Abstract
Objective
While overall success rates of bariatric surgery are high, approximately 20% of patients either regain or never lose the expected amount of weight. The purpose of this study was to determine whether, after gastric-bypass surgery, the degree of weight loss can be differentiated based on the neural response to food cues.
Design and Methods
In this functional MRI study, 31 post-surgical patients viewed food and neutral images in two counterbalanced runs during which they were either instructed to “crave” or to “resist” craving. The neural response to food cues was assessed within and between runs for all participants, and further analyzed between more successful (n = 24) and less successful (n = 7) groups. More successful was defined by meeting 50% excess weight loss.
Results
Overall, instructions to “crave” elicited significant activity in the dorsomedial prefrontal cortex (PFC) whereas “resist” elicited significant activity in the dorsolateral PFC (DLPFC). Between groups there was no brain difference when instructed to “crave.” The more successful participants however had significantly more activity in the DLPFC when instructed to “resist.”
Conclusions
These findings suggest that the ability to mobilize neural circuits involved in executive control post-gastric-bypass surgery may be a unique component of successful outcome post-surgery.
Introduction
The number of deaths attributed to obesity-related health problems is growing at an alarming rate, and obesity management remains a formidable challenge (1). Behavioral therapy, diet regimens, and pharmacologic strategies are associated with significant weight loss, but long-term success rates are often disappointing (2). Surgical therapy (including gastric banding, gastric-bypass, and sleeve gastrectomy) is associated with durable long-term success in most patients and the practice of bariatric surgery is expanding rapidly. Patients who undergo bariatric surgery can expect to lose between 60 and 70% of their excess weight, defined as the difference between their current weight and ideal weight (3).
Despite the overall success of these procedures, however, there are some patients who do not lose the expected amount of weight, and/ or regain previously lost weight post-bariatric surgery. Approximately 20% of patients regain their weight at 18-24 months post-gastric-bypass surgery (4-6). The reasons for failure after bariatric surgery are unclear, and vary based on surgery type, but are sometimes similar to those seen with traditional approaches to weight loss (e.g., increase in energy intake, obesity-related health problems, and motivation) (7). The field currently lacks assessments that may predict bariatric failure or success, and little is known about changes that occur in the brain as a consequence of obesity or following weight-loss surgery.
One factor that may predict relapse or weight regain following bariatric surgery, is a particularly strong limbic response to food-cues, accompanied by a strong sense of craving (9). At baseline obese individuals have heightened arousal to food cues (10,11). It is possible that following gastric-bypass surgery formerly obese individuals who experience intense food cravings may be more likely to regain weight (12), just as persistent drug-cravings predict relapse in former substance-dependent individuals (9,13,14). This is consistent with the suggestion by Kral (8) that binge-eating disorder, which has many similarities to addiction (9-11), may contribute to poor outcomes following bariatric surgery. Another factor that may be associated with unsuccessful weight-loss outcomes is an inability to mobilize executive control circuitry in the presence of appetitive food cues, leading to an inability to resist the urge to eat. DelParigi et al. (15) demonstrated that individuals who were successful dieters had elevated activity in executive control regions when presented with cues, relative to unsuccessful dieters.
Limbic processing and executive control are largely believed to exist in parallel, functionally segregated cortical–subcortical circuits in the brain (16), with limbic processing associated with medial and ventral regions while executive control is largely more in lateral and dorsal regions. In addiction literature it is well known that cue-induced drug craving is associated with elevated activity in multiple limbic areas including the medial prefrontal cortex (PFC), orbito-frontal cortex, ventral striatum, and thalamus (17-19). In contrast, when told to resist a drug-related cue there is significantly more activity in executive control regions including the dorsolateral PFC (DLPFC) (17,18). The obesity literature has demonstrated that obese patients (10) and individuals with bulimia (20) have dysfunctional activity within limbic reward circuitry when presented with food cues. Among the eating disorder patients, craving levels in individuals are associated with hyperactivity in the orbitofrontal and anterior cingulate cortex (20). Evidence suggests that brain activity in relation to eating behavior may differ in lean and obese individuals (15,21-24), with successful dieters having a significantly higher level of activity in executive control regions relative to those who are unsuccessful (15,25). Further, studies that compare obese individuals to controls have shown varied and inconsistent results. This remains an under-examined area among bariatric surgery patients, however, and is relevant given that up to 20% of patients regain weight following surgery.
The purpose of the current study was to examine the functional neuroanatomical and neuropsychological correlates of postsurgical outcomes. Specifically, this study assessed functional neuroanatomy and neural networks associated with gaining weight after one particular type of bariatric surgery, gastric-bypass surgery. The primary hypothesis was that individuals who have maintained expected weight-loss trajectories at least 1 year post-gastric-bypass surgery (more successful) would have significantly lower neural activity in limbic reward related regions (e.g., medial PFC) when exposed to appetitive food cues than would individuals that did not maintain the expected trajectory (less successful). Additionally, we anticipated that the more successful individuals would mobilize executive control circuitry more efficiently when instructed to resist the food cues than less successful participants.
Methods
Participants
Individuals aged 21-65, at least one year post gastric-bypass surgery (ROUX-en-Y) were recruited from the greater Charleston, South Carolina area via flyers and web broadcast advertisements. Forty one participants were initially enrolled in the study. Following screening, comprehensive psychological assessment and functional MRI (fMRI) scanning, 31 complete data sets were acquired. Mean time since surgery was 3.07 years (SD = 2.00, range 1.08-8.32). The mean age was 45.87 (SD = 11.08). The majority of participants were women (83.9%) and Caucasian (87.1%). The mean pre-surgery BMI for of the 31 participants was 51.28 kg/m2 (SD = 10.13) and the mean current BMI was 32.16 kg/m2 (SD = 7.27). The mean percent of total weight lost post-surgery based on current weight was 36.92% (SD = 10.65). Participants were divided into two groups according to the Reinhold criteria, 50% excess weight lost being the mark between “more successful” and “less successful” (26). Specifically, the less successful group included patients who either never lost 50% excess body weight or did but then regained it by the time of consent and their entry into the study. Twenty-four of the 31 participants, at the time of consent, remained on an appropriate post-surgical weight-loss trajectory (M = 73.50% excess weight loss; more successful), while 7 did not (M = 38.86% excess weight loss; less successful). Groups did not differ on depression symptomatology as measured by the CESD-10. The non-parametric Mann–Whitney test was used to compare continuous variables and Fisher's exact test was used to compare categorical variables between groups with SPSS (Version 20; IBM, Armonk, NY). Groups were significantly different only on current BMI, percent excess weight lost, and percent total weight lost (Table 1).
TABLE 1.
Participant characteristics by group
| Measures | More Successful (n = 24) | Less Successful (n = 7) |
|---|---|---|
| Gender (Men/Women) | 5/19 | 0/7 |
| Ethnicity (Caucasian/Other) | 20/4 | 7/0 |
| Age | 46.58 (11.36) | 43.43 (10.47) |
| Depression | 8.30 (6.56) | 8.14 (4.26) |
| Hours since they last ate | 5.38 (5.63) | 5.71 (3.83) |
| Years since surgery | 2.73 (1.80) | 4.22 (2.37) |
| Pre-surgery BMI (kg/m2) | 51.59 (11.22) | 50.21 (5.36) |
| Current BMI (kg/m2)a | 30.41 (7.16) | 38.16 (3.69) |
| Percent excess weight losta | 73.50 (16.17) | 38.86 (9.04) |
| Percent total weight losta | 40.81 (8.21) | 23.60 (6.46) |
Continuous variables shown are group means with standard deviations in parentheses. Depression measured with CESD-10.
Indicates a group difference (P < 0.05).
Screening/behavioral assessment
Procedures were in accordance with ethical standards and approved by the Institutional Review Board of the Medical University of South Carolina. Interested participants called and were initially screened on the telephone. Inclusion criteria included: between the age of 21 and 65, had gastric-bypass surgery at least one year ago, not suicidal, no history of brain surgery or history of loss of consciousness, and no history of autoimmune or endocrine disorder. The research procedures, risks and benefits were also explained. If individuals appeared to meet the inclusion criteria and were still interested in participating they were scheduled. If the participant was a pre-menopausal female, her session was scheduled during the luteal phase of her menstrual cycle, a time in the menstrual cycle where women experience more frequent food cravings (27). All participants were told not to eat within 4 h of their scheduled session. Participants reported eating a mean of 5.45 h before the scanning visit (SD = 5.22). Sessions were not specific to a time of day. During their visit, informed consent was presented and participants’ rights as research participants were explained. Once written informed consent was obtained, their heights and weights were measured, and their self-reported history of weight loss and weight gain since surgery was assessed.
Neuroimaging protocol
This study was performed on a Siemens 3T TIM trio scanner (Siemens, Erlangen, Germany) with a 12-channel RAPID Biomedical (Rimpar, Germany) head coil. First, high-resolution T1-weighted anatomical images were acquired for each participant (repetition time [TR] = 1750 ms, echo time [TE] = 4 ms, voxel dimensions 1.0 × 1.0 × 1.0 mm, 160 slices). Following anatomical image acquisition, participants performed the Food Craving/Resisting Task during two fMRI runs. These fMRI runs consisted of a multislice single-shot gradient echo planar imaging sequence with the following parameters: TR = 2.2 s, TE = 35 ms, 64 × 64 matrix, parallel imaging factor of 2, 3 × 3 × 3 mm voxels, 36 ascending transverse slices with approximate anterior commissure–posterior commissure alignment.
Food craving/resisting task
The fMRI task was divided into two functional runs. Twenty food images, 20 matched neutral images, and rest screens (static crosshair) were presented in a block design. Each run contained 15, 28-s blocks: five blocks each of food images, neutral images, and rest. Image blocks were composed of four randomly ordered pictures displayed for 7 s each, such that images were not repeated within each run. Blocks were pseudo-randomly ordered. During one of the two functional runs participants were told to “allow yourself to crave” (crave run). During the other run participants viewed the same images, but in a different order, and were asked to “resist food cravings” (resist run). The “crave” and “resist” runs were counterbalanced across participants. The food-images were standardized color food images from the International Affective Picture System (IAPS; 28). The foods used were high caloric foods, including sweets and carbohydrates (e.g., ice cream, cheese-burgers, pizza, and potato chips). Neutral images were matched on size, shape, and color (e.g., snowman, pennies, and stairs). The use of two-dimensional food pictures in craving studies is a widely used methodology and has been shown to produce unique cortical and subcortical activation relative to pictures of non-food items (29). At the completion of the visit participants were assessed for adverse effects of the procedures.
Functional image analysis
Statistical Parametric Mapping 8 software (SPM8; Wellcome Department of Imaging Neuroscience, London, UK), implemented in MATLAB (Mathworks, Natick, MD), was used to preprocess and analyze fMRI data. For preprocessing, functional images were first motion corrected with realignment to the first volume in the run. The T1-weighted image was co-registered to the mean realigned image, anatomical and functional images were normalized to the Montreal Neurological Institute (MNI) template and functional images were spatially smoothed spatially smoothed with an 8 mm3 Gaussian kernel full-width at half-maximum. Volumes with more than 2 voxels of movement were excluded from analyses. Functional images were modeled with a boxcar function convolved with the hemodynamic response function, using the general linear model, and included the food, neutral, and rest blocks. For each participant, first-level, fixed-effects comparisons were made to determine activation during food blocks relative to neutral blocks (food > neutral) for the crave run and the resist run. Overall effects of the crave and resist runs and group level differences (more successful versus less successful participants) were calculated using second-level, random-effects analyses of the food > neutral contrast during both the crave and resist runs. Analyses used a threshold of P < 0.01 for 10 contiguous voxels. Clusters with a P-value less than 0.05 are reported (corrected and uncorrected).
Results
Blood-oxygen-level-dependent (BOLD) response to appetitive food cues when instructed to “crave”: all participants
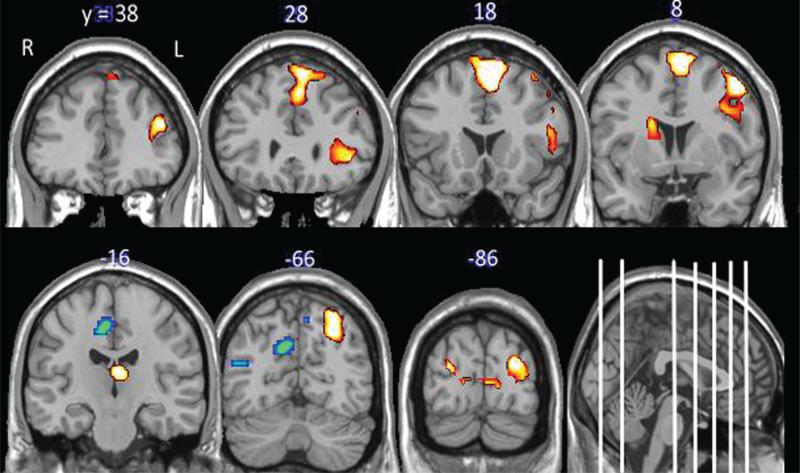
When instructed to crave, participants had significantly more activity in the left medial/superior frontal gyrus and middle frontal gyrus, anterior insula, left cuneus and bilateral middle occipital, the left superior parietal, left thalamus, and right caudate (Figure 1; Table 2) when viewing appetitive food cues relative to neutral cues. Additionally, participants had significantly less activity in the superior parietal and precuneus bilaterally, and the temporal cortex when viewing food cues relative to neutral cues.
FIGURE 1.
Brain response to “craving” appetitive food cues. The data in this figure include statistical maps of the brain regions significantly activated when viewing food cues relative to non-food cues when the participants were instructed to “Crave.” Brain regions with elevated activity to the food cues (red color map and depressed activity to the food cues (blue color map) relative to neutral are displayed (cluster-wise P < 0.05, uncorrected). The coordinates above the coronal slices refer to the anterior–posterior dimension of the standardized MNI template. They are displayed as white lines on the sagittal section for further reference. R = right hemisphere, L = left hemisphere.
TABLE 2.
Areas of elevated and suppressed response to food cues relative to neutral cues for all participants: the “Crave” run
| Region | ~BA | MNI Coordinates | t | Size |
|---|---|---|---|---|
| Elevated activity when viewing food cues | ||||
| Medial/superior frontal gyrus L | 6/8 | –6 14 52 | 8.00 | 642a |
| Middle frontal gyrus, anterior insula L | 6 | –48 2 49 | 7.44 | 608a |
| Cuneus L/middle occipital (bilateral) | 18/19 | –27 –94 22 | 5.70 | 334a |
| Superior parietal L | 7 | –27 –70 43 | 5.16 | 265a |
| Thalamus L/caudate R | –3 22 13 | 5.00 | 202 | |
| Suppressed activity when viewing food cues | ||||
| Superior parietal/precuneus/occipital (bilateral) | 5/7,19 | 9 –40 52 | 4.64 | 1549a |
| Middle temporal gyrus R | 39 | 51 –55 13 | 4.62 | 770a |
N = 31; Voxel threshold P < 0.01 for 10 contiguous voxels. Regions are significant at cluster-level P < 0.05, uncorrected
FDR corrected. Coordinates of peak activity reported.
BOLD response to appetitive food cues when instructed to “resist”: all participants
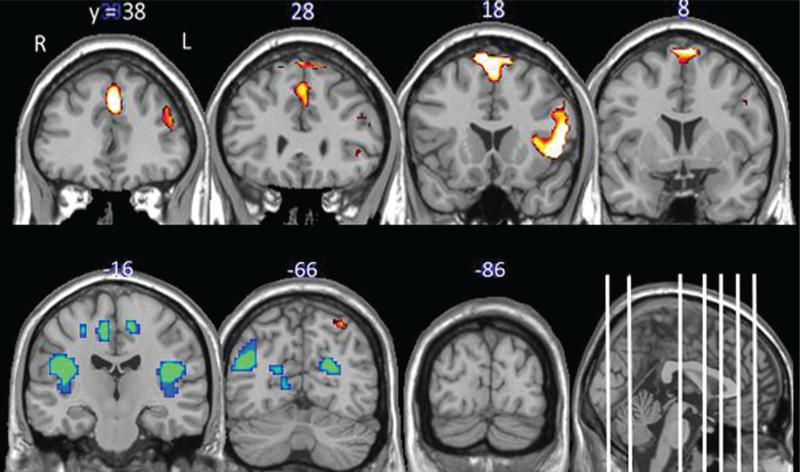
When instructed to resist craving, participants had significantly more activity throughout the PFC when viewing food cues relative to neutral cues. The effects were the largest in areas involved in cognitive processing including the left DLPFC but also included the right inferior frontal gyrus, anterior insula, and the left superior parietal cortex (Figure 2; Table 3). As with the “crave” run, participants had significantly less activity in a large cluster containing the precuneus bilaterally, the posterior insula, and the occipital lobe when viewing food cues relative to neutral cues. When the response to food cues during “crave” and “resist” were compared directly, no significant differences were found.
FIGURE 2.
Brain response to “resisting” appetitive food cues. The data in this figure include the statistical maps of the brain regions significantly activated when viewing food cues relative to non-food cues when the participants were instructed to “Resist” the cues. Brain regions with elevated activity to the food cues (red color map) and depressed activity to the food cues (blue color map) relative to neutral are displayed (cluster-wise P < 0.05, uncorrected). The coordinates above the coronal slices refer to the anterior–posterior dimension of the standardized MNI template. They are displayed as white lines on the sagittal section for further reference. R = right hemisphere, L = left hemisphere.
TABLE 3.
Areas of elevated and suppressed response to food cues relative to neutral cues for all participants: the “Resist” run
| Region | ~BA | MNI Coordinates | t | Size |
|---|---|---|---|---|
| Elevated activity when viewing food cues | ||||
| Inferior/middle frontal gyrus, anterior insula L | 9/10/13 | –51 14 –2 | 6.97 | 687a |
| Superior/medial frontal gyrus L | 6/9 | –3 32 34 | 5.84 | 526a |
| Superior parietal L | 7 | –42 –64 49 | 4.21 | 172 |
| Suppressed activity when viewing food cues | ||||
| Precuneus, posterior insula, occipital | 7,18,19 | 6 –52 43 | 5.91 | 6450a |
N = 31; Voxel threshold P < 0.01 for 10 contiguous voxels Regions are significant at cluster-level P < 0.05 uncorrected
FDR corrected. Coordinates of peak activity reported.
BOLD response to appetitive food cues: more successful versus less successful participants
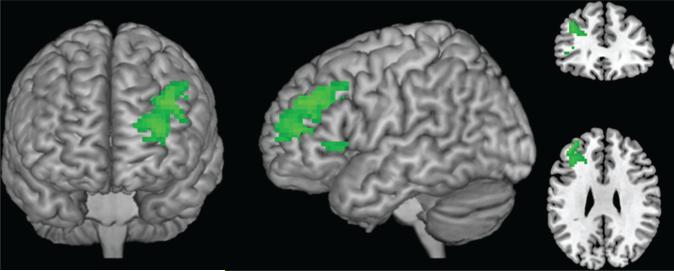
When individuals were exposed to appetitive food cues and instructed to allow themselves to crave (crave run), there were no significant differences in neural activity (BOLD response) between the groups. When individuals were exposed to cues and instructed to resist craving (resist run) however, the more successful group had significantly greater activation in the left DLPFC than the less successful group (Figure 3; Table 4). Significantly greater left DLPFC activation was still seen in the more successful than less successful group during the “resist” run when excluding the males from the more successful group (data not shown). This was the only region that was differentially activated between the groups and there were no regions in which the less successful group had greater activation than more successful participants when told to resist. We also examined the role of percent excess weight lost and current BMI in response to food cues, controlling for time since surgery. There were no significant correlations between percent excess weight lost and activation in response to cues in the “crave” or the “resist” run. There were also no negative correlations between current BMI and response to cues in the “resist” run. However, activation in the anterior cingulate cortex (peak activity in MNI space [x,y,z]:12 35 19) and insula (–42 –7 1; both P <0.05 uncorrected) was positively correlated with BMI during the “crave” run, indicating greater craving-related reward response in heavier participants.
FIGURE 3.
Brain response to “Resist”: More successful greater than less successful. This figure displays the region with significantly higher activation (left dorsolateral prefrontal cortex) in the “more successful” group relative to the “less successful” group when instructed to resist the urge to crave in response to food cues (cluster-wise P < 0.05, uncorrected).
TABLE 4.
Neural response to appetitive food cues relative to neutral cues among individuals with “more successful” and “less successful” weight loss outcomes after gastric-bypass surgery
| Region | ~BA | MNI Coordinates | t | Size |
|---|---|---|---|---|
| Crave run | ||||
| More greater than less successful | No significant clusters | |||
| More greater than less successful | No significant clusters | |||
| Resist run | ||||
| More greater than less successful | ||||
| Superior/middle frontal gyrus L | 9 | –39 38 31 | 4.00 | 343 |
| More greater than less successful | No significant clusters |
Results of two-sample t-tests comparing groups. More successful (n = 24), less successful (n = 7); Voxel threshold P < 0.01 for 10 contiguous voxels. Region is significant at cluster-level P = 0.005, uncorrected (P = .062, corrected). Coordinates of peak activity reported.
Discussion
Obesity continues to be a social problem and bariatric surgery is on the rise, as it is one of the most effective weight loss methods. Unfortunately, however, many individuals regain weight or do not lose the appropriate amount of weight after the surgery making this a potentially costly procedure for society and potentially causing physical and psychological risks to people who may not get any long-term benefit from it (4-6). A greater understanding of the underlying neurological processes associated with successful and non-successful surgical outcomes could inform therapeutic approaches to managing post-surgery weight loss. The results of this initial, cross-sectional study of participants who underwent gastric-bypass surgery demonstrated that individuals who are able to activate a brain region integral in executive control, the DLPFC, when told to resist craving, are significantly more likely to have successful outcomes. While this study is cross-sectional and preliminary, it supports and extends prior findings in gastric-bypass patients and suggests that fMRI may provide insight into the causes of weight loss failure.
There are two main conclusions from this study. The first is that when post-surgical gastric-bypass participants are exposed to appetitive food cues, a network of limbic related neural regions is activated, including the medial frontal gyrus, the anterior insula, the caudate and the thalamus. In addition to modulating reward and motivation in healthy adults, these limbic regions are also specifically activated in response to salient cues, including food cues among obese individuals and drug cues among substance-dependent individuals (30,31). The activation of the anterior insula (a brain region tightly coupled to craving) (32) when these formerly obese patients are presented with appetitive food cues is consistent with prior literature and our a priori expectations.
The second, and perhaps most interesting conclusion from this preliminary investigation is that, although among the entire study sample there were no statistically unique neural regions associated with “resisting” the food cues compared to “craving,” the ability of post-surgical individuals to recruit executive control circuitry was related to sustained weight-loss following gastric-bypass. Individual differences between those who were “more successful” versus “less successful” (as defined by meeting 50% of their excess weight loss) emerged only when we assessed their ability to “resist” the food cues. The more successful individuals had significantly greater activity in the left DLPFC, a brain region which is perhaps the primary cortical node of the executive control loop in the brain (16). Previous research demonstrated the importance of DLPFC in response to food cues, finding that the DLPFC is associated with self-control in food-related decision-making (33), and that stimulation of the DLPFC can inhibit food craving (34). In a study of bariatric patients, decreases in limbic and DLPFC response to passive viewing of food cues from one month pre to post gastric-bypass surgery were found, suggesting that when reward value of the food decreases less inhibitory control is needed (35). The current findings extend this research by highlighting the importance of the left DLPFC and inhibitory control in actively resisting food craving over 1 year post-surgery. Although these data are still preliminary given the small number of people who were not as successful at losing weight post-surgery, these data suggest that individuals that are “more successful” may be able to recruit significantly more of the DLPFC when instructed to “resist” appetitive food cues.
The field continues to lack assessments that predict bariatric surgery outcomes and little is known about changes that occur in the brain as a consequence of obesity, or following weight-loss surgery. Although results remain inconsistent when comparing obese individuals to controls, evidence suggests that brain activity in relation to eating behavior may differ in lean and obese individuals and that there is change from one month pre to post-bariatric surgery, specifically in gastric-bypass patients (15,21-24,35,36). Additionally, fMRI findings have demonstrated variability in neural responses to food cues when comparing obese, normal-weight, and obese individuals who have maintained a near normal-weight, but this remains an under examined area among bariatric surgery patients (25). The results of the current study help elucidate the differences in brain activity in response to food cues in those that are more successful at weight loss after surgery, versus those that are not. While we only have data indicating differences post-surgery, the findings from this study help clarify other results and suggest that the ability to activate the executive circuit may be a predictor of surgery outcomes. These differences in brain activation may also be used to identify individuals who need additional treatment (e.g., cognitive training) prior to surgery in order to be successful post-bariatric surgery. In addition, the knowledge that a lack of success post-surgery is partly due to an inability to effectively activate control regions when resisting food craving could inform treatment for people who have already undergone surgery, but have not been able to achieve their weight-loss goals. Therefore, these findings could lead to the development of new targets for behavioral, pharmacologic, brain stimulation, and/or cognitive interventions to improve the success rate of bariatric surgery.
There are several limitations to the current study. The sample used for the current study was small with only 31 participants, and the group difference in this study only survived a cluster-wise, uncorrected statistical threshold as a balance between Type 1 and 2 errors. Use of a small sample size can raise threats to external validity. Regarding generalizability, the sample used in this study consisted mainly of Caucasian women and it is not known if similar brain activity would be observed in a sample consisting of males and/or other ethnic backgrounds. Further, while there were five men in the group that was more successful, all of the less successful participants were female, which leads to additional questions regarding gender differences in the ability to recruit executive control circuitry when presented with a salient limbic cue. There are also potential confounding variables between groups pre- and post-surgery, such as type 2 diabetes and binge eating disorder, or possibly differences in neurovascular coupling which could have affected the results. It is unlikely however that the latter difference would specifically affect the left DLPFC. Perhaps the most important limitation to this study is the cross-sectional design, which did not allow scans prior to and post-surgery. Few studies have started to examine this, but further research is needed in this area to compare brain activation in individuals prior to and post-weight loss surgery in order to determine if there are changes that occur in direct response to surgery that can predict surgical outcomes. It is also possible that the laboratory paradigm employed in this study does not elicit responses that generalize to real-world eating or craving behaviors, as there may also be a difference between voluntary craving, and “instructed” craving, to the natural experience of craving. Furthermore, the nature of the fMRI paradigm makes it impossible to verify attention to and compliance with the task during the runs. Finally, the criteria to determine if a participant was more successful versus less successful at weight loss following gastric-bypass surgery is challenging. Here, we used a criterion that is well used within the bariatric surgery field, which is participants that have lost less than 50% of their excess weight were considered less successful (26), but certainly other strategies/algorithms could have been applied.
Taken together, data from this preliminary, cross-sectional study demonstrate that individuals who undergo gastric-bypass surgery, as a group, have the ability to increase limbic circuitry when instructed to crave in the presence of appetitive food cues, and to dampen activity in the insula, and other limbic processing areas, when instructed to resist these food cues. The difference between individuals that were more successful versus less successful, however, is in their ability to utilize executive control circuitry when told to resist. Specifically, the more successful individuals have a much larger brain response in the DLPFC. This dissociation between limbic drive and executive control circuitry is a common theme in addiction literature used to explain substance use and relapse (30) and may provide valuable insight into basis for so many unfortunate relapses to obesity following gastric-bypass surgery. Through these results and future research in this area we may be able to identify individuals who have a lower integrity of these executive control circuits and either advise them against surgery or provide additional pre- and post-operative targeted cognitive control therapy.
Acknowledgments
RLG received the funding for this research, participated in study design, data collection, interpretation, and writing of this article. MC and CAH conducted the analyses, and were involved with data interpretation and writing of the article. JJB was co-investigator on the grant and was involved in the study design, data collection, analyses, interpretation of the data, and revisions of the article. PMO and AM were co-investigators on the grant and involved in study design and revisions of the article. TKB was the surgeon of these participants and was involved in revisions of the article. MSG was involved in study design and revisions of the article. All authors had final approval of the submitted version.
Funding agencies: This study was supported by Covidien through a grant from the Obesity Society. The following grants enabled Drs. Canterberry and Hanlon to contribute to this study: K01DA027756 (to C.H.) and T32DA02788 (to M.C.).
Footnotes
Disclosures: Dr. Goldman has no conflicts of interest. Dr. Borckardt receives research support from NIAMS and NIDA at NIH, The North American Spine Society, and The American Society for Gastrointestinal Endoscopy. Drs. Canterberry, Madan, and Hanlon receive research support from NIH. Dr. Byrne has no conflicts. Dr. George has no conflicts. Dr. O'Neil receives research support from NovoNordisk, Orexigen Therapeutics, and Weight Watchers. Dr. O'Neil also receives honoraria and/or travel support from Pharmatecture, Vindico CME, Vivus, CMEducation Resources, and CMEIncite.
References
- 1.Calle EE, Thun MJ, Petrelli JM, et al. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 2.Balsige BM, Murr MM, Poggio JL, Sarr MG. Bariatric surgery: surgery for weight control in patients with morbid obesity. Med Clin North Am. 2000;84:477–489. doi: 10.1016/s0025-7125(05)70232-7. [DOI] [PubMed] [Google Scholar]
- 3.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292/14:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 4.Benotti PN, Forse A. The role of gastric surgery in the multidisciplinary management of severe obesity. Am J Surg. 1995;169:361–367. doi: 10.1016/s0002-9610(99)80177-9. [DOI] [PubMed] [Google Scholar]
- 5.Hsu L, Benotti PN, Dwyer J, et al. Nonsurgical factors that influence the outcome of bariatric surgery: a review. Psychosom Med. 1998;60:338–346. doi: 10.1097/00006842-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Shah M, Simha V, Garg A. Review: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab. 2006;91:4223–4231. doi: 10.1210/jc.2006-0557. [DOI] [PubMed] [Google Scholar]
- 7.Ray EC, Nickels MW, Sayeed S, Sax HC. Predicting success after gastric bypass: the role of psychosocial and behavioral factors. Surgery. 2003;134:555–563. doi: 10.1016/s0039-6060(03)00279-4. [DOI] [PubMed] [Google Scholar]
- 8.Kral JG. Surgical interventions for obesity. In: Brownell KD, Fairburn CG, editors. Eating Disorders and Obesity: A Comprehensive Handbook. The Guilford Press; New York: 1995. pp. 510–515. [Google Scholar]
- 9.Budak AR, Thomas SE. Food craving as a predictor of “relapse” in the bariatric surgery population: a review with suggestions. Bariat Nurs Surg Pat. 2009;4:115–121. [Google Scholar]
- 10.Davis C, Carter J C. Compulsive overeating as an addiction disorder. A review theory and evidence. Appetite. 2009;53:1–8. doi: 10.1016/j.appet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 12.Gendall KA, Joyce PR, Sullivan PF, Bulik CM. Food cravers: characteristics of those who binge. Int J Eat Disord. 1998;23:353–360. doi: 10.1002/(sici)1098-108x(199805)23:4<353::aid-eat2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Anton RF. What is craving? Alcohol Res Health. 1999;23:165–173. [PMC free article] [PubMed] [Google Scholar]
- 14.Odom J, Zalesin KC, Washington TL, et al. Behavioral predictors of weight regain after bariatric surgery. Obes Surg. 2010;20:349–356. doi: 10.1007/s11695-009-9895-6. [DOI] [PubMed] [Google Scholar]
- 15.DelParigi A, Chen K, Salbe AD, et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes. 2007;31:440–448. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]
- 16.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 17.George MS, Anton RF, Bloomer C, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- 18.Myrick H, Anton RF, Li X, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 20.Uher R, Murphy T, Brammer MJ, et al. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am J Psychiatry. 2004;161:1238–1246. doi: 10.1176/appi.ajp.161.7.1238. [DOI] [PubMed] [Google Scholar]
- 21.DelParigi A, Chen K, Salbe AD, et al. Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obes Relat Metab Disord. 2004;28:370–377. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- 22.Gautier JF, Del Parigi A, Chen K, et al. Effect of satiation on brain activity in obese and lean women. Obes Res. 2001;9:676–684. doi: 10.1038/oby.2001.92. [DOI] [PubMed] [Google Scholar]
- 23.Kilgore WD, Yurgelun-Todd DA. Body mass predicts orbitofrontal activity during visual presentations of high-calorie foods. Neuroreport. 2005;16:859–863. doi: 10.1097/00001756-200505310-00016. [DOI] [PubMed] [Google Scholar]
- 24.Martin LE, Holsen LM, Chambers R, et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity. 2010;18:254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- 25.McCaffery JM, Haley AP, Sweet LH, et al. Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. Am J Clin Nutr. 2009;90:928–934. doi: 10.3945/ajcn.2009.27924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinhold RB. Critical analysis of long-term weight loss following gastric bypass. Surg Gynecol Obstet. 1982;155:385–394. [PubMed] [Google Scholar]
- 27.Davidsen L, Vistisen B, Astrup A. Impact of the menstrual cycle on determinants of energy balance: a putative role in weight loss attempts. Int J Obes. 2007;31:1777–1785. doi: 10.1038/sj.ijo.0803699. [DOI] [PubMed] [Google Scholar]
- 28.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. NIMH Center for the Study of Emotion and Attention; Gainsville, FL: 1997. [Google Scholar]
- 29.Killgore WDS, Young AD, Femia LA, et al. Cortical and limbic activation during viewing of high versus low-calorie foods. Neuroimage. 2003;19:1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carnell S, Gibson C, Benson L, et al. Neuroimaging and obesity: current knowledge and future directions. Obes Rev. 2012;13:43–56. doi: 10.1111/j.1467-789X.2011.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:351–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 34.Uher R, Yoganathan D, Mogg A, et al. Effect of left prefrontal repetitive transcranial magnetic stimulation on food craving. Biol Psychiatry. 2005;58:840–842. doi: 10.1016/j.biopsych.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 35.Ochner CN, Stice E, Hutchins E, et al. Relation between changes in neural responsivity and reductions in desire to eat high-calorie foods following gastric bypass surgery. Neuroscience. 2012;209:128–135. doi: 10.1016/j.neuroscience.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochner CN, Kwok Y, Conceição E, et al. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg. 2011;253:502–507. doi: 10.1097/SLA.0b013e318203a289. [DOI] [PMC free article] [PubMed] [Google Scholar]