Abstract
Background
Animal studies have linked in utero vitamin D exposure to various aspects of offspring brain development. Limited research has translated these findings to humans, and none have employed cord blood to measure exposure late in gestation.
Methods
Our objective was to examine associations between maternal 25(OH)D measured at ≤26 weeks’ gestation or cord blood 25(OH)D and offspring global development, IQ, achievement, and behavior in the U.S. Collaborative Perinatal Project (1959–73). This was a secondary analysis of data from a case-cohort study, with 3896 women and children who participated in at least one outcome assessment. Psychologists assessed global development at eight months, IQ and behavior at four and seven years, and achievement at seven years. Multiple linear and logistic regression was used to examine associations between 25(OH)D and child outcomes, controlling for maternal education, age, parity, race, maternal BMI, marital status, smoking, gestational age and month of blood draw, and study site.
Results
Positive associations for many outcomes were greatly attenuated upon adjustment for confounders and were generally null. Only IQ at age 7 was associated with both maternal and cord blood 25(OH)D, but the effect estimates were very small (β for 5 nmol/L increment of maternal 25(OH)D=0.10; 95% CI: 0.00, 0.19).
Conclusions
We observed very little indication that maternal or cord blood 25(OH)D are associated with cognitive development, achievement and behavior between 8 months and 7 years of age.
Reduced sunlight exposure and low dietary intake contribute to poor vitamin D status among pregnant women in the U.S.1, 2 Maternal vitamin D insufficiency has been associated with poor fetal growth3 and offspring skeletal development.4 Vitamin D may also be important in fetal brain development by supporting neuronal differentiation and maturation, synthesis of neurotransmitters, and regulation of damaging reactive oxygen species, among other functions based on the results of animal studies.5–10
Limited research has examined associations between in utero vitamin D exposure and brain development, and most studies have relied on season of birth as a proxy for vitamin D because of the strong seasonal variation of vitamin D status.11 However, serum 25(OH)-vitamin D (25(OH)D) is a measure that integrates vitamin D synthesized in the skin from sunlight exposure as well as vitamin D taken in through diet and supplements, and therefore is the best marker of vitamin D nutritional status.2 Maternal 25(OH)D diffuses across the placental barrier during pregnancy.12 Cord blood 25(OH)D concentrations are 75–90% of maternal concentrations at delivery.12, 13 In fact, the fetus relies entirely on the vitamin D stores/intakes of the mother; if the mother is deficient, so is the fetus.14 However, previous studies of in utero vitamin D have relied on measurement of maternal 25(OH)D and not cord blood which may be a better measure of fetal 25(OH)D status in late gestation. Cord blood 25(OH)D may also be more relevant to pediatric outcomes.
Several rodent studies have observed abnormal behaviors in offspring exposed to low maternal 25(OH)D,10, 15 and three human studies have examined various cognitive or behavioral outcomes in relation to maternal 25(OH)D.16–18 Gale et al. measured third trimester 25(OH)D and IQ and parent-reported behavior at age nine in 178 offspring and found no association with IQ or with behavior problems with the exception of fewer peer problems with increasing maternal 25(OH)D.16 An Australian study of vitamin D status at 18 weeks’ gestation and parent-reported child behavior and receptive vocabulary at various ages 2–18 years (n=412–592) observed no association with internalizing or externalizing behavior at any age, but found the odds of language impairment to be almost twice as high for the children with maternal 25(OH)D in the lowest quartile (15–46 nmol/L) compared to the highest quartile (72–154 nmol/L).17 Loss to follow-up resulted in reduced sample sizes in these studies which may have limited the ability to detect subtle associations. Also, relying only on parent reports of behavior may be an incomplete assessment. Morales et al. observed positive linear associations between maternal 25(OH)D usually measured during the second trimester and mental and psychomotor development in 1820 infants at 14 months of age but did not report on later outcomes.18 Differences in the timing of blood collection and variation in outcome measures may partly explain the heterogeneity of previous results.
Even if vitamin D insufficiency during pregnancy has only subtle effects on fetal brain development, the potential impact at the population level is great because 75% of black women and 49% of white women have 25(OH)D <50 nmol/L—concentrations that may be suboptimal1—and differences of a few IQ points are meaningful for long-term educational outcomes.19 Our objective was to examine associations between maternal 25(OH)D measured at ≤26 weeks’ gestation or cord blood 25(OH)D and psychologist-assessed global infant development assessed at eight months of age, IQ and behavior at ages four and seven years, and achievement at seven years. We carried out this study using data collected as part of the U.S. Collaborative Perinatal Project (CPP), a large and racially diverse cohort of pregnant women and their offspring born in 1959–66.
METHODS
Study Population
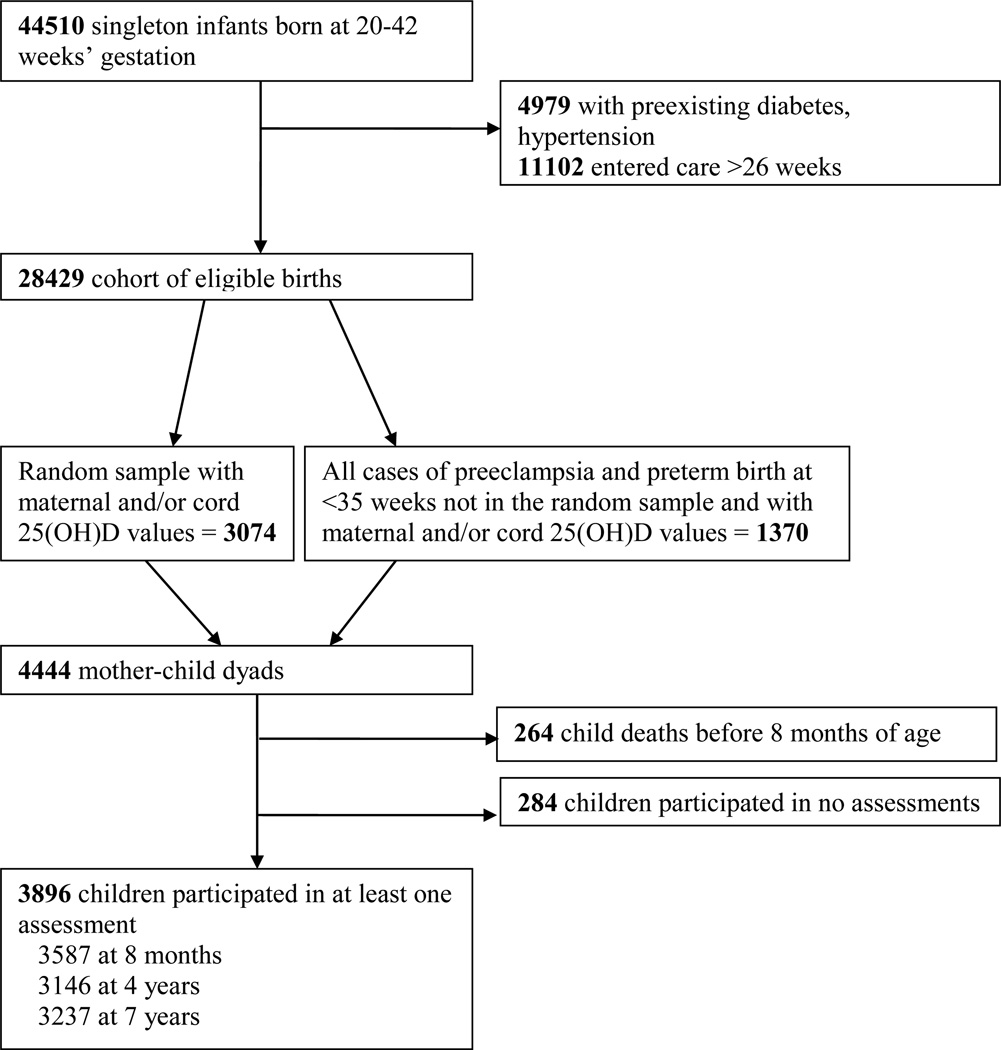
The CPP enrolled women (55,000 pregnancies) at the first prenatal visit at 12 US sites (1959 –1965).20 Women were followed prospectively through delivery, and their offspring were followed to age seven. A recent secondary analysis was carried out to study vitamin D and pregnancy outcomes (see Supporting Information Figure 1). This analysis randomly sampled 3074 pregnancies from the total 28,429 that met the following criteria: singleton gestation; white, black, or Puerto Rican maternal race/ethnicity; no preexisting diabetes or hypertension; entry to prenatal care at ≤26 weeks; available stored serum sample collected at ≤26 weeks; and gestational age at delivery of 20–42 weeks. That sample of 3074 was augmented with all eligible cases of preeclampsia and preterm birth <35 weeks (totaling n=1370 cases) for a total sample size of 4444.21, 22 Of the 4444 selected, 4371 had maternal 25(OH)D values, 2123 had cord blood 25(OH)D values, and 2050 had both. From these 4444 pregnancies, 4180 offspring lived beyond the neonatal period, but 284 children failed to participate in any assessments, leaving the final sample of 3896 mother-child dyads with at least one 25(OH)D value and including a child who participated in at least one development or behavior assessment at eight months or four or seven years (3825 with maternal 25(OH)D, 1997 with cord blood, 1926 with both).
Figure 1.
Selection of Study Participants, U.S. Collaborative Perinatal Project (1959–73)
Vitamin D
Non-fasting blood samples were collected from mothers at enrollment and every eight weeks thereafter, and cord blood was collected at delivery. Maternal and cord serum samples were stored in glass at −20°C with no recorded thaws. From each woman, we randomly selected one banked serum sample drawn at ≤26 weeks. A DEQAS (Vitamin D External Quality Assessment Scheme)-proficient laboratory was used to assay the maternal and cord serum for total 25(OH)D [25(OH)D2 + 25(OH)D3] using liquid-chromatography-tandem mass spectrometry according to the requirements of the National Institute of Standards and Technology.23 The intra-assay coefficient of variation was 6.0%. Serum 25(OH)D is extremely stable at −20°C for years and not sensitive to exposure to UV light or repeated freezing and thawing.24 In a pilot study comparing 25(OH)D in CPP serum with serum frozen for ≤ two years, we found that 25(OH)D in the CPP samples is unlikely to show significant degradation.25
Outcome variables
Child development and behavior was assessed at eight months and four and seven years of age by trained psychologists during in-person study visits. The measures and time points for these assessments were chosen by the original CPP investigators to study outcomes including cerebral palsy and intellectual disability.
Child cognitive development and achievement
The eight-month assessment included the Bayley Scales of Mental and Motor Development to measure global infant development.26, 27 IQ was estimated at ages four and seven using the Stanford-Binet Intelligence Scale and the Wechsler Intelligence Scale for Children (WISC), respectively.28, 29 The Wide Range Achievement Test (WRAT) was used to measure student achievement in arithmetic, reading and spelling at age seven.30 We converted raw IQ and WRAT scores to continuous standard scaled scores based on published norms.28–30
Child behavior
At the conclusion of the lengthy ages four and seven assessments, psychologists completed a series of standardized behavioral symptom ratings based on child behavior during the study visit. This rating scheme has been validated in several ways previously. Teachers rated a sub-sample of CPP children using the same rating system for the externalizing behaviors. High concordance between psychologist and teacher ratings was observed.31 The Berkeley Growth Study used a version of these scales and found them to be reliable (reliability coefficients ranged 0.68–0.90 for the age 7 visit).32
Donatelli et al. previously used these data to identify specific, clinically-relevant problem behaviors in the CPP cohort. They used principal components analysis to identify behavioral symptoms that cluster together into specific problem behaviors and developed five scales (Table 1).33 As per Donatelli et al., we classified children as to whether they exhibited each component behavior (yes/no) and then each item was summed to generate a score. Also per Donatelli et al., we formed five binary outcome variables by assigning children scoring greater than one SD above the mean for the scale as having that behavior problem; children with lower scores were classified as having no problem in that domain.
Table 1.
Behavior Scales and Component Rating Itemsa, U.S. Collaborative Perinatal Project (1959–73)
| Age 4 | Age 7 | |||
|---|---|---|---|---|
| Internalizing behaviors | Hyperactive behaviors | Oppositional | Internalizing behaviors | Externalizing behaviors |
| Flat affect | Brief attention span | Unstable emotional response | Shy/withdrawn | Negative/resistive |
| Phlegmatic | No or brief effort toward goal | Irritable | Flat affect | Assertive/willful |
| Passive | Overactive | Negative/resistive | Inactive | Hostile |
| Rigid | Impulsive/uncontrolled behavior | Demands a lot of attention | Little or no communication | Unstable emotional response |
| Little or no communication | Unwilling to follow directions | Fearful/apprehensive | Acts out/becomes upset | |
| Passive | Impulsive/uncontrolled behavior | |||
Developed by Donatelli et al (28)
Covariates
Interviewers recorded demographic and health information at the first prenatal visit, including maternal years of education, age, parity (continuous), race/ethnicity (White, Black, Puerto Rican), marital status (married vs unmarried), and current smoking status at the time of enrollment (yes or no). Maternal pre-pregnancy body mass index (BMI)(kg/m2) was calculated from measured height and self-reported weight. Gestational age of the offspring at delivery was based on last menstrual period. Study site (a proxy for latitude and other possible subtle differences by city) was recorded for each pregnancy. Dates when blood was drawn were categorized into months, and trimester of blood draw was calculated from the date of the last menstrual period. A non-verbal IQ test (Scientific Research Associates) was administered to a sub-sample of 45% of women when their child was age four.34
Statistical Analysis
We used both continuous (in 5 nmol/L increments) and categorical measures of 25(OH)D in the analyses. Categories were set at <25, 25–<50, 50–<75, and ≥75 nmol/L to be relevant to existing clinical recommendations for vitamin D status. We employed χ2 tests and linear regression to examine the relationships between measures of 25(OH)D and each covariate. To explore potential non-linear associations between 25(OH)D (continuous) and each outcome, we used a published SAS macro for restricted cubic splines to model the associations.35 For associations found to be linear, we used simple and multivariable linear and logistic regression. A set of possible confounders was identified a priori based on previous studies and refined based on relationships we hypothesized to exist with both exposure and outcomes. Adjusted models included maternal education, age, parity, race, maternal pre-pregnancy BMI, marital status, smoking, gestational age and month of blood draw, and study site. In a second set of models we included maternal IQ to explore the potential for confounding in the sub-sample of those with non-missing maternal IQ. Also, based on our previous research, we considered preeclampsia and spontaneous preterm birth to be on the causal path between maternal 25(OH)D and child outcomes.21, 22 However, as 25(OH)D may act both through pregnancy complications as well as through other pathways, we built a third set of models restricted to term births without preeclampsia. Finally, we built models including maternal IQ and restricted to term births without preeclampsia. We used complete case analysis throughout. To assess how children evaluated for outcomes differed from those who were not evaluated, we used t and χ2 tests to examine whether loss to follow-up at age 7 was differential by exposures and outcomes at 8 months or 4 years.
Analyses were carried out using SAS 9.3 and STATA 12.36, 37 The study used de-identified, publicly-available data and was therefore exempt from human subjects review.
RESULTS
The median maternal 25(OH)D was 45 nmol/L (inter-quartile range (IQR)=34); median cord blood 25(OH)D was 32nmol/L (IQR=28). Maternal and cord blood 25(OH) concentrations were moderately correlated (Pearson r=0.26, p<0.01); correlations increased as the week of gestation of the maternal 25(OH)D measurement increased. Maternal blood collection occurred at a median 21 weeks of pregnancy (IQR=7.3, range 2–26). Standard outcome scores were distributed as expected with the exception of slightly lower means for all measures, reflecting the below average socioeconomic circumstances of CPP participants. In bivariate analyses, maternal 25(OH)D concentration was positively associated with years of maternal education, maternal age, lower parity, white race, lower pre-pregnancy BMI, being married, gestational age at delivery, and trimester and month of blood collection, but they were unassociated with pre-eclampsia (Table 2).
Table 2.
Characteristics of Mothers and Children by Categories of Maternal 25(OH)-vitamin D (n=3,825), U.S. Collaborative Perinatal Project (1959–73)
| Categories of maternal 25(OH)-vitamin D (nmol/l) | ||||
|---|---|---|---|---|
| Category 1 (<25) |
Category 2 (25–<50) |
Category 3 (50–<75) |
Category 4 (≥75) |
|
| 25(OH)-vitamin D concentration (nmol/l) (mean, SD) | 18 (5) | 37 (7) | 61 (7) | 98 (23) |
| n (%) | ||||
| Maternal educationa - <12 years | 436 (67.9) | 950 (60.3) | 491 (50.0) | 262 (44.9) |
| 12 years | 169 (26.3) | 490 (31.1) | 349 (35.5) | 206 (35.3) |
| ≥13 years | 37 (5.8) | 135 (8.6) | 142 (14.5) | 115 (19.7) |
| Maternal agea - <20 | 215 (33.3) | 485 (30.4) | 240 (24.0) | 128 (21.8) |
| 20–29 | 323 (50.2) | 855 (53.6) | 591 (59.2) | 357 (60.9) |
| 30–34 | 64 (9.9) | 159 (10.0) | 111 (11.1) | 61 (10.4) |
| ≥35 | 42 (6.5) | 97 (6.1) | 57 (5.7) | 40 (6.8) |
| Parityb - 0 | 233 (36.3) | 557 (34.9) | 386 (38.7) | 208 (35.6) |
| 1 | 127 (19.8) | 367 (23.0) | 238 (23.9) | 152 (26.0) |
| ≥2 | 282 (43.9) | 671 (42.1) | 373 (37.4) | 225 (38.5) |
| Race and ethnicitya – Black | 481 (74.7) | 909 (57.0) | 361 (36.1) | 152 (25.9) |
| White | 127 (19.7) | 559 (35.0) | 569 (57.0) | 398 (67.9) |
| Puerto Rican | 36 (5.6) | 128 (8.0) | 69 (6.9) | 36 (6.1) |
| Maternal pre-pregnancy BMIb - Underweight (<18.5) | 61 (10.1) | 180 (11.9) | 96 (10.5) | 60 (11.0) |
| Normal weight (18.5–24.9) | 414 (68.4) | 1037 (68.3) | 676 (73.6) | 402 (73.9) |
| Overweight (25.0–29.9) | 92 (15.2) | 226 (14.9) | 111 (12.1) | 59 (10.9) |
| Obese (≥30) | 38 (6.3) | 76 (5.0) | 36 (3.9) | 23 (4.2) |
| Marital status – marrieda | 439 (68.2) | 1225 (76.8) | 835 (83.6) | 508 (86.7) |
| Smoker - yes | 323 (50.5) | 753 (47.3) | 459 (46.2) | 252 (43.0) |
| Gestational age at birtha,c - <32 weeks’ | 43 (6.7) | 106 (6.6) | 53 (5.3) | 23 (3.9) |
| 32–36 | 192 (29.8) | 437 (27.4) | 239 (23.9) | 139 (23.7) |
| 37–40 | 260 (40.4) | 619 (38.8) | 411 (41.1) | 235 (40.1) |
| ≥41 | 149 (23.1) | 434 (27.2) | 296 (29.6) | 189 (32.3) |
| Trimester of blood collectiona – 1st trimester | 88 (13.7) | 231 (14.5) | 167 (16.7) | 67 (11.4) |
| 2nd trimester | 556 (86.3) | 1365 (85.5) | 832 (83.3) | 519 (88.6) |
| Season of blood collectiona – Winter | 215 (33.4) | 401 (25.1) | 190 (19.0) | 79 (13.5) |
| Spring | 241 (37.4) | 432 (27.1) | 226 (22.6) | 81 (13.8) |
| Summer | 100 (15.5) | 395 (24.8) | 295 (29.5) | 218 (37.2) |
| Fall | 88 (13.7) | 368 (23.1) | 288 (28.8) | 208 (35.5) |
| Maternal pre-eclampsia | 108 (16.8) | 271 (17.0) | 154 (15.4) | 98 (16.8) |
43 women were missing data for education, 6 for parity, 238 for BMI, 14 for smoking, 4 for pre-eclampsia.
χ2 p-value <0.01,
p<0.05
median gestational age was 39 weeks (inter-quartile range=5)
Children who participated at age 7 had Bayley mental scores 0.8-points higher (p=0.03) and were less likely to show internalizing problems at age 4 (OR=0.63, 95% CI: 0.45, 0.87) compared to those who were lost to followup by age 7. However, they did not differ on other outcomes at ages 8 months and 4 years and did not differ on mean 25(OH)D.
Associations between maternal 25(OH)D and outcomes
For the cognitive development and achievement scales, unadjusted analyses indicated positive associations between maternal 25(OH)D and IQ at ages four and seven, and arithmetic, reading, and spelling achievement at age seven (Tables 3, 4). Of the behavior scales, maternal 25(OH)D concentration was associated only with a lower odds of internalizing problems at age 4 and only for the highest category of 25(OH)D. Upon adjustment for maternal education, age, parity, race, maternal BMI, marital status, smoking, gestational age and month of blood draw, and study site, maternal 25(OH)D concentration was very modestly associated with child IQ at age 7 and no other outcomes. No associations were non-linear based on Wald χ2 tests (p<0.05).
Table 3.
Mean (SD) Scores on Cognitive Assessments and the Number (%) of Children with Behavioral Problems by Categories of Maternal 25(OH)-vitamin D (n=3825), U.S. Collaborative Perinatal Project (1959–73)
| Categories of maternal 25(OH)-vitamin D (nmol/l) | ||||
|---|---|---|---|---|
| Category 1 (<25) |
Category 2 (25–<50) |
Category 3 (50–<75) |
Category 4 (≥75) |
|
| mean (SD) | ||||
| Cognitive Development and Achievement | ||||
| 8 months | ||||
| Bayley Mental Score | 78.1 (8.5) | 78.6 (7.6) | 79.1 (6.2) | 78.7 (7.2) |
| Bayley Motor Score | 32.5 (5.3) | 32.4 (5.3) | 32.7 (5.1) | 32.6 (5.6) |
| 4 years | ||||
| Stanford-Binet IQa | 92.2 (15.8) | 95.1 (16.7) | 98.7 (17.7) | 100.2 (17.6) |
| 7 years | ||||
| WISC IQ | 90.5 (15.0) | 94.2 (14.9) | 98.3 (14.9) | 100.1 (17.0) |
| WRAT Arithmetic | 94.3 (12.1) | 95.9 (11.6) | 97.3 (11.0) | 97.7 (11.1) |
| WRAT Reading | 95.9 (14.7) | 98.2 (16.0) | 101.1 (17.0) | 100.9 (17.1) |
| WRAT Spelling | 93.2 (13.6) | 95.5 (13.4) | 98.1 (14.2) | 98.2 (14.0) |
| n (%) | ||||
| Behavior Scales | ||||
| 4 years | ||||
| Internalizing behaviors | 97 (18.6) | 248 (19.6) | 141 (18.0) | 61 (13.2) |
| Hyperactive behaviors | 119 (22.8) | 272 (21.5) | 147 (18.7) | 103 (22.2) |
| Oppositional | 73 (14.0) | 178 (14.1) | 101 (12.8) | 72 (15.4) |
| 7 years | ||||
| Internalizing behaviors | 77 (14.7) | 181 (14.0) | 119 (14.8) | 75 (15.0) |
| Externalizing behaviors | 82 (15.6) | 188 (14.6) | 125 (15.5) | 84 (17.0) |
Table 4.
Associations between Maternal 25(OH)-vitamin D and Cognitive Assessment Scores and Behavioral Problems (n=3825), U.S. Collaborative Perinatal Project (1959–73)
| Maternal 25(OH)-vitamin D (nmol/l) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Continuous measure (in 5 nmol/L increments) |
Categorical measure (reference=Category 1, <25) | |||||||
| Category 2 (25–<50) | Category 3 (50–<75) | Category 4 (≥75) | ||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Cognitive Development and Achievement | β [95% CI] | |||||||
| 8 months | ||||||||
| Bayley Mental Score (n=3308) | 0.04[−0.00, 0.08] | 0.02 [−0.03, 0.07] | 0.42 [−0.29, 1.12] | 0.35 [−0.36, 1.05] | 0.94 [0.17, 1.70] | 0.66 [−0.14, 1.47] | 0.54 [−0.32, 1.40] | 0.17 [−0.78, 1.12] |
| Bayley Motor Score (n=3311) | 0.02[−0.01, 0.05] | 0.01 [−0.03, 0.04] | −0.06 [−0.56, 0.46] | −0.14[−0.65, 0.36] | 0.27 [−0.27, 0.82] | −0.04[−0.62, 0.54] | 0.14 [−0.48, 0.76] | −0.09 [−0.77, 0.59] |
| 4 years | ||||||||
| Stanford-Binet IQ (n=2893) | 0.51 [0.40, 0.62] | 0.07 [−0.05, 0.18] | 2.87 [1.14, 4.60] | −0.17[−1.70, 1.36] | 6.44 [4.57, 8.31] | −0.11[−1.88, 1.67] | 8.00 [5.89, 10.11] | 0.22 [−1.85, 2.29] |
| 7 years | ||||||||
| WISC IQ (n=2987) | 0.57 [0.47, 0.66] | 0.10 [0.00, 0.19] | 3.72 [2.18, 5.26] | 0.67 [−0.63, 1.97] | 7.84 [6.17, 9.50] | 1.14 [−0.37, 2.65] | 9.65 [7.79, 11.51] | 1.50 [−0.26, 3.27] |
| WRAT Arithmetic (n=2968) | 0.17 [0.10, 0.24] | −0.01 [−0.09, 0.07] | 1.57 [0.41, 2.73] | 0.55 [−0.54, 1.64] | 2.97 [1.71, 4.22] | 0.45 [−0.81, 1.72] | 3.39 [1.99, 4.80] | 0.67 [−0.81, 2.16] |
| WRAT Reading (n=2968) | 0.28 [0.18, 0.39] | −0.04 [−0.15, 0.07] | 2.38 [0.73, 4.02] | 0.78 [−0.71, 2.27] | 5.19 [3.41, 6.97] | 0.92 [−0.82, 2.65] | 4.94 [2.95, 6.92] | −0.11 [−2.14, 1.92] |
| WRAT Spelling (n=2964) | 0.30 [0.22, 0.39] | 0.05 [−0.05, 0.15] | 2.23 [0.84, 3.62] | 0.82 [−0.48, 2.12] | 4.90 [3.39, 6.41] | 1.27 [−0.24, 2.80] | 4.92 [3.24, 6.61] | 0.77 [−0.99, 2.54] |
| Behavior Scales | OR [95% CI] | |||||||
| 4 years | ||||||||
| Internalizing behaviors (n=2825) | 0.98 [0.96, 1.00] | 0.98 [0.96, 1.01] | 1.07 [0.82, 1.39] | 1.01 [0.76, 1.34] | 0.96 [0.72, 1.28] | 0.94 [0.67, 1.31] | 0.66 [0.47, 0.94] | 0.70 [0.47, 1.06] |
| Hyperactive behaviors (n=2829) | 0.99 [0.98, 1.01] | 1.00 [0.98, 1.02] | 0.93 [0.73, 1.18] | 1.03 [0.78, 1.34] | 0.78 [0.59, 1.02] | 0.96 [0.70, 1.32] | 0.97 [0.72, 1.31] | 1.17 [0.82, 1.69] |
| Oppositional (n=2827) | 1.01 [0.99, 1.03] | 1.00 [0.98, 1.03] | 1.00 [0.75, 1.35] | 0.99 [0.73, 1.39] | 0.90 [0.65, 1.24] | 0.89 [0.62, 1.29] | 1.11 [0.78, 1.58] | 0.99 [0.65, 1.51] |
| 7 years | ||||||||
| Internalizing behaviors (n=2903) | 1.00 [0.98, 1.02] | 1.01 [0.99, 1.04] | 0.95 [0.71, 1.27] | 0.99 [0.72, 1.36] | 1.01 [0.74, 1.38] | 1.16 [0.80, 1.66] | 1.03 [0.73, 1.45] | 1.27 [0.83, 1.93] |
| Externalizing behaviors (n=2896) | 1.00 [0.99, 1.02] | 1.00 [0.98, 1.02] | 0.93 [0.70, 1.23] | 1.04 [0.76, 1.42] | 1.00 [0.74, 1.35] | 1.15 [0.80, 1.64] | 1.11 [0.80, 1.55] | 1.23 [0.82, 1.85] |
Adjusted for maternal education, age, parity, race, maternal BMI, marital status, smoking, gestational age and month of blood draw, and study site.
We included maternal IQ in models for about one-half the cohort that had non-missing maternal IQ and observed no associations with the exception of a small negative association with WRAT Reading (β=−0.16, 95% CI: −0.32, −0.00)(Supplemental Table 1). Maternal IQ and child IQ were correlated (Pearson r for Stanford-Binet IQ=0.35, p<0.01; for WISC IQ=0.41, p<0.01). We also built adjusted models that were restricted to term births unaffected by preeclampsia (both without and with maternal IQ included as a confounder) to compare to the results in Table 4 to evaluate associations in uncomplicated pregnancies. All associations were null except for the model including maternal IQ and restricted to term, non-preeclamptic births where increasing 25(OH)D was associated with a slightly lower odds of internalizing behaviors (OR=0.95, 95% CI: 0.91, 1.00).
We decided a priori to test two interactions, in the adjusted models in Table 4. First, we tested the interaction between a variable indicating which trimester blood was drawn and 25(OH)D using an interaction term to explore whether any associations were specific to the first or second trimester given the conflicting results in prior studies and found no interaction. Based on our previous work with this cohort which observed an interaction with race, we tested the interaction between race and 25(OH)D concentrations and observed no interaction.21
Associations between cord blood 25(OH)D and outcomes
Cord blood 25(OH)D concentration was positively associated with IQ at ages four and seven and arithmetic, reading, and spelling achievement at age seven, but unassociated with Bayley scores or behavior scales (Supplemental Tables 2, 3). Adjusted models included maternal education, age, parity, race, maternal BMI, marital status, smoking, gestational age and month of blood draw, and study site. Upon adjustment, each 5nmol/L increment increase in cord blood 25(OH)D was associated with a small increase in WISC scores (β=0.16, 95% CI: 0.03,0.29); this was most apparent for the highest 25(OH)D category compared to the lowest (β=2.54, 95% CI: 0.07, 5.01). (Supplemental Table 3). No associations were non-linear based on Wald χ2 tests (p<0.05). Inclusion of maternal IQ resulted in a similar result for WISC IQ as in Supplemental Table 3 (Supplemental Table 4). This association was no longer apparent in models restricted to term pregnancies without preeclampsia, but a positive association was observed for Bayley motor scores (β=0.09, 95% CI: 0.01, 0.17).
COMMENTS
In this large prospective study of maternal and cord blood 25(OH)D concentrations and offspring cognitive and behavioral outcomes, we observed little indication that maternal or cord blood 25(OH)D is associated with better outcomes. Effect estimates were greatly attenuated upon adjustment, which suggests that variation in choice of confounders may underlie the differences in results across published studies. No associations were consistently observed across measurement time points (ages 4 and 7). Only WISC IQ was very modestly associated with both maternal and cord blood 25(OH)D. Results for models that included maternal IQ and that were restricted to term pregnancies without pre-eclampsia differed slightly from the previous models, but effect estimates were close to the null and should be interpreted with caution as they were based on a much reduced sample size. It is difficult to assess the role of preterm birth or pre-eclampsia in the associations under study because so many of the effect estimates were near the null before adjustment for those factors.
Our results contrast one previous study of early childhood global development in that we observed virtually no association with Bayley scores at 8 months of age, while Morales et al. recently reported positive associations with Bayley mental and psychomotor scores at 14 months.18 The difference in the age at testing may be one reason for the inconsistent results. Gale et al. observed no association with IQ at age 9; we consider our results to be generally consistent with those findings. To our knowledge, ours is the first study to have examined 25(OH)D relative to tests of achievement in children. Our adjusted models indicated virtually no relationship between 25(OH)D and achievement. We had expected to observe an association with reading or spelling achievement, given the findings from the Raine cohort which found low maternal 25(OH)D to be associated with poorer receptive language ability.17
Our findings for behavioral outcomes were fairly consistent with those of Gale et al. and Whitehouse et al. which observed no important associations with offspring behavior. Those studies relied on parent reported behaviors, while the present study used measures from psychologist assessments. Taken together, there is little evidence for protective effects of high 25(OH)D in pregnancy for problem behaviors.
In this study maternal 25(OH)D was measured at ≤26 weeks of pregnancy, most similar to the Raine (18 weeks’ pregnancy) and INMA cohorts (mean=13.5 weeks’ pregnancy) and contrasting the study by Gale et al. (third trimester).16–18 The most active period of fetal brain growth and development lies in the third trimester, but the Raine and INMA cohorts’ findings suggest vitamin D may also exert influence on basic structures forming earlier. While we had access to measures of maternal and fetal vitamin D status from different points in gestation, our findings do not suggest a possible critical window for exposure. Because we did not observe clear differences between the results for maternal and cord blood 25(OH)D, this suggests either may serve as a general, global indicator of vitamin D status.
Animal and in vitro studies indicate a wide variety of potential roles for vitamin D in early brain development including in early neuronal differentiation and protection from inflammatory factors.38 Based on the findings of previous studies, these functions may have more direct relevance to cognitive development than for later behavior, although the precise mechanisms are likely extremely varied and not yet well-characterized.
The size and diversity of the participant sample lend strength to this study. This is the largest study to date on this topic which permitted greater statistical power to detect subtle associations than previous studies, and this is the first major study to include a racially and geographically diverse sample living at U.S. latitudes. Median maternal 25(OH)D was 44 nmol/L in this study, which is lower than the Raine and INMA cohorts and the women in the study by Gale et al. Thus, over half of the women in this study may be considered vitamin D deficient, depending on cutpoint.39 This permitted examination of associations across a wide distribution of vitamin D status, making these results highly applicable to the many women who remain vitamin D deficient today. A wide array of potentially confounding variables was available to include in the analysis, including maternal BMI, smoking, and sociodemographic factors, thereby reducing bias from these factors. Our use of outcome assessments covering numerous developmental and behavioral domains carried out by trained psychologists with high reliability is a major strength of the study. Developmental assessment began at eight months of age which is earlier than previous studies in this area. Our null findings, even for unadjusted models, for infant development are not surprising given the often limited sensitivity and predictive value of very early assessment.40 Our measurement of both maternal and cord blood 25(OH)D using the gold standard method also adds strength to the study in that it permitted rigorous study of vitamin D exposure in pregnancy and the neonatal period. There were no differences in 25(OH)D by follow-up status at age 7. Unless the associations differed between those followed and lost, our results should not be biased.
The generalizability of our results may be limited because these were data from the 1960’s. In addition, our results should be considered in light of possible unmeasured confounding by skin color within the sub-group of black women, as a subtle marker of social class and a plausible common cause of both vitamin D status and the outcomes under study. Another limitation is our use of outcome assessments that have been superseded by more contemporary versions. However, the behavior ratings have been validated against the Child Behavior Checklist,41 and the cognitive development and achievement measures remain in use today in later version. Additionally, we carried out multiple comparisons which may have contributed to our observing a small number of positive, and potentially spurious, associations. However, we elected not to adjust our confidence intervals for multiple comparisons per published recommendations.42 Finally, we could not assess vitamin D status in the children concurrent with the outcome assessments because blood was not collected after the neonatal period, so we could not evaluate the role of child vitamin D status on development and behavior.
In the largest study to date on this topic, we observed no meaningful relationships between neurodevelopment, achievement, or behavior and maternal and cord blood 25(OH)D. Future studies that include important covariates like parental IQ and large sample sizes will help clarify conflicting results reported to date among studies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jill Diesel for data management support, Loïc Desquillbet for the use of his SAS macro and the women and children who participated in the CPP. This work was supported by NIH grant HD R01056999 (PI: Bodnar).
Footnotes
The authors have no conflicts of interest to disclose.
STATEMENT OF AUTHORS’ CONTRIBUTIONS TO MANUSCRIPT
Designed research – SK, MK, LB
Conducted research – SK, MK, LB
Provided essential materials – LB
Analyzed data – SK, MK
Wrote paper – SK, MK, LB
Had primary responsibility for final content – SK
All authors read and approved the final manuscript.
References
- 1.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008;88:1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Gernand AD, Simhan HN, Klebanoff MA, Bodnar LM. Maternal serum 25-hydroxyvitamin D and measures of newborn and placental weight in a U.S. multicenter cohort study. J Clin Endocrinol Metab. 2013;98:398–404. doi: 10.1210/jc.2012-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 5.Garcion E, Thanh XD, Bled F, Teissier E, Dehouck MP, Rigault F, et al. 1,25-Dihydroxyvitamin D3 regulates gamma 1 transpeptidase activity in rat brain. Neurosci Lett. 1996;216:183–186. doi: 10.1016/0304-3940(96)87802-5. [DOI] [PubMed] [Google Scholar]
- 6.Kesby JP, Burne TH, McGrath JJ, Eyles DW. Developmental vitamin D deficiency alters MK 801-induced hyperlocomotion in the adult rat: An animal model of schizophrenia. Biol Psychiatry. 2006;60:591–596. doi: 10.1016/j.biopsych.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 7.Kesby JP, Cui X, O'Loan J, McGrath JJ, Burne TH, Eyles DW. Developmental vitamin D deficiency alters dopamine-mediated behaviors and dopamine transporter function in adult female rats. Psychopharmacology (Berl) 2010;208:159–168. doi: 10.1007/s00213-009-1717-y. [DOI] [PubMed] [Google Scholar]
- 8.Brown J, Bianco JI, McGrath JJ, Eyles DW. 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. 2003;343:139–143. doi: 10.1016/s0304-3940(03)00303-3. [DOI] [PubMed] [Google Scholar]
- 9.Marini F, Bartoccini E, Cascianelli G, Voccoli V, Baviglia MG, Magni MV, et al. Effect of 1alpha,25-dihydroxyvitamin D3 in embryonic hippocampal cells. Hippocampus. 2010;20:696–705. doi: 10.1002/hipo.20670. [DOI] [PubMed] [Google Scholar]
- 10.O'Loan J, Eyles DW, Kesby J, Ko P, McGrath JJ, Burne TH. Vitamin D deficiency during various stages of pregnancy in the rat; its impact on development and behaviour in adult offspring. Psychoneuroendocrinology. 2007;32:227–234. doi: 10.1016/j.psyneuen.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Davies G, Welham J, Chant D, Torrey EF, McGrath J. A systematic review and meta-analysis of Northern Hemisphere season of birth studies in schizophrenia. Schizophr Bull. 2003;29:587–593. doi: 10.1093/oxfordjournals.schbul.a007030. [DOI] [PubMed] [Google Scholar]
- 12.Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: Institute of Medicine, National Academies Press; 2010. [PubMed] [Google Scholar]
- 13.Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137:447–452. doi: 10.1093/jn/137.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollis BW, Pittard WB., 3rd Evaluation of the total fetomaternal vitamin D relationships at term: evidence for racial differences. J Clin Endocrinol Metab. 1984;59:652–657. doi: 10.1210/jcem-59-4-652. [DOI] [PubMed] [Google Scholar]
- 15.Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience. 2003;118:641–653. doi: 10.1016/s0306-4522(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 16.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, et al. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2008;62:68–77. doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Kusel MM, Hart PH. Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics. 2012;129:485–493. doi: 10.1542/peds.2011-2644. [DOI] [PubMed] [Google Scholar]
- 18.Morales E, Guxens M, Llop S, Rodriguez-Bernal CL, Tardon A, Riano I, et al. Circulating 25-hydroxyvitamin D3 in pregnancy and infant neuropsychological development. Pediatrics. 2012;130:e913–e920. doi: 10.1542/peds.2011-3289. [DOI] [PubMed] [Google Scholar]
- 19.Needleman HL, Leviton A, Bellinger D. Lead-associated intellectual deficit. N Engl J Med. 1982;306:367. doi: 10.1056/NEJM198202113060618. [DOI] [PubMed] [Google Scholar]
- 20.Niswander KR, Gordon M. The women and their pregnancies: the Collaborative Perinatal Study of the National Institute of Nueorlogical Diseases and Stroke. Washington, DC: National Institutes of Health; 1972. [Google Scholar]
- 21.Bodnar LM, Klebanoff MA, Gernand AD, Platt RW, Parks WT, Catov JM, et al. Maternal Vitamin D Status and Spontaneous Preterm Birth by Placental Histology in the US Collaborative Perinatal Project. Am J Epidemiol. 2013 doi: 10.1093/aje/kwt237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodnar LM, Simhan HN, Catov JM, Roberts JM, Platt RW, Diesel JC, et al. Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiology. doi: 10.1097/EDE.0000000000000039. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005;90:3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 24.Zerwekh JE. The measurement of vitamin D: analytical aspects. Ann Clin Biochem. 2004;41:272–281. doi: 10.1258/0004563041201464. [DOI] [PubMed] [Google Scholar]
- 25.Bodnar LM, Catov JM, Wisner KL, Klebanoff MA. Racial and seasonal differences in 25-hydroxyvitamin D detected in maternal sera frozen for over 40 years. Br J Nutr. 2009;101:278–284. doi: 10.1017/S0007114508981460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayley N. Mental growth during the first three years: A developmental study of sixty-one children by repeated tests. Genetic Psychology Monographs. 1933;114:1–92. [Google Scholar]
- 27.Bayley N. Manual for the Bayley Scales of Infant Development. New York: Psychological Corporation; 1969. [Google Scholar]
- 28.Terman LM, Merrill MA. Stanford-Binet Intelligence Scale: Manual for the Third Revision Form L-M. Boston: Houghton Mifflin; 1960. [Google Scholar]
- 29.Wechsler D. Wechsler Intelligence Scale for Children. New York, NY: The Psychological Corporation; 1949. [Google Scholar]
- 30.Jastak JF, Jastak SR. The Wide Range Achievement Test Manual. Wilmington, DE: Guidance Associates of Delaware, Inc.; 1965. [Google Scholar]
- 31.Nichols PL, Chen TC. Minimal Brain Dysfunction: A Prospective Study. Hillsdale, NJ: Lawrence Erlbaum Associates; 1981. [Google Scholar]
- 32.Schaefer ES, Bayley N. Maternal Behavior, Child Behavior and Their Intercorrelation. Monographs of the Society for Research in Child Development. 1963;87 [PubMed] [Google Scholar]
- 33.Donatelli JA, Seidman LJ, Goldstein JM, Tsuang MT, Buka SL. Children of parents with affective and nonaffective psychoses: a longitudinal study of behavior problems. Am J Psychiatry. 2010;167:1331–1338. doi: 10.1176/appi.ajp.2010.09020241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Non-verbal IQ Test. Science Research Associates, Inc.; 1947. [Google Scholar]
- 35.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 36.SAS software, v9.3. Cary, NC: SAS Institute Inc.; 2000–2010. [Google Scholar]
- 37.STATA 12.0 Statistics/Data Analysis. College Station, TX: 1985–2011. [Google Scholar]
- 38.Eyles D, Burne T, McGrath J. Vitamin D in fetal brain development. Semin Cell Dev Biol. 2011;22:629–636. doi: 10.1016/j.semcdb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Holick MF, Binkley NC, Bischoff-Ferrari H, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. The Endocrine Society; 2011. [DOI] [PubMed] [Google Scholar]
- 40.Shonkoff JP, Phillips DA, editors. From neurons to neighborhoods: the science of early childhood development. Washington, DC: National Research Council and Institute of Medicine; 2000. [PubMed] [Google Scholar]
- 41.Kubzansky LD, Martin LT, Buka SL. Early manifestations of personality and adult emotional functioning. Emotion. 2004;4:364–377. doi: 10.1037/1528-3542.4.4.364. [DOI] [PubMed] [Google Scholar]
- 42.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.