean ± SDd | 55.8 ± 7.0 | 53.9 ± 8.4 | <.001 |
Percentages are of the number with data for a given covariate and counts may not sum to the total N for the column because of missing data (<2% missing for all variables, except apolipoprotein E (APOE) ε4 carrier, see below).
Analytical sample is 1,947 participants without cognitive impairment at baseline and with necessary inflammation and cognitive follow-up data. Table compares those who developed incident cognitive impairment at 5- or 10-year follow-up with those who did not.
P-values are adjusted for age and sex, except the P-values for sex and age, which are adjusted for age only and sex only, respectively.
APOE genotype data were available for 1,691 (86.9%) of the 1,947 included in the analyses.
Range 0 to 100, higher scores representing better mental health.
Figure 1.
Proportion without cognitive impairment according to 10-year interleukin-6 profile (based on estimated survival curves). No participants were cognitively impaired at baseline (1998–2000).
Figure 2.
Proportion without cognitive impairment according to 10-year C-reactive protein profile (based on estimated survival curves). No participants were cognitively impaired at baseline (1998–2000).
As shown in Table 2, those in the high-high CRP group were less likely to develop cognitive impairment in the subsequent 10 years than those with low-low CRP. There were no statistically significant differences in cognitive impairment risk according to 10-year IL-6 profile or baseline inflammation categories in the whole cohort (Table 2). In sensitivity analyses restricted to statin nonusers, only the results for 10-year IL-6 profile were substantially different from the results in the whole cohort (Table 2). Of statin nonusers, those in the high-high IL-6 group were estimated to have more than triple the risk of cognitive impairment than those with low-low IL-6 (Table 2). The results with the MCI/dementia outcome (Table 3) were generally consistent with those for the cognitive impairment outcome. Participants with high-high CRP had lower odds of MCI/dementia than those with low-low CRP, and no significant associations were observed with baseline inflammation categories or 10-year IL-6 profiles (Table 3). Results were not substantially different in sensitivity analyses restricted to statin nonusers (Table 3). The primary results were not considerably different when analyses excluded participants with CRP levels >10 mg/L, except the lower odds of MCI/dementia for those with high-high CRP were no longer statistically significant (data not shown). No significant interactions with APOE ε4 carrier status were observed.
Table 2.
Cox Regression Models for the Associations Between Inflammatory Marker Categories and Incident Cognitive Impairment in 2003–2005 or 2009–2010 in Participants without Cognitive Impairment at Baseline
| Inflammatory Marker | At Risk, na | Follow-Up, % | Incident Cases, n | Cumulative Incidence, %b | Hazard Ratio (95% Confidence Interval) | ||
|---|---|---|---|---|---|---|---|
| Model 1c | Model 2d | Statin Nonuserse | |||||
| Baseline CRP | n = 1,947 | n = 1,904 | n = 1,513 | ||||
| Tertile 1 | 764 | 86.0 | 67 | 11.2 | Reference | Reference | Reference |
| Tertile 2 | 760 | 87.2 | 54 | 9.1 | 0.72 (0.49–1.06) | 0.72 (0.48–1.08) | 0.85 (0.53–1.35) |
| Tertile 3 | 763 | 82.3 | 57 | 10.7 | 0.92 (0.63–1.36) | 0.83 (0.55–1.25) | 0.83 (0.52–1.34) |
| Baseline IL-6 | n = 1,947 | n = 1,904 | n = 1,513 | ||||
| Tertile 1 | 762 | 92.5 | 59 | 9.0 | Reference | Reference | Reference |
| Tertile 2 | 762 | 86.0 | 63 | 10.9 | 0.89 (0.61–1.31) | 0.96 (0.64–1.42) | 0.96 (0.61–1.51) |
| Tertile 3 | 763 | 77.1 | 56 | 11.6 | 0.99 (0.67–1.48) | 0.95 (0.62–1.45) | 0.95 (0.59–1.54) |
| Baseline TNF- α | n = 1,947 | n = 1,904 | n = 1,513 | ||||
| Tertile 1 | 653 | 99.9f | 60 | 10.1 | Reference | Reference | Reference |
| Tertile 2 | 643 | 100.0f | 50 | 8.7 | 0.75 (0.50–1.12) | 0.73 (0.49–1.11) | 0.65 (0.41–1.04) |
| Tertile 3 | 655 | 99.7f | 68 | 12.3 | 0.82 (0.56–1.21) | 0.78 (0.53–1.15) | 0.68 (0.43–1.06) |
| 10-year CRP | n = 1,941 | n = 1,899 | n = 1,470 | ||||
| Low-low | 378 | 88.6 | 38 | 12.5 | Reference | Reference | Reference |
| All other | 1,402 | 86.0 | 110 | 10.2 | 0.80 (0.54–1.20) | 0.74 (0.49–1.13) | 0.74 (0.46–1.19) |
| High-high | 500 | 80.4 | 29 | 8.9 | 0.57 (0.34–0.96) | 0.46 (0.26–0.80) | 0.41 (0.21–0.79) |
| 10-year IL-6 | n = 801 | n = 779 | n = 604 | ||||
| Low-low | 222 | 93.2 | 10 | 5.4 | Reference | Reference | Reference |
| All other | 573 | 82.9 | 57 | 13.6 | 1.75 (0.85–3.64) | 1.81 (0.85–3.87) | 1.93 (0.78–4.82) |
| High-high | 164 | 72.6 | 14 | 16.5 | 2.35 (0.96–5.73) | 2.35 (0.90–6.14) | 3.35 (1.09–10.30) |
CRP= C-reactive protein; IL-6= interleukin-6.
Participants without cognitive impairment at baseline (1998–2000).
Cumulative incidence using Kaplan–Meier method.
Model 1 covariates: baseline age, sex, and education.
Model 2 covariates: Model 1 + smoking status, ever heavy drinking, Medical Outcomes Study 36-item Short-Form Health Survey Mental Component Subscale score, body mass index, and stroke.
For baseline categories, includes only participants reporting statin nonuse at baseline; for 10-year categories, includes only participants reporting statin nonuse at baseline and 5 and 10 years before baseline (Model 2 covariates).
Tumor necrosis factor alpha (TNF-α) was only measured in participants with some follow-up.
Table 3.
Logistic Regression Models for the Associations Between Inflammatory Marker Categories and Mild Cognitive Impairment (MCI)/Dementia in 2009–2010 in Participants without Cognitive Impairment at Baseline
| Inflammatory Marker | At Risk, na | Follow-Up, % | MCI/Dementia N (%) | OR (95% CI) | ||
|---|---|---|---|---|---|---|
| Model 1b | Model 2c | Statin Nonusersd | ||||
| Baseline CRP | n = 1,490 | n = 1,456 | n = 1,152 | |||
| Tertile 1 | 764 | 68.7 | 66 (12.6) | Reference | Reference | Reference |
| Tertile 2 | 760 | 64.1 | 47 (9.7) | 0.70 (0.46–1.06) | 0.75 (0.48–1.16) | 0.64 (0.38–1.08) |
| Tertile 3 | 763 | 62.8 | 56 (11.7) | 0.94 (0.63–1.41) | 0.87 (0.56–1.35) | 0.69 (0.41–1.14) |
| Baseline IL-6 | n = 1,490 | n = 1,456 | n = 1,152 | |||
| Tertile 1 | 762 | 77.6 | 52 (8.8) | Reference | Reference | Reference |
| Tertile 2 | 762 | 66.5 | 69 (13.6) | 1.37 (0.92–2.03) | 1.42 (0.93–2.16) | 1.37 (0.85–2.22) |
| Tertile 3 | 763 | 51.5 | 48 (12.2) | 1.34 (0.87–2.06) | 1.28 (0.80–2.05) | 1.22 (0.71–2.10) |
| Baseline TNF-α | n = 1,489 | n = 1,455 | n = 1,151 | |||
| Tertile 1 | 653 | 78.6e | 63 (12.3) | Reference | Reference | Reference |
| Tertile 2 | 643 | 78.5e | 54 (10.7) | 0.80 (0.54–1.20) | 0.76 (0.49–1.16) | 0.72 (0.45–1.17) |
| Tertile 3 | 655 | 72.1e | 51 (10.8) | 0.73 (0.48–1.10) | 0.70 (0.46–1.08) | 0.62 (0.37–1.03) |
| 10-year CRP | n = 1,486 | n = 1,453 | n = 1,125 | |||
| Low-low | 378 | 72.8 | 41 (14.9) | Reference | Reference | Reference |
| All other | 1,402 | 65.5 | 98 (10.7) | 0.67 (0.44–1.01) | 0.65 (0.41–1.01) | 0.61 (0.37–1.02) |
| High-high | 500 | 58.8 | 30 (10.2) | 0.62 (0.36–1.05) | 0.49 (0.27–0.90) | 0.42 (0.21–0.85) |
| 10-year IL-6 | n = 593 | n = 578 | n = 443 | |||
| Low-low | 222 | 80.2 | 16 (9.0) | Reference | Reference | Reference |
| All other | 573 | 61.3 | 42 (12.0) | 1.01 (0.52–1.94) | 0.90 (0.44–1.84) | 1.03 (0.43–2.45) |
| High-high | 164 | 39.0 | 9 (14.1) | 1.57 (0.62–3.98) | 1.36 (0.44–4.27) | 2.42 (0.66–8.98) |
CRP= C-reactive protein; IL-6= interleukin-6.
Participants without cognitive impairment at baseline (1998–2000).
Model 1 covariates: baseline age, sex, and education.
Model 2 covariates: Model 1 + smoking status, ever heavy drinking, Medical Outcomes Study 36-item Short-Form Health Survey Mental Component Subscale score, body mass index, hypertension, diabetes mellitus, myocardial infarction, and arthritis.
For baseline categories, includes only participants reporting statin nonuse at baseline; for 10-year categories, includes only participants reporting statin nonuse at baseline and 5 and 10 years before baseline (Model 2 covariates).
TNF-α was measured only in participants with some follow-up.
In the secondary analyses using continuous variables for change in inflammation over the 20-year period (1988– 1990 to 2009–2010), each doubling in IL-6 change over 20 years was positively associated with cognitive impairment (odds ratio (OR) = 1.40, 95% confidence interval (CI) = 1.04–1.89; Model 2; n = 560) but not MCI/dementia (data not shown). Twenty-year CRP change was not associated with either outcome in the whole cohort (data not shown), although in sensitivity analyses restricted to statin nonusers, each doubling in CRP change over 20 years was positively associated with cognitive impairment (OR = 1.32, 95% CI = 1.06–1.65; Model 2; n = 586) and MCI/dementia (OR = 1.24; 95% CI = 1.03–1.49; Model 2; n = 585). IL-6 results in statin nonusers were similar to those in the whole cohort, except the association with cognitive impairment was no longer statistically significant in the reduced sample (OR = 1.55 per doubling in IL-6 change, 95% CI = 0.93–2.60; Model 2; n = 251).
DISCUSSION
High CRP level in 1988–1990 and 1998–2000 was associated with lower risk of cognitive impairment over the subsequent 10 years. High IL-6 level at both time points was not significantly associated with cognitive impairment risk in the whole cohort but was associated with triple the risk of cognitive impairment in those not using statins at or before baseline. Baseline inflammation categories were not associated with either outcome. Increasing IL-6 over 20 years was associated with greater odds of concurrent onset of cognitive impairment, and in statin non-users, a similar positive association was observed for 20- year change in CRP. Consistent results were observed with the MCI/dementia outcome, except 20-year IL-6 change was not associated with MCI/dementia.
The IL-6 results are generally consistent with previous studies, although few studies have included IL-6 levels measured at multiple times, and to the knowledge of the authors, none have compared the risk of cognitive impairment between those with repeatedly high levels of IL-6 and those with repeatedly low levels of IL-6. Statin nonusers had a greater risk associated with having high IL-6 levels at two time points, supporting a link between chronic systemic inflammation and cognitive impairment. In the whole cohort, the association was weaker and not significant, which might reflect effect dilution due to misclassification of long-term inflammation exposure in statin users. It is also possible that statin use may attenuate the association, although this observational study could not distinguish the influence of statin use per se from underlying differences in statin users, such as greater vascular risk or CVD burden. It was also found that each doubling in IL-6 change over 20 years was associated with 40% greater odds of cognitive impairment, which is similar to the 35% greater odds of cognitive impairment observed in the Cardiovascular Health Study All Stars with 9 years of follow-up.18 In contrast, a study in Uppsala, Sweden, had IL-6 measures from men at age 70 and 77 and found that longitudinal change was not associated with risk of AD or dementia.19 No association was observed for IL-6 measured at a single time point, consistent with some previous large cohort studies,20,21 although positive associations have also been reported.17,19 When classified using measures from multiple time points, greater IL-6 exposure is associated with greater risk of cognitive impairment.
The CRP results are more difficult to interpret. High CRP level in 1988–1990 and 1998–2000 was associated with lower risk of cognitive impairment than consistently low levels, whereas 20-year change in CRP was not associated with cognitive impairment in the whole cohort. In statin nonusers, 20-year CRP change was significantly associated with cognitive impairment (37% greater odds per doubling in CRP change). The lack of association for 20-year CRP change in the whole cohort probably reflects a substantial increase in statin use, which reportedly affects CRP levels. In this population, between 1998–2000 and 2009–2010, CRP levels decreased on average in statin users but not in nonusers.42 Two studies previously examined change in CRP with cognitive impairment, and one was consistent with the current study findings. The Cardiovascular Health Study found that each doubling in CRP change over a 9-year period was associated with 18% greater odds of cognitive impairment,18 whereas the Uppsala, Sweden, study found no association with 7-year CRP change.19 Other prospective studies investigated CRP levels measured at a single time point. They reported positive,16 marginal, 17 or null19,20,22,23 associations with dementia or AD risk, although two of these observed significant inverse associations in models adjusted for fewer covariates.20,23 Thus, despite no previous studies comparing individuals with multiple high CRP levels with those with multiple low CRP levels, the current study’s observed lower risk in individuals with high-high CRP was unexpected based on the existing literature.
Inflammation can be beneficial or harmful. Low CRP levels could be a marker of poor immune function in less healthy participants who may have a higher risk of cognitive impairment. Thus, it is possible that the choice of the low-low CRP category for the reference group may have contributed to the observed lower risk for high-high CRP. When the “all other” category was used as the reference, the high-high CRP group still had significantly lower cognitive impairment risk, although their lower odds of MCI/dementia was no longer significant. It is possible that higher inflammation levels protect against dementia, as a brain tissue study that indicated that very old people with normal cognition at death had upregulation of genes related to inflammatory and immune function suggested.46 Yet such findings could also reflect a hardy survivor effect. Several others have suggested that high CRP with normal cognition at older ages may characterize a resilient subgroup with countervailing protective factors that have not been identified.47 A recent study of cognitively intact adults aged 75 and older found lower dementia risk in relatives of those with high CRP, suggesting that familial, possibly genetic, factors may contribute to resiliency.48
Several potential mechanisms might explain an association between inflammation and cognitive impairment. It may be that inflammation is associated with dementia through vascular mechanisms. Inflammation is associated with cardiovascular5,6,14 and cerebrovascular disease,49 and both these diseases could contribute to dementia. It is also possible that inflammation is associated with dementia independent of vascular-related conditions. Accumulating evidence indicates that cytokines play a central and complex role in numerous healthy-state cognitive processes on the molecular level through pathways such as synaptic plasticity, neurogenesis, and neuromodulation. 50 Cytokines mediate cellular mechanisms involved in cognition such as cholinergic and dopaminergic pathways and can facilitate neurodegeneration or regeneration. 51 Some evidence indicates that peripheral cytokines can penetrate the blood–brain barrier, for example, through circumventricular regions less protected by the blood–brain barrier51 or through stimulation of the vagal nerve.52,53
Strengths and limitations of the current study should be considered when interpreting the results. In particular, some of the observed null associations could be due to lack of power, hardy survivor effects, or use of systemic inflammatory markers rather than measures of brain inflammation. Levels of IL-6 from 1988–90 were available only for a subset, limiting power to detect modest associations. Included participants were generally healthier than those who were not included because of missing data, as expected in a long-term study of older adults. Thus, these results may only apply to those who are healthier and survive to older ages. In addition, in those with exposure data, greater proportions were lost from the higher inflammation categories. Given that cognitive impairment is a major factor related to attrition in population-based studies of older adults54 and that attrition was greater in higher inflammation categories, it is possible that differential losses to follow-up (e.g., hardy survivor effect) affected the findings. It is likely that such potential bias would lead to underestimated associations and could theoretically explain the unexpected lower risk observed in those with high-high CRP. However, this was a large, prospective, population-based cohort with consistently high participation rates, and home and nursing home visits were provided to minimize loss to follow-up. This cohort was predominantly non-Hispanic white, which may limit the generalizability of results or potentially explain different results in studies of other racial or ethnic groups (e.g., evidence linking high CRP and dementia risk is largely based on results from a Japanese-American cohort16). No depression- specific measure was collected at baseline, and although analyses were adjusted for baseline SF-36 MCS (generic mental health), residual confounding could exist. The cognitive impairment outcome was based on MMSE performance or reported AD diagnosis rather than diagnostic evaluations, yet results were largely similar for a second outcome available only in 2009–2010, MCI/dementia, which was based on additional cognitive tests and memory concerns. The 10-year follow-up for incident cognitive impairment provides a biologically plausible time period for a potential causal role of inflammation in impairment and reduces the chances that the observed associations reflect a reverse causal direction. Unlike most previous studies, CRP and IL-6 levels were obtained from three time points over a 20-year period, enabling a better proxy measure of long-term inflammation.
In conclusion, this study supports existing evidence of an association between IL-6 and cognitive impairment and demonstrates the importance of multiple measures over time. There was also a suggestion that a long-term increase in CRP levels may be associated with cognitive impairment. Additional prospective population-based studies with multiple inflammatory marker measures are needed to confirm or refute these findings.
Acknowledgments
This work was supported by R37AG011099 (Karen J. Cruickshanks) from the National Institute on Aging (NIA); U10EY06594 (Ronald Klein, Barbara E.K. Klein) from the National Eye Institute; DK073217 (Ronald Klein) from the National Institute of Diabetes and Digestive and Kidney Diseases; R01AG021917 (Karen J. Cruickshanks) from the NIA, National Eye Institute, and National Institute on Deafness and Other Communication Disorders; and an unrestricted grant from Research to Prevent Blindness. Margarete A. Wichmann is currently supported by a postdoctoral traineeship (T32AG000213). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Sponsor’s Role: None.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Wichmann M.A.: acquisition of data, design and performance of analyses, interpretation of results, writing of the article. Cruickshanks K.J.: study conceptualization, acquisition of subjects and data, analysis and interpretation of data, writing of the article. Fischer M.E., Klein R., Klein B.E.K., Schubert C.R.: acquisition of subjects and data, analyses and interpretation of results, writing of the article. Carlsson C.M., Chappell R.: analysis and interpretation of data, writing of the article. Tsai M.Y.: provided advice on data collection and quality, writing of the article. All authors read the final manuscript and accept responsibility for the contents.
References
- 1.Prince M, Bryce R, Albanese E, et al. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Launer LJ. Demonstrating the case that AD is a vascular disease: Epidemiologic evidence. Ageing Res Rev. 2002;1:61–77. doi: 10.1016/s0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 3.Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer’s disease: Inflammation, cholesterol, and misfolded proteins. Lancet. 2004;363:1139–1146. doi: 10.1016/S0140-6736(04)15900-X. [DOI] [PubMed] [Google Scholar]
- 4.Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: How to move forward? Neurology. 2009;72:368–374. doi: 10.1212/01.wnl.0000341271.90478.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease application to clinical and public health practice —a statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 6.Tracy RP. Emerging relationships of inflammation, cardiovascular disease and chronic diseases of aging. Int J Obes. 2003;27:S29–S34. doi: 10.1038/sj.ijo.0802497. [DOI] [PubMed] [Google Scholar]
- 7.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Duncan BB, Schmidt MI, Pankow JS, et al. Low-grade systemic inflammation and the development of type 2 diabetes—the Atherosclerosis Risk in Communities Study. Diabetes. 2003;52:1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 10.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860– 867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 12.De Martinis M, Franceschi C, Monti D, et al. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80:219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Jenny NS, Yanez ND, Psaty BM, et al. Inflammation biomarkers and nearterm death in older men. Am J Epidemiol. 2007;165:684–695. doi: 10.1093/aje/kwk057. [DOI] [PubMed] [Google Scholar]
- 14.Libby P, Ridker PM. Inflammation and atherothrombosis—from population biology and bench research to clinical practice. J Am Coll Cardiol. 2006;48:A33–A46. [Google Scholar]
- 15.Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt R, Schmidt H, Curb JD, et al. Early inflammation and dementia: A 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52:168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 17.Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: The Rotterdam Study. Arch Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 18.Jenny NS, French B, Arnold AM, et al. Long-term assessment of inflammation and healthy aging in late life: The Cardiovascular Health Study All Stars. J Gerontol A Biol Sci Med Sci. 2012;67A:970–976. doi: 10.1093/gerona/glr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundelof J, Kilader L, Helmersson J, et al. Systemic inflammation and the risk of Alzheimer’s disease and dementia: A prospective population-based study. J Alzheimers Dis. 2009;18:79–87. doi: 10.3233/JAD-2009-1126. [DOI] [PubMed] [Google Scholar]
- 20.Tan ZS, Beiser AS, Vasan RS, et al. Inflammatory markers and the risk of Alzheimer disease: The Framingham Study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 21.Ravaglia G, Forti P, Maioli F, et al. Blood inflammatory markers and risk of dementia: The Conselice Study of Brain Aging. Neurobiol Aging. 2007;28:1810–1820. doi: 10.1016/j.neurobiolaging.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson UK, Pedersen NL, Reynolds CA, et al. Associations of gene sequence variation and serum levels of C-reactive protein and interleukin- 6 with Alzheimer’s disease and dementia. J Alzheimers Dis. 2011;23:361–369. doi: 10.3233/JAD-2010-101671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Himbergen TM, Beiser AS, Ai M, et al. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and Alzheimer disease: Results from the Framingham Heart Study. Arch Neurol. 2012;69:594–600. doi: 10.1001/archneurol.2011.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koyama A, O’Brien J, Weuve J, et al. The role of peripheral inflammatory markers in dementia and Alzheimer’s disease: A meta-analysis. J Gerontol A Biol Sci Med Sci. 2013;68A:433–440. doi: 10.1093/gerona/gls187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein R, Klein BEK, Linton KLP, et al. The Beaver Dam Eye Study: Visual acuity. Ophthalmology. 1991;98:1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 26.Cruickshanks KJ, Wiley TL, Tweed TS, et al. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin: The Epidemiology of Hearing Loss Study. Am J Epidemiol. 1998;148:879–886. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- 27.Cruickshanks KJ, Nondahl DM, Tweed TS, et al. Education, occupation, noise exposure history and the 10-yr cumulative incidence of hearing impairment in older adults. Hear Res. 2010;264:3–9. doi: 10.1016/j.heares.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruickshanks KJ, Tweed TS, Wiley TL, et al. The 5-year incidence and progression of hearing loss: The Epidemiology of Hearing Loss Study. Arch Otolaryngol Head Neck Surg. 2003;129:1041–1046. doi: 10.1001/archotol.129.10.1041. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell JL, Cruickshanks KJ, Klein BEK, et al. Postmenopausal hormone therapy and its association with cognitive impairment. Arch Intern Med. 2003;163:2485–2490. doi: 10.1001/archinte.163.20.2485. [DOI] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Zhong WJ, Cruickshanks KJ, Schubert CR, et al. Carotid atherosclerosis and 10-year changes in cognitive function. Atherosclerosis. 2012;224:506– 510. doi: 10.1016/j.atherosclerosis.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivnik RJ, Malec JF, Smith GE, et al. Mayo’s Older Americans Normative Studies: WAIS-R norms for ages 56–97. Clin Neuropsychol. 1992;6:1–30. [Google Scholar]
- 33.Ivnik RJ, Malec JF, Smith GE, et al. Neuropsychological tests’ norms above age 55: COWAT, BNT, MAE token, WRAT-R reading, AMNART, STROOP, TMT, and JLO. Clin Neuropsychol. 1996;10:262–278. [Google Scholar]
- 34.Ivnik RJ, Malec JF, Smith GE, et al. Mayo’s Older Americans Normative Studies: Updated AVLT norms for ages 56 to 97. Clin Neuropsychol. 1992;6:83–104. [Google Scholar]
- 35.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14:167–177. [PubMed] [Google Scholar]
- 36.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270– 279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shankar A, Sun L, Klein BEK, et al. Markers of inflammation predict the long-term risk of developing chronic kidney disease: A population-based cohort study. Kidney Int. 2011;80:1231–1238. doi: 10.1038/ki.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 40.Borhani NO, Kass EH, Langford HG, et al. The hypertension detection and follow-up program. Prev Med. 1976;5:207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 41.Landis JR, Heyman ER, Koch GG. Average partial association in 3-way contingency-tables: A review and discussion of alternative tests. Int Stat Rev. 1978;46:237–254. [Google Scholar]
- 42.Nash SD, Cruickshanks KJ, Klein R, et al. Long-term variability of inflammatory markers and associated factors in a population-based cohort. J Am Geriatr Soc. 2013;61:1269–1276. doi: 10.1111/jgs.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ridker PM, Rifai N, Pfeffer MA, et al. Long-term effects of pravastatin on plasma concentration of C-reactive protein. Circulation. 1999;100:230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 44.Yoon SS, Dillon CF, Carroll M, et al. Effects of statins on serum inflammatory markers: The U.S. National Health and Nutrition Examination Survey 1999–2004. J Atheroscler Thromb. 2010;17:1176–1182. doi: 10.5551/jat.5652. [DOI] [PubMed] [Google Scholar]
- 45.Lyngdoh T, Vollenweider P, Waeber G, et al. Association of statins with inflammatory cytokines: A population-based Colaus study. Atherosclerosis. 2011;219:253–258. doi: 10.1016/j.atherosclerosis.2011.07.117. [DOI] [PubMed] [Google Scholar]
- 46.Katsel P, Tan WL, Haroutunian V. Gain in brain immunity in the oldest-old differentiates cognitively normal from demented individuals. PLoS One. 2009;4:15. doi: 10.1371/journal.pone.0007642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buchman AS, Bennett DA. High CRP with “normal” cognition: A resilient phenotype in old age. Neurology. 2012;79:1078–1079. doi: 10.1212/WNL.0b013e3182698df4. [DOI] [PubMed] [Google Scholar]
- 48.Silverman JM, Schmeidler J, Beeri MS, et al. C-reactive protein and familial risk for dementia: A phenotype for successful cognitive aging. Neurology. 2012;79:1116–1123. doi: 10.1212/WNL.0b013e3182698c89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tarkowski E, Rosengren L, Blomstrand C, et al. Intrathecal release of proand anti-inflammatory cytokines during stroke. Clin Exp Immunol. 1997;110:492–499. doi: 10.1046/j.1365-2249.1997.4621483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 51.Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition—the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- 52.Dantzer R, Bluthe RM, Laye S, et al. Cytokines and sickness behavior. Ann N Y Acad Sci. 1998;840:586–590. doi: 10.1111/j.1749-6632.1998.tb09597.x. [DOI] [PubMed] [Google Scholar]
- 53.Holmes C, Cunningham C, Zotova E, et al. Proinflammatory cytokines, sickness behavior, and Alzheimer disease. Neurology. 2011;77:212–218. doi: 10.1212/WNL.0b013e318225ae07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chatfield MD, Brayne CE, Matthews FE. A systematic literature review of attrition between waves in longitudinal studies in the elderly shows a consistent pattern of dropout between differing studies. J Clin Epidemiol. 2005;58:13–19. doi: 10.1016/j.jclinepi.2004.05.006. [DOI] [PubMed] [Google Scholar]