Abstract
Background
Repetitive transcranial magnetic stimulation (rTMS) can temporarily interrupt or facilitate activity in a focal brain region. Several lines of evidence suggest that rTMS of the dorsolateral prefrontal cortex (DLPFC) can affect processes involved in drug addiction. We hypothesized that a single session of low-frequency rTMS of the left DLPFC would modulate cue-induced craving for methamphetamine (MA) when compared to a sham rTMS session.
Methods
In this single-blind, sham-controlled crossover study, 10 non-treatment seeking MA-dependent users and 8 healthy controls were randomized to receive 15 min of sham and real (1 Hz) DLPFC rTMS in two experimental sessions separated by 1 h. During each rTMS session, participants were exposed to blocks of neutral cues and MA-associated cues. Participants rated their craving after each cue block.
Results
In MA users, real rTMS over the left DLPFC increased self-reported craving as compared to sham stimulation (17.86 ± 1.46 vs. 24.85 ± 1.57, p = 0.001). rTMS had no effect on craving in healthy controls. One Hertz rTMS of the left DLPFC was safe and tolerable for all participants.
Conclusions
Low frequency rTMS of the left DLPFC transiently increased cue-induced craving in MA participants. These preliminary results suggest that 1 Hz rTMS of the left DLPFC may increase craving by inhibiting the prefrontal cortex or indirectly activating subcortical regions involved in craving.
Keywords: Methamphetamine, Craving, Transcranial magnetic stimulation, Dorsolateral prefrontal cortex
1. Introduction
Methamphetamine (MA) abuse is a substantial public health problem in the United States and in other parts of the world. Each year, 24.7 million people use amphetamine or methamphetamine (MA) worldwide, which represents more consumers than that for heroin or cocaine (United Nations Office on Drugs and Crime, 2008; http://www.unodc.org/documents/about-unodc/AR08WEB.pdf). Approximately 13 million people 12 years and older have abused MA in their lifetimes, with approximately 353,000 current users in the US in 2010 (National Survey on Drug Use and Health, 2010; http://www.drugabuse.gov/publications/topics-in-brief/metham-phetamine-addiction-progress-need-to-remain-vigilant). Unfortunately, there are no Food and Drug Administration (FDA) approved medications for MA dependence and thus behavioral interventions remain the mainstay of treatment programs (Colfax et al., 2010; Karila et al., 2010). These data emphasize the importance of developing new treatment approaches for MA users.
Chronic MA abuse is associated with profound alterations in brain circuits and neurochemical markers, particularly in early abstinence (Baicy and London, 2007; Chang et al., 2007). These changes include higher activity in the amygdala and lower activity in the infralimbic cortex, deficits in global metabolism, and altered neural integrity (Volkow et al., 2001a). Previous imaging studies also reported that MA users showed reduced activation in frontal cortex regions while they performed a color-word Stroop task, which requires cognitive control (Nestor et al., 2011; Salo et al., 2013, 2009). In a recent animal study by our group, we reported that prefrontal cortex-specific alterations in neuronal function might play a key role in MA induced attentional deficits and drug seeking (Parsegian et al., 2011). Together, these data suggest that dysfunction in prefrontal cortical areas that are important for executive function underlies cognitive control deficits associated with MA dependence (Nestor et al., 2011).
Craving for an addictive substance may be described as an intense subjective urge to acquire and ingest drug(s), and may be elicited even after periods of sustained abstinence by exposure to stress, to a priming dose of the drug, or to environmental cues previously associated with use of the drug (Carter and Tiffany, 1999; Mahoney et al., 2007). Craving for MA is commonly reported by heavy users of the drug and may increase the risk of relapse in newly abstinent individuals (Tolliver et al., 2010). MA cravings have been shown to involve activation of the prefrontal cortex, nucleus accumbens, and the anterior insula, similar to cravings for other addictive substances such as cocaine, opiates, and alcohol (Berman et al., 2008; Brody et al., 2002; George et al., 2001; Myrick et al., 2004). Recently, a treatment study showed that bupropion reduced acute MA-induced subjective effects and reduced cue-induced craving (Newton et al., 2006). As such, reducing cue craving might be a strategy to help prevent relapse and treat MA dependence.
Transcranial magnetic stimulation (TMS) is a noninvasive brain stimulation technology that can focally stimulate the brain in awake individuals (Barker et al., 1985). This relatively new method allows modulation of discrete brain areas of the awake and conscious subject under study. The pulsatile electromagnetic field generated around the coil crosses the skull and directly depolarize neurons in the underlying cortices, with immediate excitatory effects (Padberg and George, 2009). In humans, repetitive TMS (rTMS) can induce changes in cortical excitability. Distinct from the immediate effects of TMS, rTMS leads to different cumulative effects within the region of the brain being stimulated (Fitzgerald et al., 2006). Depending on the frequency of the pulsed magnetic fields, rTMS can be used to either stimulate (high frequency) or suppress (low frequency) neural activity in a particular cortical region (Chen et al., 1997; Pascual-Leone et al., 1994). Growing evidence generally indicates that serial rTMS at 1 Hz has an overall inhibitory effect on the region stimulated. An example of this is temporary inhibition of the motor cortex during digit movement and on the size of motor evoked potentials (Chen, 2000; Hallett, 2000). In contrast, some evidence has shown that high-frequency (≥5 Hz) rTMS is excitatory in nature (Fitzgerald et al., 2006; Haraldsson et al., 2004). In addition, the effects of rTMS are not limited to the exact site of stimulation and can induce changes in distant interconnected sites of the brain, including subcortical regions (Bohning et al., 1999; Li et al., 2004). In clinical depression, studies have reported opposite effects of high and low frequency rTMS on regional brain activity, with high frequency leading to increased regional cerebral blood flow (rCBF) and low frequency producing decreased rCBF (Speer et al., 2000).
Commonly employed as a clinical research tool, daily rTMS for 4–6 weeks is a recently approved US Food and Drug Administration (FDA) treatment for depression (George et al., 2010; George and Post, 2011). While some studies have shown potential efficacy in treating some aspects of drug addiction (Barr et al., 2011; Feil and Zangen, 2010), it has not been studied before in MA addiction. As noted above, low frequency (≤1 Hz) rTMS inhibits neuronal firing in a localized area and is used to induce virtual lesions in order to examine a brain region’s role in different tasks (Chen et al., 1997; Iyer et al., 2003), while high frequency rTMS (≥5 Hz) tends to be excitatory and can cause an increase in neuronal depolarization under the stimulating coil (Haraldsson et al., 2004). Previous studies of rTMS over the DLPFC support the ability of rTMS to transiently reduce the level of craving in tobacco (Li et al., 2013), alcohol (Mishra et al., 2010), and cocaine (Camprodon et al., 2007) addicted patients. To the best of our knowledge, no study has used rTMS to modulate cue-induced craving in a MA dependent population. As such, it would be very important as a first step to evaluate whether a single session of rTMS is safe, tolerable, and efficacious for craving modulation in MA users.
The purpose of this randomized, single blind sham-controlled study was to test whether low frequency rTMS of the left DLPFC would modulate cue-induced craving in adult MA users. We hypothesized that low frequency active rTMS would modulate self-reported MA cravings more than sham rTMS in MA users. In the current study, we used low frequency rTMS (and not high frequency rTMS) to investigate cue craving in MA users. This choice was primarily done for safety reasons. Individuals with a history of MA use exhibit significantly increased cortical excitability (Flavel et al., 2012) and MA users often show increased seizure susceptibility (Slamberova et al., 2011). Thus, the potential exists that high frequency TMS in MA users may cause seizures. Moreover, no study has been done in an addicted population with low frequency rTMS. If the theory of prefrontal governance over craving is correct, then low frequency rTMS, which is inhibitory, might influence craving and perhaps even worsen it.
2. Methods and materials
2.1. Participants
Ten healthy, non-treatment seeking individuals who met DSM-IV-TR (First and Tasman, 2004) criteria for current MA dependence participated in this study. Eight healthy control participants who had never used MA were also recruited. Control subjects were matched to the MA group for gender, race, and other biographical characteristics. All control subjects had negative urine drug screens during screening. No control subjects currently used tobacco products or had a lifetime history of any drugs of abuse. All participants were financially compensated for their participation.
Participants were recruited through local television, radio advertisements, and word-of-mouth. Participants underwent 1–2 weeks of telephone and in-person screening. The first in-person screening was preceded by oral and written informed consent approved by the Institutional Review Board (IRB) of the Medical University of South Carolina (MUSC). Screening included the MINI diagnostic psychiatric interview (Sheehan et al., 1998), general medical history, general physical and neurologic assessments, timeline follow back for multiple drugs and alcohol prior to informed consent, and concurrent medication history. Laboratory studies included the following: hematology, comprehensive blood chemistries, routine urinalyses, and daily urine drug screens for amphetamine, MA, opiates, marijuana, benzodiazepines, cocaine and barbiturates. Participants with significant hepatic, renal, cardiac or neurological (including stroke, seizure, migraine, head trauma) impairment or a history of major Axis I disorders such as bipolar disorder, schizophrenia, dementia, or current depressive disorder were excluded. Participants were also excluded if they had ferromagnetic implants or if they had taken any medication during the previous thirty days that might alter central nervous system (CNS) function or CNS blood supply (e.g., calcium channel agonists, sedative-hypnotics, over-the-counter CNS agents). Control subjects were subjected to daily urine drug screens and breathalyzers. Demographic and MA using-habits profile data were collected at baseline.
2.2. Experimental design
A randomized, single-blind, sham and healthy controlled study was employed in 10 MA users and 8 healthy controls who received 2 different types of brain stimulation during one visit: sham rTMS and real rTMS of the DLPFC, with an hour interval between treatment sessions (see Fig. 1 for details). The order of stimulation was randomized and counterbalanced across participants. The randomizations were performed with a web-based randomization generator (www.randomization.com). Participants were blind to the treatment arm.
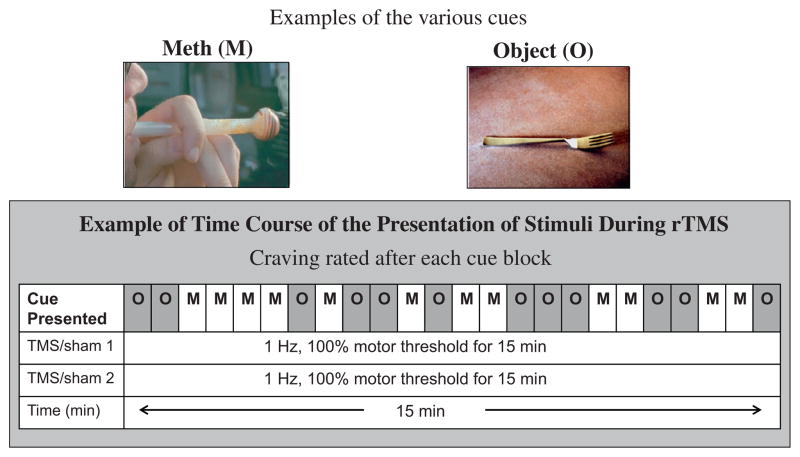
Fig. 1.
Diagram showing the stimulation and assessments that were performed during this study. M = methamphetamine, O = object.
2.3. Cue craving presentation and assessments
Each cue craving presentation lasted for approximately 15 min. The cue exposure presentation consisted of 40 MA pictures and 40 neutral pictures. These pictures of MA-related (drug, paraphernalia, or persons using the drug) and neutral pictures were selected as described previously (Tolliver et al., 2010). Each picture was presented twice for 4 s. Participants were instructed to pay close attention to the pictures. Standard visual analog scales (VAS) that consisted of 100 mm lines with anchoring statements at both ends were completed after each block of picture presentation on a desktop computer. Subjects were asked to rate craving with 0 mm being “no craving at all” and 100 mm representing “the most craving I have ever had”. Cue craving presentation and VAS were conducted during real rTMS or sham stimulation as well as pre experiment baseline.
2.4. rTMS procedure
2.4.1. Determining motor threshold and locating cortical targets
Focal TMS was delivered by a focal figure-of-eight magnetic air-cooled coil (each wing 70 mm in diameter) connected to a MAGSTIM Super Rapid stimulator (Magstim Co., Whitland, Dyfed, UK) that generated biphasic electrical pulses of approximately 250 μs duration. Resting motor thresholds (rMT) were performed via visual twitch in the contralateral (right) abductor policis brevis (APB) at the beginning of each experiment. The coil was positioned over the area of the skull corresponding to the motor cortex, adjusted until each pulse resulted in isolated movement of the right APB, and then adjusted for the lowest intensity that reliably produced thumb or hand movement. rMT was defined as the lowest output to produce thumb movement 50% of the time (Ziemann and Hallett, 2000). The position of the coil used for rMT assessment was identified as the motor cortex target (M1). The standardized stimulation localization was over the left prefrontal cortex, determined by moving the TMS coil 6 cm anterior to M1 along a parasagittal line (George and Post, 2011; Herbsman et al., 2009).
2.4.2. Real rTMS
Subjects received 1 Hz, at 100% rMT for 15 min with 900 pulses over the left DLPFC. This setting falls well within the safety guidelines (Rossi et al., 2009).
2.4.3. Sham rTMS
During sham stimulation, we raised the lateral wing of the figure eight coil 45° off the head with the edge of the medial wing of the coil still touching the scalp. The sham TMS system was connected to an electrical generator on a 9 V battery and electrodes were placed over the medial prefrontal cortex. The regulator was triggered by the TMS machine to allow brief, microsecond pulses of the electrical current through to the skin on the subjects’ forehead. Electrical stimulation was triggered by the TMS machine to correspond to the sham TMS pulses. The intensity of the electrical stimulation was matched to subjects’ subjective experience of real TMS at 100% of rMT.
2.5. Data analyses
Analyses were completed with SPSS statistical software, version 20.0 (IBM corporation, Endicott, New York). A mixed model analysis of variance (ANOVA) was used to assess the main effects of groups (healthy controls vs. MA users), treatments (baseline vs. real rTMS vs. sham rTMS), and cue exposure conditions (neutral vs. MA cue) on MA craving ratings. T tests and one-way ANOVA were used for post hoc analyses.
3. Results
3.1. Participant demographics
Table 1 shows the demographic information for study subjects. Ten healthy, non-treatment seeking individuals who met DSM-IV-TR criteria for current MA dependence (3 men and 7 women; the average age was 34.7 ± 10.6) participated in this study. Eight control participants (1 man and 7 women; the average age was 32.5 ± 12.6) who had never used MA were also recruited for study participation. There were no significant difference between the MA and control groups in terms of average age, gender, and race.
Table 1.
Demographics and methamphetamine use (mean ± SD).
| Methamphetamine (n = 10) | Control (n = 8) | Statistics | |
|---|---|---|---|
| Age | 34.7 ± 10.6 | 32.5 ± 12.6 | p = 0.68, NS |
| Education | 10.7 ± 1.8 | 14.4 ± 2.5 | p = 0.002 |
| Gender (male/female) | 4/8 | 1/7 | p = 0.29, NS |
| Race (%Caucasian/other) | 100/0 | 100/0 | NS |
| Years of meth use | 10.7 ± 7.1 | NA | |
| Baseline % days abstinent | 88.0 ± 62.7 | NA |
3.2. Cue exposure paradigm validity and reliability
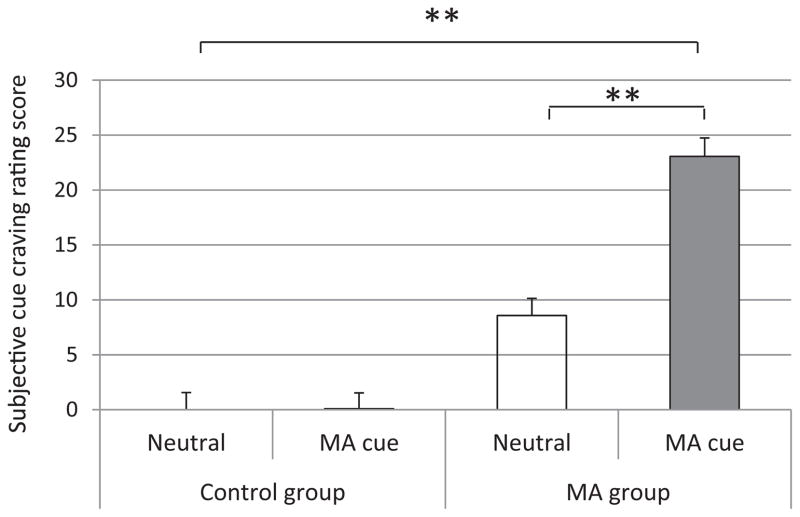
Baseline cue exposure assessment: Mixed model ANOVA results showed a main effect of cue pictures (MA cue: 11.57 ± 1.11 vs. neutral cue: 4.29 ± 1.18; F1,297 = 20.18, p < 0.0001) and a main effect of group (control: 0.04 ± 1.07 vs. MA: 15.82 ± 1.22; F1,297 = 94.69, p < 0.00001). Post hoc results showed that in the healthy control group, there was no significant difference in subjective cue craving rating between MA cues and neutral cues (0.08 ± 1.45 vs. 0.00 ± 1.56, t = 1.05, p = 0.29). In the MA users group, the craving rating following MA cue exposure was significantly higher than it was after neutral cue exposure (23.06 ± 3.3 vs. 8.58 ± 1.4, t = 3.89, p < 0.001; see Fig. 2 for details).
Fig. 2.
Comparisons of cue-induced craving rating between neutral cue and MA cue in the healthy control group and the MA dependent user group on baseline assessment. Mixed model analysis of variance revealed a significant main group (control vs. MA) effect (p < 0.0001) and a significant main cue exposure effect (p < 0.00001). Post hoc t test showed that MA cue exposure induced significant higher subjective cue craving ratings (p < 0.001), while no significant difference was found between neutral and MA cue in control group (p = 0.29). Mean ± SEM; *p < 0.05, **p < 0.01.
3.3. Modulation of rTMS on MA cue craving
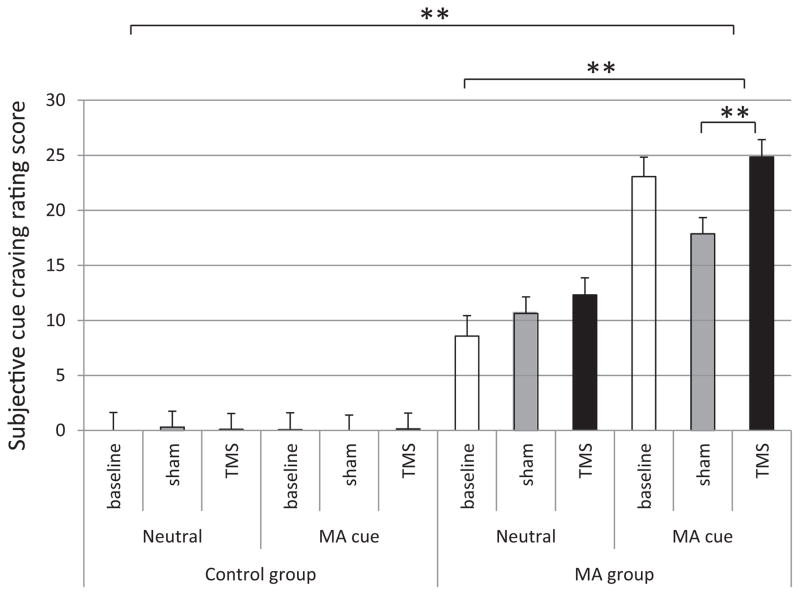
Comparisons of cue-induced cravings were performed between real rTMS and sham stimulation, between neutral cues and MA cues, and between healthy controls and MA users. With respect to subjective cue rating, the results showed significant main effects for group and for cues. Mixed model ANOVA showed that the cue craving of MA users group was significantly higher than the control group (0.11 ± 0.60 vs. 16.22 ± 0.66; F1,1074 = 321.8, p < 0.0000001); MA cue craving was significant higher than neutral cue craving (11.0 ± 0.63 vs. 5.33 ± 0.65; F1,1074 = 39.97, p < 0.000001). Although the main effect of treatment was not significant (7.92 ± 0.85 vs. 7.21 ± 0.73 vs. 9.36 ± 0.75; F2,1074 = 2.17, p = 0.14), post hoc results showed that the cue craving during real rTMS was significantly higher than it was during sham stimulation (7.92 ± 0.85 vs. 9.36 ± 0.75, p = 0.04). The results also showed a significant two-way interaction “group (healthy controls vs. MA) and treatment (baseline vs. sham vs. real stimulation)”, p < 0.001. Post hoc t-test showed a significant difference between sham TMS and real TMS in MA group (14.25 ± 1.05 vs. 18.58 ± 1.11, p = 0.005). No significant difference was found in healthy control group. Further, post hoc t-test was conducted for MA cues data in MA group. The results in the MA group showed that real TMS induced significantly increased cue craving than did sham TMS (24.85 ± 1.57 vs. 17.86 ± 1.46, p = 0.001; see Fig. 3 for details).
Fig. 3.
Comparisons of cue-induced craving rating between TMS and sham; neutral cue and MA cue; control group and the MA group. Mixed model analysis of variance showed that the cue craving of MA group was significant higher than control group (p < 0.0000001); MA cue craving was significant higher than neutral cue (p < 0.000001). Although main effect of treatment was not significant (p = 0.14), post hoc results showed that the cue craving during TMS was significant higher than that during sham stimulation (7.93 vs. 9.36, p = 0.04). Further, post hoc analysis did not show a significant difference of cue craving between real and sham TMS in healthy controls, while the post hoc results of MA group showed that real TMS induced significantly cue craving than sham TMS (17.86 ± 1.46 vs. 24.85 ± 1.57, p = 0.001). Mean ± SEM; *p < 0.05, **p < 0.01.
3.4. Safety
The major safety concern in rTMS studies is induction of seizures. No seizures occurred nor were any serious adverse events recorded. The only adverse events were that some participants experienced mild scalp discomfort at the start of stimulation, but this was mild and transient and equal across the sham and active sessions.
4. Discussion
This study found that a lab-based paradigm of MA-related cue exposure reliably increased subjective craving in MA participants, but had no effect in healthy control participants. With this background, we found that low-frequency rTMS of the left DLPFC significantly increased MA participants’ self-reported cue-induced cravings for MA when compared to sham rTMS of the same region. To the best of our knowledge, this is the first demonstration that low-frequency rTMS of left DLPFC can modulate cue-induced craving for MA.
The mechanism by which low frequency rTMS affects cue-elicited craving in MA is likely multifaceted. One possible explanation is that low frequency rTMS inhibits DLPFC function (Figner et al., 2010; Knoch et al., 2006). The dopaminergic mesocorticolimbic pathway, which arises in the ventral tegmental area (VTA) and connects brain structures involved with reward (e.g., nucleus accumbens) and cognitive control (e.g., prefrontal cortex), appears to be a critical substrate of drug craving and relapse (Di Chiara, 2000; Diana, 2011; Melis et al., 2005). Chronic MA abuse is associated with profound alterations in these brain circuits, especially during early abstinence. Elevated activity in the amygdala and diminished activity in the infralimbic cortex are among the changes induced by MA abuse. Moreover, MA abuse may also induce structural deficits in cingulate, limbic and paralimbic regions and deplete markers of dopaminergic and serotonergic neurotransmitter systems. These neurochemical findings are consistent with changes in neural integrity as well as glial cell proliferation or metabolism (McCann et al., 1998; Volkow et al., 2001b; Wilson et al., 1996). Drug-dependent individuals also exhibit lower resting activity in the prefrontal cortex. When such individuals are exposed to drug-related cues, there is marked activation in the prefrontal cortex and ventral striatum (Goldstein et al., 2009). Furthermore, MA-dependent individuals show impairments in prefrontal-dependent cognition, including deficits in self-regulation and impulse control, which contribute to relapse vulnerability (Nestor et al., 2011). The assumption in most preclinical and clinical studies is that changes in these prefrontal-striatal circuits (and the accompanying impulsivity) result from chronic drug use rather than preexisting genetically or epigenetically determined deficits. In addition, previous studies combining TMS and fMRI done by our group have confirmed that rTMS of the DLPFC induces brain activation in sub-cortical regions (Bohning et al., 1999; Li et al., 2011, 2004) linked with appetitive drive. These TMS/fMRI findings support the idea that rTMS of the DLPFC causes functional changes in subcortical regions, such as the reward system. The DLPFC is associated with executive functions as decision-making, behavioral inhibition, and repetitive behavior. Low frequency rTMS of the DLPFC may affect craving through its influence on decision making (Fecteau et al., 2010) and inhibitory control (Feil and Zangen, 2010) as risky decision making and difficulty with inhibitory control are traits common to people who suffer from addiction.
In nicotine dependent individuals, we previously reported that 15 min of excitatory, high frequency (10 Hz) rTMS of the left DLPFC significantly reduced cigarette cue-induced craving as compared to sham stimulation (Li et al., 2013). However, we did not compare high frequency rTMS with low frequency rTMS. Rose and colleagues reported that cue-induced craving was elevated after 10 Hz stimulation of the superior frontal gyrus, whereas craving after neutral cue presentations was reduced (Rose et al., 2011). Repeated low-frequency stimulation of a single neuron in culture produces long-lasting inhibition of cell–cell communication (Bear, 1999; Stanton and Sejnowski, 1989; High frequency stimulation, by contrast, can improve or enhance neurotransmission Malenka and Nicoll, 1999). It has been hypothesized that TMS can produce sustained inhibitory or excitatory effects in a way analogous to single-cell electrical stimulation (Wang et al., 1996). Our results are consistent with the model that suppression of the left DLPFC by low frequency rTMS reduces inhibitory control, leading to enhance cue-induced cravings for drugs like MA. Consistent with this notion, in nicotine dependence, our previous data suggest that 10 Hz rTMS of the left DLPFC leads to a significant suppression of cue-induced craving (Li et al., 2013).
The current study also provides evidence that low frequency (1 Hz) TMS can be safely used in well-screened MA-dependent subjects. Importantly, there were no severe adverse events such as seizure activity. However, more studies are needed to fully explore the safety profile of TMS parameters in MA participants (Tassinari et al., 2003; Wassermann, 1998).
There are several limitations of the current study. First, we were cautious about the number and frequency of TMS pulses administered to our participants, given the elevated risk of seizure activity in MA-dependent subjects (Flavel et al., 2012; Slamberova et al., 2011). We only used low frequency stimulation, as we presumed that low frequency TMS would be safest due to the association of MA with increased seizure susceptibility (Slamberova et al., 2011). Future work should evaluate the effects and safety of higher frequencies (e.g., 10 and 20 Hz) in MA-dependent populations, as these higher frequencies have been used to treat other drug addictions (Barr et al., 2011; Feil and Zangen, 2010). Also, an increased number of stimuli may be warranted in future craving studies. Second, the current study does not examine more than the left DLPFC, so it does not determine that the left DLPFC is the optimal area for TMS stimulation. However, one previous MRI study reported that MA dependence showed reduced gray matter density in the left middle frontal gyrus, which is modulated by the DLPFC (Hare et al., 2009; Schwartz et al., 2010). Furthermore, one rTMS in smokers conducted by our group showed that left DLPFC rTMS reduced cue craving for smoking (Li et al., 2013). Third, the small sample size of the current study may have led to Type II errors. However, ratings in both healthy controls and pre-experiment baseline cue-induced cravings were measured. Additionally, the current cue-craving paradigm has a high validity and reliability (see Section 3 and Fig. 2 for details). Finally, cue-induced cravings were assessed only at baseline testing and during rTMS session and sham stimulation session. As such, the current study could only investigate the immediate temporal effects of rTMS. In future studies, we plan to measure the longer-term effects of high frequency rTMS in MA participants.
The results of this preliminary study demonstrate that low frequency rTMS of the left DLPFC can temporarily increase cue-induced craving in MA users. This finding suggests that low frequency rTMS suppresses prefrontal cortex function to enhance cue-induced craving in MA users. Future studies may test whether high frequency rTMS can reduce cue craving through increasing prefrontal cortex functions.
Acknowledgments
Role of funding source
The study was funded by a grant from the National Institutes of Health (Grant no. P20 DA022658 (RE See)).
Footnotes
ClinicalTrials.gov brief title
Transcranial Magnetic Stimulation Used to Both Measure Cortical Excitability and Explore Methamphetamine Cue Craving.
ClinicalTrials.gov Link http://clinicaltrials.gov/ct2/results?term=NCT01685463.
Conflict of interest
None of the authors has a conflict of interest to declare.
Contributors
Xingbao Li contributed in study design, performing experiment, data analysis, drafting the manuscript. Robert Malcolm supervised patients enrolled. Kristina Huebner helped in enrollment. Colleen Hanlon helped in drafting the manuscript. Joseph Taylor helped in drafting the manuscript. Kathleen Brady supervised in study design. Mark George supervised in study and helped in drafting the manuscript. Ronald See contributed in obtaining grant funding and helped draft the manuscript. All authors contributed to and approved the final manuscript.
References
- Baicy K, London ED. Corticolimbic dysregulation and chronic methamphetamine abuse. Addiction. 2007;102(Suppl 1):5–15. doi: 10.1111/j.1360-0443.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Wing VC, George TP, Fitzgerald PB, Daskalakis ZJ. Repetitive transcranial magnetic stimulation and drug addiction. Int Rev Psychiatry. 2011;23:454–466. doi: 10.3109/09540261.2011.618827. [DOI] [PubMed] [Google Scholar]
- Bear MF. Homosynaptic long-term depression: a mechanism for memory? Proc Natl Acad Sci USA. 1999;96:9457–9458. doi: 10.1073/pnas.96.17.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SM, Voytek B, Mandelkern MA, Hassid BD, Isaacson A, Monterosso J, Miotto K, Ling W, London ED. Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse. Mol Psychiatry. 2008;13:897–908. doi: 10.1038/sj.mp.4002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohning DE, Shastri A, McConnell KA, Nahas Z, Lorberbaum JP, Roberts DR, Teneback C, Vincent DJ, George MS. A combined TMS/fMRI study of intensity-dependent TMS over motor cortex. Biol Psychiatry. 1999;45:385–394. doi: 10.1016/s0006-3223(98)00368-0. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Camprodon JA, Martinez-Raga J, Alonso-Alonso M, Shih MC, Pascual-Leone A. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend. 2007;86:91–94. doi: 10.1016/j.drugalcdep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chen R. Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve Suppl. 2000;9:S26–S32. doi: 10.1002/1097-4598(2000)999:9<::aid-mus6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Colfax G, Santos GM, Chu P, Vittinghoff E, Pluddemann A, Kumar S, Hart C. Amphetamine-group substances and HIV. Lancet. 2010;376:458–474. doi: 10.1016/S0140-6736(10)60753-2. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Diana M. The dopamine hypothesis of drug addiction and its potential therapeutic value. Front Psychiatry. 2011;2:64. doi: 10.3389/fpsyt.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Fregni F, Boggio PS, Camprodon JA, Pascual-Leone A. Neuromodulation of decision-making in the addictive brain. Subst Use Misuse. 2010;45:1766–1786. doi: 10.3109/10826084.2010.482434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil J, Zangen A. Brain stimulation in the study and treatment of addiction. Neurosci Biobehav Rev. 2010;34:559–574. doi: 10.1016/j.neubiorev.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Figner B, Knoch D, Johnson EJ, Krosch AR, Lisanby SH, Fehr E, Weber EU. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 2010;13:538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- First MB, Tasman A. DSM-IV-TR mental disorders: diagnosis etiology and treatment. John Wiley & Sons; West Sussex, England: 2004. [Google Scholar]
- Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117:2584–2596. doi: 10.1016/j.clinph.2006.06.712. [DOI] [PubMed] [Google Scholar]
- Flavel SC, White JM, Todd G. Motor cortex and corticospinal excitability in humans with a history of illicit stimulant use. J Appl Physiol. 2012;113:1486–1494. doi: 10.1152/japplphysiol.00718.2012. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, Anderson B, Nahas Z, Bulow P, Zarkowski P, Holtzheimer PE, 3rd, Schwartz T, Sackeim HA. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67:507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- George MS, Post RM. Daily left prefrontal repetitive transcranial magnetic stimulation for acute treatment of medication-resistant depression. Am J Psychiatry. 2011;168:356–364. doi: 10.1176/appi.ajp.2010.10060864. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- Haraldsson HM, Ferrarelli F, Kalin NH, Tononi G. Transcranial magnetic stimulation in the investigation and treatment of schizophrenia: a review. Schizophr Res. 2004;71:1–16. doi: 10.1016/j.schres.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Herbsman T, Avery D, Ramsey D, Holtzheimer P, Wadjik C, Hardaway F, Haynor D, George MS, Nahas Z. More lateral and anterior prefrontal coil location is associated with better repetitive transcranial magnetic stimulation antidepressant response. Biol Psychiatry. 2009;66:509–515. doi: 10.1016/j.biopsych.2009.04.034. [DOI] [PubMed] [Google Scholar]
- Iyer MB, Schleper N, Wassermann EM. Priming stimulation enhances the depressant effect of low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23:10867–10872. doi: 10.1523/JNEUROSCI.23-34-10867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila L, Weinstein A, Aubin HJ, Benyamina A, Reynaud M, Batki SL. Pharmacological approaches to methamphetamine dependence: a focused review. Br J Clin Pharmacol. 2010;69:578–592. doi: 10.1111/j.1365-2125.2010.03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D, Gianotti LR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, Brugger P. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J Neurosci. 2006;26:6469–6472. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hartwell KJ, Owens M, Lematty T, Borckardt JJ, Hanlon CA, Brady KT, George MS. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry. 2013;73:714–720. doi: 10.1016/j.biopsych.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Large CH, Ricci R, Taylor JJ, Nahas Z, Bohning DE, Morgan P, George MS. Using interleaved transcranial magnetic stimulation/functional magnetic resonance imaging (fMRI) and dynamic causal modeling to understand the discrete circuit specific changes of medications: lamotrigine and valproic acid changes in motor or prefrontal effective connectivity. Psychiatry Res. 2011;194:141–148. doi: 10.1016/j.pscychresns.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Li X, Nahas Z, Kozel FA, Anderson B, Bohning DE, George MS. Acute left prefrontal transcranial magnetic stimulation in depressed patients is associated with immediately increased activity in prefrontal cortical as well as subcortical regions. Biol Psychiatry. 2004;55:882–890. doi: 10.1016/j.biopsych.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Mahoney JJ, 3rd, Kalechstein AD, De La Garza R, 2nd, Newton TF. A qualitative and quantitative review of cocaine-induced craving: the phenomenon of priming. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:593–599. doi: 10.1016/j.pnpbp.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation – a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Spiga S, Diana M. The dopamine hypothesis of drug addiction: hypodopaminergic state. Intl Rev Neurobiol. 2005;63:101–154. doi: 10.1016/S0074-7742(05)63005-X. [DOI] [PubMed] [Google Scholar]
- Mishra BR, Nizamie SH, Das B, Praharaj SK. Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction. 2010;105:49–55. doi: 10.1111/j.1360-0443.2009.02777.x. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacol. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Nestor LJ, Ghahremani DG, Monterosso J, London ED. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194:287–295. doi: 10.1016/j.pscychresns.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacol. 2006;31:1537–1544. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- NSDUH. Methamphetamine Addiction: Progress, but Need to Remain Vigilant. 2010. [accessed on 20.03.13]. [Google Scholar]
- Padberg F, George MS. Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. Exp Neurol. 2009;219:2–13. doi: 10.1016/j.expneurol.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Parsegian A, Glen WB, Jr, Lavin A, See RE. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry. 2011;69:253–259. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Grafman J, Hallett M. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science. 1994;263:1287–1289. doi: 10.1126/science.8122113. [DOI] [PubMed] [Google Scholar]
- Rose JE, McClernon FJ, Froeliger B, Behm FM, Preud’homme X, Krystal AD. Repetitive transcranial magnetic stimulation of the superior frontal gyrus modulates craving for cigarettes. Biol Psychiatry. 2011;70:794–799. doi: 10.1016/j.biopsych.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Fassbender C, Buonocore MH, Ursu S. Behavioral regulation in methamphetamine abusers: an fMRI study. Psychiatry Res. 2013;211:234–238. doi: 10.1016/j.pscychresns.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:706–709. doi: 10.1016/j.biopsych.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH, Hoffman WF. Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. Neuroimage. 2010;50:1392–1401. doi: 10.1016/j.neuroimage.2010.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. (quiz 34–57) [PubMed] [Google Scholar]
- Slamberova R, Hruba L, Matejovska I, Bernaskova K, Rokyta R. Increased seizure susceptibility induced by prenatal methamphetamine exposure in adult female rats is not affected by early postnatal cross-fostering. Epilepsy Behav. 2011;20:6–11. doi: 10.1016/j.yebeh.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Speer AM, Kimbrell TA, Wassermann EMJ, Willis DR, Herscovitch MW, Post PRM. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry. 2000;48:1133–1141. doi: 10.1016/s0006-3223(00)01065-9. [DOI] [PubMed] [Google Scholar]
- Stanton PK, Sejnowski TJ. Associative long-term depression in the hippocampus induced by hebbian covariance. Nature. 1989;339:215–218. doi: 10.1038/339215a0. [DOI] [PubMed] [Google Scholar]
- Tassinari CA, Cincotta M, Zaccara G, Michelucci R. Transcranial magnetic stimulation and epilepsy. Clin Neurophysiol. 2003;114:777–798. doi: 10.1016/s1388-2457(03)00004-x. [DOI] [PubMed] [Google Scholar]
- Tolliver BK, McRae-Clark AL, Saladin M, Price KL, Simpson AN, DeSantis SM, Baker NL, Brady KT. Determinants of cue-elicited craving and physiologic reactivity in methamphetamine-dependent subjects in the laboratory. Am J Drug Alcohol Abuse. 2010;36:106–113. doi: 10.3109/00952991003686402. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Wong C, Logan J. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychiatry. 2001a;158:383–389. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001b;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang X, Scheich H. LTD and LTP induced by transcranial magnetic stimulation in auditory cortex. Neuroreport. 1996;7:521–525. doi: 10.1097/00001756-199601310-00035. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Hallett M. Basic neurophysiological studies with TMS. In: George M, Belmaker R, editors. Transcranial Magnetic Stimulation In Neuropsychiatry. American Psychiatric Press, Inc; Washington, DC, USA: 2000. p. 45. [Google Scholar]