Abstract
There is evidence that having a stronger sense of positive well-being may be a potential resource for healthier aging as represented by slower physical decline, reduced risk of frailty and longer survival. However, it is unclear whether positive well-being is protective of another crucial component of healthy aging, cognitive function, or whether it has a bidirectional relationship with cognitive function. We use multilevel models with within-person centering to estimate the within- and between-person association between cognitive function and positive well-being in 4 waves of data from the English Longitudinal Study of Ageing (ELSA), (N = 10985, aged 50–90 years at wave 1). Our findings show that, although most variation in cognitive function was explained by age, and most variation in well-being was explained by depression, small but significant associations between cognition and well-being remained after variation in age and depression were controlled. In models where cognition was the outcome, the association was mainly because of variation in mean levels of well-being between persons. In models where well-being was the outcome, the association was mainly because of within-person fluctuation in cognitive test performance. Exercise and depression were the most important moderating influences on the association between cognition and positive well-being. Depression had greater effect upon this association for those with higher well-being, but exercise protected cognitive performance against the adverse effects of lower well-being.
Keywords: positive well-being, cognitive function, multilevel model, within-person change
Maintaining positive psychological well-being in the face of the changes and losses of later life is generally considered a crucial part of “healthy” ageing (Baltes & Baltes, 1990; Rowe & Kahn, 1997; Jeste, Depp, & Vahia, 2010). In recent years there has been growing evidence to suggest that well-being may also be a potential resource for ageing well. Prospective studies have found that older people with greater well-being are less likely to develop problems with mobility or other activities of daily life (Collins, Goldman, & Rodriguez, 2008; Ostir, Markides, Black, & Goodwin, 2000; Boyle, Buchman, & Bennett, 2010), or to become physically frail (Gale, Cooper, Deary, & Aihie Sayer, 2014; Ostir, Ottenbacher, & Markides, 2004). In a meta-analysis of 35 prospective studies where greater positive well-being was linked with a reduced risk of mortality both in healthy populations and those already ill at baseline, the survival benefit associated with greater well-being was particularly marked in people aged 60 and over (Chida & Steptoe, 2008). In all these studies the protective effect of positive well-being persisted after adjustment for negative affect, and so was not due merely to the absence of depression.
It is unclear whether positive well-being might be a protective factor with regard to another crucial component of healthy ageing, cognitive function (Fiocco & Yaffe, 2010; Rowe & Kahn, 1997; Baltes & Baltes, 1990). Most early studies, largely carried out in small, nonrepresentative samples, found no link between positive well-being and cognition (Diener, 1984). More recent cross-sectional analyses in several cohorts of older people have found either small to moderate positive correlations (Isaacowitz & Smith, 2003; Gale, Cooper, et al. 2012) or no association (Gow et al., 2005). Whether these significant cross-sectional associations reflect an effect of well-being on cognition or vice versa is uncertain. Most prospective studies that have examined whether mental states might influence subsequent cognitive function have focused on the negative mental state of depression, and their findings have been mixed (see review in Gale et al., 2012). Whereas it is likely that depression may be accompanied by lower positive well-being, it is increasingly recognized that positive well-being and negative mental states are to some degree independent of each other (Diener & Emmons, 1984; Huppert & Whittington, 2003), as suggested by the findings that positive well-being is predictive of various health outcomes after controlling for the presence of depression (Chida & Steptoe, 2008; Ostir et al., 2000; Ostir et al., 2004; Collins et al., 2008; Boyle, Buchman, Barnes, & Bennett, 2010). As yet, very few prospective studies have examined whether higher positive well-being might protect against cognitive decline. In one such study of people aged 70 and over, greater positive well-being—assessed using a measure based on items about life satisfaction and satisfaction with ageing—was linked with slower decline in perceptual speed (Gerstorf, Lovden, Rocke, Smith, & Lindenberger, 2007). There is some evidence linking a stronger sense of perceived control—sometimes viewed as a facet of eudaimonic well-being—with better performance on cognitive tests (Welch & West, 1995; West, Bagwell, & Dark-Freudeman, 2008). Longitudinal evidence linking a stronger sense of perceived control with less cognitive decline is inconsistent. In a study that examined within-person changes in perceived control in relation to rates of change in cognition, increases in control were only weakly and not significantly linked to reduced rates of cognitive decline (Windsor & Anstey, 2008). But older people with a stronger sense of perceived control had fewer difficulties in performing cognitive tasks 20 years later (Caplan & Schooler, 2003). Having a stronger sense of purpose in life—also viewed as a facet of eudaimonic well-being (Ryff, 1989)—has been associated with a reduced risk of incident Alzheimer’s disease and mild cognitive impairment (Boyle, Buchman, Barnes, & Bennett, 2010).
Positive well-being might influence cognitive function in later life through several mechanisms. There is considerable evidence from experimental studies that induced states of positive well-being improve performance on a range of cognitive tasks, possibly because of raised dopamine levels (Isen, Rosenzweig, & Young, 1991; Ashby et al., 1999; Isen, 2008 and 2009). Higher levels of positive well-being have been associated with reduced neuroendocrine activity, lower concentrations of inflammatory factors and triglycerides, higher concentrations of high-density-lipoprotein cholesterol, lower heart rate and blood pressure and less central obesity (Steptoe, Wardle, & Marmot, 2005; Steptoe, Demakakos, de Oliveira, & Wardle, 2012), and a reduction in risk of incident coronary heart disease (Davidson, Mostofsky, & Whang, 2010). All of these occurred independently of depressive symptoms. Associations between positive well-being and cognitive decline could potentially be moderated by these factors.
It is plausible that the relationship between positive well-being and cognitive function is bidirectional. At older ages impaired cognition may constrain the ability to manage usual activities of daily life and hence cause decline in well-being. Longitudinal evidence in support of this is limited. One study of people aged 70 and over found no evidence that increases in cognitive limitations, as measured by the Mini Mental State Examination (MMSE), over a 54-month period influenced the trajectory of positive well-being (Kurland, Gill, Patrick, Larson, & Phelan, 2006). However, the MMSE is a crude estimate of cognitive function, and has a marked ceiling effect in nonclinical samples. In the Berlin Ageing Study no association was found between decline in perceptual speed and trajectory of well-being over a 13-year period (Gerstorf et al., 2007), but perceptual speed is just one domain of cognitive functioning. In a longitudinal study of people aged 78–98 where participants took tests of executive function, processing speed, episodic memory, sematic memory, spatial ability and working memory, better performance on tests of processing speed and spatial ability, but not on tests of other cognitive domains, were significantly associated with slightly higher scores on a measure of life satisfaction 3 years later after adjustment for potential confounding factors including depressive symptoms (Enkvist, Ekstrom, & Elmstahl, 2013). However, no adjustment was made for levels of life satisfaction at baseline, so it is impossible to be certain whether processing speed or spatial ability were linked with trajectory of life satisfaction or merely with its level at follow-up. In a study using two waves of data from the Mid Life in the United States survey (MIDUS), Rocke and Lachman (2008) used cluster analysis to identify profiles of subjective change in life satisfaction from past to future and investigated whether cognition was associated with these profiles. Three different profiles were identified based on participants’ perceived trajectories of life satisfaction, but after adjustment for sociodemographic variables, there was no difference in score on a composite measure of cognitive function between participants in the three groups. Some rather more persuasive evidence that poorer cognitive function might have an adverse effect on well-being came from a study where participants from MIDUS completed positive and negative mood reports and a diary of stressors over eight consecutive days (Stawski, Almeida, Lachman, & Tun, 2010). Participants with better cognition tended to experience smaller decreases in positive mood and smaller increases in negative mood after exposure to stressors, suggesting that they may be more emotionally resilient in the face of daily stress. In an investigation of change in depressive symptoms before and after the onset of Alzheimer’s disease, scores on a subset of items in the Center for Epidemiologic Studies Depression scale that asks about positive affect decreased before and after diagnosis, but the change was very small (Wilson et al., 2010) It has been suggested that cognitive decline might have a greater adverse impact on eudaimonic aspects of well-being than on the hedonic aspects of positive affect or life satisfaction (Wilson et al., 2013). Some evidence that this might be the case came from a 5-year follow-up study where more rapid cognitive decline was associated with greater decline in scores for several domains of eudaimonic well-being, as measured by Ryff’s Scales of Psychological Wellbeing (Ryff, 1989), especially purpose in life (Wilson et al., 2013). Rate of decline of three of the four specific cognitive domains assessed (episodic memory, semantic memory and perceptual speed) was also associated with rate of decline in purpose in life, and the association between rates of decline in working memory and purpose in life was of borderline significance (Wilson et al., 2013).
In the present study we used mixed-effects modeling of data from four waves of the English Longitudinal Study of Ageing (ELSA) to investigate the within- and between-persons associations between positive well-being—assessed using a measure that reflects both hedonic and eudaimonic domains—and cognition based upon general cognitive function and markers of three different important domains of cognitive function—processing speed, memory and executive function— in community-dwelling, nondemented older people. In a previous analysis using this cohort, we examined whether there was a bidirectional relationship between depressive symptoms and general cognitive ability and found some evidence that depression might influence cognitive decline, but there was no indication of a reverse effect (Gale, Allerhand, & Deary, 2012). No attempt was made in that study to distinguish between the within-person and between-person effects of depression and cognition that could have led to incorrect inferences about change and its determinants (Sliwinski, Hoffman, & Hofer, 2010) and we did not examine the domains of cognition separately. In the present study we aimed to build on our earlier work in this cohort by exploring the within- and between-persons associations between positive well-being and cognitive function and investigating whether any such associations were independent of depression. Based on the evidence of previous work, we hypothesized that this would be the case. In addition to depressive symptoms, we investigated whether physical health (Bond, Dickinson, Matthews, Jagger, & Brayne, 2006; Pressman & Cohen, 2005), exercise (Fratiglioni, Paillard- Borg, & Winblad, 2004; Netz, Wu, Becker, & Tenenbaum, 2005), smoking (Nooyens, van Gelder, & Verschuren, 2008), education (Anstey & Christensen, 2000; Dear, Henderson, & Korten, 2002), household wealth or difficulties in activities of daily living (Koster et al., 2005; Dear et al., 2002; Rajan, Hebert, Scherr, Mendes de Leon, & Evans, 2012) moderated any such associations. These factors have been associated with both positive well-being and cognitive function in the cited previous studies.
Method
The Data
The English Longitudinal Study of Ageing (ELSA) is an ongoing longitudinal study of adults aged 50 and over (Marmot et al., 2011). In total, 11,392 sample members took part in the first ELSA follow-up in 2002–2003. There were further follow-up surveys of the cohort in 2004–2005, 2006–2007, and 2008–2009. Everyone was measured at roughly the same time at each follow-up (within a year of each other), and were followed up at roughly the same 2-year intervals. In particular, for each participant at each wave, cognition and well-being were assessed during the same interview on the same day. The mean age at baseline was 65 years. People entering the study ranged in age from 50 to over 90 years. The variables we extracted from the ELSA data set are broadly divided into time-varying measures of cognitive ability and positive well-being taken at four waves of measurements, and time-invariant observations of several covariates taken at or before the first wave. We excluded cases who had reported by wave 1 that a doctor had told them that they had Alzheimer’s disease, Parkinson’s disease, dementia, organic brain syndrome or serious memory impairment (N = 120), and those aged over 90 at wave 1 (N = 93). After these exclusions our analytical sample was based on 10,985 people.
Measurement of Cognitive Function
The ELSA data include scores on four tests of cognitive function: verbal fluency, immediate and delayed verbal memory, and attention (Steel, Huppert, McWilliams, & Melzer, 2003). Verbal (semantic) fluency was assessed by asking participants to name as many animals as they could think of in 1 minute. Immediate and delayed verbal memory was assessed using lists of nouns presented aurally. Attention was assessed using a letter cancellation task. Scores on these tests were used as measures of three kinds of cognitive function: the scores on the animal naming task were taken as a measure of executive function, the sum of the scores on the immediate and delayed recall tasks were taken as a measure of memory, and the scores on the letter cancellation task were taken as a measure of processing speed. The Pearson correlation between the scores on the immediate and delayed recall tasks at each wave were: 0.70, 0.70, 0.73, and 0.75. These three cognitive measures were separately modeled to explore their different associations with well-being, but they were also combined into a single measure to explore the association between general cognitive function and well-being. The composite measure was derived as the score on the first principal component. The standardized loadings of executive function, memory, and processing speed were respectively: 0.80, 0.80, and 0.69, and the first component explained 58% of the variance. The distributions of these cognitive variables at each wave had no significant skewness. Each variable was centered on the variable’s mean at wave 1 and scaled into units of its standard deviation at wave 1.
Measurement of Positive Well-Being
The CASP-19 is a self-reported summative index consisting of 19 items designed to measure well-being (Wiggins, Netuveli, Hyde, Higgs, & Blane, 2008). The items cover four theoretical domains: control, autonomy, self-realization, and pleasure. In order to obtain a measure of positive well-being from this scale, we used the 13 items of the CASP-19 that are positively worded, following the example of Huppert and Whittington (2003) who created a measure of positive well-being from the positively worded items of the General Health Questionnaire. These 13 items assess both hedonic and eudaimonic aspects of well-being (Ryan & Deci, 2001). Examples include: “I enjoy the things that I do,” and “I feel that my life has meaning.” Cronbach’s alpha for the 13 items at each wave was respectively: 0.875, 0.882, 0.884, and 0.885. The positive well-being score was derived as the equally weighted sum of the responses to these 13 items. Over 92% of all respondents had no missing items, and over 97% had no more than one missing item, which was judged acceptable to be ignored. The response to each item was a 4-point Likert scale coded as 0 to 3, so the aggregate of the 13 items was a score between 0 and 39. The direction of the scale was adjusted so that a higher score represented greater positive well-being. The scores at each wave were scaled into units of the standard deviation at wave 1, and the mean score at each wave was measured from the mean score at wave 1. The overall distribution of positive well-being scores had mild negative skewness. Transformations to eliminate skewness had no substantial effect on the final estimates or their precision so these data were not transformed.
Age
The study sample members were followed up for four waves of measurement occasions at intervals of about 2 years. People entering the study ranged in age from 50 to over 90 years. The mean age at wave 1 was nearly 65 years with a standard deviation of nearly 10 years, (see Table 1). Age was used as the time variable in modeling. The age variable was centered on 70 years to reduce collinearity between linear and quadratic terms in the models.
Table 1. Characteristics of the Study Participants According to wave, (N = 10985).
| Wave 1 | Wave 2 | Wave 3 | Wave 4 | |
|---|---|---|---|---|
| N | 10985 | 8560 | 7345 | 6476 |
| Age: mean (SD) | 64.95 (9.99) | 66.7 (9.66) | 68.07 (9.47) | 69.62 (9.08) |
| Range: min, max | 50,90 | 52,92 | 54,94 | 56,95 |
| Interval: mean (SD) | 2.3 (0.46) | 1.82 (0.44) | 2.08 (0.42) | |
| Depression: mean | 1.60 | 1.54 | 1.51 | 1.47 |
| Sex: count(%age) | ||||
| female 0 | 6025 (54.8) | 4741 (55.4) | 4097 (55.8) | 3649 (56.3) |
| male 1 | 4960 (45.2) | 3819 (44.6) | 3248 (44.2) | 2827 (43.7) |
| Education: count(%age) | ||||
| 1 (least) | 44 (0.4) | 31 (0.4) | 28 (0.4) | 27 (0.4) |
| 2 | 2551 (23.8) | 1801 (21.5) | 1460 (20.4) | 1176 (18.6) |
| 3 | 3671 (34.2) | 2833 (33.9) | 2405 (33.5) | 2156 (34.1) |
| 4 | 1922 (17.9) | 1552 (18.6) | 1347 (18.8) | 1205 (19) |
| 5 | 727 (6.8) | 616 (7.4) | 550 (7.7) | 502 (7.9) |
| 6 | 539 (5) | 433 (5.2) | 383 (5.3) | 353 (5.6) |
| 7 (most) | 1282 (11.9) | 1095 (13.1) | 1001 (14) | 911 (14.4) |
| Wealth: count(%age) | ||||
| 1 (least) | 2087 (19.2) | 1456 (17.2) | 1188 (16.3) | 996 (15.5) |
| 2 | 2182 (20) | 1658 (19.5) | 1386 (19) | 1183 (18.4) |
| 3 | 2189 (20.1) | 1731 (20.4) | 1492 (20.5) | 1290 (20.1) |
| 4 | 2187 (20.1) | 1798 (21.2) | 1556 (21.4) | 1407 (21.9) |
| 5 (most) | 2243 (20.6) | 1844 (21.7) | 1657 (22.8) | 1541 (24) |
| Health: count(%age) | ||||
| 0 (fewest) | 3227 (29.6) | 2595 (30.6) | 2287 (31.4) | 2071 (32.2) |
| 1 | 3575 (32.8) | 2790 (32.8) | 2392 (32.8) | 2162 (33.6) |
| 2 | 2328 (21.4) | 1821 (21.4) | 1551 (21.3) | 1336 (20.8) |
| 3 | 1034 (9.5) | 764 (9) | 656 (9) | 536 (8.3) |
| 4 | 453 (4.2) | 332 (3.9) | 267 (3.7) | 227 (3.5) |
| 5 | 181 (1.7) | 121 (1.4) | 84 (1.2) | 62 (1) |
| 6 | 70 (0.6) | 55 (0.6) | 45 (0.6) | 27 (0.4) |
| 7 | 17 (0.2) | 12 (0.1) | 7 (0.1) | 6 (0.1) |
| 8 (most) | 8 (0.1) | 4 (0) | 2 (0) | 1 (0) |
| Smoking: count(%age) | ||||
| never smoked 1 | 3972 (36.2) | 3130 (36.6) | 2720 (37) | 2457 (37.9) |
| don’t smoke now 2 | 5071 (46.2) | 3964 (46.3) | 3397 (46.2) | 2971 (45.9) |
| smoke now 3 | 1942 (17.7) | 1466 (17.1) | 1228 (16.7) | 1048 (16.2) |
| Physical activity: count(%age) | ||||
| 1 (least) | 2730 (25.1) | 1900 (22.3) | 1538 (21) | 1254 (19.5) |
| 2 | 4256 (39.1) | 3410 (40) | 2932 (40.1) | 2597 (40.3) |
| 3 | 1241 (11.4) | 992 (11.6) | 874 (11.9) | 794 (12.3) |
| 4 (most) | 2652 (24.4) | 2214 (26) | 1971 (26.9) | 1799 (27.9) |
| Difficulties with activities of daily living | 2.76 | 2.55 | 2.47 | 2.31 |
Covariates
We used eight covariates that we grouped into three kinds. The first was depression. The second was demographic variables: sex, age at finishing full-time education, and household wealth. The third was health-related variables (besides depression): physical health, smoking status, physical exercise, and difficulties in activities of daily living. Symptoms of depression were assessed at waves 1–4 using an 8-item version of the Center for Epidemiological Studies-Depression Scale (CES-D). This has an internal consistency and factor structure that are comparable to longer versions of the scale (Turvey, Wallace, & Herzog, 1999). We derived a time-invariant depression score by summing the items at each wave and taking each person’s average across the waves to capture trait depression. A higher score represented greater depression. The data were log transformed to reduce the skew in the sample’s data, because the majority of people did not have much depressive symptomatology. Information on education and household wealth was collected at the initial interview. The wealth measure, which has been identified as the most accurate indicator of long-term socioeconomic circumstances in ELSA (Banks, Karlsen, & Oldfield, 2003), includes savings and investments, value of any property or business assets, net of debt, and excluding pension assets. For this analysis we used data on quintiles of wealth that were supplied with the data set. The education measure was age at leaving full-time education, and this was grouped into a 7-point scale in which the highest score represents the most educated. The physical health measure was derived as the count of the number of chronic physical health conditions. A simple unweighted count of the number of chronic health problems has been shown to be almost as effective as complex severity-weighted measures in predicting most outcomes, (Huntley, Johnson, Purdy, Valderas, & Salisbury, 2012). During wave 1, participants were asked whether a doctor had ever told them that they had any of the following conditions: high blood pressure/hypertension, angina, heart attack, congestive heart failure, diabetes or high blood sugar, a stroke, chronic lung disease, asthma, arthritis or rheumatism, osteoporosis, or cancer. We added the number of chronic conditions present to get a measure of physical health. The lowest score corresponds with the most healthy, in the sense of having the fewest chronic conditions. The physical exercise measure was derived from three ELSA variables describing frequency of vigorous, moderate and mild exercise. Combinations of the response to these variables were ranked according to the amount of exercise involved, and the results grouped into quartiles to form a 4-point scale representing increasing exercise: never or hardly ever; mild exercise about once a week; moderate but fairly regular exercise, such as a good walk; vigorous exercise fairly regularly. The measure of difficulties with activities of daily living (ADL) was derived from the participant’s response to a question asking whether they had any difficulty doing a range of activities. We created a score for difficulties in activities of daily living by summing the number of difficulties reported.
The covariates were treated as time-invariant (having the same value at each wave), either because the variable is intrinsically constant within-person throughout the study (such as sex), or because it was assumed to remain substantively constant. All covariates except sex were centered on their mean and scaled for unit standard deviation. (Sex was coded 0 = female, 1 = male). The direction of the scale of each of the covariates is shown in Table 1. To summarize, a higher score for each of the variables respectively represents: greater depression, male, more years in education, greater wealth, more chronic health problems, more smoking, more physical exercise, and more difficulties with activities of daily living.
Missing Values
Cases with missing values were excluded only where there were no measures at all on any of the variables of interest. No attempt was made to impute missing values except for sensitivity analysis, but rather we aim to show the missingness was ignorable under maximum likelihood estimation. Table 1 shows the count of participants at each wave, and shows the proportions of response levels within covariates for the participants still in the study at each wave. Despite falling numbers the proportions within each covariate remain reasonably constant across the waves, indicating that the sample remained reasonably similarly balanced on the covariates. In other words nothing in the covariates indicated selective dropout.
The effects of patterns of missing values in the outcome variables of cognitive function and positive well-being were tested using the procedure described by Hedeker and Gibbons (1997). We regressed the cognition and well-being outcome variables separately onto dummy variables indicating observations belonging to persons with particular patterns of missing values. Three dummy variables represented patterns of monotone missingness: dropout at the fourth, third, and second waves, and one dummy variable indicated any of the four nonmonotone patterns of missingness found in the data. The dummy variables were coded so that each coefficient was a mean outcome difference from the reference category representing completers. They entered regression models as main effects on the outcome intercept and as interactions with time. Covariates were included in blocks to explore how missingness effects were attenuated by covariate adjustment. About 39% of the sample were persons with monotone dropout, and about 6% had some nonmonotone missingness. There was no significant difference in initial status or slope of cognition or well-being because of nonmonotone missingness. In addition because there were relatively few cases of nonmonotone missingness we judged this to be ignorable. Persons who dropped out at some stage had a lower mean score and steeper decline in both cognition and well-being. The difference in mean score was greater for persons who dropped out earlier, but this was attenuated by covariate adjustment, primarily age and wealth. Persons who dropped out after wave 1 were 0.29 SDs of cognition and 0.37 SDs of well-being lower than the average completer. Those who dropped out after the second or third wave were respectively 0.16 and 0.12 SDs of cognition lower, and 0.12 and 0.02 SDs of well-being lower. There were no significant differences in the slope of cognition or well-being, or in the mean levels of well-being, because of monotone missingness. However the difference in mean cognition was significant (p = .004) for persons who dropped out for the last one or two waves. Therefore we carried out a sensitivity analysis to estimate variation and bias in the parameter estimates for models of cognition using data imputed for missing values in the last two waves. In a two-stage procedure we first estimated prediction intervals for each missing value using individual growth curves based on all available information from shrinkage estimates of each person’s linear growth parameters. In the second stage we created 20 data sets each with different imputed missing values by randomly sampling with uniform probability within the prediction intervals. The final full models were fitted to each of these data sets and the variation in the resulting parameters was assessed. The sign and significance of model parameters was the same across the multiple data sets. Therefore we judged the occurrence of monotone missingness to be ignorable when the covariates were included in the model.
Models
Our overall aim was to explore the within- and between-person associations between well-being and cognition, and how these change with age, and with levels of depression, demographic covariates, and other health-related covariates. We used two-level mixed effects models instead of latent growth models for this application because disentangling within- and between-person effects is difficult with a latent growth model (e.g., Curran & Bauer, 2011), and it is straightforward to incorporate age as a continuous time variable in a multilevel model, which suited the wide age range of our data.
Several checks were carried out to assess whether the data met the assumptions required for analysis of within- and between-person associations: normality and homogeneity of within-person sampling variability across age. Violations of the latter are known to attenuate between-person effects (Ludtke et al., 2008). The proportions of within- and between-person variability were assessed using the intraclass correlation for each of the four time-varying variables. Additionally, their within-person sampling variability was explored (cf. Salthouse, 2007). These results are given in Table 2. Intraclass correlation was between 0 and 1, indicating that outcome variation was not exclusively within-person or between-person, but a mixture of these things. Within-person variability was about half the magnitude of the variability in between-person mean levels in standard deviations. There was positive correlation between the mean level and within-person variability for processing speed and executive function, but little correlation for memory. Interestingly there was negative correlation for well-being, indicating variability was smaller for people with higher mean levels of well-being. Correlations between mean levels and age were all negative, but correlations between within-person variability and age were all small, indicating little systematic change in within-person sampling variability with age. Regressing within-person standard deviation onto mean age indicated the rate of linear change in variability was less than 0.003 standard deviations per year for all time-varying variables.
Table 2. Intraclass Correlation and Indices of Within-Person Sampling Variability.
| ICC | M(SD)/SD(M) | M.SD | M.age | S.age | |
|---|---|---|---|---|---|
| Note. ICC = intraclass correlation; M(SD)/SD(M) = ratio of the average within-person standard deviation to the standard deviation of the person mean; M.SD = correlation between person mean and within-person standard deviation; M.age = correlation between person mean and age; S.age = correlation between within-person standard deviation and age. | |||||
| Well-being | 0.674 | 0.495 | −0.392 | −0.136 | 0.074 |
| Executive function | 0.605 | 0.585 | 0.121 | −0.404 | −0.066 |
| Memory | 0.611 | 0.605 | −0.041 | −0.501 | 0.031 |
| Processing speed | 0.588 | 0.533 | 0.281 | −0.385 | 0.031 |
Initially eight mixed-effects models were fitted: four models of well-being each predicted by either the composite cognitive measure or one of the three time-varying measures of cognition, and four models of cognition, one for each of the measures, each predicted by the time-varying measure of well-being. All models had random intercept and slope within persons that were allowed to covary. The residuals were assessed graphically for normality and judged to be acceptable. The models were then respecified using group-mean centering (here person-mean centering, Hoffman & Stawski, 2009). Each time-varying independent variable was replaced by two independent variables: a within-person centered (WP) variable and a person mean (PM) variable. The WP variable entered as a time-varying (level-1) variable. The PM variable was centered on its mean, and entered as a time-invariant (level-2) variable in the equations for the random intercept (the PM main effect), and the random slope of the WP variable, (the WP*PM interaction). The models were fitted by R function lmer under REML (equivalent to SAS PROC MIXED or STATA xtmixed). The simple main effects of the WP and PM variables, and the contextual effect of their difference (PM-WP), were tested using R function glht from package multcomp (equivalent to SAS ESTIMATE or STATA lincom). In all models the simple main effects were positive and significant, the PM effect was larger than the WP effect, and their difference was significant.
The eight models were each fitted in six stages so that a predictor or block of predictors could be added at each stage to assess its relative contribution. The models at the first stage were baseline growth models with independent variables age (centered on 70 years), and age squared. Subsequent stages added predictors successively: first the WP variable, then the corresponding PM variable, then the depression covariate, then a block of demographic covariates, and finally a block of health-related covariates. The final full models included the main effects of age and age squared, the main effects of WP and PM and their interactions with each other and with age, the main effects of the covariates and their interactions with the WP and PM variables. The models were specified with random intercept, and slopes of age and WP, which were allowed to covary.
Results
Analysis of the variance components of the eight models at each stage is shown in Table 3 and Table 4. These results show how much variation was uniquely accounted for by predictors included progressively in six stages. Table 3 shows the proportional reduction in within-person residual variance and Table 4 the proportional reduction in between-person intercept variance of each model relative to the empty (intercept only) model (Raudenbush & Bryk, 2002; Singer & Willett, 2003). In both tables the results of the second stage model (B) show the additional effect of the time-varying covariate with age controlled. In all models, the additional contribution at each stage was significant (p < .01, model comparison with the previous stage by likelihood ratio).
Table 3. Percentage Reduction in Within-Person Residual Variance Relative to the Empty Model.
| A | B | C | D | E | F | |
|---|---|---|---|---|---|---|
| Note. Model A is the baseline model with age and age2. Models B to F successively include: the WP variable (B), the PM variable (C), depression (D), demographic covariates (E), and health covariates (F). | ||||||
| Well-being on cognition | 4.18 | 8.29 | 7.51 | 6.19 | 6.92 | 7.5 |
| Well-being on executive function | 4.18 | 5.89 | 5.54 | 3.96 | 4.58 | 5.36 |
| Well-being on memory | 4.18 | 6.54 | 6.07 | 4.50 | 4.91 | 5.53 |
| Well-being on processing speed | 4.18 | 7.84 | 7.45 | 5.77 | 6.63 | 6.96 |
| Cognition on well-being | 2.13 | 2.83 | 3.14 | 3.03 | 4.13 | 4.12 |
| Executive function on well-being | 0.43 | 0.39 | 0.14 | 0.22 | 1.02 | 0.96 |
| Memory on well-being | 0.06 | 1.52 | 1.75 | 1.84 | 2.48 | 2.43 |
| Processing speed on well-being | 2.06 | 3.01 | 3.10 | 3.09 | 2.97 | 2.98 |
Table 4. Percentage Reduction in Between-Person Intercept Variance Relative to the Empty Model.
| A | B | C | D | E | F | |
|---|---|---|---|---|---|---|
| Note. Model A is the baseline model with age and age2. Models B to F successively include: the WP variable (B), the PM variable (C), depression (D), demographic covariates (E), and health covariates (F). | ||||||
| Well-being on cognition | 4.04 | 5.28 | 10.97 | 43.83 | 46.21 | 48.87 |
| Well-being on executive function | 4.04 | 3.52 | 7.62 | 42.06 | 45.25 | 48.04 |
| Well-being on memory | 4.04 | 3.78 | 7.84 | 42.21 | 44.99 | 47.50 |
| Well-being on processing speed | 4.04 | 5.05 | 8.11 | 43.21 | 46.23 | 48.61 |
| Cognition on well-being | 27.75 | 33.57 | 38.48 | 38.71 | 49.34 | 50.07 |
| Executive function on well-being | 21.80 | 28.55 | 32.34 | 33.04 | 40.34 | 40.41 |
| Memory on well-being | 28.55 | 36.06 | 40.06 | 40.45 | 51.45 | 51.74 |
| Processing speed on well-being | 17.89 | 22.17 | 25.22 | 25.19 | 32.11 | 32.91 |
The results of Table 3 indicate that, although relatively little within-person residual variance was explained by these models, more well-being variation within-person was explained by cognition than cognitive variation within-person was explained by well-being. Up to 7% of within-person variation in well-being was explained by these models: about 4% by age but a further 2% to 3% by time-varying cognitive scores on all three cognitive measures. However, less than 1% of executive function and between 2.5% and 3% of within-person variation in memory and processing speed was explained by these models. Of the variation explained in memory most was accounted for by within-person variation in well-being and very little by age. Of the variation explained in processing speed most, about 2%, was accounted for by age although 1% was because of within-person variation in well-being.
The results of Table 4 indicate that between-person differences in mean levels on the covariates accounted for between 30% and 50% of intercept variation for cognitive and well-being outcomes. Most variation in cognitive function between persons, between 20% and 30%, was explained by age. However a further significant 5% to 10% was explained uniquely by mean levels of well-being. Intercept variation in cognition was barely reduced by accounting for depression, but the demographic covariates contributed 8%. Further contribution from health-related covariates was negligible. Most variation in well-being between persons, between 30% and 40%, was explained by depression. Mean levels of cognition contributed about 4% to well-being, with the greatest from executive function and the least from processing speed. The blocks of demographic and health covariates contributed about a further 3% each.
In the following paragraphs we describe the results for the fixed effects estimated for the final eight full models. The results for the models of well-being are shown in Table 5 and the results for the models of cognition are shown in Table 6.
Table 5. Fixed Effects in the Full Models of Well-Being.
| Outcome: | Well-being | Well-being | Well-being | Well-being |
|---|---|---|---|---|
| Focal predictor: | Cognition | Executive function | Memory | Processing speed |
| Note. Estimates are in standard deviation units, with standard errors in brackets. WP is the effect of the average person’s deviation from their own within-person average score on the focal predictor. PM is the effect of individual difference in the person-mean of the focal predictor. Estimates labeled a*b are interactions. Only significant (p < .05) estimates are shown. Significance was based on standard errors. All WP*covariate and PM*covariate interactions were included in the models, but interactions that were non-significant in any model are not included in the table. The intercept is not included in the table. Models were fitted by R function lmer under REML. | ||||
| Age | −0.0103 (0.001) | −0.0109 (0.0009) | −0.0121 (0.0009) | −0.0102 (0.0009) |
| Age^2 | −0.0006 (0.0001) | −0.0006 (0.0001) | −0.0006 (0.0001) | −0.0005 (0.0001) |
| WP | 0.0693 (0.013) | 0.0458 (0.0105) | 0.0339 (0.0101) | 0.0447 (0.0124) |
| PM | 20.0358 (0.0133) | |||
| WP*PM | −0.0323 (0.012) | −0.0263 (0.008) | −0.0214 (0.0081) | |
| WP*age | 0.0029 (0.0012) | 0.0019 (0.0009) | ||
| PM*age | −0.0047 (0.001) | −0.0037 (0.001) | −0.0045 (0.001) | −0.0026 (0.001) |
| WP*depression | 0.0399 (0.0112) | 0.0171 (0.0087) | 0.0278 (0.0098) | |
| PM*depression | −0.0267 (0.0095) | −0.0309 (0.0099) | −0.0321 (0.0101) | |
| PM*exercise | −0.0249 (0.0093) | −0.0309 (0.0095) | −0.0196 (0.0099) | −0.0206 (0.0102) |
| PM*adl | 0.0217 (0.0106) | 0.0243 (0.0113) | ||
| Depression | −0.4483 (0.0089) | −0.4504 (0.0088) | −0.4497 (0.0089) | −0.4499 (0.0089) |
| Sex | −0.2171 (0.0154) | −0.2271 (0.0152) | −0.2241 (0.0155) | −0.214 (0.0155) |
| Education | ||||
| Wealth | 0.0866 (0.0085) | 0.0846 (0.0085) | 0.0888 (0.0086) | 0.0902 (0.0085) |
| Smoking | −0.0347 (0.0077) | −0.034 (0.0076) | −0.0346 (0.0077) | −0.0336 (0.0076) |
| Health | ||||
| Exercise | 0.0617 (0.0084) | 0.0621 (0.0083) | 0.0633 (0.0085) | 0.0595 (0.0082) |
| ADL | −0.1125 (0.01) | −0.1175 (0.0099) | −0.118 (0.0099) | −0.1114 (0.01) |
Table 6. Fixed Effects in the Full Models of Cognition.
| Outcome: | Cognition | Executive function | Memory | Processing speed |
|---|---|---|---|---|
| Focal predictor: | Well-being | Well-being | Well-being | Well-being |
| Note. Estimates are in standard deviation units, with standard errors in brackets. WP is the effect of the average person’s deviation from their own within-person average score on the focal predictor. PM is the effect of individual difference in the person-mean of the focal predictor. Estimates labeled a*b are interactions. Only significant (p < .05) estimates are shown. Significance was based on standard errors. All WP*covariate and PM*covariate interactions were included in the models, but interactions that were non-significant in any model are not included in the table. The intercept is not included in the table. Models were fitted by R function lmer under REML. | ||||
| Age | −0.0371 (0.0009) | −0.0242 (0.0009) | −0.0335 (0.0009) | −0.0274 (0.0009) |
| Age^2 | −0.001 (0.0001) | −0.0006 (0.0001) | −0.0009 (0.0001) | −0.0005 (0.0001) |
| WP | ||||
| PM | 0.0982 (0.0139) | 0.0871 (0.0144) | 0.0602 (0.0133) | 0.0735 (0.0142) |
| WP*PM | ||||
| WP*age | ||||
| PM*age | 0.0021 (0.0009) | |||
| WP*depression | 0.0199 (0.01) | |||
| PM*depression | −0.0242 (0.0094) | −0.0213 (0.01) | −0.0243 (0.0091) | |
| PM*exercise | −0.0303 (0.0099) | −0.0301 (0.0105) | −0.0212 (0.0094) | −0.0285 (0.0101) |
| PM*adl | ||||
| Depression | −0.0497 (0.0103) | −0.0293 (0.011) | −0.0611 (0.0099) | −0.0279 (0.0106) |
| Sex | −0.2221 (0.0157) | 0.0558 (0.0166) | −0.2354 (0.0149) | −0.334 (0.0161) |
| Education | 0.2221 (0.0084) | 0.1916 (0.0088) | 0.1898 (0.008) | 0.0962 (0.0085) |
| Wealth | 0.1017 (0.0087) | 0.0884 (0.0092) | 0.0977 (0.0083) | 0.0393 (0.0089) |
| Smoking | ||||
| Health | ||||
| Exercise | 0.0661 (0.0084) | 0.0603 (0.0089) | 0.0506 (0.008) | 0.0358 (0.0086) |
| ADL | ||||
The coefficients labeled age and age2 respectively show the slope of the outcome trajectory for the average person at age 70 and the rate of change or curvature of that slope. The direction of these effects indicates that well-being and cognition are both in decline at age 70, and the rate of decline accelerates between ages 50 and 90 years. Model intercepts are not shown in the tables, but the covariate main effects are shown and each represents the difference in expected outcome level at age 70 because of individual difference in the covariate between otherwise average people. The direction of the significant covariate effects for people with average mean levels of cognitive function at age 70 (in Table 5) indicates that higher levels of well-being would be expected for people with lower levels of depression, who are female, with greater wealth, less smoking, more physical exercise, and fewer problems with daily activities. Likewise for people with average mean levels of well-being at age 70 (see Table 6), higher levels of cognitive function were associated with female sex, more education, greater wealth, and more exercise. The main effect of sex, for example, represents the expected change in outcome level between women and men. The average man would have about 0.2 standard deviations lower well-being at age 70 (see Table 5), and about 0.2 to 0.3 standard deviations lower cognition (see Table 6). However, men had slightly higher scores than women on cognition measured as executive function.
The results of main interest in Table 5 and Table 6 are the association between the outcome and the focal predictor, represented by the WP and PM variables, and their interactions with each other and with age and with covariates. The association represented by the WP effect is the expected change from a person’s usual (within-person average) outcome score on a testing occasion when measurement of their focal predictor is one unit higher than it usually is for them. This person is otherwise average in the sense defined by zero on all covariates (thus aged 70, female, and average on all other covariates including the mean level of the focal predictor). The association represented by the PM effect is the expected difference in outcome level between persons whose mean level of the focal predictor differs by one unit, but who are otherwise average people. Although most variation in cognitive function was explained by age, and most variation in well-being was explained by depression, small but significant associations between cognition and well-being remained after variation in age and depression were controlled. In models where cognition was the outcome the association was mainly because of variation in mean levels of well-being between persons. In models where well-being was the outcome the association was mainly because of within-persons variation in cognitive function. Figure 1 shows sample average trajectories of age-related change in cognition and well-being, and how these were moderated by well-being or by cognitive functioning, respectively.
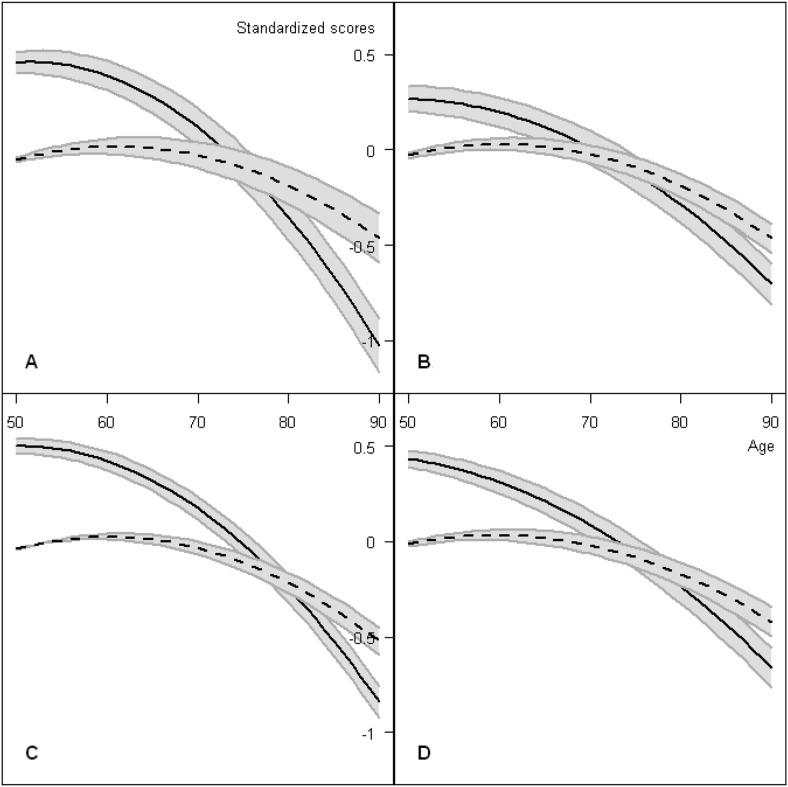
Figure 1.
Model-predicted trajectories of age-related change in cognition and well-being. Note: The trajectories were predictions of the full models (reported in Tables 5 and 6) with all other covariates held constant at their respective means (equivalent to residualizing). The four panels show: A, General cognition; B, Executive function; C, Memory; D, Processing speed. Solid lines show trajectories of cognition with gray bands indicating how this was moderated by a difference of one standard deviation in person-mean well-being (the PM effects reported in Table 6). For example, the vertical distance at age 70 from the solid line to the upper band in panel A is 0.098 (see Table 6). Dashed lines show trajectories of well-being with gray bands indicating a difference of one standard deviation in within-person cognition (the WP effects reported in Table 5).
The fixed effects estimated for full models where well-being was the outcome are shown in Table 5. The results show a positive and significant within-person effect of cognition, indicating that a person who performed better than they usually did, on any of the three cognitive tests, could also be expected to score higher on well-being than they usually would. The cross-level interaction between WP and PM was negative for executive function and processing speed, indicating that for these measures the within-person effect was stronger for people whose mean level of cognition was lower than others. For these measures the interaction between WP and depression was positive, indicating the within-person effect was stronger for people whose mean level of depression was higher than others. The PM effect of mean levels of cognition upon well-being was positive and significant for executive function but nonsignificant for memory and processing speed. However all three were positive and significant in models at a previous stage that included depression but not the demographic or health covariates (detailed results not shown), indicating that between-person variation in well-being because of memory and processing speed was accounted for by covariates. The interaction between the PM effect of all cognitive measures and the physical exercise covariate was negative and significant, indicating that the between-person variation in well-being because of cognitive function was moderated primarily by exercise. The interaction between WP and age indicated that the within-person associations did not vary substantially with age (although the effect was borderline (p = .04) for memory), but the between-person (PM) associations interacted negatively with age, suggesting that difference between people’s well-being that was because of difference in their mean levels of cognition would become less with age.
The fixed effects estimated for full models where cognition was the outcome are shown in Table 6. The association between cognition and well-being was mainly because of variation in mean levels of well-being between persons. Little within-person variation was explained by these models at any stage, indicating a person who scored higher on well-being than usual for them on a particular occasion would not necessarily be expected to perform better than usual on a cognitive test. However, the effect of between-persons (PM) variation in the mean level of well-being was significant and positive for all cognitive outcomes at every stage, indicating the variation in mean levels of well-being between persons was not accounted for by the demographic and health covariates including depression. Thus women (for whom sex was coded 0), aged 70 who were average on all other covariates but who differ in their mean level of well-being would be expected to differ in the same direction in their cognitive function. The interaction between PM and sex was not significant, indicating the effect of individual difference in mean levels of well-being upon cognitive function was not significantly different for men. However, the interaction between PM and depression and between PM and physical exercise were both significant, suggesting these variables were the most important moderating influences on the association between cognition and positive well-being. The interactions with depression and with exercise have the same sign, both negative, but it is important to interpret each in conjunction with the corresponding main effect, noting that the PM variable was centered on its average. Exercise had a positive main effect indicating its generally beneficial effect on the outcome. In this case the negative interaction with well-being indicates that more exercise makes the association between well-being and cognition diverge more positively from the average when well-being is below average. We interpret this as indicating the benefits of exercise for cognitive function are relatively greater for those with lower well-being. Conversely, depression had a negative main effect indicating its detrimental effect on the outcome. Here the negative interaction makes the association diverge more negatively from the average when well-being is above average. Therefore we interpret this as indicating the adverse effect of depression is greater for those with higher well-being. These interactions are illustrated in Figure 2. However, depression had no significant effect on the association when cognitive function was measured solely in terms of processing speed. Neither the WP nor the PM effects interacted significantly with age, suggesting the effect of mean levels of well-being upon cognitive function did not change significantly with age.
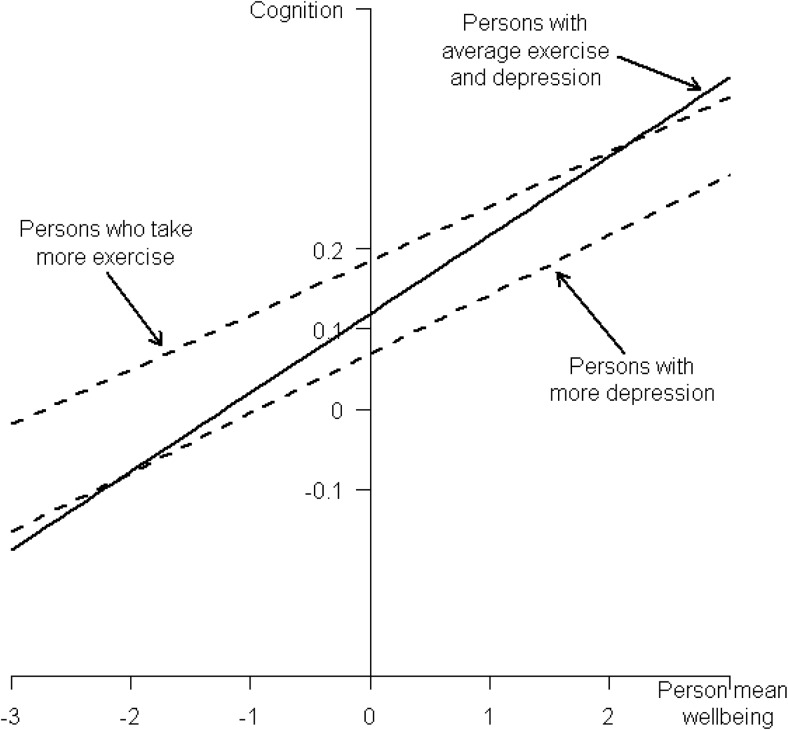
Figure 2.
The effects of exercise and depression on the relationship between cognition and well-being. Note: The graph shows the cognition outcome for a range of person-mean well-being predicted using the fitted model of cognition reported in Table 6. The scales are in standard deviation units and person-mean well-being was mean-centered so 0 represents the average. The solid line represents the predicted outcome with all other independent variables held constant at their centered values. The dashed lines represent the effect for persons with 1 standard deviation greater exercise (upper dashed line) and depression (lower dashed line). The slope of each dashed line is the slope of the solid line (0.0982) plus the respective interaction effects (−0.0303 and −0.0242, see Table 6). The intercept differences between the solid and dashed lines are the respective main effects (0.0661 and −0.0497, see Table 6).
Discussion
In this large study of people aged 50 to 90 years, cognitive function and positive well-being were assessed four times over a 6-year period. We used multilevel models with within-person centering to model the within- and between-persons associations between cognition and positive well-being, how these change with age, and how they are moderated by levels of depression, demographic, and health-related covariates. Cognition and well-being in the study sample were both in decline at age 70, and the rate of decline accelerated between ages 50 and 90 years. Many studies have shown that cognitive decline begins early, in the 50s or 60s or even earlier. There have been few studies of the long-term trajectory of well-being, but the evidence suggests that in later life it tends to be relatively stable or to decline slightly (Charles, Reynolds, & Gatz, 2001; Kurland et al., 2006), with a steeper decline as death approaches (Mroczek & Spiro, 2005). We found higher levels of well-being associated with lower levels of depression, female, greater wealth, less smoking, more physical exercise, and fewer problems with daily activities. Higher levels of cognition were associated with female, more education, greater wealth, and more exercise. We found that most of the variation in cognitive function was explained by age, and most of the variation in well-being was explained by depression. However, small but significant associations between cognition and well-being remained after variation in age and depression were controlled.
In models where cognition was the outcome the association was mainly because of variation in mean levels of well-being between persons: higher levels of average well-being were associated with better cognitive function after differences in demographic and health covariates, and in particular depression, were accounted for. However the population averaged effect of variation within persons was relatively insignificant. This suggests a person can be expected to score higher on cognitive tests than a person with similar cognitive function, if they generally have a higher level of positive well-being; their gain would depend upon their mean level of well-being rather than a transient higher mood state. This was observed for three different kinds of cognitive test, which could suggest the effect may be because of overall performance rather than function in particular domains of cognition.
By contrast when well-being was the outcome of interest, the association between cognition and well-being was almost entirely because of within-person fluctuation in cognitive test performance, suggesting that when a person’s cognitive function is better than usual for them they will also feel greater well-being. The effect of mean levels of well-being upon cognitive function did not change significantly with age. But the effect of mean levels of cognition upon well-being did change with age, suggesting that difference between people’s well-being because of difference in their mean levels of cognition would become less with age.
We found some differential effects between the three domains of cognitive function that were measured: executive function, memory, and processing speed. The association of between-person differences in average well-being with cognition was consistent in all three domains of cognition examined. However depression had no significant effect on this association when cognitive function was measured solely in terms of processing speed. With well-being and the other covariates controlled, women scored higher than men when cognition was measured by memory and by processing speed, but men scored higher than women for executive function. In follow-up analyses we fitted a model including all three cognitive variables as predictors and found that effects that were significant when the cognitive variables were included individually became attenuated but remained significant when all three cognitive variables were included. The association of within-person change in cognition with the well-being outcome was consistent in all three domains of cognition. There was some association because of cognitive variation between persons, but only measured as executive function. There was no difference between individuals in general cognitive ability, and in particular when cognition was measured as memory or as processing speed. Few previous studies have examined whether change in or level of specific domains of cognition is linked with later well-being. In the study by Wilson et al. (2013), decline in episodic memory, semantic memory and perceptual speed, as well as general cognitive ability, were all associated with change in purpose in life, and baseline levels of semantic memory, working memory and perceptual speed, and general cognitive ability, were associated with later purpose in life. Our finding that between-person change in executive function was associated with well-being is consistent with the observation in that study on the effect of change in executive functions on purpose in life. Wilson et al. (2013) suggest that the impact of cognitive decline on well-being may be primarily on those aspects of eudaimonic well-being that require executive control skills, such as purpose in life, because they involve intentions and regulating behavior in accordance with those intentions. The measure of well-being used in our study incorporated items on both eudaimonic and hedonic aspects, although the former predominated. We think it is the presence of eudaimonic items in our well-being scale that explains our finding that well-being appeared to be influenced by between-person change in executive function but not by change in other cognitive domains or general cognitive ability. To our knowledge, most previous longitudinal studies into the potential influence of level of or change in cognition on well-being in older people have used measures of well-being based on hedonic aspects alone, and their results have been negative. For example, in the study by Gerstorf et al. referred to above (2007), no association was found between change in perceptual speed and trajectory of scores on the Philadelphia Center Geriatric Morale Scale. Similarly, there was no indication that increases in cognitive limitations, as measured by the Mini Mental State Examination, over a 54-month period influenced the trajectory of positive affect (Kurland et al., 2006). In the Lothian Birth Cohort, 1921 there was no association between change in cognitive function between ages 11 and 79 and scores on Diener’s Satisfaction with Life scale (Gow et al., 2005). One previous study used a newly developed measure of well-being that consists of positively worded items on both hedonic and eudaimonic well-being—the Warwick-Edinburgh Mental Wellbeing Scale (Gale, Cooper, et al., 2012). In a meta-analysis of estimates from four cohorts whose members had completed this scale, no significant association was found between change in cognition between age 11 and later life and well-being. Although Wilson et al. (2013) found evidence that greater decline in cognition has an adverse effect on some eudaimonic aspects of well-being, our findings in the current study suggest that any influences of cognition on subsequent well-being occur primarily at the individual level. It is possible of course that our ability to detect between-person effects of cognition on well-being were hindered by the relative stability of individual differences in well-being over the short follow-up period of our study (Charles et al., 2001).
Exercise and depression were the most important moderating influences on the association between cognition and positive well-being. Our results suggest exercise has a beneficial effect upon cognitive function that is relatively greater for those with lower well-being. Differences in cognition would be expected to be less influenced by differences in mean levels of well-being between persons taking more exercise. In other words more exercise protects cognitive performance against the detrimental effects of lower well-being. Indeed exercise could provide a kind of well-being. This would be consistent with evidence from randomized controlled trials in older people, which found that being more physically active has a small beneficial effect on well-being (Netz et al., 2005). The adverse effect of depression upon the cognition-well-being association was greater for those with higher well-being.
The strengths of the present study lie in its size, the fact that it is representative of the community-dwelling English population aged 50 and over (Taylor, Conway, Calderwood, & Lessof, 2003), and the assessment of three different cognitive domains. A further strength is the use of within-person centering to distinguish between the within-person and between-person effects of cognition and well-being, which is necessary to avoid incorrect inferences about change and its determinants (Sliwinski et al., 2010). Within-group centering (here within-person centering Hoffman & Stawski, 2009) provides a method for disaggregating these effects that leads not only to greater precision but also to models that explicitly account for both stable trait-like differences between persons and transient state-like changes within persons. The study has some limitations. First, there were only four waves of repeated measures of each individual, with just 2 years between waves, which may have hindered more accurate measurement of within-person change. Second, there were no data on personality traits such as neuroticism or extraversion. This prevented our testing whether these factors confounded the links between cognition and well-being (Gale, Cooper, et al., 2012).
In a previous analysis of data from this cohort, we examined whether there was a bidirectional relationship between depressive symptoms and cognitive ability (Gale, Allerhand, et al., 2012). We found that greater depression was associated with a slightly faster rate of cognitive decline but only in those aged 60 to 80 years. There was no support for the hypothesis that there might be reciprocal dynamic influences between cognitive ability and depressive symptoms. In this earlier analysis we found no consistent associations between physical health, smoking, exercise, social class or education and the rate of change of cognitive decline. The methods of analysis used in the present study were different from those used in our earlier paper (Gale, Allerhand, et al., 2012). There we found little evidence that any of the covariates influenced the rate of cognitive decline. The present study provides greater precision by disaggregating the within- and between-persons effects of the focal predictor. We now find that the slope of cognition at age 70 is in less decline (more positive) for greater wealth and physical exercise and for less smoking. These findings are consistent with observations in other cohorts (Fratiglioni et al., 2004; Koster et al., 2005; Nooyens et al., 2008). We found no evidence to link better physical health or better physical function, as measured by number of difficulties with activities of daily living, with the slope of cognition.
Few longitudinal studies have examined the potential influence of well-being on subsequent cognitive performance. In one such study, having a stronger sense of purpose in life—a facet of eudaimonic well-being—was linked with a reduced risk of incident Alzheimer’s disease and mild cognitive impairment (Boyle, Buchman, Barnes, & Bennett, 2010). Gerstorf et al. (2007) examined 13 years of data from the Berlin Ageing Study on well-being, measured using the Philadelphia Geriatric Center Morale Scale that assesses satisfaction with life, satisfaction with ageing and lack of agitation, and perceptual speed. Using a latent change score model they found a link between greater well-being and less average decline in perceptual speed. We found a link between greater average well-being and better performance in three cognitive domains. However, the PM effects we found cannot directly be compared with lagged dynamic change effects in a latent change score model. There is some longitudinal evidence that sense of perceived control—a facet of eudaimonic well-being—may be predictive of later cognition. In one study, older people with a stronger sense of perceived control had fewer difficulties in performing cognitive tasks 20 years later (Caplan & Schooler, 2003). In another study of young, middle-aged and older adults, Windsor and Anstey (2008) found that for each age group, there were significant positive associations between between-person variation in perceived control at baseline and subsequent performance on tests of memory, verbal intelligence, and processing speed, but within-person changes in perceived control were not associated with changes in cognitive test performance over the study period. This is consistent with the findings of the present study where the within-person effects of well-being were nonsignificant for all cognitive outcomes at every stage, whereas the effect of between-person variation in the mean level of well-being was significant and positive for all cognitive outcomes.
Our findings illustrate the value of distinguishing between within-person and between-person effects in longitudinal models of change and suggest that although at a population level, greater well-being may be linked with a reduced risk of cognitive decline, this apparently protective relationship does not appear to hold at an individual level.
References
- Anstey K., & Christensen H. (2000). Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: A review. Gerontology, 46, 163–177. doi: 10.1159/000022153 [DOI] [PubMed] [Google Scholar]
- Ashby F. G., Isen A. M. &, Turken A. U. (1999). A neuropsychological theory of positive affect and its influence on cognition. Psychological Review, 106, 529–550. doi: 10.1037/0033-295X.106.3.529 [DOI] [PubMed] [Google Scholar]
- Baltes P. B., & Baltes M. M. (Eds.). (1990). Successful aging: Perspectives from the behavioural sciences. New York: Cambridge University Press; (No DOI) [Google Scholar]
- Banks J., Karlsen S., & Oldfield Z. (2003). Socio-economic position In Marmot M., Banks J., Blundell R., Lessof C., & Nazroo J. (Eds.), Health, wealth and lifestyles of the older population in England (pp. 71–125). London: Institute of Fiscal Studies [Google Scholar]
- Bond J., Dickinson H. O., Matthews F. Jagger C., & Brayne C. (2006). Self-rated health as a predictor of death, functional and cognitive impairment: A longitudinal cohort study. European Journal of Ageing, 3, 193–206. doi: 10.1007/s10433-006-0039-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. A., Buchman A. S., Barnes L. L., & Bennett D. A. (2010). Effect of purpose in life on risk of incident Alzheimer’s disease and mild cognitive impairment in community dwelling older people. Archives of General Psychiatry, 67, 304–310. doi: 10.1001/archgenpsychiatry.2009.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle P. A., Buchman A. S., & Bennett D. A. (2010). Purpose in life is associated with a reduced risk of incident disability among community-dwelling older people. American Journal of Geriatric Psychiatry, 18, 1093–1102. doi: 10.1097/JGP.0b013e3181d6c259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan L. J., & Schooler C. (2003). The roles of fatalism, self-confidence, and intellectual resources in the disablement process in older adults. Psychology and Aging, 18, 551–561. doi: 10.1037/0882-7974.18.3.551 [DOI] [PubMed] [Google Scholar]
- Charles S. T., Reynolds C. A., & Gatz M. (2001). Age-related differences and change in positive and negative affect over 23 years. Journal of Personality and Social Psychology, 80, 136–151. doi: 10.1037/0022-3514.80.1.136 [DOI] [PubMed] [Google Scholar]
- Chida Y. C., & Steptoe A. (2008). Positive psychological well-being and mortality: A quantitative review of prospective observational studies. Psychosomatic Medicine, 70, 741–756. doi: 10.1097/PSY.0b013e31818105ba [DOI] [PubMed] [Google Scholar]
- Collins A. L., Goldman N., & Rodriguez G. (2008). Is positive well-being protective of mobility limitations among older adults? Journal of Gerontology: Psychological Sciences, 63B, 321–327. doi: 10.1093/geronb/63.6.P321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran P. J., & Bauer D. J. (2011). The disaggregation of within-person and between-person effects in longitudinal models of change. Annual Review of Psychology, 62, 583–619. doi: 10.1146/annurev.psych.093008.100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson K. W., Mostofsky E., & Whang W. (2010). Don’t worry, be happy: Positive affect and reduced 10-year incident coronary heart disease: The Canadian Nova Scotia Health Survey. European Heart Journal, 31, 1065–1070. doi: 10.1093/eurheartj/ehp603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dear K., Henderson S., & Korten A. (2002). Well-being in Australia - Findings from the National Survey of Mental Health and Well-Being. Social Psychiatry and Psychiatric Epidemiology, 37, 503–509. doi: 10.1007/s00127-002-0590-3 [DOI] [PubMed] [Google Scholar]
- Diener E. (1984). Subjective wellbeing. Psychological Bulletin, 95, 542–575. doi: 10.1037/0033-2909.95.3.542 [DOI] [PubMed] [Google Scholar]
- Diener E., & Emmons R. A. (1984). The independence of positive and negative affect. Journal of Personality and Social Psychology, 47, 1105–1117. doi: 10.1037/0022-3514.47.5.1105 [DOI] [PubMed] [Google Scholar]
- Enkvist A., Ekstrom H., & Elmstahl S. (2013). Associations between cognitive abilities and life satisfaction in the oldest-old. Results from the longitudinal population study Good Aging in Skane. Clinical Interventions in Aging, 8, 845–853. doi: 10.2147/CIA.S45382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocco A. J., & Yaffe K. (2010). Defining successful aging: The importance of including cognitive function over time. Archives of Neurology, 67, 876–880. doi: 10.1001/archneurol.2010.130 [DOI] [PubMed] [Google Scholar]
- Fratiglioni L., Paillard-Borg S., & Winblad B. (2004). An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurology, 3, 343–353. doi: 10.1016/S1474-4422(04)00767-7 [DOI] [PubMed] [Google Scholar]
- Gale C. R., Allerhand M., & Deary I. J. (2012). Is there a bidirectional relationship between depressive symptoms and cognitive ability in older people? A prospective study using the English Longitudinal Study of Ageing. Psychological Medicine, 42, 2057–2069. doi: 10.1017/S0033291712000402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale C. R., Cooper C., Deary I. J., & Aihie Sayer A. (2014). Psychological wellbeing and incident frailty in men and women: The English Longitudinal Study of Ageing. Psychological Medicine, 44, 697–706. doi: 10.1017/S0033291713001384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale C. R., Cooper R., Craig L., Elliott J., Kuh D., Richards M., et al. Deary I. J. (2012). On behalf of the HALCyon Study Team. Cognitive function in childhood and lifetime cognitive change in relation to mental wellbeing in four cohorts of older people. PLOS ONE, 7, . doi: 10.1371/journal.pone.0044860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D., Lovden M., Rocke C., Smith J., & Lindenberger U. (2007). Well-being affects changes in perceptual speed in advanced old age: Longitudinal evidence for a dynamic link. Developmental Psychology, 43, 705–718. doi: 10.1037/0012-1649.43.3.705 [DOI] [PubMed] [Google Scholar]
- Gow A. J., Whiteman M. C., Pattie A., Whalley L., Starr J., & Deary I. J. (2005). Lifetime intellectual function and satisfaction with life. British Medical Journal, 331, 141–142. doi: 10.1136/bmj.38531.675660.F7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeker D., & Gibbons R. D. (1997). Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychological Methods, 2, 64–78. doi: 10.1037/1082-989X.2.1.64 [DOI] [Google Scholar]
- Hoffman L., & Stawski R. (2009). Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Research in Human Development, 6, 97–100. doi: 10.1080/15427600902911189 [DOI] [Google Scholar]
- Huntley A. L., Johnson R., Purdy S., Valderas J. M., & Salisbury C. (2012). Measures of multimorbidity and morbidity burden for use in primary care and community settings: A systematic review and guide. Annals of Family Medicine, 10, 134–141. doi: 10.1370/afm.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert F. A., & Whittington J. E. (2003). Evidence for the independence of positive and negative wellbeing: Implications for quality of life assessment. British Journal of Health Psychology, 8, 107–122. doi: 10.1348/135910703762879246 [DOI] [PubMed] [Google Scholar]
- Isaacowitz D. M., & Smith J. (2003). Positive and negative affect in very old age. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 58, P143–P152. doi: 10.1093/geronb/58.3.P143 [DOI] [PubMed] [Google Scholar]
- Isen A. M. (2008). Some ways in which positive affect influences decision making and problem solving In Lewis M., Haviland-Jones J. M., & Feldman-Barrett L. (Eds.), Handbook of emotions, 3rd ed. (pp. 548–573). New York: Guilford Press [Google Scholar]
- Isen A. M. (2009). A role for neuropsychology in understanding the facilitating influence of positive affect on social behavior and cognitive processes In Snyder C. R. & Lopez S., (Eds.), Handbook of positive psychology (pp. 503–518). New York: Oxford. doi: 10.1093/oxfordhb/9780195187243.013.0048 [DOI] [Google Scholar]
- Isen A. M., Rosenzweig A. S., & Young M. J. (1991). The influence of positive affect on clinical problem solving. Medical Decision Making, 11, 221–227. doi: 10.1177/0272989X9101100313 [DOI] [PubMed] [Google Scholar]
- Jeste D. V., Depp C. A., & Vahia I. V. (2010). Successful cognitive and emotional aging. World Psychiatry, 9, 78–84 (No DOI) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster A., Penninx B. W., Bosma H., Kempen G. I., Newman A. B., Rubin S. M., et al. Kritchevsky S. B. (2005). Socioeconomic differences in cognitive decline and the role of biomedical factors. Annals of Epidemiology, 15, 564–571. doi: 10.1016/j.annepidem.2005.02.008 [DOI] [PubMed] [Google Scholar]
- Kurland B. F., Gill T. M., Patrick D. L., Larson E. B., & Phelan E. A. (2006). Longitudinal change in positive affect in community-dwelling older persons. Journal of the American Geriatrics Society, 54, 1846–1853. doi: 10.1111/j.1532-5415.2006.00970.x [DOI] [PubMed] [Google Scholar]
- Lüdtke O., Marsh H. W., Robitzsch A., Trautwein U., Asparouhov T., & Muthén B. (2008). The multilevel latent covariate model: A new, more reliable approach to group-level effects in contextual studies. Psychological Methods, 13, 203–229. doi: 10.1037/a0012869 [DOI] [PubMed] [Google Scholar]
- Marmot M., Nazroo J., Banks J., Blundell R., Erens B., Lessof C., & Huppert F. A. (2011). English Longitudinal Study of Ageing: Wave 0 (1998, 1999 and 2001) and waves 1–4 (2002–2009) [computer file], 15th ed., SN: 5050 UK Data Archive: Colchester [Google Scholar]
- Mroczek D. K., & Spiro A. (2005). Change in life satisfaction during adulthood: Findings from the veterans affairs normative aging study. Journal of Personality and Social Psychology, 88, 189–202. doi: 10.1037/0022-3514.88.1.189 [DOI] [PubMed] [Google Scholar]
- Netz Y., Wu M. J., Becker B. J., & Tenenbaum G. (2005). Physical activity and psychological well-being in advanced age: A meta-analysis of intervention studies. Psychology of Aging, 20, 272–284. doi: 10.1037/0882-7974.20.2.272 [DOI] [PubMed] [Google Scholar]
- Nooyens A. C., van Gelder B. M., & Verschuren W. M. (2008). Smoking and cognitive decline among middle-aged men and women: The Doetinchem Cohort Study. American Journal of Public Health, 98, 2244–2250. doi: 10.2105/AJPH.2007.130294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostir G. V., Markides K. S., Black S. A., & Goodwin J. S. (2000). Emotional well-being predicts disability, subsequent functional independence and survival. Journal of the American Geriatrics Society, 48, 473–478 (No DOI) [DOI] [PubMed] [Google Scholar]
- Ostir G. V., Ottenbacher K. J., & Markides K. S. (2004). Onset of frailty in older adults and the protective role of positive affect. Psychology and Aging, 19, 402–408. doi: 10.1037/0882-7974.19.3.402 [DOI] [PubMed] [Google Scholar]
- Pressman S. D., & Cohen S. (2005). Does positive affect influence health? Psychological Bulletin, 131, 925–971. doi: 10.1037/0033-2909.131.6.925 [DOI] [PubMed] [Google Scholar]
- Rajan K. B., Hebert L. E., Scherr P. A., Mendes de Leon C. F., & Evans D. A. (2012). Disability in basic and instrumental activities of daily living is associated with faster rate of decline in cognitive function of older adults. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. Oct 25. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush S. W., & Bryk A. S. (2002). Hierarchical linear models (2nd ed.). Thousand Oaks, CA: Sage [Google Scholar]
- Rocke C., & Lachman M. E. (2008). Perceived trajectories of life satisfaction across past, present and future: Profiles and correlates of subjective change in young, middle-aged, and older adults. Psychology and Aging, 23, 833–847. doi: 10.1037/a0013680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J. W., & Kahn R. L. (1997). Successful aging. Gerontologist, 37, 433–440. doi: 10.1093/geront/37.4.433 [DOI] [PubMed] [Google Scholar]
- Ryan R. M., & Deci E. L. (2001). On happiness and human potentials: A review of research on hedonic and eudaimonic well-being. Annual Reviews of Psychology, 52, 141–166. doi: 10.1146/annurev.psych.52.1.141 [DOI] [PubMed] [Google Scholar]
- Ryff C. D. (1989). In the eye of the beholder - Views of psychological wellbeing among middle-aged and older adults. Psychology and Aging, 4, 195–210 [DOI] [PubMed] [Google Scholar]
- Salthouse T. A. (2007). Implications of within-person variability in cognitive and neuropsychological functioning for the interpretation of change. Neuropsychology, 4, 401–411. doi: 10.1037/0894-4105.21.4.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J. D., & Willett J. B. (2003). Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press. doi: 10.1093/acprof:oso/9780195152968.001.0001 [DOI] [Google Scholar]
- Sliwinski M., Hoffman L., & Hofer S. M. (2010). Evaluating convergence of within-person change and between-person age differences in age-heterogeneous longitudinal studies. Research in Human Development, 7, 45–60. doi: 10.1080/15427600903578169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawski R. S., Almeida D. A., Lachman M. E., & Tun P. A. (2010). Fluid cognitive ability is associated with greater exposure and smaller emotional reactions to daily stressors. Psychology and Aging, 25, 330–342. doi: 10.1037/a0018246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel N., Huppert F. A., McWilliams B., & Melzer D. (2003). Physical and cognitive function In Marmot M., Banks J., Blundell R., Lessof C., & Nazroo J. (Eds.), Health, wealth and lifestyles of the older population in England: The 2002 English longitudinal study of ageing (pp. 249–300). London: Institute of Fiscal Studies [Google Scholar]
- Steptoe A., Demakakos P., de Oliveira C., & Wardle J. (2012). Distinctive biological correlates of positive psychological wellbeing in older men and women. Psychosomatic Medicine, 74, 501–508. doi: 10.1097/PSY.0b013e31824f82c8 [DOI] [PubMed] [Google Scholar]
- Steptoe A., Wardle J., & Marmot M. (2005). Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc Natl Acad Sci USA, 102, 6508–6512. doi: 10.1073/pnas.0409174102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R., Conway L., Calderwood L., & Lessof C. (2003). Methodology In Marmot M., Banks J., Blundell R., Lessof C., & Nazroo J. (Eds.), Health, wealth and lifestyles of the older population in England: The 2002 English longitudinal study of ageing (pp. 357–374). London: Institute of Fiscal Studies [Google Scholar]
- Turvey C. L., Wallace R. B., & Herzog R. (1999). A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. International Psychogeriatrics, 11, 139–148. doi: 10.1017/S1041610299005694 [DOI] [PubMed] [Google Scholar]
- Welch D. C., & West R. L. (1995). Self-efficacy and mastery: Its application to issues of environmental control, cognition, and aging. Developmental Review, 15, 150–171. doi: 10.1006/drev.1995.1007 [DOI] [Google Scholar]
- West R. L., Bagwell D. K., & Dark-Freudeman A. (2008). Self-efficacy and memory aging: The impact of a memory intervention based on self-efficacy. Aging, Neuropsychology and Cognition, 15, 302–309. doi: 10.1080/13825580701440510 [DOI] [PubMed] [Google Scholar]
- Wiggins R. D., Netuveli G., Hyde M., Higgs P., & Blane D. (2008). The evaluation of a self-reported scale of quality of life (CASP-19) in the context of research on ageing: A combination of exploratory and confirmatory approaches. Social Indicators Research, 89, 61–87. doi: 10.1007/s11205-007-9220-5 [DOI] [Google Scholar]
- Wilson R. S., Boyle P. A., Segawa E., Yu L., Begeny C. T., Anagnos S. E., & Bennett D. A. (2013). The influence of cognitive decline on well-being in old age. Psychology and Aging, 28, 304–313. doi: 10.1037/a0031196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. S., Hoganson G. M., Rajan K. B., Barnes L. L., Mendes de Leon C. F., & Evans D. A. (2010). Temporal course of depressive symptoms during the development of Alzheimer disease. Neurology, 75, 21–26. doi: 10.1212/WNL.0b013e3181e620c5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windsor T. D., & Anstey K. J. (2008). A longitudinal investigation of perceived control and cognitive performance in young, midlife and older adults. Neuropsychology, Development, and Cognition Section B, Aging, Neuropsychology and Cognition, 15, 744–763. doi: 10.1080/13825580802348570 [DOI] [PubMed] [Google Scholar]