Abstract
Deposition of amyloid-β (Aβ) in Alzheimer’s disease (AD) is strongly correlated with the APOE genotype. However, the role of apolipoprotein E (apoE) in Aβ aggregation has remained unclear. Here we have used different apoE preparations, such as recombinant protein or protein isolated from cultured astrocytes, to examine the effect of apoE on the aggregation of both Aβ1–40 and Aβ1–42. The kinetics of aggregation, measured by the loss of fluorescence of tetramethylrhodamine-labeled Aβ, is shown to be dramatically slowed by the presence of substoichiometric concentrations of apoE. Using these concentrations, we conclude that apoE binds primarily to and affects the growth of oligomers that lead to the nuclei required for fibril growth. At higher apoE concentrations, the protein also binds to Aβ fibrils, resulting in fibril stabilization and a slower rate of fibril growth. The aggregation of Aβ1–40 is dependent on the apoE isoform, being the most dramatic for apoE4 and less so for apoE3 and apoE2. Our results indicate that the detrimental role of apoE4 in AD could be related to apoE-induced stabilization of the soluble but cytotoxic oligomeric forms and intermediates of Aβ, as well as fibril stabilization.
Alzheimer’s disease (AD) is characterized by the extracellular accumulation of amyloid-β (Aβ) in compact plaques of the brain. These plaques are hypothesized to be directly related to the development of this disease. There are, however, many additional factors implicated in the development of Alzheimer’s disease. Among these is the protein apolipoprotein E4 (apoE4), which is known to be the major genetic risk factor for developing late-onset AD.1,2 How apoE4 influences the pathology of AD is not fully understood, but a large body of literature points to its role in influencing the aggregation and clearance of Aβ from the brain.3−11 The presence of both soluble complexes12−14 and insoluble deposits4,15 of the apoE–Aβ complex in the mouse and human brain suggests the importance of a direct interaction between apoE and Aβ in the development of AD. There are many conflicting reports in the literature about the nature of this interaction.1,3,5,6,9,11,16−18 We believe that these apparent contradictions can be traced to the complex properties of both apoE and Aβ themselves. For example, most reported biophysical experiments have used recombinant lipid-free apoE that is both structurally and functionally different from apoE complexed with lipids.19−21 Lipid-free apoE has a compact structure comprising a four-helix bundle N-terminal domain and a C-terminal helical domain that displays extensive interactions with the N-terminal domain.20 There is no high-resolution structure available for lipidated apoE, but a low-resolution structure obtained by X-ray diffraction of dipalmitoylphosphatidylcholine (DPPC)-bound apoE particles shows that the DPPC–apoE complex adopts an extended conformation forming a beltlike structure around the lipid core.21 ApoE lipoproteins derived from cultured cells, plasma, or cerebrospinal fluids vary widely in terms of their content of lipids, sterols, and other associated lipoproteins. Similarly, both common alloforms of Aβ, Aβ1–40 and Aβ1–42, are intrinsically disordered peptides with strong tendencies to aggregate. Thus, biophysical experiments with apoE and Aβ need to consider the different forms of apoE as well as the various self-associated forms of Aβ. Particularly important are the interactions of apoE with the soluble oligomeric intermediates of Aβ that are believed to be the major toxic species in AD; the monomers are considered to be nontoxic.9,22−25 In this study, we examine the interactions between apoE and Aβ in detail by investigating the effects of the apoE obtained from different sources on the aggregation of Aβ in vitro.
To understand the effect of apoE on Aβ aggregation, it is important to understand the mechanism of aggregation of Aβ itself. In a recent publication, we analyzed the full time course of self-association of Aβ by using Aβ labeled with tetramethylrhodamine (TMR).26 We observed that aggregation of Aβ can be divided into three phases, an early oligomerization phase, an intermediate phase, and a growth phase. Here we examine the apoE–Aβ interactions by measuring the effect of apoE on the different phases of aggregation of Aβ. Our data, which show strong effects of apoE on the aggregation kinetics of Aβ, are consistent with high-affinity binding of apoE to oligomeric intermediates and, at higher concentrations, to fibrils of Aβ, leading to an enhancement of the stability of the amyloid aggregates.
Experimental Procedures
Preparation of Aβ and ApoE
Unlabeled (WT-Aβ) and TMR-labeled Aβ (TMR-Aβ) peptides were chemically synthesized and purchased from the Keck Foundation (Yale University, New Haven, CT). The TMR was chemically attached to the N-terminus of Aβ using FMOC-Lys(5-TMR)-OH.26 We have previously verified that N-terminal labeling of Aβ with TMR does not appreciably alter the aggregation properties of Aβ.26 The peptides were further purified according to protocols described previously.26 Stock solutions of purified WT-Aβ and TMR-Aβ were prepared in 3 mM NaOH, 150 mM NaCl, 5 mM β-mercaptoethanol (βME), and 1 mM EDTA. Recombinant lipid-free apoE was prepared according to the protocol described previously.27 Lipidation of recombinant apoE was performed by incubating 30 μM apoE with 2 mg/mL small unilamellar vesicles (SUV) of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) in 20 mM phosphate, pH 7.4 buffer containing 150 mM NaCl and 5 mM β-mercaptoethanol (βME).28 The solution was incubated at 25 °C overnight. The unbound apoE was removed by ultracentrifugation of the apoE–lipid sample in a KBr gradient.11 The unbound lipids were then removed by size exclusion chromatography using a Superdex 200 column (GE Healthcare) in 20 mM phosphate, pH 7.4 buffer containing 150 mM NaCl, 5 mM βME, and 1 mM EDTA. ApoE containing lipoproteins from astrocytes stably expressing human apoE isoforms were collected and purified by immunoaffinity chromatography as described previously.11
Fluorescence Time Course Measurements
The TMR-Aβ stock solution (20 μM) prepared in 3 mM NaOH was diluted to an appropriate final concentration in 20 mM phosphate, pH 7.4 buffer containing 150 mM NaCl, 1 mM EDTA, and 5 mM βME and incubated in a clean glass test tube while being continuously stirred in a temperature-controlled cuvette holder.26 Aggregation of TMR-Aβ samples was monitored continuously using TMR fluorescence (λex = 520 nm; λem = 600 nm). To increase the rate of fibrillization, all samples were stirred using a stir bar (3 mm × 6 mm).
Electron Microscopy of the ApoE–Aβ Protein
Aggregation of 5 μM WT-Aβ1–42 in 20 mM phosphate, pH 7.4 buffer containing 150 mM NaCl, 1 mM EDTA, and 5 mM βME at 37 °C that was being continuously stirred was monitored in the spectrofluorometer using Thioflavin T (ThT) fluorescence (λex = 438 nm; λem = 480 nm). Aliquots (20 μL) of an Aβ solution were collected at time zero, after 4 h (in the intermediate phase), and after 24 h (in the growth phase). These aliquots were mixed with apoE4 at a 10:1 (Aβ:apoE) molar ratio and incubated at room temperature for 30 min. The apoE4 used in this experiment had a six-His tag at the N-terminal end for labeling with Ni-NTA gold nanoparticles for visualization via electron microscopy (EM). There are five additional mutations (F257A, W264R, V269A, L279Q, and V287E) in the C-terminal domain to keep apoE monomeric.29 For EM measurements, a Formvar carbon-coated 200 mesh copper grid (Electron Microscopy Sciences, Hatfield, PA) was placed inverted on a 12 μL drop of an Aβ–apoE sample for 1 min. The grid was then washed with water followed by incubation on a 10 μL drop of a 20 nM solution of 5 nm Ni-NTA nanogold (Nanoprobes, Yaphank, NY) for 5 min at room temperature. The grid was then washed twice with 20 mM Tris, pH 7.4 buffer containing 30 mM imidazole and 150 mM NaCl. Washing the grid with an imidazole solution prevents nonspecific attachment of the nanoparticles to the Aβ aggregates (see Figure S4 of the Supporting Information). The grid was further washed with water followed by negative staining with 0.5% uranyl acetate for 1 min. The images were recorded with a JEOL 100CX transmission electron microscope (TEM) equipped with an AMT digital camera.
Stability of the Fibrils following Sonication
Aliquots of a fibrillized Aβ1–42 solution obtained at the end of the experiment described in the legend of Figure 1 were incubated with or without WT-apoE4 (5:1 Aβ:apoE molar ratio) at room temperature for 30 min. These solutions were then subjected to sonication in a water bath sonicator for 5 min. The sonicated samples were then imaged using EM.
Figure 1.
Time course of TMR-Aβ aggregation in the presence of various forms of the apoE proteins. Panels A–C show the aggregation of TMR-Aβ1–40: (A) 4 μM TMR-Aβ1–40 in the presence of 0, 50, 100, and 200 nM recombinant lipid-free apoE4 and (B and C) 4 μM TMR-Aβ1–40 in the absence or presence of 150 nM recombinant lipid-free apoE (B) and DMPC–apoE (C), respectively. Panels D–F show the aggregation of TMR-Aβ1–42: 2 μM TMR-Aβ1–42 in the absence or presence of recombinant lipid-free apoE (50 nM), DMPC–apoE (50 nM), and astrocyte-derived apoE (11 nM) respectively.
Fluorescence Time Course of Urea-Induced Dissociation of the Fibrils
A 50 μL aliquot of fibrillized TMR-Aβ1–42 (4 μM) was diluted 20-fold into 2 M urea in 20 mM phosphate, pH 7.4 buffer containing 150 mM NaCl, 1 mM EDTA, and 5 mM βME. The solution was continuously stirred at room temperature in the spectrofluorometer. The dissociation of the fibrils was monitored by the change in TMR fluorescence.
Results
Effects of ApoE on Aβ Aggregation
We examined the role of apoE on Aβ aggregation by observing the effects of recombinant lipid-free and lipidated apoE isoforms on the aggregation of both Aβ1–40 and Aβ1–42. Aggregation of Aβ was monitored by TMR fluorescence when using TMR-labeled Aβ or by ThT fluorescence when using unlabeled Aβ.26 As previously shown using TMR-labeled Aβ,26 the aggregation of Aβ can be described by three phases: an oligomeric phase resulting in the formation of dimers, trimers, and higher-order oligomers, an intermediate phase during which there is little change in monomer concentration but rather clustering of oligomers, and the final fibrillization phase reflecting the formation and growth of fibrils.26 In this work, the term “aggregation” refers to all three phases. Figure 1A shows the effect of different concentrations of recombinant lipid-free apoE4 on the aggregation of TMR-labeled Aβ1–40. In this experiment, the concentration of apoE4 (0.05–0.2 μM) was considerably lower than that of TMR-labeled Aβ1–40 (4 μM) but the overall aggregation process was much slower in the presence of apoE4 because of a longer intermediate phase and a slower fibrillization phase. As discussed elsewhere, the oligomerization phase of Aβ1–40 is hardly noticeable at these concentrations (4 μM).26 The effect on the intermediate phase is evident at all apoE concentrations, but the effect on the fibrillization phase is observed only at relatively higher concentrations of apoE4. Figure 1B compares the effects of the three isoforms of apoE (0.15 μM) on the aggregation of TMR-Aβ1–40 (4 μM). The apoE isoforms differ in terms of their effect, with apoE4 showing the strongest and apoE2 the weakest effect on the overall aggregation of Aβ1–40. Figure 1C shows that the differential effects of the apoE isoforms on the overall aggregation of Aβ1–40 are similar in the presence of apoE lipidated with 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC–apoE). While a published low-resolution structure of lipidated apoE was obtained using DPPC–apoE lipoproteins, we have used DMPC–apoE in our experiments because DMPC–apoE particles form readily at room temperature. Additionally, DMPC–apoE and DPPC–apoE lipoprotein particles are shown to be similar both functionally and structurally.21,30 The data presented in panels A–C of Figure 1 use TMR-Aβ1–40. Consistent with our previous results, the formation of oligomers that occurs within approximately 1 h is much poorer for Aβ1–40 than for Aβ1–42.26 Similar observations are made using unlabeled Aβ1–40 as shown in Figure S1A–C of the Supporting Information.
Panels D–F of Figure 1 show the effects of substoichiometric amounts of recombinant lipid-free and lipidated apoE, as well as lipoproteins derived from cultured astrocyte cells, on the time course of aggregation of TMR-Aβ1–42. With Aβ1–42, and in the absence of apoE, the oligomerization phase is clearly observed.26 As described elsewhere, observation of the early oligomerization step is a consequence of self-quenching of TMR moieties from two or more Aβ monomers coming into direct contact.26 This early phase is not observed in the presence of apoE (Figure 1D,E). We discuss this issue later. Both the intermediate phase and the fibrillization phase of Aβ1–42 aggregation are strongly influenced by apoE irrespective of the source of origin of the apoE lipoproteins present. Differential effects, if any, between the apoE isoforms on Aβ1–42 aggregation are small (see Figure 1D–F). Figure S1D of the Supporting Information provides data using recombinant lipid-free apoE isoforms that differ in terms of their effects on the aggregation of unlabeled Aβ1–42, although again the differences appear to be small. We have also used the human cerebral spinal fluid-derived apoE lipoproteins that have strong effects on the aggregation of Aβ1–42 (see Figure S2 of the Supporting Information). Taken together, the results described above indicate that all three isoforms of apoE derived from different sources strongly alter the aggregation of both Aβ1–40 and Aβ1–42.
Effects on Fibrillization
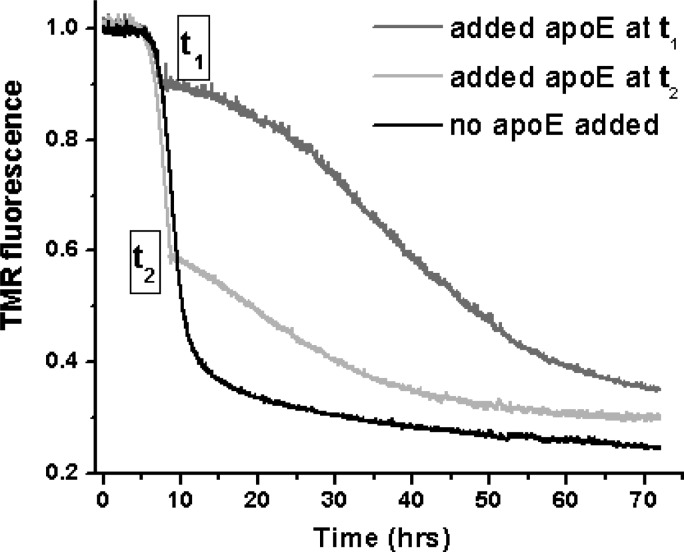
The pronounced effects observed at the substoichiometric concentrations of apoE suggest that apoE is binding to some form(s) of Aβ other than the unstructured monomers, i.e., to prefibrillar oligomers and/or fibrils. The data presented above may be explained by assuming that apoE may bind to the oligomers to slow their conversion to fibrils, as proposed previously.17,18 Additionally, apoE may alter fibril growth because of its interactions with the fibrillar intermediates of Aβ. To verify whether apoE binds to fibrils, we examined the time course of the aggregation of Aβ upon addition of apoE in the fibrillar phase. Figure 2 shows that the time course of TMR-Aβ1–40 (4 μM) fluorescence is dramatically altered when a substoichiometric amount of apoE4 (200 nM) is added to this solution both at the beginning of the fibrillization phase (corresponding to an ∼10% loss of TMR fluorescence) and in the middle of the fibrillization phase (corresponding to an ∼40% loss of TMR fluorescence). Because this phase is coincident with the appearance of β-structure fibrillar forms of Aβ,26 pronounced effects of apoE in this phase indicate interactions of apoE with the Aβ fibrils. Similar effects on the growth of the Aβ1–40 fibrils can also be observed upon addition of DMPC–apoE4 (see Figure S3 of the Supporting Information).
Figure 2.
Effect of apoE on the Aβ growth phase. The black line represents the time course of aggregation of TMR-Aβ1–40 (4 μM) in the absence of apoE. The dark gray and light gray lines represent the time courses when 200 nM recombinant lipid-free apoE4 is added during the growth phase at times t1 and t2, respectively.
Electron Microscopy of the ApoE–Aβ Complexes
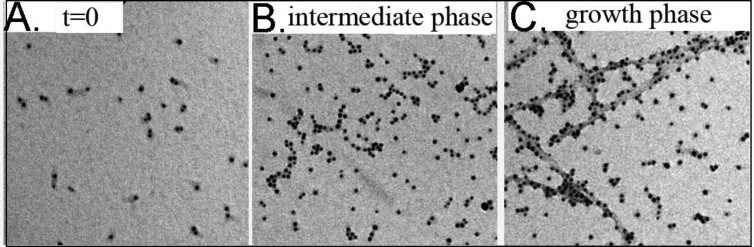
Electron microscopy (EM) was used to examine the nature of apoE–Aβ complexes. In these experiments, the apoE molecules were visualized by being labeled with Ni-NTA gold nanoparticles that attach to a six-His tag at the N-terminal end of apoE4. To rule out effects from self-association of apoE, we used a monomeric mutant of apoE4 for this experiment.27,29,31 The experiments were conducted by taking an aliquot of unlabeled Aβ1–42 (5 μM) at different stages of aggregation and incubating the aliquot with apoE4 (final concentration of 500 nM) at room temperature for 15 min. We note that labeling of apoE with the gold nanoparticles is performed following plating of the sample on the EM grids. Figure 3A represents an EM image of the apoE4–Aβ1–42 solution at time zero. The gold nanoparticles, which represent the location of apoE molecules, are seen as black dots in the image. Clearly, the isolated nanoparticles indicate that most of apoE exists as monomers at time zero. Figure 3B is a representative image of the apoE4–Aβ1–42 solutions during the intermediate phase of Aβ aggregation. Here, in addition to the monomers, a significant fraction of apoE is found to be clustered in a linear or random geometry. Because self-association of apoE is ruled out under our experimental conditions, the presence of clusters of apoE molecules indicates binding of apoE with the oligomers and/or protofilaments of Aβ1–42 in this phase. Figure 3C represents an image of the apoE4–Aβ1–42 complex in the fibrillization phase of aggregation. Numerous fibrils can be observed in this phase with apoE bound to the surface of the fibrils. It may be noted here that the preparation of grids for EM requires extensive washing of the grids with both buffer and water, which prevents nonspecific labeling of the Aβ fibrils with the gold nanoparticles and most likely washes away weakly bound apoE molecules from the apoE–Aβ complexes. Figure S4 of the Supporting Information shows that the nonspecific labeling of the Aβ fibrils in the absence of apoE is minimal under our experimental conditions. Thus, data presented in Figure 3 indicate binding of apoE to the soluble oligomeric and insoluble fibrillar aggregates of Aβ.
Figure 3.
Electron microscopy of the apoE–Aβ complexes. Representative images of the apoE4–Aβ1–42 solution collected (A) at time zero, (B) during the intermediate phase, and (C) during the growth phase of aggregation. The black dots represent gold nanoparticles attached to the molecules of apoE4 via a six-His tag at the N-terminus of apoE.
Effect of ApoE on the Stability of the Aβ Fibrils
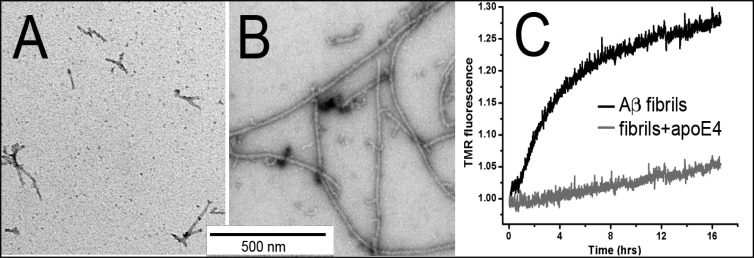
We then asked how the interactions with apoE might alter the stability of Aβ fibrils. It has previously been shown that continuous stirring (a commonly used method for accelerating fibril formation) of the Aβ solution causes fragmentation of the amyloid fibrils.32 Hence, under our conditions, the rate of fragmentation of the fibrils can strongly influence the rate of formation of new fibrils in the growth phase. As noted in Experimental Procedures, all of our experiments have been performed under continuous stirring of the samples. To examine whether interaction of apoE alters the stability of the Aβ fibrils, we tested whether apoE changes (i) the fragmentation of the fibrils induced by sonication and/or (ii) the denaturation of the fibrils induced by urea. Figure 4A shows a representative EM image of the Aβ1–42 fibrils following sonication for 4 min in a water bath sonicator. The fibrils are much shorter than those prior to sonication (see Figure S5 of the Supporting Information), indicating fragmentation caused by sonication. Figure 4B shows EM images of Aβ1–42 fibrils that were incubated with apoE4 for 30 min prior to sonication. Clearly, these fibrils can be seen to be long and networked, quite similar to the fibrils prior to sonication (Figure S5 of the Supporting Information), indicating that apoE increases the mechanical stability of the fibrils. We then examined whether Aβ fibrils could be denatured or dissociated using urea and whether apoE can affect this process. In this experiment, fibrils of TMR-Aβ1–42 (4 μM) were incubated without or with apoE4 (1 μM) prior to dilution in 2 M urea. Figure 4C shows that TMR fluorescence increases with time in both cases, indicating dissociation of the fibrils as a result of denaturation. Clearly, the rate of dissociation of the fibrils incubated with apoE4 is considerably slower than the rate of those without any apoE. Thus, interaction of apoE with the Aβ fibrils increases both the mechanical and conformational stability of fibrils.
Figure 4.
Stability of the fibrils. Electron microscopy images following sonication of Aβ1–42 fibrils that have been incubated in the absence (A) or presence (B) of apoE4. (C) Fluorescence of TMR-Aβ1–42 following dilution into 2 M urea of fibrillized TMR-Aβ1–42 that had been previously incubated without (black line) or with apoE4 (gray line) at a 10:1 (Aβ:apoE) molar ratio.
Discussion
According to the amyloid hypothesis, a major causative factor in the development of Alzheimer’s disease (AD) is the presence of extracellular plaques that contain amyloid-β. It is well-established that the major genetic risk factor for the development of late-onset Alzheimer’s disease is the presence of the apoE4 isoform. The apoE isoforms strongly influence amyloid deposition in the human brain (apoE4 > noncarriers)33 and in amyloid precursor protein (APP) mouse models expressing human apoE isoforms (apoE4 > apoE3 > apoE2).7 Several mechanisms of action for the role of apoE in AD have been proposed. The most relevant one in relation to this study involves apoE–Aβ interactions. A large body of literature data suggests the importance of apoE in modulating the clearance, degradation, and aggregation of Aβ in the brain.1,7,8,10,11,13,34−37 The goal of this study is to clarify the interactions between apoE and Aβ by examining the kinetic behavior of apoE on Aβ aggregation.
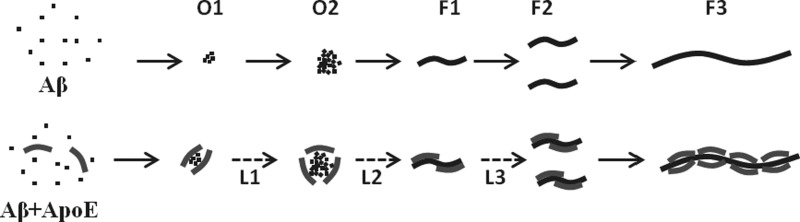
The data presented here suggest that in vitro Aβ aggregation is slowed by all three isoforms of apoE irrespective of the source or lipidation status of apoE. We have used recombinant apoE, in both lipid-free and lipidated forms, and astrocyte-secreted apoE to show that the overall nature of the effects of apoE on Aβ aggregation is independent of the preparation of apoE (Figure 1). ApoE lipoproteins derived from human cerebral spinal fluid also exhibit strong effects on the aggregation of Aβ1–42 (see Figure S2 of the Supporting Information). A putative model for the role of apoE in aggregation of Aβ based on our experimental results is presented in Figure 5. In this model, Aβ monomers self-associate to form small oligomers that then grow larger, presumably by forming an ensemble of clusters.26 Fibrils are formed following nucleation within the larger clusters. The fibrils then grow by monomer addition and by fragmentation. Our model of Aβ aggregation is consistent with the recent observations that the rate of fibril formation of Aβ peptides in vitro is not determined by homogeneous nucleation but rather by processes such as formation of oligomers from the monomers, followed by conversion of the oligomers to the fibrils and the fragmentation and secondary nucleation of the fibrils.26,32,38−40 We have omitted secondary nucleation from our model because fragmentation is more important under our experimental conditions involving, as it does, continuous stirring of the samples.41 The EM data presented in Figure 3 suggest that apoE binds both to the oligomeric intermediates and to the surface of the fibrils. We propose in the model presented in Figure 5 that apoE alters Aβ aggregation primarily by two possible mechanisms: (1) by binding to the oligomeric intermediates, perhaps altering the nature of the intermediates with respect to fibril formation, and (2) by reducing the rate of fibril fragmentation. The increase in the duration of the intermediate phase at all concentrations of apoE is consistent with binding to the oligomeric intermediates.17,18 The slow kinetics of growth of the fibrils at higher apoE concentrations is mediated by the interaction of apoE with the Aβ fibrils and subsequent fibril stabilization. The data presented in Figure 4 clearly show that apoE reduces both the rate of fragmentation of the fibrils induced by sonication and the rate of dissociation of the fibrils induced by urea.
Figure 5.

Effect of apoE on Aβ aggregation. The top panel shows Aβ monomers self-assemble to form small oligomers (O1) that grow to larger oligomers (O2) by monomer addition and/or by clustering. The fibrils (F1) are formed within the large oligomers following nucleation. The fibrils subsequently undergo fragmentation (F1 → F2) and growth (F2 → F3). The bottom panel shows that at low concentrations apoE interacts with the Aβ oligomers (O1 and O2) only, but at higher concentrations, it binds to the fibrils (F1–F3), as well. The broken lines indicate slow growth (L1) and nucleation (L2) of the Aβ oligomers and a reduced rate of fragmentation (L3) of the Aβ fibrils due to interactions with apoE. Stabilization of the oligomers increases the duration of the intermediate phase, while stabilization of the fibrils slows the growth phase.
Several previous in vitro studies have shown that apoE isoforms delay Aβ aggregation,6,17,18,42−44 consistent with the observations reported here. In contrast, some early studies reported the role of apoE as accelerating the fibrillization of Aβ.3,5,45 These latter studies, however, used only the lipid-free form of apoE and unusually high concentrations (∼100 μM) of Aβ at which almost all Aβ exists as a mixture of oligomers.26,46,47 Under these conditions, apoE may bind to the oligomers and promote fibril formation by inhibiting nonspecific growth of the oligomers to amorphous aggregates.
ApoE Binding to Aβ Oligomers
The fact that substoichiometric amounts of apoE (1:200 apoE:Aβ) affect Aβ aggregation (see Figure 1) indicates that apoE either does not bind or binds only poorly to the monomeric form of Aβ. As noted earlier, TMR-Aβ1–42 shows a clear oligomerization phase that arises because of quenching of the TMR fluorescence in the oligomers.26 This phase is abolished by apoE (Figure 1D,E). This could happen for two reasons: either the Aβ oligomers are not forming, or the fluorescence of the oligomers is not quenched when they are bound to apoE. We favor the latter that is most likely a consequence of a change in the spatial proximity of the TMR moieties of TMR-Aβ, suggesting that the TMR moieties are not in direct contact on the surface of the apoE molecules. Previous in vitro studies have reported that apoE binds to the Aβ oligomers and stabilizes them.17,18 Furthermore, complexes of apoE and Aβ oligomers have been observed in the synapses in the human brain using an Aβ oligomer specific antibody such as NAB61 and apoE specific antibody WU-E4.48 Thus, we would suggest that binding to apoE leads to stabilization of the Aβ oligomers that are in a different orientation.
While apoE–Aβ interactions have been studied extensively, there are considerable differences in the published results on the nature of these interactions.25 Many studies have reported strong interactions between apoE and Aβ,9,16−18,25 but a recent study by Verghese et al. suggests that the apoE proteins do not bind to soluble (i.e., monomeric) Aβ.11 Our results summarized in the model presented in Figure 5 illustrate that apoE interacts with the Aβ oligomers and fibrils that form in a time- and concentration-dependent manner. Hence, little interaction may be observed at low Aβ concentrations that consist predominantly of monomeric populations of Aβ.11 While the biophysical properties of the Aβ oligomers that interact with apoE are still unclear, the EM images of the nanogold-apoE and Aβ complexes shown in Figure 3B indicate that there is considerable heterogeneity in the size and structures of the Aβ oligomers. It is likely that the interactions are strongly dependent on both the size of the oligomers and the conformations of Aβ in the oligomers.
ApoE4 is the strongest risk factor for AD and is also associated with a higher amyloid plaque load in the brain.33 In amyloid precursor protein (APP) mouse models, the presence of human apoE4 has been associated with accelerated deposition of Aβ in the brain.7,36,49 This apparently contradicts the in vitro observations reported here that show delayed aggregation of Aβ in the presence of apoE4. However, recent experiments suggest that soluble intermediates and not the fibrillar deposits of Aβ are the primary cytotoxic species in AD.9,23−25,27 Hence, we believe that the detrimental effects of apoE4 are mediated by the stabilization of the toxic intermediates of Aβ. Fibrillar deposition of Aβ in the brain is possibly a later event that occurs as a consequence of the accumulation of the toxic intermediates. The effects of apoE4 could be further exacerbated because of the retarded clearance of soluble Aβ from the extracellular space in the brain in the presence of apoE4.11 Our results are consistent with the in vivo observations that apoE proteins are colocalized with the amyloid plaques in the brains of those with late-onset Alzheimer’s disease. In particular, apoE is found associated with the dense core plaques.7 It has been observed that transgenic AD mouse models show an abundance of dense core plaques in the brain, but apoE (−/−) knockout mice show only diffuse plaques.7 We speculate that stabilization of the amyloid fibrils by apoE leads to the formation of the dense plaques as opposed to the formation of diffuse plaques in vivo.
The Issue of Lipidation of ApoE
An important issue is the role of lipid with respect to binding of Aβ to apoE. Lipid-free apoE has a compact structure comprising a four-helix bundle N-terminal domain and a C-terminal domain containing stretches of helical and unstructured regions.20 The proposed models for binding of apoE to lipid particles (DPPC), using X-ray and small angle X-ray scattering measurements, are clearly different from the structure of lipid-free apoE.21 Using various C-terminally truncated forms of apoE, Strittmatter et al. postulated that the Aβ binding region is localized in the C-terminal region of apoE.50 This is consistent with our preliminary data presented in Figure S6 of the Supporting Information that indicate that the C-terminal region of apoE has a much stronger effect on the aggregation of Aβ than does the N-terminal domain. The C-terminal region is also shown to be required in the initiation of lipid binding of apoE.51 Thus, the initiation of lipid binding and Aβ binding may occur in the same region of the apoE structure. Why lipid-free and lipid-bound forms of apoE have similar effects on Aβ aggregation is still unclear. It is possible that apoE monomers may dissociate from the lipid surface to bind to the Aβ fibrils. However, establishing or eliminating such possibilities requires further investigation. We note here that although Aβ is known to interact with lipids52 we have verified that the low concentrations of lipids used in our experiments do not alter the aggregation kinetics of Aβ (data not shown).
Differences between the ApoE Isoforms
The data of Figure 1 indicate that effects of apoE on the aggregation of Aβ1–40 are isoform specific, with apoE4 having the strongest and apoE2 the weakest effects. As mentioned above, data shown in Figure S5 of the Supporting Information suggest that the interaction with Aβ most likely involves the C-terminal region of apoE, and it is certainly possible that there are structural differences between the isoforms in this region. This would be consistent with the structural differences that occur at the regions distant from the Cys-Arg mutation sites between the apoE isoforms as postulated by Frieden and Garai on the basis of hydrogen–deuterium exchange and mutation data.53 The structural differences may lead to differences in the binding with the Aβ oligomers or fibrils. It is surprising that apoE isoform specific differences were less pronounced in the case of Aβ1–42 than in the case of Aβ1–40. Why apoE isoforms differ in their interactions with Aβ1–40 but not with Aβ1–42 is unclear. However, it should be pointed out that the aggregation of Aβ1–40 is considerably slower than that of Aβ1–42 in part because of the less extensive formation of oligomers.26 Interesting speculation would be that the oligomeric intermediates of Aβ1–42 differ from those of Aβ1–40.
Characterization of Oligomers and Fibrils
We have used quenching of TMR fluorescence and enhancement of thioflavin T fluorescence to detect the appearance of the oligomers and/or the fibrils of Aβ. We have previously shown that during the early phase of aggregation there is quenching of TMR-Aβ fluorescence but no increase in the thioflavin T fluorescence, indicating the appearance of unstructured oligomers.26 However, during the growth phase of aggregation, there is both quenching of TMR fluorescence and an increase in ThT fluorescence indicating the appearance of the fibrils.26 The presence of oligomers and/or fibrils could also be detected by oligomer and fibril specific antibodies that are regularly used in cell culture and in vivo studies in which the assays mentioned above cannot be applied because of a lack of both sensitivity and specificity.
Conclusions
The results presented here clearly indicate that apoE interacts with both oligomeric intermediates and fibrils of Aβ with high affinity, which is evident from the striking effects observed even with very low concentrations (nanomolar) of apoE used in our experiments. The binding of apoE affects the kinetics of fibrillization of Aβ by altering the mechanism of growth of the intermediates and the fibrils. Particularly important is the apoE–fibril interaction because the fibrils have a defined structure. Our results open the possibility for future studies toward identification and hence therapeutic targeting of the residues at the interface of the apoE–Aβ complexes.
Acknowledgments
We thank Drs. Scott Crick and Jan Bieschke for useful discussions.
Supporting Information Available
Time course of ThT fluorescence of WT-Aβ1–40 and Aβ1–42 in the presence of the apoE isoforms (Figure S1), effect of apoE lipoproteins derived from human cerebrospinal fluid (CSF) on the aggregation of TMR-Aβ1–42 (Figure S2), effect of DMPC–apoE on the growth of the Aβ fibrils (Figure S3), control image for examination of nonspecific labeling of the Aβ1–42 fibrils (Figure S4), EM images of the WT-Aβ1–42 fibrils before sonication (Figure S5), and a comparison of the effects of the N- and C-terminal fragments of the apoE isoforms on the aggregation of Aβ (Figure S6). This material is available free of charge via the Internet at http://pubs.acs.org.
Author Present Address
∥ P.B.V.: C2N Diagnostics, Center for Emerging Technologies, St. Louis, MO 63108.
This work was supported by the BrightFocus Foundation (to C.F.), a BrightFocus Fellowship Grant (to P.B.V.), and National Institutes of Health Grant AG13956 and the Cure Alzheimer’s Fund (to D.M.H.).
The authors declare the following competing financial interest(s): D.M.H. is a co-founder and scientific advisor to C2N Diagnostics, LLC, as well as a consultant for Genentech, AstraZeneca, and Forum pharmaceuticals. For other authors, there is no conflict of interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Strittmatter W. J.; Saunders A. M.; Schmechel D.; Pericak-Vance M.; Enghild J.; Salvesen G. S.; Roses A. D. (1993) Apolipoprotein E: High-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 90, 1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder E. H.; Saunders A. M.; Pericak-Vance M. A.; Roses A. D. (1995) There is a pathologic relationship between ApoE-ε4 and Alzheimer’s disease. Arch. Neurol. 52, 650–651. [DOI] [PubMed] [Google Scholar]
- Ma J.; Yee A.; Brewer H. B. Jr.; Das S.; Potter H. (1994) Amyloid-associated proteins α1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer β-protein into filaments. Nature 372, 92–94. [DOI] [PubMed] [Google Scholar]
- Wisniewski T.; Frangione B. (1992) Apolipoprotein E: A pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci. Lett. 135, 235–238. [DOI] [PubMed] [Google Scholar]
- Wisniewski T.; Castano E. M.; Golabek A.; Vogel T.; Frangione B. (1994) Acceleration of Alzheimer’s fibril formation by apolipoprotein E in vitro. Am. J. Pathol. 145, 1030–1035. [PMC free article] [PubMed] [Google Scholar]
- Evans K. C.; Berger E. P.; Cho C. G.; Weisgraber K. H.; Lansbury P. T. Jr. (1995) Apolipoprotein E is a kinetic but not a thermodynamic inhibitor of amyloid formation: Implications for the pathogenesis and treatment of Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 92, 763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman D. M.; Bales K. R.; Tenkova T.; Fagan A. M.; Parsadanian M.; Sartorius L. J.; Mackey B.; Olney J.; McKeel D.; Wozniak D.; Paul S. M. (2000) Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 97, 2892–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinson H.; Lev D.; Masliah E.; Michaelson D. M. (2008) Activation of the Amyloid Cascade in Apolipoprotein E4 Transgenic Mice Induces Lysosomal Activation and Neurodegeneration Resulting in Marked Cognitive Deficits. J. Neurosci. 28, 4690–4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T.; Serrano-Pozo A.; Hori Y.; Adams K. W.; Takeda S.; Banerji A. O.; Mitani A.; Joyner D.; Thyssen D. H.; Bacskai B. J.; Frosch M. P.; Spires-Jones T. L.; Finn M. B.; Holtzman D. M.; Hyman B. T. (2012) Apolipoprotein E, especially apolipoprotein E4, increases the oligomerization of amyloid β peptide. J. Neurosci. 32, 15181–15192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer P. E.; Cirrito J. R.; Wesson D. W.; Lee C. Y.; Karlo J. C.; Zinn A. E.; Casali B. T.; Restivo J. L.; Goebel W. D.; James M. J.; Brunden K. R.; Wilson D. A.; Landreth G. E. (2012) ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science 335, 1503–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese P. B.; Castellano J. M.; Garai K.; Wang Y.; Jiang H.; Shah A.; Bu G.; Frieden C.; Holtzman D. M. (2013) ApoE influences amyloid-β (Aβ) clearance despite minimal apoE/Aβ association in physiological conditions. Proc. Natl. Acad. Sci. U.S.A. 110, E1807–E1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biere A. L.; Ostaszewski B.; Stimson E. R.; Hyman B. T.; Maggio J. E.; Selkoe D. J. (1996) Amyloid β-Peptide Is Transported on Lipoproteins and Albumin in Human Plasma. J. Biol. Chem. 271, 32916–32922. [DOI] [PubMed] [Google Scholar]
- Fagan A. M.; Younkin L. H.; Morris J. C.; Fryer J. D.; Cole T. G.; Younkin S. G.; Holtzman D. M. (2000) Differences in the Aβ40/Aβ42 ratio associated with cerebrospinal fluid lipoproteins as a function of apolipoprotein E genotype. Ann. Neurol. 48, 201–210. [PubMed] [Google Scholar]
- Tai L. M.; Bilousova T.; Jungbauer L.; Roeske S. K.; Youmans K. L.; Yu C.; Poon W. W.; Cornwell L. B.; Miller C. A.; Vinters H. V.; Van Eldik L. J.; Fardo D. W.; Estus S.; Bu G.; Gylys K. H.; LaDu M. J. (2013) Levels of soluble apolipoprotein E/amyloid-β complex are reduced and oligomeric Aβ increased with APOE4 and Alzheimer disease in a transgenic mouse model and human samples. J. Biol. Chem. 288, 5914–5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba Y.; Tomonaga M.; Kawasaki H.; Otomo E.; Ikeda K. (1991) Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 541, 163–166. [DOI] [PubMed] [Google Scholar]
- LaDu M. J.; Falduto M. T.; Manelli A. M.; Reardon C. A.; Getz G. S.; Frail D. E. (1994) Isoform-specific binding of apolipoprotein E to β-amyloid. J. Biol. Chem. 269, 23403–23406. [PubMed] [Google Scholar]
- Cerf E.; Gustot A.; Goormaghtigh E.; Ruysschaert J. M.; Raussens V. (2011) High ability of apolipoprotein E4 to stabilize amyloid-β peptide oligomers, the pathological entities responsible for Alzheimer’s disease. FASEB J. 25, 1585–1595. [DOI] [PubMed] [Google Scholar]
- Ly S.; Altman R.; Petrlova J.; Lin Y.; Hilt S.; Huser T.; Laurence T. A.; Voss J. C. (2013) Binding of apolipoprotein E inhibits the oligomer growth of amyloid-β peptide in solution as determined by fluorescence cross-correlation spectroscopy. J. Biol. Chem. 288, 11628–11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatters D. M.; Peters-Libeu C. A.; Weisgraber K. H. (2006) Apolipoprotein E structure: Insights into function. Trends Biochem. Sci. 31, 445–454. [DOI] [PubMed] [Google Scholar]
- Chen J.; Li Q.; Wang J. (2011) Topology of human apolipoprotein E3 uniquely regulates its diverse biological functions. Proc. Natl. Acad. Sci. U.S.A. 108, 14813–14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters-Libeu C. A.; Newhouse Y.; Hall S. C.; Witkowska H. E.; Weisgraber K. H. (2007) Apolipoprotein E-dipalmitoylphosphatidylcholine particles are ellipsoidal in solution. J. Lipid Res. 48, 1035–1044. [DOI] [PubMed] [Google Scholar]
- Garai K.; Sahoo B.; Kaushalya S. K.; Desai R.; Maiti S. (2007) Zinc lowers amyloid-β toxicity by selectively precipitating aggregation intermediates. Biochemistry 46, 10655–10663. [DOI] [PubMed] [Google Scholar]
- Cleary J. P.; Walsh D. M.; Hofmeister J. J.; Shankar G. M.; Kuskowski M. A.; Selkoe D. J.; Ashe K. H. (2005) Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat. Neurosci. 8, 79–84. [DOI] [PubMed] [Google Scholar]
- Jin M.; Shepardson N.; Yang T.; Chen G.; Walsh D.; Selkoe D. J. (2011) Soluble amyloid β-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc. Natl. Acad. Sci. U.S.A. 108, 5819–5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D. B. (2005) The interaction of amyloid-β with ApoE. Subcell. Biochem. 38, 255–272. [DOI] [PubMed] [Google Scholar]
- Garai K.; Frieden C. (2013) Quantitative analysis of the time course of Aβ oligomerization and subsequent growth steps using tetramethylrhodamine-labeled Aβ. Proc. Natl. Acad. Sci. U.S.A. 110, 3321–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garai K.; Mustafi S. M.; Baban B.; Frieden C. (2010) Structural differences between apolipoprotein E3 and E4 as measured by 19F NMR. Protein Sci. 19, 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garai K.; Baban B.; Frieden C. (2011) Dissociation of apoE oligomers to monomers is required for high affinity binding to phospholipid vesicles. Biochemistry 50, 2550–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Vasudevan S.; Sojitrawala R.; Zhao W.; Cui C.; Xu C.; Fan D.; Newhouse Y.; Balestra R.; Jerome W. G.; Weisgraber K.; Li Q.; Wang J. (2007) A monomeric, biologically active, full-length human apolipoprotein E. Biochemistry 46, 10722–10732. [DOI] [PubMed] [Google Scholar]
- Schneeweis L. A.; Koppaka V.; Lund-Katz S.; Phillips M. C.; Axelsen P. H. (2005) Structural Analysis of Lipoprotein E Particles. Biochemistry 44, 12525–12534. [DOI] [PubMed] [Google Scholar]
- Huang R. Y. C.; Garai K.; Frieden C.; Gross M. L. (2011) Hydrogen/Deuterium Exchange and Electron-Transfer Dissociation Mass Spectrometry Determine the Interface and Dynamics of Apolipoprotein E Oligomerization. Biochemistry 50, 9273–9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W. F.; Radford S. E. (2013) An imaging and systems modeling approach to fibril breakage enables prediction of amyloid behavior. Biophys. J. 105, 2811–2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. C.; Roe C. M.; Xiong C.; Fagan A. M.; Goate A. M.; Holtzman D. M.; Mintun M. A. (2010) APOE predicts amyloid-β but not tau Alzheimer pathology in cognitively normal aging. Ann. Neurol. 67, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R.; Sagare A.; Hamm K.; Parisi M.; Lane S.; Finn M. B.; Holtzman D. M.; Zlokovic B. V. (2008) apoE isoform-specific disruption of amyloid β peptide clearance from mouse brain. J. Clin. Invest. 118, 4002–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDu M. J.; Shah J. A.; Reardon C. A.; Getz G. S.; Bu G.; Hu J.; Guo L.; Van Eldik L. J. (2001) Apolipoprotein E and apolipoprotein E receptors modulate Aβ-induced glial neuroinflammatory responses. Neurochem. Int. 39, 427–434. [DOI] [PubMed] [Google Scholar]
- Bien-Ly N.; Gillespie A. K.; Walker D.; Yoon S. Y.; Huang Y. (2012) Reducing human apolipoprotein E levels attenuates age-dependent Aβ accumulation in mutant human amyloid precursor protein transgenic mice. J. Neurosci. 32, 4803–4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski M. J.; Pankiewicz J.; Scholtzova H.; Mehta P. D.; Prelli F.; Quartermain D.; Wisniewski T. (2006) Blocking the apolipoprotein E/amyloid-β interaction as a potential therapeutic approach for Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 103, 18787–18792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Culyba E. K.; Powers E. T.; Kelly J. W. (2011) Amyloid-β forms fibrils by nucleated conformational conversion of oligomers. Nat. Chem. Biol. 7, 602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles T. P.; Waudby C. A.; Devlin G. L.; Cohen S. I.; Aguzzi A.; Vendruscolo M.; Terentjev E. M.; Welland M. E.; Dobson C. M. (2009) An analytical solution to the kinetics of breakable filament assembly. Science 326, 1533–1537. [DOI] [PubMed] [Google Scholar]
- Cohen S. I.; Linse S.; Luheshi L. M.; Hellstrand E.; White D. A.; Rajah L.; Otzen D. E.; Vendruscolo M.; Dobson C. M.; Knowles T. P. (2013) Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proc. Natl. Acad. Sci. U.S.A. 110, 9758–9763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W.-F.; Hellewell A. L.; Gosal W. S.; Homans S. W.; Hewitt E. W.; Radford S. E. (2009) Fibril Fragmentation Enhances Amyloid Cytotoxicity. J. Biol. Chem. 284, 34272–34282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster S.; Rogers J. (1996) Relative efficacies of amyloid β peptide (Aβ) binding proteins in Aβ aggregation. J. Neurosci. Res. 46, 58–66. [DOI] [PubMed] [Google Scholar]
- Chauhan A.; Pirttila T.; Mehta P.; Chauhan V. P.; Wisniewski H. M. (1996) Effect of cerebrospinal fluid from normal and Alzheimer’s patients with different apolipoprotein E phenotypes on in vitro aggregation of amyloid β-protein. J. Neurol. Sci. 141, 54–58. [DOI] [PubMed] [Google Scholar]
- Beffert U.; Poirier J. (1998) ApoE associated with lipid has a reduced capacity to inhibit β-amyloid fibril formation. NeuroReport 9, 3321–3323. [DOI] [PubMed] [Google Scholar]
- Castano E. M.; Prelli F.; Wisniewski T.; Golabek A.; Kumar R. A.; Soto C.; Frangione B. (1995) Fibrillogenesis in Alzheimer’s disease of amyloid β peptides and apolipoprotein E. Biochem. J. 306(Part 2), 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan G.; Lomakin A.; Teplow D. B. (2001) Amyloid β-protein oligomerization: Prenucleation interactions revealed by photo-induced cross-linking of unmodified proteins. J. Biol. Chem. 276, 35176–35184. [DOI] [PubMed] [Google Scholar]
- Bernstein S. L.; Dupuis N. F.; Lazo N. D.; Wyttenbach T.; Condron M. M.; Bitan G.; Teplow D. B.; Shea J. E.; Ruotolo B. T.; Robinson C. V.; Bowers M. T. (2009) Amyloid-β protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat. Chem. 1, 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie R. M.; Hashimoto T.; Tai H. C.; Kay K. R.; Serrano-Pozo A.; Joyner D.; Hou S.; Kopeikina K. J.; Frosch M. P.; Lee V. M.; Holtzman D. M.; Hyman B. T.; Spires-Jones T. L. (2012) Apolipoprotein E4 effects in Alzheimer’s disease are mediated by synaptotoxic oligomeric amyloid-β. Brain 135, 2155–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Eltorai A. E.; Jiang H.; Liao F.; Verghese P. B.; Kim J.; Stewart F. R.; Basak J. M.; Holtzman D. M. (2012) Anti-apoE immunotherapy inhibits amyloid accumulation in a transgenic mouse model of Aβ amyloidosis. J. Exp. Med. 209, 2149–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W. J.; Weisgraber K. H.; Huang D. Y.; Dong L. M.; Salvesen G. S.; Pericak-Vance M.; Schmechel D.; Saunders A. M.; Goldgaber D.; Roses A. D. (1993) Binding of human apolipoprotein E to synthetic amyloid β peptide: Isoform-specific effects and implications for late-onset Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 90, 8098–8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M.; Vedhachalam C.; Sakamoto T.; Dhanasekaran P.; Phillips M. C.; Lund-Katz S.; Saito H. (2006) Effect of Carboxyl-Terminal Truncation on Structure and Lipid Interaction of Human Apolipoprotein E4. Biochemistry 45, 4240–4247. [DOI] [PubMed] [Google Scholar]
- Hoshino T.; Mahmood I.; Mori K.; Matsuzaki K. (2013) Binding and Aggregation Mechanism of Amyloid β-Peptides onto the GM1 Ganglioside-Containing Lipid Membrane. J. Phys. Chem. B 117, 8085–8094. [DOI] [PubMed] [Google Scholar]
- Frieden C.; Garai K. (2012) Structural differences between apoE3 and apoE4 may be useful in developing therapeutic agents for Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 109, 8913–8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.