Abstract
Having a history of infection with one pathogen may sometimes provide a level of T cell-dependent protective heterologous immunity to another pathogen. This immunity was initially thought due to cross-reactive T cell epitopes, but recent work has suggested that such protective immunity can be initiated nonspecifically by the action of cytokines on memory T cells. We retested this concept using two small and well-defined arenaviruses, lymphocytic choriomeningitis virus (LCMV) and Pichinde virus (PV), and found that heterologous immunity in these systems was indeed linked to T cell epitopes and the major histocompatibility complex.
Keywords: arenaviruses, cross-reactivity CD8 T cell, epitopes, heterologous immunity, lymphocytic choriomeningitis virus, Pichinde virus
INTRODUCTION
The term heterologous immunity refers to the phenomenon whereby a previous exposure of an immune system to one pathogen will alter the host response to a second heterologous pathogen (Welsh et al., 2010). The mechanisms of heterologous immunity can be varied and involve both innate and adaptive components of the immune system. T cell-dependent heterologous immunity refers to the ability of memory T cells to provide beneficial or detrimental immunity to another pathogen. T cell cross-reactivity against epitopes expressed by heterologous pathogens is quite common, and initial studies on T cell-dependent heterologous immunity implicated such cross-reactive T cells in this process (Welsh & Selin, 2002). However, there have been a series of reports indicating that a number of cytokines, such as IL-12 and IL-18, may be able to “non-specifically” activate memory T cells, thereby enabling them to provide resistance to certain pathogens by the generation of interferon (IFN)γ (Gilbertson et al., 2004;Raue et al., 2004;Berg et al., 2002). Recent reports have suggested that these T cells may also express the receptor NKG2D and be able to recognize stress-related ligands expressed on pathogen-infected cells (Chu et al., 2013;Hamerman et al., 2004).
Proving that T cell cross-reactivity is not occurring in these types of studies is challenging, given that cross-reactivity becomes increasingly more difficult to disprove as the functional avidity between putative cross-reactive epitopes declines. A recent study, using a green fluorescent protein (GFP)-nur77 transgenic mouse, whose T cells up-regulate GFP expression if their TCR is stimulated, showed some greening of the memory T cells putatively nonspecifically stimulated by pathogen-induced cytokines (Hamerman et al., 2004). Cytokines like type 1 IFN can up-regulate MHC and costimulatory antigens and cause a partial activation of naïve T cells in the form of expression of the effector cell transcription factor eomesodermin (Marshall et al., 2010). This effect is seen only with transgenic T cells that have enough reactivity with self-antigens to undergo homeostatic proliferation and is inhibited by cyclosporine, a potent inhibitor of TCR signaling. Hence, one mechanism of a putative “non-specific” activation of memory cells could be by elevating the effector function of cells already being signaled by self antigen.
We have found that vaccinia virus (VACV), a strong inducer of IL-12 and a pathogen highly sensitive to IFNγ, replicates more poorly in mice immune to LCMV, PV, influenza A virus, or bacillus Calmette-Guerin (BCG) than in naïve mice (Selin et al., 1998;Chen et al., 2001;Mathurin et al., 2009). One might initially conclude that this had to be explained by a non-specific or self-antigen-stimulated phenomenon, but the patterns of IFNγ production in vivo during the first three days of VACV infection differed with the immunizing pathogen. After VACV infection of BCG-immune mice memory CD4 T cells made much more IFNγ than did memory CD8 T cells, but memory CD8 T cells in LCMV-immune mice made much more IFNγ than did memory CD4 T cells. Statistically, there are many possibilities for cross-reactive T cell epitopes between these very large pathogens, and, in fact, protective cross-reactive CD8 T cell epitopes have been defined between VACV and LCMV (Kim et al., 2005;Cornberg et al., 2007). However, just because T cell cross-reactivity can be shown does not mean that it is the predominant mechanism of protective heterologous immunity.
To address the mechanism of pathogen-cross-reactive T cells in protective heterologous immunity we focused on much less antigenically diverse pathogens, LCMV and PV. LCMV and PV are arenaviruses that each encode only four proteins, making studies on cross-reactive T cell epitopes much more manageable (Peters et al., 1996). In C57BL/6 (B6) mice, LCMV and PV encode cross-reactive MHC Kb –restricted epitopes that have 6 of 8 amino acids in common: LCMV-NP205-212 (YTVKYPNL) and PV-NP205-212 (YTVKFPNM) (Brehm et al., 2002). T cell responses to these epitopes are normally weak, but in mice immune to one virus and challenged by the other, they become immunodominant, and protective T cell-dependent heterologous immunity is seen. Studies with naturally selected or genetically engineered LCMV mutants in this epitope showed that the protective heterologous immunity between LCMV and PV was mostly lost (Chen et al., 2012). This was evidence that true T cell cross-reactivity was responsible for heterologous immunity in this system. However, given the new findings on “non-specific” heterologous immunity, we thought it important to address this in more detail and used these viruses to address the MHC basis of heterologous immunity. To examine this process we first employed commonly used laboratory strains of mice (B6, BALB/c, CBA) harboring differing MHC complexes. Then to determine whether any differences seen in pathogenesis were related to the MHC, we employed MHC-diverse congenic mice on the same C57BL/10 (B10) genetic background.
RESULTS AND DISCUSSION
Initially we examined the ability of a history of an LCMV infection to provide protective heterologous immunity to acute PV infection. Table 1, Expt. 1, lists a representative experiment done at the time of these studies showing that PV replicates to over 10-fold higher titers in naïve mice than in LCMV-immune B6 (H2b) mice. This heterologous immunity in B6 mice has been documented extensively in previous studies (Brehm et al., 2002;Cornberg et al., 2006;Chen et al., 2012). It is associated with a change in CD8 T cell immunodominance such that the normally subdominant cross-reactive NP205 epitope becomes immunodominant. Further, LCMV variants mutant in the NP205 epitope fail to provide heterologous immunity against PV. This heterologous immunity is reciprocal, though LCMV-immune mice protect better against PV than do PV-immune mice against LCMV, probably because more NP205-specific T cells are in the LCMV-induced memory pool.
Expt. 2 and 3 show two similar experiments with B10 mice. These mice are closely related to B6, and they exhibited the same pattern of heterologous immunity, where a history of an LCMV infection strongly protected against PV. Of note is that in expt. 1-3 the variation in PV titers was higher in the LCMV-immune mice than in the non-immunized mice. This is a commonly observed phenomenon in heterologous immunity studies and is a consequence of variations in the memory pools caused by the private specificities of the immune repertoires in the immune mice (Cornberg et al., 2006;Kim et al., 2005). We have shown that, depending on the individual immune B6 mouse, the magnitude of the cross-reactive response may vary from 5 to 30% (Cornberg et al., 2006), making the assessment of mean titers not always the best way to measure the protective capacity. However, since T cell cross-reactivity and protective heterologous immunity are so strong between these arenaviruses in mice with an MHC haplotype of b, such protection can easily be demonstrated.
Experiments 4-8 examine heterologous immunity in two strains of mice expressing an MHC of k: CBA and B10.BR. The B10.BR mice are congenic with B10 mice, except for the MHC region, which contains immune system genes with k alleles rather than b alleles. None of the five experiments showed any statistical differences in the mean viral titers between PV-challenged naïve mice and LCMV-immune groups. In three of the experiments titers were slightly higher in the naïve group, and in two of the experiments titers were slightly higher in the immune group, but none of the differences approached significance. Given that the B10.BR mice differ from the B10 mice only in the MHC, this experiment would argue that protective heterologous immunity between these two viruses was linked to the H2b vs. H2k MHC differences of these strains of mice.
Studies done with BALB/c and B10.D2 mice, which have an MHC haplotype of d, were a bit more complex and revealed a phenotype that suggested heterologous immunity but at a weaker level than that seen with the H2b mice. In each of the 5 experiments (#9-13) the means of the PV titers in LCMV-immune mice were lower than those in the naïve mice, averaging to about a 0.7 log (5-fold) difference in titer. The individual experiments differed in their levels of significance. Two (expt. 10 and 13) were highly significant, one (expt. 9) was borderline, and two (expt. 11 and 12) registered as non-significant. However the two non-significant experiments had very high standard deviations in the LCMV-immune group, an event commonly seen with heterologous immunity due to the private specificities of the immune repertoires, as mentioned above. Given this suggestion of heterologous immunity between LCMV and PV in H2d mice, we tested its reciprocal nature by challenging PV-immune H2d mice with LCMV. Experiments 14 and 15 in Table 1 show that a history of PV infection did provide heterologous immunity to LCMV in these H2d –expressing mice, supporting the idea that heterologous immunity between these two viruses does occur in the context of MHC of d, but perhaps not as strongly as in the context of MHC of b. In contrast, one experiment (16) showed very little protective heterologous immunity in this PV + LCMV virus sequence in CBA (H2k) mice, much like the lack of differences seen in the LCMV + PV sequence in other H2k mice. While the degree of protection in H2d mice was variable and modest compared to what was observed in the H2b haplotype, it is important to note that no protection was ever observed in H2k mice, indicating that heterologous immunity between these viruses is an MHC-dependent phenomenon that indeed exists in the H2d haplotype although not as robustly as in the H2b haplotype.
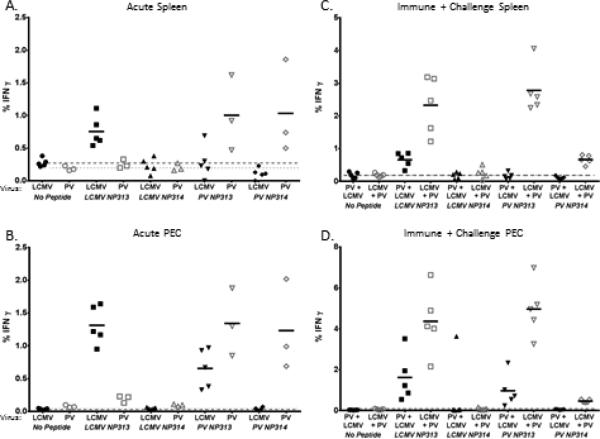
Based on the results shown in Table 1 indicating modest MHC-linked heterologous immunity in H2d mice, we hypothesized that there may be H2d-restricted T cell epitopes cross-reactive between LCMV and PV. LCMV- specific CD8 T cell epitopes have been identified on an H2d background, but no H2d - epitopes for PV had previously been identified. Initial screens done with BALB/c mice used Elispots and intracellular cytokine assays for detecting IFNγ after exposure of splenocytes or PEC to overlapping peptides of the LCMV and PV GP and NP proteins. These initially yielded a number of positive results and potential cross reactive candidates, but subsequent examination of these peptides resulted in only an individual CD8 peptide from both viruses that scored consistently over the no peptide background. The positive epitope was contained within the LCMV peptide NP309-326 and the PV peptide NP311-330. The nucleoprotein (NP) is the most highly expressed protein for each of these viruses. Using predicted binding motifs for class 1 MHC epitopes we defined two possible epitopes located within the LCMV and PV peptides. These consisted of the putatively Ld –restricted epitopes LCMV NP313-322 (WPYIACRTSI) and PV NP313-322 (WPYIGSRSQV) as well as putative Kd –restricted epitopes LCMV NP314-322 (PYIACRTSI) and PV NP314 (PYIGSRSQV), with the underlined being anchoring amino acids. Previous work with LCMV had identified the subdominant epitope NP313 through the use of DNA minigene protection assays (Rodriguez et al., 2001). However, the LCMV peptide beginning at NP314 was not considered a true epitope (van der Most et al., 1996). We synthesized all four peptides and were able to confirm the previously published findings for these epitopes during an acute LCMV infection (Fig. 1A,B).
Figure 1 shows the LCMV- and PV-induced CD8 T cell responses to the four epitopes in the spleen (A) and PEC (B) of BALB/c mice infected for 8 days with either LCMV or PV. In general stronger results were seen within the PEC, reflecting the inoculation route of the virus. The LCMV and PV NP313 epitopes were both recognized by LCMV- or PV-induced T cell populations, though the PV-induced populations only weakly recognized the LCMV NP313, while more strongly recognizing the PV313. The PV-induced populations also recognized PV-encoded NP314, and it is unclear whether some of the PV-encoded NP313 was cleaved into the NP314 form. Recognition of NP314 by LCMV-induced populations was, as previously reported (van der Most et al., 1996), negligible.
Figure 1 C,D displays the day 8 T cell response to these four peptides in mice immune to one virus and challenged with another. Again the PEC T cell responses were much stronger than those in the spleen. LCMV-immune mice challenged with PV developed a strong dominant T cell response to each of the NP313 epitopes, ranging in the 4-5% range. This was not as strong as that seen against the NP205 epitope in H2b mice, perhaps consistent with the heterologous immunity in these H2d mice being not as profound. The CD8 T cell response was also greater in LCMV-immune mice challenged with PV compared to PV-immune mice challenged with LCMV (Fig. 1C, 1D), perhaps reflecting the fact that the frequency of spleen LCMV NP313-specific T cells was higher in LCMV-immune (~0.26%) mice than in PV-immune mice (<0.1%). The response against the PV-encoded NP313 was much stronger than that against the PV-encoded NP314, arguing that it was probably truly against the intact Ld-restricted NP313 and not a NP314 breakdown product presented by Kd. The heterologous immune response in PV-immune mice challenged with LCMV was not as strong (Fig. 2) and was not much different at this day 8 time point from non-immune mice acutely infected with LCMV (Fig. 1).
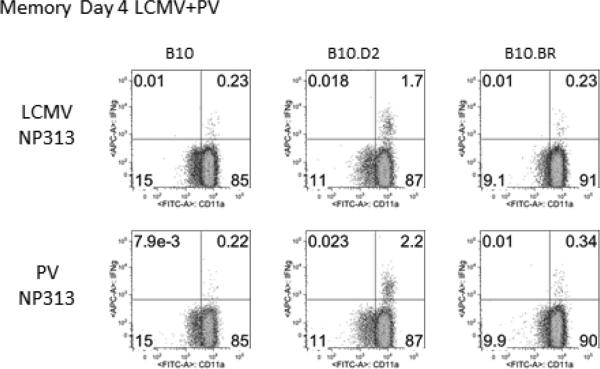
The design of our experiments in Table 1 to measure heterologous immunity was to titrate virus at day 4, which we also found was a good time point to examine the development of the early heterologous CD8 T cell responses from the PEC, which had migrated into the site of initial viral inoculation. To confirm the MHC-restricted nature of the heterologous immune response to the defined peptide epitopes, we show in Fig. 2 the PEC T cell response to the cross-reactive NP313 epitopes encoded by LCMV and PV in MHC congenic mice immune to LCMV and challenged with PV. Readily detectable responses were made against both epitopes in the immune B10.D2 mice, whereas the responses in the B10 and B10.BR mice were negligible and at background levels. This use of the congenic B10-series mice further demonstrates the MHC-restriction of the cross-reactive epitope.
The results of this study support the argument that protective heterologous immunity between distantly related agents can be mediated rather selectively, be dependent on the MHC of the host, and be correlated with the presence of cross-reactive epitopes. Further analysis in this system with mutants in the cross-reactive H2d epitopes, as we have done previously with the H2b haplotype (Chen et al., 2012), will be needed to provide the definitive link between cross-reactive CD8 T cell populations and heterologous immunity between LCMV and PV in H2d mice. These results are inconsistent with the frequently promoted idea that memory cells get non-specifically stimulated and provide non-specific protection against infection through the release of cytokines like IFNγ or through non-specific attack on stressed target cells mediated through innate sensors (Gilbertson et al., 2004;Raue et al., 2004;Berg et al., 2002;Chu et al., 2013;Hamerman et al., 2004). However, our study does not mean that those situations do not occur. Rather, it means that they do not have to occur for the protective capacities of heterologous immunity to be manifested. LCMV and PV may not induce enough of the appropriate cytokines, such as IL-12, to non-specifically activate memory cells. Further, these viruses may not be sensitive enough to IFNγ to be affected by that mechanism, and, being relatively non-cytopathic, they may not stress infected cells sufficiently to induce ligands for stress-detecting receptors. These viruses do, however, provide a simple system to more clearly define the mechanisms of heterologous immunity. In B6 mice the specific role of CD4 and CD8 T cells in mediating heterologous immunity between these pathogens is well established and helped by the fact that each virus encodes only four proteins (Brehm et al., 2002;Cornberg et al., 2006;Chen et al., 2012;Selin et al., 1998). With larger and more complicated viral or bacterial pathogens, it may be tempting to conclude that the heterologous immunity is a non-specific function because it is so difficult to define the cross-reactive elements between the pathogens.
We believe that the more one looks for T cell cross-reactivity, the more one finds it, but the evidence for non-specific factors regulating heterologous immunity must be considered and appreciated. We predict that the mechanisms of heterologous immunity will be diverse and depend greatly with the pathogens being examined.
Research Highlights.
Heterologous immunity between LCMV and Pichinde virus (PV) was MHC-dependent.
Heterologous immunity between LCMV and PV was a specific phenomenon.
CD8 T cell cross-reactivity was demonstrated between LCMV and PV in H2d mice.
LCMV and PV encode putative Ld-restricted NP313-NP322 epitopes.
The NP313 epitopes of LCMV and PV are cross-reactive with CD8 T cells.
Figure 1. Cross-reactive epitopes in BALB/c (H2d) mice.
A,B. After preliminary screening efforts to detect cross-reactive epitopes, BALB/c mice were inoculated with LCMV or PV as described in Table 1. At day 8 post-infection splenocytes (A) or peritoneal exudate cells (PEC) (B) were exposed to the four putative cross-reactive epitopes encoded by LCMV and by PV and defined in the text. These were tested in intracellular cytokine assays after stimulation with 1 μM peptide and gated on CD8 T cells. Solid horizontal lines mark the means of the plotted samples showing reactivity. Horizontal dashed lines show the levels of background staining without peptide. C and D show enhanced reactivity to the NP313 epitopes in mice immune to one virus and then challenged with the other at 8 days post-infection. For clarity of the figure, data for unchallenged immune controls are not plotted. However, in unchallenged LCMV-immune or PV-immune BALB/c mice, less than 0.5% of the spleen CD8 cells reacted with any of the peptides. Less than 2.5% of CD8 T cells in the PEC reacted with any of the peptides, and the number of CD8 T cells in the PEC expanded greater than 10-fold by day 8.
Figure 2. MHC restriction of the cross-reactive epitopes.
This figure plots the CD8 T cell responses to the defined cross-reactive LCMV and PV NP313 epitopes under conditions of LCMV + PV heterologous immunity, as described in Table 1. This plots the activity in PEC CD8 T cells at day 4 post-infection. Here, the B10 series of mice is examined, and only those expressing H2d (B10.D2, like the H2d –expressing BALB/c mice (Figure 1)), show positive reactivity to the two peptides, thereby demonstrating MHC-restriction.
Table 1.
Legend Age-matched male mice 5-10 weeks of age were immunized i.p. with 100 μl of either 5 × 104 PFU of LCMV diluted 70-fold from stock virus into serum-free Hank's Balanced Salt Solution (HBSS), 107 PFU of PV purified in sucrose gradients and diluted in HBSS, or HBSS only (Naïve control). A higher dose of PV was used because this stock of PV does not replicate as well in mice as LCMV. After at least 6 weeks the mice were given heterologous challenges with similar doses of LCMV or PV. Four days after challenge spleens were harvested, and spleen suspensions were analyzed for PFU. Data presented are the means ± standard deviation, with the number of mice per group in parentheses. Experiments were designed with n=4-5 per group. In three experiments (#3, 7, and 12) there was unexpected death of some mice prior to challenge. In this table we decided to present all the data we had rather than to delete those experiments. Replication of Virus in the Spleen of Naïve or Immune Mice
| Expt. | Mouse | (MHC) | Naïve/PV | LCMV-imm/PV | P |
|---|---|---|---|---|---|
| 1. | C57BL/6 | (b) | 2.9±0.2 (5) | 1.4±0.7 (4) | .002 |
| 2. | B10 | (b) | 3.3±0.5 (5 ) | 1.5±0.7 (5) | .002 |
| 3. | B10 | (b) | 3.8±0.3 (2) | 1.9±0.7 (4) | .025 |
| 4. | CBA | (k) | 5.4±.04 (5) | 5.5±0.2 (5) | .30 (NS) |
| 5. | CBA | (k) | 5.8±0.5 (5) | 5.2±0.8 (5) | .60 (NS) |
| 6. | B10.BR | (k) | 3.7±0.6 (4) | 3.2±0.5 (4) | .25 (NS) |
| 7. | B10.BR | (k) | 3.5±0.6 (2) | 3.7±0.5 (4) | .68 (NS) |
| 8. | B10.BR | (k) | 3.8±0.5 (5) | 3.7±0.4 (5) | .35 (NS) |
| 9. | BALB/c | (d) | 5.6±0.4 (4) | 5.1±0.2 (4) | .067 |
| 10. | BALB/c | (d) | 5.6±0.2 (4) | 5.3±0.1 (4) | .036 |
| 11. | B10.D2 | (d) | 3.3±0.6 (5) | 2.9±1.1 (5) | .49 (NS) |
| 12. | B10.D2 | (d) | 4.5±0.5 (2) | 3.4±1.3 (4) | .33 (NS) |
| 13. | B10.D2 | (d) | 4.2±0.5 (5) | 3.1±0.3 (4) | .04 |
| Naïve/LCMV PV-imm/LCMV | |||||
| 14. | BALB/c | (d) | 4.5±0.63 (4) | 3.8±0.3 (4) | .08 |
| 15. | BALB/c | (d) | 5.1±0.02 (4) | 4.0±0.7 (4) | .02 |
| 16. | CBA | (k) | 4.6±.05 (5) | 4.4±.05 (5) | .0002 |
ACKNOWLEDGMENTS
These studies were supported by U.S. P.H.S. National Institutes of Health grants AI 081675 and AI 046629. The opinions expressed are those of the authors and not necessarily those of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Berg RE, Cordes CJ, Forman J. Contribution of CD8+ T cells to innate immunity: IFN-gamma secretion induced by IL-12 and IL-18. Eur.J.Immunol. 2002;32:2807–2816. doi: 10.1002/1521-4141(2002010)32:10<2807::AID-IMMU2807>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat.Immunol. 2002;3:627–634. doi: 10.1038/ni806. [DOI] [PubMed] [Google Scholar]
- Chen AT, Cornberg M, Gras S, Guillonneau C, Rossjohn J, Trees A, Emonet S, de la Torre JC, Welsh RM, Selin LK. Loss of anti-viral immunity by infection with a virus encoding a cross-reactive pathogenic epitope. PLoS.Pathog. 2012;8:e1002633. doi: 10.1371/journal.ppat.1002633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HD, Fraire AE, Joris I, Brehm MA, Welsh RM, Selin LK. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat.Immunol. 2001;2:1067–1076. doi: 10.1038/ni727. [DOI] [PubMed] [Google Scholar]
- Chu T, Tyznik AJ, Roepke S, Berkley AM, Woodward-Davis A, Pattacini L, Bevan MJ, Zehn D, Prlic M. Bystander-activated memory CD8 T cells control early pathogen load in an innate-like, NKG2D-dependent manner. Cell Rep. 2013;3:701–708. doi: 10.1016/j.celrep.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornberg M, Chen AT, Wilkinson LA, Brehm MA, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Welsh RM, Selin LK. Narrowed TCR repertoire and viral escape as a consequence of heterologous immunity. J Clin.Invest. 2006;116:1443–1456. doi: 10.1172/JCI27804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornberg M, Sheridan BS, Saccoccio FM, Brehm MA, Selin LK. Protection against vaccinia virus challenge by CD8 memory T cells resolved by molecular mimicry. J Virol. 2007;81:934–944. doi: 10.1128/JVI.01280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson B, Germano S, Steele P, Turner S, Fazekas de St GB, Cheers C. Bystander activation of CD8+ T lymphocytes during experimental mycobacterial infection. Infect.Immun. 2004;72:6884–6891. doi: 10.1128/IAI.72.12.6884-6891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerman JA, Ogasawara K, Lanier LL. Cutting edge: Toll-like receptor signaling in macrophages induces ligands for the NKG2D receptor. J.Immunol. 2004;172:2001–2005. doi: 10.4049/jimmunol.172.4.2001. [DOI] [PubMed] [Google Scholar]
- Kim SK, Cornberg M, Wang XZ, Chen HD, Selin LK, Welsh RM. Private specificities of CD8 T cell responses control patterns of heterologous immunity. J Exp Med. 2005;201:523–533. doi: 10.1084/jem.20041337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall HD, Prince AL, Berg LJ, Welsh RM. IFN-alpha beta and self-MHC divert CD8 T cells into a distinct differentiation pathway characterized by rapid acquisition of effector functions. J.Immunol. 2010;185:1419–1428. doi: 10.4049/jimmunol.1001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathurin KS, Martens GW, Kornfeld H, Welsh RM. CD4 T-cell-mediated heterologous immunity between mycobacteria and poxviruses. J.Virol. 2009;83:3528–3539. doi: 10.1128/JVI.02393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters CJ, Buchmeier M, Rollin PE, Ksiazek TG. Arenaviruses. In: Fields BN, Knipe BN, Howley PM, editors. Fields Virology. Lippincott-Raven; Philadelphia, pp: 1996. pp. 1521–1551. [Google Scholar]
- Raue HP, Brien JD, Hammarlund E, Slifka MK. Activation of virus-specific CD8+ T cells by lipopolysaccharide-induced IL-12 and IL-18. J Immunol. 2004;173:6873–6881. doi: 10.4049/jimmunol.173.11.6873. [DOI] [PubMed] [Google Scholar]
- Rodriguez F, Slifka MK, Harkins S, Whitton JL. Two overlapping subdominant epitopes identified by DNA immunization induce protective CD8(+) T-cell populations with differing cytolytic activities. J Virol. 2001;75:7399–7409. doi: 10.1128/JVI.75.16.7399-7409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selin LK, Varga SM, Wong IC, Welsh RM. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J.Exp.Med. 1998;188:1705–1715. doi: 10.1084/jem.188.9.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Most RG, Sette A, Oseroff C, Alexander J, Murali-Krishna K, Lau LL, Southwood S, Sidney J, Chesnut RW, Matloubian M, Ahmed R. Analysis of cytotoxic T cell responses to dominant and subdominant epitopes during acute and chronic lymphocytic choriomeningitis virus infection. J.Immunol. 1996;157:5543–5554. [PubMed] [Google Scholar]
- Welsh RM, Che JW, Brehm MA, Selin LK. Heterologous immunity between viruses. Immunol.Rev. 2010;235:244–266. doi: 10.1111/j.0105-2896.2010.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RM, Selin LK. No one is naive: the significance of heterologous T-cell immunity. Nat Rev Immunol JID - 101124169. 2002;2:417–426. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]