Abstract
The molecular events underlying the immediate steps of retroviral uncoating, occurring after membrane fusion and leading to the formation of an active reverse transcription complex, are not known. To better understand these processes, we have developed a cell-free system that recapitulates these early steps of retroviral replication by using avian sarcoma and leukosis virus as a model retrovirus. The substrates used in this system are viral particles that are trapped before completing membrane fusion. These virions are induced to fuse out of endosomes and the viral cores are released into solution where they are amenable to biochemical manipulation. This system revealed that membrane fusion is not sufficient to stimulate the formation of a reverse transcription complex. Instead, ATP hydrolysis and cellular factors >5 kDa in size are required. Furthermore, later steps of avian sarcoma and leukosis virus reverse transcription were stimulated by nuclear factors. The cell-free system should now allow for the definition of retroviral uncoating mechanisms and facilitate the identification and characterization of the cellular factors involved.
The first steps of retroviral infection involve virus interaction with specific cell surface receptors followed by the fusion of viral and cell membranes, delivering the viral ribonucleoprotein core particle into the cytoplasm (1–3). The viral core particle is then presumed to undergo an uncoating step leading to the activation of reverse transcription within a reverse transcription complex (RTC) (4, 5). However, the precise steps involved in the formation of the RTC remain unknown.
It has been hypothesized that cellular factors may be involved in the earliest steps of retroviral uncoating (5). Indeed, in the case of HIV-1, the cellular protein cyclophilin A appears to be important (6). Cyclophilin A is incorporated into assembling virions by virtue of binding the capsid (CA) domain of the viral Gag polyprotein (7–9). Cyclosporin A, an immunosuppressant that disrupts the CA-cyclophilin A interaction, inhibits HIV-1 infection at a step before the initiation of reverse transcription (6, 9, 10). Although cyclophilin A is a peptidyl-prolyl cis–trans isomerase, its enzymatic activity is not required for its function during HIV-1 uncoating (11). Instead, cyclophilin A seems to counteract the inhibitory effects of Ref-1, a restriction factor(s) expressed in human cells that would otherwise interfere with the onset of reverse transcription (12).
In addition to cellular factors that are assembled into virions, those present in the target cell may also influence the earliest events in retroviral uncoating (5). In support of this hypothesis, a mutant cell line has been isolated that supports virus–cell membrane fusion but not reverse transcription (13). In addition, certain simian cell lines exhibit a partial, innate resistance to this step of HIV-1 infection (14, 15). This phenomenon, termed Lv-1 restriction, is analogous to Ref-1 restriction in human cells (15, 16). In certain primates, Lv-1 restriction is modulated by cyclophilin A present in the HIV-1 particle (12). Recently, TRIM-5α was identified as a candidate component of Lv-1 restriction (17).
Although cellular factors have been implicated in retroviral uncoating, their role and the precise events surrounding the formation of the RTC remain poorly understood. A major hurdle in efforts to understand these processes has been the lack of a cell-free system that reproduces retroviral fusion, uncoating, and the initiation of reverse transcription in vitro. We report here the development of such a system, which derives from the remarkable stability of avian sarcoma and leukosis virus (ASLV) particles when viral penetration into the cytosol is blocked by treatment with lysosomotropic agents that elevate endosomal pH (18–21). Subgroup A ASLV (ASLV-A) remains highly infectious for many hours when cells that express the glycosylphosphatidylinositol-linked form of the TVA viral receptor are treated with ammonium chloride (20). This feature of ASLV-A sets this virus apart from all other low pH-dependent viruses, which are rapidly degraded and inactivated under similar conditions (22).
We have exploited this unique stability of fusion-arrested ASLV-A to generate transiently trapped virions as substrates for an in vitro retroviral fusion and uncoating system. The block to viral replication is removed by inhibitor withdrawal, and the viral cores are released into solution, where they are amenable to biochemical manipulation. We have used this system to obtain direct evidence that a cellular factor(s) as well as ATP hydrolysis promote early ASLV DNA synthesis and that a nuclear factor(s) stimulates viral late DNA synthesis.
Materials and Methods
Cells, Viruses, and Materials. All chemicals were from Sigma unless otherwise stated. Stock solutions of 25 mM MgCl2,10mM d NTPs (Stratagene), and 500 mM NH4Cl were made and stored at 4°C, -20°C, or prepared fresh for every experiment, respectively. Homogenization buffer (HB, 250 mM sucrose/3 mM imidazole, pH 7.4) was stored at 4°C, and 1× protease inhibitor mixture (Roche Diagnostics) was added just before each use. The construction of human 293(TVA800) cells has been described (20). The subgroup A-specific ASLV vector RCASBP(A)-EGFP virus, encoding the enhanced green fluorescent protein (EGFP), was harvested from extracellular supernatants of chronically infected DF-1 cells as described (23, 24).
Cell-Free Fusion and Uncoating Reactions. 293(TVA800) cells were challenged with the RCASBP(A)-EGFP vector at the indicated multiplicities of infection (MOIs) in the presence of 30 mM NH4Cl as described (20). Infection was initiated for 6 h by a temperature shift from 4°C to 37°C. The cells were then dislodged from the plate by repeated pipetting and centrifuged at 1,000 × g for 5 min at 4°C. Approaches previously used to establish cell-free systems for endosome-endosome fusion (25–27) were adapted to establish the virus fusion/uncoating assay. Specifically, the cell pellet was resuspended at a concentration of 1.67 × 107 cells per ml in HB containing 30 mM NH4Cl at 4°C. Approximately 1.25 × 107 cells were then passed eight times through a prechilled ball-bearing cell cracker [10-μm clearance, European Molecular Biology Laboratory, as has been described (26, 27)] by using 1-ml syringes. These conditions routinely allowed for >80% of the cells being broken with minimal nuclear lysis as judged by light microscopy as described (27). The cell lysate was then subjected to centrifugation at 1,000 × g for 10 min at 4°C to pellet unbroken cells and nuclei and generate the viral postnuclear supernatant (VPNS). Endosomal latency in these preparations was measured at ≈70% by using horseradish peroxidase as an endosomal marker (27) (data not shown).
The standard fusion/uncoating reaction consisted of 30 μl of freshly prepared VPNS (i.e., from 5 × 105 cells) in a total volume of 300 μl of HB+/-(3 mM MgCl2/50 μM dNTPs/60 mM NH4Cl/100–200 μg of S10 fraction representing the supernatant from a 1,000 × g and then 10,000 × g spin of a lysate prepared in HB from uninfected 293 cells by Dounce homogenization). As indicated, in some reactions the 293 S10 fraction was substituted with a yeast cell S10 extract (28). Alternatively, reactions were set up with the 293 S10 fraction or with an equivalent amount of 293 S10 fraction that had either been subjected to gel filtration over a PD-10 desalting column (Mr > 5,000) (Amersham Pharmacia), to dialysis against HB using a Slide-A-Lyzer (3.5-kDa membrane cutoff) (Pierce), or to membrane filtration (5-kDa membrane cutoff) (Vivaspin). In some reactions, the 293 S10 fraction was substituted with the same cell equivalent amounts of either a P100 or S100 cell fraction representing the pellet and supernatant, respectively, of a 100,000 × g spin of the 293 cell homogenate. In others, 2.5 mM adenosine 5′-[γ-thio]triphosphate or 10 units/ml apyrase was added either before initiating the reaction or at the indicated times after shifting to 37°C, respectively.
A similar protocol was used when examining late DNA products of ASLV reverse transcription with the exception that the 293(TVA800) cells were challenged with the RCASBP(A)-EGFP vector at MOIs of ≈1.0 EGFP-transducing unit. Standard fusion/uncoating reactions were set up as described above either with or without an aliquot of a nuclear extract (representing that derived from 1.6 × 106 293 cells prepared as described in ref. 29) added before initiating infection by temperature shift.
All reactions were performed at 37°C for 6–12 h unless otherwise stated, and stopped by a 15-min incubation with 100 μg of Proteinase K (Boehringer Mannheim) at room temperature before shifting samples to -80°C. The DNA contained in these samples was purified by using phenol/chloroform/isoamylalcohol extraction (28) and the precipitated DNA was resuspended in 50 μl of distilled water and stored at -20°C as described (19, 20). Both early and late DNA products of ASLV reverse transcription were measured by a quantitative real-time PCR amplification method as described (19, 20).
Results
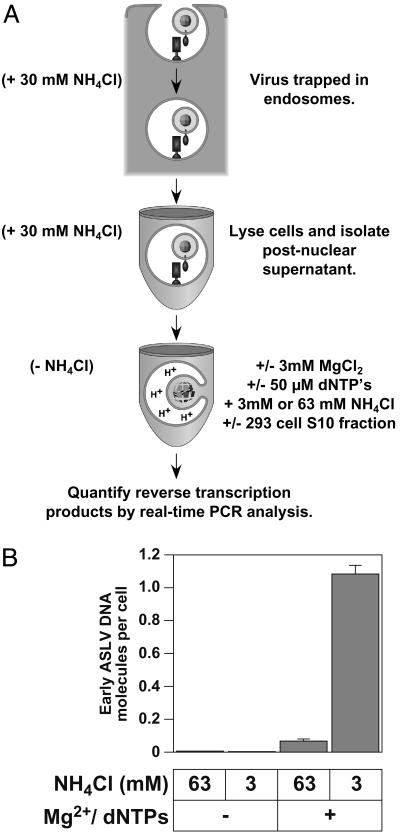
A Cell-Free System That Reconstitutes Retroviral Fusion, Uncoating, and Reverse Transcription. The first step in generating the cell-free system involved trapping ASLV-A virions at a stage before the completion of fusion within an intracellular fusion compartment, presumably endosomes (Fig. 1A). This procedure involved binding a subgroup A ASLV vector to human 293(TVA800) cells expressing glycosylphosphatidylinositol-anchored TVA. Viral entry was then initiated by shifting the cells from 0°C to 37°C in the presence of 30 mM NH4Cl, allowing for accumulation of virions within endosomes. The cells were then shifted back to 4°C in the continued presence of NH4Cl and lysed by using a ball-bearing cell cracker to minimize damage to endosomes (26, 30, 31). A VPNS was then isolated and incubated under conditions that were either permissive (3 mM NH4Cl) or not permissive (63 mM NH4Cl) for virus–cell membrane fusion. ASLV fusion and entry into cells is effectively blocked by 30 mM or higher concentrations of NH4Cl (19, 20) but is unaffected by the presence of 3 mM NH4Cl (data not shown).
Fig. 1.
A cell-free system to study ASLV fusion and uncoating. (A) A schematic diagram of the cell-free system described in the text. (B) Low pH-dependent membrane fusion and added magnesium ions and dNTPs are critical for ASLV early DNA production. 293(TVA800) cells were challenged with RCASBP(A)-EGFP at an estimated MOI of 0.15 EGFP transducing unit. The ASLV fusion/uncoating reaction was then set up at 37°C as described in Materials and Methods with 150 μg of 293 S10 fraction and was performed under permissive (3 mM NH4Cl) or nonpermissive (63 mM NH4Cl) conditions for ASLV Env-dependent fusion. Samples were incubated with or without 3 mM MgCl2 and 50 μM dNTPs. Viral DNA was then quantified by using a real-time PCR amplification method. The results are shown as the number of viral DNA molecules synthesized per cell that was used to make the VPNS. A representative example of an experiment that was performed at least three independent times, each time in triplicate, is shown, with the standard deviation of the data indicated with error bars.
To assess whether or not viral cores that were released from endosomes were competent for reverse transcription, and to investigate the possible role of cellular factors, the samples were incubated in a buffer that included 3 mM MgCl2, a physiological level (50 μM) of dNTPs, and the supernatant of a 10,000 × g spin of a 293 cell extract (293 S10 fraction). A real-time PCR amplification-based assay was then used to quantify the viral DNA products that arose in the cell-free system (Fig. 1 A). The oligonucleotide primers used were derived from the U3 and U5 regions of the viral LTR to detect newly synthesized minus-strand DNA products resulting from the first strand-transfer event of reverse transcription (20).
These studies revealed that early reverse transcription products were synthesized in vitro, but only under fusion-permissive conditions (Fig. 1B). Moreover, viral DNA synthesis was absolutely dependent on added Mg2+ ions and dNTPs (Fig. 1B). A small number of viral DNA products were observed under fusion nonpermissive conditions in the presence of Mg2+ ions and dNTPs (Fig. 1B). These DNA products are probably derived from viral particles released from endosomes, which are broken during preparation of the VPNS.
The maximum amount of viral DNA that was obtained in the cell-free system under fusion-permissive conditions was ≈20% of that seen within infected cells (20), demonstrating that the cell-free system is highly efficient.
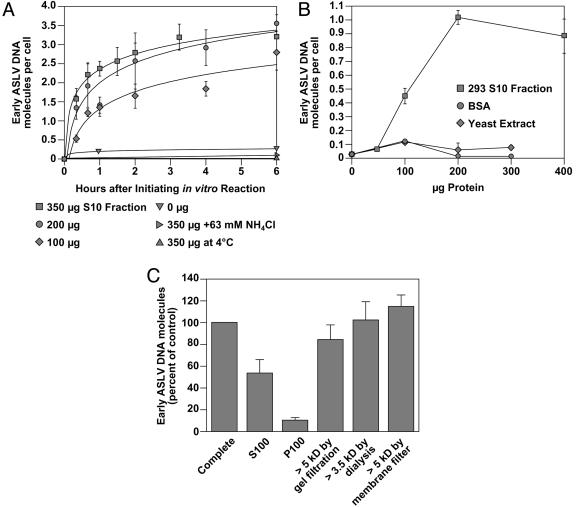
Cellular Factor(s) Stimulate ASLV Fusion/Uncoating in the Cell-Free System. In the experiment shown in Fig. 1B, cellular factors contained within a 293 S10 fraction were included in the cell-free reaction. To test the contribution of these factors, they were either included or omitted from the cell-free reactions. These studies showed that the 293 S10 fraction increased the initial rate as well as the final extent of viral DNA synthesis in a dose-dependent manner (Fig. 2A). This effect was specific because it was not seen with addition of a similar amount of yeast cell S10 fraction or BSA (Fig. 2B). A preliminary characterization of the 293 S10 factor(s) has revealed that they are soluble in nature and are >5 kDa in size (Fig. 2C). Viral DNA synthesis in the cell-free system was also temperature-dependent because it was not observed with samples incubated at 4°C (Fig. 2 A). Taken together, these results indicate that the virus–cell membrane fusion reaction is not sufficient to initiate reverse transcription but instead cellular factors are required.
Fig. 2.
Cellular factor(s) stimulate ASLV fusion/uncoating in the cell-free system. (A) The 293 S10 fraction enhances both the rate and extent of ASLV early viral DNA production. 293(TVA800) cells were challenged with RCASBP(A)-EGFP at an estimated MOI of 0.4 EGFP transducing unit, and fusion/uncoating reactions were set up at 37° C with 3 mM NH4Cl and with different amounts (0–350 μg) of 293 S10 fraction added. For control purposes, samples containing the maximum amount of 293 S10 fraction (350 μg) were also incubated under nonpermissive fusion conditions (63 mM NH4Cl or at 4°C). Reactions were then stopped at the indicated times and early viral DNA products were measured as in Fig. 1B. A representative example of an experiment that was performed two independent times, each time in triplicate, is shown, with the standard deviation of the data indicated with error bars. (B) ASLV early viral DNA production in the cell-free system is specifically enhanced by a 293 S10 fraction. 293(TVA800) cells were challenged with RCASBP(A)-EGFP at an estimated MOI of 0.1 EGFP transducing unit. The ASLV fusion/uncoating reaction was then set up at 37°C with 3 mM NH4Cl and with different amounts of the 293 S10 fraction, BSA, or yeast cell S10 fraction added. Early viral DNA products were then measured as in Fig. 1B. A representative example of an experiment that was performed two independent times, each time in triplicate, is shown with the standard deviation of the data indicated with error bars. (C) The 293 cell factor(s) are soluble and >5 kDa in size. 293(TVA800) cells were challenged with RCASBP(A)-EGFP at an estimated MOI of 0.15 EGFP transducing unit. Fusion/uncoating reactions were then set up at 37°C with either 150 μg of 293 S10 fraction or equivalent amounts of either 293 P100 or S100 fractions, or 293 S10 fractions that had been subjected to gel filtration (Mr > 5 kDa), dialysis (molecular mass cutoff, 3.5 kDa) or membrane filtration (molecular mass cutoff, 5 kDa). Early viral DNA products were measured as described in Fig. 1B. The results are shown as a percentage of the data obtained with 150 μg of 293 S10 fraction added (designated as 100%). A representative example of an experiment that was performed twice, both times in triplicate, is shown, with the standard deviation of the data indicated with error bars.
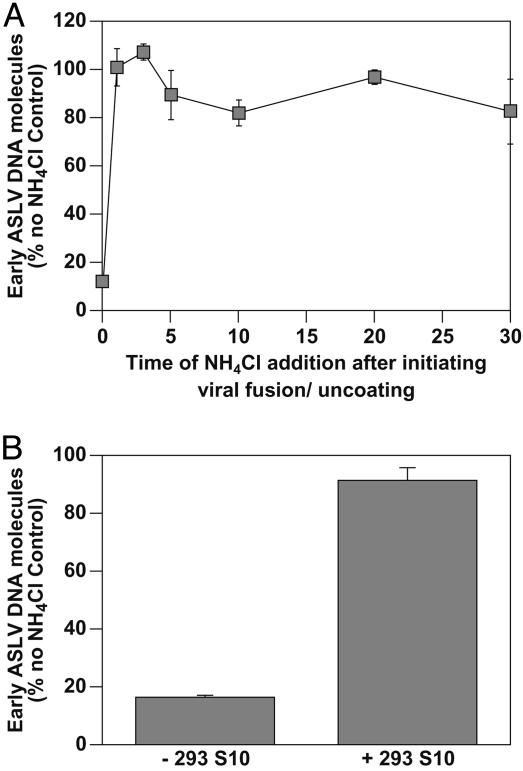
The 293 Cell Factor(s) Act After Completion of the Low pH-Dependent Step. Consistent with a role for low pH in virus-cell membrane fusion, ASLV-A overcomes the NH4Cl-induced block in infected cells within only 1 min after inhibitor removal (20). To determine whether virions are trapped at the same stage of infection in the cell-free system, a similar experiment was performed in vitro by releasing the NH4Cl-induced block for different periods of time before restoring the block. These studies showed that virions traversed the NH4Cl-sensitive step in the cell-free system with the same kinetics seen previously with infected cells (Fig. 3A). Therefore, the trapped virus particles used as substrates in the cell-free reaction seem to be blocked at the same stage of entry as those located within intact NH4Cl-treated cells, i.e., at membrane fusion.
Fig. 3.
The 293 S10 factor(s) act after completion of low pH-dependent fusion. (A) ASLV Env-dependent fusion occurs immediately after removal of the NH4Cl-imposed block. 293(TVA800) cells were challenged with RCASBP(A)-EGFP at an estimated MOI of 0.4 EGFP transducing unit. Cell-free ASLV fusion/uncoating reactions were then set up with 150 μg of 293 S10 fraction and 3 mM NH4Cl, and fusion was initiated by temperature shift from 4°C to 37°C. At different time points, the concentration of NH4Cl was increased to 63 mM to block any further virus–cell membrane fusion. ASLV early viral DNA products were then measured by real-time PCR amplification as described in Fig. 1B. The results are shown relative to those obtained from a parallel sample that was allowed to proceed to completion after the initiation of fusion (defined here as 100%). A representative example of an experiment that was performed twice, each time in triplicate, is shown, with the standard deviation of the data indicated with error bars. (B) The 293 S10 factor(s) act after low pH-dependent membrane fusion has been completed. 293(TVA800) cells were challenged with RCASBP(A)-EGFP at an estimated MOI of 0.08 EGFP transducing unit, and an ASLV cell-free fusion/uncoating reaction was set up with 3 mM NH4Cl and without the 293 S10 fraction. Fusion was induced for 1 min by shifting the samples to 37°C, and then NH4Cl was added to a final concentration of 63 mM to block any further fusion. Samples were then incubated with or without a 150-μg aliquot of the 293 S10 fraction for an additional 6 h at 37°C before early viral DNA products were measured by real-time PCR amplification. The results are shown relative to those obtained from a control sample that had the 293 S10 fraction added before induction of fusion and was maintained under fusion-permissive conditions (3 mM NH4Cl) (defined here as 100%). A representative example of an experiment that was performed three times, each time in triplicate, is shown, with the standard deviation of the data indicated with error bars.
To test whether the 293 S10 factor(s) act after the low pH-dependent step, cell-free reactions were performed by first initiating fusion under permissive conditions (3 mM NH4Cl) in the absence of these factors. NH4Cl was then added to a final concentration of 63 mM to block subsequent rounds of fusion and the 293 S10 fraction was then added to some samples. We reasoned that if the cellular factors were required to stimulate the low pH-dependent step of fusion, then viral DNA should not be synthesized under these conditions. Instead, we found that the cellular factors stimulated viral DNA synthesis (Fig. 3B). Therefore, the 293 cell factor(s) are not required for the low pH-dependent step of viral penetration from endosomes, but instead act at a subsequent step leading to reverse transcription.
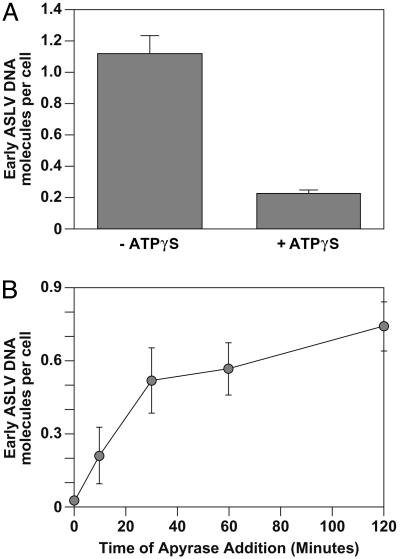
ATP Hydrolysis Is Required for Stimulating ASLV Early DNA Synthesis. To determine whether ATP hydrolysis is required for viral DNA synthesis in the cell-free system, adenosine 5′-[γ-thio]triphosphate, a nonhydrolyzable ATP analogue, was included in some samples. This nucleotide analogue markedly reduced the stimulation of early DNA synthesis in the cell-free system (Fig. 4A). Furthermore, addition of apyrase to reduce the levels of endogenous ATP present also markedly reduced the amount of ASLV early DNA products (Fig. 4B). Intriguingly, this treatment had little or no effect when apyrase was added 30 min or later after initiating the cell-free reaction (Fig. 4B). Together, these data suggest that ATP hydrolysis is required for events leading to the initiation of ASLV early DNA synthesis.
Fig. 4.
ATP hydrolysis is required for the 293 S10 fraction-stimulation of ASLV early DNA synthesis. 293(TVA800) cells were challenged with RCASBP(A)-EGFP at an estimated MOI of 0.15 EGFP transducing unit. Fusion/uncoating reactions were then set up as described for Fig. 1B in the presence of 150 μgof293 S10 fraction and with or without 2.5 mM adenosine 5′-[γ-thio]triphosphate (A). Alternatively, 10 units/ml apyrase was added to reaction samples at the indicated times after shifting to 37°C (B). After a 6-h incubation, early viral DNA was quantified by real-time PCR amplification as in Fig. 1B. A representative experiment performed in triplicate is shown, with the standard deviation of the data indicated by error bars.
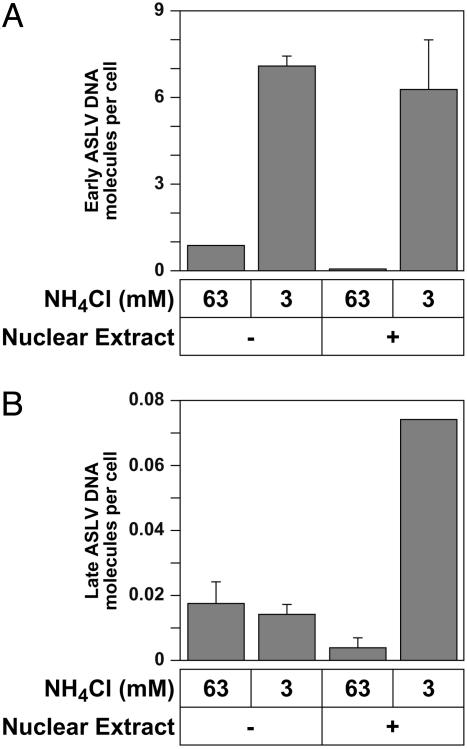
A Nuclear Extract Promotes ASLV Late DNA Synthesis. Previously, it was shown that most ASLV DNA molecules found in the cytoplasm are incompletely synthesized, and that integration of this DNA does not occur unless reverse transcription is more or less completed (32). These observations led to the proposal that nuclear factors may be required for completing reverse transcription of the ASLV genome. We have tested this hypothesis directly by adding a nuclear extract to some samples and assessing its effect on early and late DNA production. These experiments revealed that the nuclear extract enhanced late but not early ASLV DNA synthesis (Fig. 5). The level of late viral DNA synthesis in the experiment shown in Fig. 5B was ≈4% of that expected from infected cells, i.e., a lower efficiency than that seen previously with early DNA synthesis. Nevertheless, these results indicate that 293 cell nuclear factors may specifically promote ASLV late DNA synthesis and suggest that the cell-free system may be useful for studying steps of retroviral replication after the earliest uncoating events.
Fig. 5.
A 293 cell nuclear extract promotes late ASLV DNA synthesis. 293(TVA800) cells were challenged with RCASBP(A)-EGFP at an estimated MOI of 1.0 EGFP transducing unit. Fusion/uncoating reactions were then set up at 37°C with 150 μg of 293 S10 fractions, 3 or 63 mM NH4Cl, and either with (+) or without (-) 1.6 × 106 cell equivalents of a 293 cell nuclear extract. Approximately 20 h later, early (A) and late (B) ASLV DNA products were measured by real-time PCR analysis as described in Materials and Methods. An experiment that was performed in triplicate is shown with the standard deviation of the data indicated with error bars. A similar ratio of early/late viral DNA molecules (600:1) was observed in a separate experiment that was performed in triplicate under the same conditions, except at a 20-fold lower apparent MOI (data not shown).
Discussion
We have described a cell-free system that efficiently reconstitutes retroviral membrane fusion, uncoating and reverse transcription in vitro. We have used this system to obtain evidence that cytosolic factors and ATP hydrolysis promote the synthesis of ASLV early DNA products, whereas nuclear factors enhance synthesis of late DNA products. These data imply an active, dynamic mechanism for retroviral uncoating involving the recruitment of cellular factors to incoming cores. The cell-free system should now allow for the identification and characterization of these cellular factors.
This cell-free system should allow for an investigation of the molecular events that surround the formation of the viral RTC. Because the system monitors steps that follow immediately after virus-cell membrane fusion leading up to the activation of reverse transcription, it is distinguished from others which have characterized the behavior of viral cores under nonphysiological conditions, e.g., using the endogenous reverse transcription reaction (ERT). The ERT assay involves the artificial disruption of virions with detergents (5, 33). However, in addition to viral membrane removal, detergent addition affects the integrity of viral cores, raising doubts that the behavior of viral particles in this system reflects that of intracellular viral complexes (34). Other approaches have been described that monitor the behavior of RTCs that are produced within infected cells before they are isolated and characterized (34, 35). However, these approaches will not detect the uncoating steps that occur immediately after the membrane fusion reaction.
Although the cell-free system is specific and efficient, we previously observed an ≈100-fold increase in ASLV DNA synthesis in intact cells under fusion-permissive conditions (3 mM NH4Cl), as compared to non-fusion-permissive conditions (30 mM NH4Cl) (20). However, in the cell-free system, the level of viral DNA that was obtained under fusion-permissive conditions was only ≈7- to 10-fold higher than that seen under non-fusion-permissive conditions (Figs. 1 and 2). Presumably, this effect is caused by the release of ASLV cores during the mechanical cell lysis of infected cells, where some endosomal/viral membranes may be ruptured. Consistent with this hypothesis, a higher level of viral DNA was synthesized under fusion nonpermissive conditions when the level of dNTPs was increased to a nonphysiological concentration (i.e., 500 μM) (data not shown). Presumably, the particles that are released from ruptured endosomes are unable to efficiently synthesize DNA in the presence of physiological dNTP levels.
We also note that a small level of viral DNA was produced in the cell-free system under fusion-permissive conditions in the absence of any added 293 cellular factors (Figs. 2 A and 3B). This effect is probably caused by the carryover of cellular factors contained in the VPNS, which is diluted 10-fold into the in vitro reaction. Future experiments will address this possibility by attempting to isolate more purified endosome populations that contain fusion-arrested virions as the starting material for the cell-free fusion/uncoating reaction.
The cellular factor(s) that stimulated ASLV early DNA synthesis acted after the low pH-dependent step of viral entry (membrane fusion) was completed. This suggests that the retroviral core may recruit cellular factors and dNTPs to stimulate uncoating events involved in initiating reverse transcription. Therefore, retroviral uncoating may not simply be a passive disassembly process, but rather it may be an active, dynamic process. Furthermore, the nuclear factor enhancement of ASLV late DNA synthesis suggests that uncoating and reverse transcription may occur via discrete intermediates involving different cellular factors as the RTC progresses to form the preinte-gration complex. Indeed, the notion that cellular factors are involved in these postfusion steps is consistent with previous observations that incoming retroviral particles associate with components of the host cell cytoplasm. For example, HIV-1 cores have been reported to associate with the host cell cytoskeleton, including actin and microtubules (36, 37). Also, partial purification of subviral complexes from cells suggest that retroviral cores may undergo changes in composition during entry (34, 35). It is now imperative to monitor the biochemical changes in RTCs formed in the cell-free system and relate these changes to those observed within intact cells during an actual infection. The cell-free system may also prove useful in identifying the potential biochemical and structural intermediates in retroviral uncoating through the analysis of virions that contain mutant ASLV capsid proteins, which are arrested at an early step of replication before reverse transcription (38).
In the cell-free system, ATP hydrolysis was required during the first 30 min after membrane fusion. However, it remains to be determined exactly how cellular factors and ATP hydrolysis may function to trigger, and perhaps orchestrate, the earliest steps of retroviral uncoating. One possibility is that ATP is used as a substrate to phosphorylate viral proteins. Indeed, for HIV-1, phosphorylation of serine residues in the viral matrix and capsid proteins has been implicated in these early events (39, 40). A second possibility is that ATP is required for the function of a molecular chaperone that may play a role in viral uncoating. In support of this idea, it has been reported that HIV-1 particles contain substantial amounts of certain members of the Hsp70 family of ATPases (41). With the cell-free system now in hand, it should be possible to distinguish between these and other possible roles for ATP in viral uncoating.
Early postfusion events remain largely unexplored for most enveloped viruses (42). In principle, it should be possible to apply the ASLV-based cell-free system to study the uncoating mechanism of any viral core that can be coassembled with ASLV Env. Indeed, we have reconstituted HIV-1 uncoating with pseudotyped (mixed) HIV-1 [ASLV-A Env] particles (S.N., T. Gibson, and J.A.T.Y., unpublished data). Thus, using these and other pseudotyped particles in the cell-free system, it should be possible to reconstitute uncoating events of a number of other viruses. In the case of HIV-1, this would be particularly important because uncoating remains a thus far unexplored target for therapeutics in the viral life cycle.
Acknowledgments
We thank Ned Landau, Rick Bushman, and Richard Barnard for critical comments on the work in this article and members of the Young laboratory for helpful discussions. We also thank John Naughton for assistance in preparing the figures. This work was supported by National Institutes of Health Grant CA 70810.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RTC, reverse transcription complex; ASLV, avian sarcoma and leukosis virus; EGFP, enhanced GFP; HB, homogenization buffer; VPNS, viral postnuclear supernatant; MOI, multiplicity of infection.
References
- 1.Eckert, D. M. & Kim, P. S. (2001) Annu. Rev. Biochem. 70, 777-810. [DOI] [PubMed] [Google Scholar]
- 2.Barnard, R. J. & Young, J. A. T. (2003) Curr. Top. Microbiol. 281, 107-136. [DOI] [PubMed] [Google Scholar]
- 3.Pierson, T. C. & Doms, R. W. (2003) Curr. Top. Microbiol. 281, 1-28. [DOI] [PubMed] [Google Scholar]
- 4.Dvorin, J. D. & Malim, M. H. (2003) Curr. Top. Microbiol. 281, 179-208. [DOI] [PubMed] [Google Scholar]
- 5.Goff, S. P. (2001) J. Gene Med. 3, 517-528. [DOI] [PubMed] [Google Scholar]
- 6.Braaten, D., Franke, E. K. & Luban, J. (1996) J. Virol. 70, 3551-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franke, E. K., Yuan, H. E. & Luban, J. (1994) Nature 372, 359-362. [DOI] [PubMed] [Google Scholar]
- 8.Luban, J., Bossolt, K. L., Franke, E. K., Kalpana, G. V. & Goff, S. P. (1993) Cell 73, 1067-1078. [DOI] [PubMed] [Google Scholar]
- 9.Thali, M., Bukovsky, A., Kondo, E., Rosenwirth, B., Walsh, C. T., Sodroski, J. & Gottlinger, H. G. (1994) Nature 372, 363-365. [DOI] [PubMed] [Google Scholar]
- 10.Karpas, A., Lowdell, M., Jacobson, S. K. & Hill, F. (1992) Proc. Natl. Acad. Sci. USA 89, 8351-8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saphire, A. C., Bobardt, M. D. & Gallay, P. A. (2002) J. Virol. 76, 2255-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Towers, G. J., Hatziioannou, T., Cowan, S., Goff, S. P., Luban, J. & Bieniasz, P. D. (2003) Nat. Med. 9, 1138-1143. [DOI] [PubMed] [Google Scholar]
- 13.Gao, G. & Goff, S. P. (1999) Mol. Biol. Cell 10, 1705-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munk, C., Brandt, S. M., Lucero, G. & Landau, N. R. (2002) Proc. Natl. Acad. Sci. USA 99, 13843-13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bieniasz, P. D. (2003) Trends Microbiol. 11, 286-291. [DOI] [PubMed] [Google Scholar]
- 16.Towers, G. J. & Goff, S. P. (2003) AIDS Rev. 5, 156-164. [PubMed] [Google Scholar]
- 17.Stremlau, M., Owens, C. M., Perron, M. J., Kiessling, M., Autissier, P. & Sodroski, J. (2004) Nature 427, 848-853. [DOI] [PubMed] [Google Scholar]
- 18.Melikyan, G. B., Barnard, R. J. O., Markosyan, R. M., Young, J. A. T. & Cohen, F. S. (2004) J. Virol. 78, 3753-3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mothes, W., Boerger, A. L., Narayan, S., Cunningham, J. M. & Young, J. A. (2000) Cell 103, 679-689. [DOI] [PubMed] [Google Scholar]
- 20.Narayan, S., Barnard, R. J. & Young, J. A. (2003) J. Virol. 77, 1977-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith, J. G., Mothes, W., Blacklow, S. C. & Cunningham, J. M. (2004) J. Virol. 78, 1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsh, M. & Helenius, A. (1989) Adv. Virus Res. 36, 107-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snitkovsky, S., Niederman, T. M., Carter, B. S., Mulligan, R. C. & Young, J. A. (2000) J. Virol. 74, 9540-9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snitkovsky, S., Niederman, T. M., Mulligan, R. C. & Young, J. A. (2001) J. Virol. 75, 1571-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruenberg, J. & Howell, K. E. (1989) Annu. Rev. Cell Biol. 5, 453-481. [DOI] [PubMed] [Google Scholar]
- 26.Gorvel, J. P., Chavrier, P., Zerial, M. & Gruenberg, J. (1991) Cell 64, 915-925. [DOI] [PubMed] [Google Scholar]
- 27.Robinson, L. J. & Gruenberg, J. (1998) in Cell Biology: A Laboratory Handbook, ed. Celis, J. (Academic, New York), Vol. 2, pp. 248-257. [Google Scholar]
- 28.Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Smith, J. A. & Struhl, K. (2000) Current Protocols in Molecular Biology (Wiley, New York), Vol. 1.
- 29.Farrell, M. L. & Mertz, J. E. (2002) J. Virol. 76, 6762-6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davey, J., Hurtley, S. M. & Warren, G. (1985) Cell 43, 643-652. [DOI] [PubMed] [Google Scholar]
- 31.Gruenberg, J. E. & Howell, K. E. (1986) EMBO J. 5, 3091-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee, Y. M. & Coffin, J. M. (1991) Mol. Cell. Biol. 11, 1419-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Temin, H. M. & Baltimore, D. (1972) Adv. Virus Res. 17, 129-186. [DOI] [PubMed] [Google Scholar]
- 34.Fassati, A. & Goff, S. P. (1999) J. Virol. 73, 8919-8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fassati, A. & Goff, S. P. (2001) J. Virol. 75, 3626-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonald, D., Vodicka, M. A., Lucero, G., Svitkina, T. M., Borisy, G. G., Emerman, M. & Hope, T. J. (2002) J. Cell Biol. 159, 441-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bukrinskaya, A., Brichacek, B., Mann, A. & Stevenson, M. (1998) J. Exp. Med. 188, 2113-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cairns, T. M. & Craven, R. C. (2001) J. Virol. 75, 242-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cartier, C., Sivard, P., Tranchat, C., Decimo, D., Desgranges, C. & Boyer, V. (1999) J. Biol. Chem. 274, 19434-19440. [DOI] [PubMed] [Google Scholar]
- 40.Kaushik, R. & Ratner, L. (2004) J. Virol. 78, 2319-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurer, C., Cimarelli, A. & Luban, J. (2002) J. Virol. 76, 4666-4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whittaker, G. R., Kann, M. & Helenius, A. (2000) Annu. Rev. Cell Dev. Biol. 16, 627-651. [DOI] [PubMed] [Google Scholar]