Abstract
Developing oligodendrocytes (OLs) are highly vulnerable to excitotoxicity and oxidative stress, both of which are important in the pathogenesis of many brain disorders. OL excitotoxicity is mediated by ionotropic glutamate receptors (iGluRs) of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate type on these cells. Here we report that metabotropic GluRs (mGluRs) are highly expressed in OL precursors but are down-regulated in mature OLs. Activation of group 1 mGluRs attenuates OL excitotoxicity by controlling downstream oxidative stress after iGluR overactivation and also prevents nonexcitotoxic forms of oxidative stress by inhibiting reactive oxygen species accumulation and intracellular glutathione loss. The modulating effect of group 1 mGluRs on hypoxic-ischemic OL injury is not due to iGluR endocytosis that occurs in neurons in response to mGluR activation but requires activation of PKCα after G protein coupling to phospholipase C. Our results reveal a previously undescribed role for mGluRs in limiting OL injury and suggest that targeting group 1 mGluRs may be a useful therapeutic strategy for treating disorders that involve excitotoxic injury and/or oxidative stress to OLs.
Perinatal hypoxic-ischemic brain injury leads to devastating neurological consequences that are highly age-dependent. In term infants, hypoxia-ischemia predominantly affects cerebral cortex with characteristic neuronal injury. In contrast, hypoxic-ischemic injury in premature infants selectively affects cerebral white matter with prominent oligodendrocyte (OL) injury, a disorder termed periventricular leukomalacia (PVL) (1). PVL is the predominant form of brain injury in premature infants and the major antecedent of cerebral palsy. The prevalence of low-birth-weight infants is increasing due to improved survival rates of premature newborns. Indeed, PVL affects ≈25-50% of the 55,000 premature infants born in the U.S. every year, yet currently no specific treatment exists for this serious pediatric problem (2).
PVL involves primarily OL precursor cells (OPCs) that populate fetal cerebral white matter during the human developmental period of greatest risk for the lesion (3). OPCs share with neurons a high vulnerability to glutamate excitotoxicity and oxidative stress (4-6), which are putatively the two major mechanisms of the pathogenesis of PVL (1). Hypoxic-ischemic injury to OPCs is primarily mediated by ionotropic glutamate receptors (iGluRs) of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate type on these cells (5, 7-9), whereas hypoxic-ischemic injury to neurons is predominantly mediated by iGluRs of the N-methyl-d-aspartate type (10). Previous neuronal studies showed that metabotropic GluRs (mGluRs) can modulate iGluR-mediated excitotoxicity (11, 12). Little is known, however, about the expression of mGluRs in OLs and the role of mGluRs in hypoxic-ischemic OL injury.
Unlike iGluRs that couple directly to ion channels, mGluRs couple to effector mechanisms via G proteins. Eight subtypes of mGluRs have been cloned and classified into three groups according to sequence similarities, intracellular signaling mechanisms, and pharmacological profiles (11). Group 1 mGluRs (mGluR1 and -5) stimulate phospholipase C (PLC) to increase levels of diacylglycerol and inositol triphosphate, which activate PKC and release Ca2+ from intracellular stores, respectively. Group 2 (mGluR2 and -3) and group 3 (mGluR4 and -6-8) receptors negatively couple to adenylyl cyclase. Additionally, a fourth yet unassigned group of mGluRs is thought to positively link to phospholipase D. Nonetheless, it is possible that many functions of mGluRs expressed on various types of brain cells under normal or pathological conditions remain to be discovered. In this study, we seek to address whether mGluRs are involved in regulating OPC injury, because such involvement may be related to both novel mechanisms and therapeutic strategies relevant to PVL.
Materials and Methods
OL Culture and Immunocytochemical Characterization. Highly enriched OPCs were prepared from mixed glial cultures of the forebrains of newborn Sprague-Dawley rats using a selective detachment procedure as described in detail elsewhere (5, 13, 14). OPCs were allowed to differentiate for different days to produce stage-specific cultures (5). Cultures were routinely characterized by immunocytochemical detection of a panel of stage-specific OL markers: A2B5, O4, O1, and myelin basic protein (MBP).
Western Blot Analysis of mGluR Expression. Crude membrane proteins were prepared from stage-specific OL cultures, resolved by electrophoresis, and transferred to polyvinylidene difluoride membranes. The membrane was first probed with anti-GluR1, -5, -2/3, -4a (Upstate Biotechnology, Lake Placid, NY; 1 μg/ml), or MBP (1:1,000) and then with a horseradish peroxidase-conjugated secondary antibody (1:20,000). The antibody conjugates were detected by using a chemiluminescence immunoblotting kit (Pierce).
Pharmacological Treatment, Kainate Exposure, Oxygen-Glucose Deprivation (OGD), Cystine Deprivation, and Cell Viability Assay. Pharmacological agents were applied 10 min before exposure of the cells to kainate, OGD, or cystine deprivation, and cell survival was assessed at 24 h, as described (5, 13).
Acid-Stripping Immunocytochemical Staining. Surface AMPA receptors were labeled on live cells with an antibody directed against the extracellular N terminus of the GluR1 (amino acids 271-285; 5 μg/ml, Oncongene Sciences) or GluR2 (amino acids 175-430; 5 μg/ml, Chemicon) subunit. The cells were then treated with mGluR agonists for 60 min, chilled in 4°C Tris-buffered saline (TBS) to stop endocytosis, and exposed to 0.5 M NaCl/0.2 M acetic acid (pH 3.5) for 4 min on ice to remove antibody bound to extracellular GluR1 or -2. Cultures were rinsed and fixed, and nonspecific staining was blocked. Cells were first immunostained with the OPC marker A2B5, followed by incubation with a rhodamine-labeled secondary antibody for 1 h (1:100). Cells were then permeabilized in TBS containing 0.1% Triton X-100 and 4% goat serum, and internalized primary anti-GluR1 or -GluR2 antibody was made visible by incubation with a fluorescein-labeled secondary antibody for 1 h (1:100).
Biochemical Measurements of Surface-Expressed Receptors. Cultures of rat hippocampal neurons were made as described (15). Biotinylation of cell-surface protein, in combination with immunoblotting of total vs. surface receptors, was performed in OPCs or 2-week-old high-density cultured hippocampal neurons, as described (5).
45Ca2+ Uptake. Calcium influx was measured as described (5).
Measurement of Intracellular Reactive Oxygen Species (ROS) and Reduced Glutathione (GSH) Levels. ROS accumulation was determined with dihydrorhodamine 123 (4, 13), and fluorescence intensity in the cytoplasm was quantified by using the nih image program. GSH was quantitatively assayed by measuring monochlorobimane fluorescence, as described (13, 16).
Infection of Cultured OPCs with Recombinant Retrovirus. Dominant-negative type PKCs contained a point mutation in the kinase domain of PKCα (K368R) or PKCβII (T500V) (17). Cells were exposed to retrovirus carrying wild-type or dominant-negative PKCs (17) in regular culture medium containing polybrene (1:2,000 dilution) for 4 h. After a complete change of the medium, the cultures were maintained for an additional 24 h before any experiments were performed.
Data Analysis. All data represent mean ± SEM. All experiments were repeated at least three times. Statistical differences were assessed by ANOVA with Tukey's post hoc analysis for multiple comparisons or by Student's t test when only two independent groups were compared.
Results
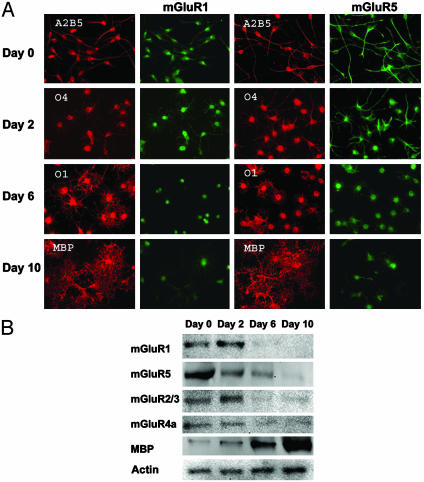
Expression of mGluRs in OL Lineage Cells Is Developmentally Regulated. A recent study showed that functional mGluRs are expressed in the OL precursor cell line CG-4 (18). We examined the expression of mGluRs in primary OL cultures. OPCs were allowed to differentiate for 0, 2, 6, or 10 days to obtain OL lineage cells at various maturational stages. During lineage progression, cells exhibited a series of distinct morphologies (Fig. 1A), characterized by the sequential expression of stage-specific OL markers: A2B5 (early precursors), O4 (later precursors), O1 (immature OLs), and MBP (mature OLs). A representative immunocytochemical characterization of such stage-specific cultures revealed the following composition: undifferentiated cultures (day 0 cultures): 95% A2B5+, 30% O4+,6%O1+, and 1% MBP+; cultures after 2 days of differentiation (day 2 cultures): 82% A2B5+, 92% O4+, 70% O1+, and 7% MBP+; day 6 cultures: 37% A2B5+, 95% O4+, 92% O1+, and 44% MBP+; day 10 cultures: 22% A2B5+, 96% O4+, 91% O1+, and 85% MBP+. All cultures contained <5% glial fibrillary acidic protein-positive astrocytes and <2% CD11+ microglia.
Fig. 1.
mGluR expression in OL lineage cells is developmentally regulated. (A) Immunocytochemical staining of mGluRs in stage-specific OL cultures. Cells were double-labeled with the stage-specific OL marker A2B5, O4, O1, or MBP and anti-mGluR1 or -mGluR5. (B) Immunoblots of mGluRs in stage-specific OL cultures. The expression of mGluR1, -5, -2/3, or -4a is developmentally down-regulated, in contrast to that of MBP, which is up-regulated during OL maturation. Actin serves as sample loading control.
We first examined mGluR expression in these cultures using immunocytochemical double labeling of mGluRs and stage-specific OL markers (Fig. 1 A). Day 0 cultures were mostly bipolar and strongly A2B5+, and exhibited high expression of the group 1 mGluRs, mGluR1 and -5. Day 2 cultures had more cellular processes, were strongly O4+, and also exhibited high levels of mGluRs. Day 6 cultures were multibranched and strongly O1+, whereas mGluR expression was down-regulated in these cells. Day 10 cultures demonstrated a complex network of cellular processes typical of mature OLs, were strongly MBP+, and had very little or no mGluR expression in the cellular processes, with only modest mGluR expression in the cell bodies. The developmental expression of group 2 (mGluR2/3) and group 3 (mGluR4a) receptors (data not shown) was similar to that observed for mGluR1. Western blot analysis confirmed that mGluR expression in cultured OL lineage cells was developmentally regulated. The mGluRs were strongly expressed in day 0 and 2 cultures but were dramatically down-regulated in day 6 and 10 cultures (Fig. 1B). Because mGluRs are transiently overexpressed in day 0-2 cultures, and day 2 OPCs best represent the cells seen in selective white-matter injury in PVL (3), we used these cultures for further experiments detailed below.
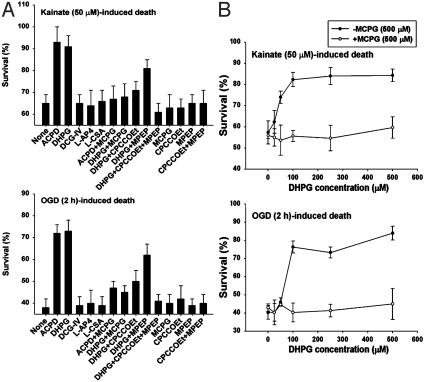
Activation of Group 1 mGluRs Attenuates Excitotoxic Injury to OPCs. We first investigated the role of mGluRs in excitotoxic OPC injury. A brief report showed that group 1 mGluRs can modulate kainate toxicity in OPCs (19). We examined the effects of the broad-spectrum mGluR agonist, (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (ACPD), and the selective mGluR agonists for groups 1-3 and the fourth undetermined group, (R,S)-3,5-dihydroxyphenylglycine (DHPG), (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl) glycine (DCG-IV), L(+)-2-amino-4-phosphonobutyric acid (l-AP4), and l-cysteinesulfinic acid (L-CSA), respectively. We evaluated the effects of these agents in two well-established excitotoxic paradigms, kainate-(50 μM, 24 h) or brief OGD-(2 h) induced OPC death assessed at 24 h (5, 14). ACPD (100 μM), and DHPG (100 μM) both markedly increased OPC survival in these excitotoxic paradigms, whereas DCG-IV (1 μM), l-AP4 (100 μM), and L-CSA (10 μM) had no effect (Fig. 2A). The protective effects of ACPD and DHPG were reversed by the broad-spectrum mGluR antagonist, (S)-methyl-4-carboxyphenylglycine (MCPG, 500 μM). In addition, the protective effects of DHPG were partially prevented by the selective mGluR1 antagonist, 7-hydroxyiminocyclopropanchromen-1a-carboxylic acid ethyl ester (CPCCOEt, 50 μM) or the selective mGluR5 antagonist, 2-methyl-6-(phenylethynyl)-pyridine (MPEP, 1 μM), and fully abolished by the combination of the two agents, whereas the antagonists per se (MCPG, CPCCOEt, MPEP, or CPCCOEt plus MPEP) had no effect (Fig. 2 A). Furthermore, DHPG exhibited a dose-dependent protective effect on kainate-(50 μM) or OGD-(2 h) induced OPC toxicity, and MCPG (500 μM) prevented the DHPG effect (Fig. 2B). Neither ACPD (100 μM) nor DHPG (100 μM) significantly protected OPCs from death induced by a higher concentration of kainate (300 μM, for 24 h), although this cell death was blocked by the AMPA/kainate receptor antagonist, 6-nitro-7-sulfamoylbenzo(f)quinoxaline-2,3-dione (NBQX, 50 μM) (data not shown), suggesting that severe excitotoxicity overwhelmed the ability of mGluRs to modulate the injury.
Fig. 2.
Activation of group 1 mGluRs attenuates OL excitotoxicity. (A) Effect of various mGluR modulators on kainate-(50 μM) or OGD-(2 h) induced OPC death at 24 h. (B) Dose response of the effect of DHPG on kainate-(50 μM) or OGD-(2 h) induced OPC death at 24 h in the absence or presence of the mGluR antagonist MCPG (500 μM).
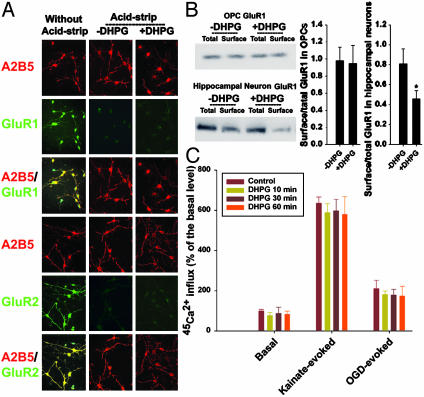
DHPG Does Not Cause iGluR Internalization from the Surface of OPCs. We next addressed potential mechanisms by which mGluRs limit iGluR-mediated excitotoxicity in OPCs. A recent study showed that mGluR activation in neurons results in a rapid loss of iGluRs from synapses due to receptor internalization from the cell surface (20). We examined whether DHPG could limit excitotoxic OPC injury by inducing endocytosis of AMPA/kainate receptors from the surface of OPCs. First, we used an acid-stripping immunocytochemical staining protocol to examine the effect of mGluR activation by DHPG on AMPA receptors expressed on the surface of OPCs. Surface receptors on living cultured OPCs were labeled with antibodies directed against the extracellular N terminus of the AMPA receptor subunit GluR1 or -2. The cultures were treated with control medium or DHPG (100 μM) for 60 min, and the remaining surface antibodies were stripped away with an acetic acid wash. The cells were fixed and permeabilized, and appropriate fluorescent secondary antibodies were used to detect primary antibodies bound to internalized GluR1 or -2. We observed no difference in immunocytochemical staining for either GluR1 or -2 in the absence or presence of DHPG (Fig. 3A). Alternatively, to confirm the above finding with a biochemical approach, we treated OPC cultures with control medium or DHPG (100 μM) for 60 min, and surface proteins were labeled with biotin. Biotinylated proteins were precipitated with immobilized avidin, and the ratio of surface to total specific iGluRs was determined by quantitative Western blotting. Cultures of hippocampal neurons (14 days in culture) treated with DHPG (100 μM, for 60 min), which responded with iGluR endocytosis (20), were used as a positive control. We found that surface GluR1 was reduced by DHPG treatment in hippocampal neurons by ≈40%, but no changes were found in OPCs (Fig. 3B). Furthermore, the surface expression of other AMPA/kainate receptor subunits, when probed with antibodies against GluR2, GluR3, GluR4, GluR5/6/7, KA1, or KA2, did not change with or without DHPG treatment (data not shown). In addition, we measured 45Ca2+ uptake as a functional assay for receptor internalization. Because AMPA/kainate receptors are the major route of Ca2+ entry with exposure of OPCs to kainate or OGD (5), we reasoned that a consequence of AMPA/kainate receptor internalization in OPCs should be a decrease in kainate- or OGD-evoked Ca2+ influx. Exposure of OPCs to kainate (300 μM) for 10 min or OGD for 2 h elicited an ≈6- or 2-fold Ca2+ influx compared to the basal level, respectively (Fig. 3C). DHPG treatment for 10, 30, or 60 min did not affect either kainate- or OGD-evoked Ca2+ uptake compared to uptake in controls (Fig. 3C). Taken together, these data indicate that the effect of DHPG is unlikely to be due to iGluR internalization from the cell surface of OPCs in response to group 1 mGluR activation.
Fig. 3.
DHPG does not cause iGluR internalization from the surface of OPCs. (A) Acid-stripping immunocytochemistry of GluR1 or -2 in A2B5+ OPCs. (B) Biochemical measurements of surface expressed GluR1 on OPCs and hippocampal neurons. *, P < 0.001 between the absence and presence of DHPG. (C) Negligible effect of DHPG on kainate-(300 μM for 10 min) or OGD-(2 h) evoked 45Ca2+ influx.
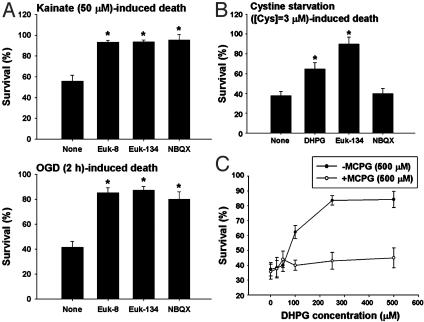
DHPG Protects OPCs from Both Excitotoxic and Nonexcitotoxic Forms of Oxidative Stress. Given the lack of mGluR regulation of AMPA/kainate receptors at the cell surface level, we next examined whether mGluR activation by DHPG could suppress downstream intracellular events leading to cell death during excitotoxicity. A previous study suggested that oxidative stress could be downstream of iGluR activation in OPCs (21). To evaluate this possibility in our experimental paradigms, we found that the synthetic superoxide dismutase/catalase mimetic, Euk-8 or -134 (30 μM each, Eukarion, Bedford, MA), prevented kainate-(50 μM) or OGD-(2 h) induced OPC death at 24 h to a degree similar to that produced by the AMPA/kainate antagonist NBQX (50 μM) (Fig. 4A). We next asked whether the cytoprotective effect of DHPG was limited to excitotoxic injury, or whether DHPG could protect OPCs from nonexcitotoxic forms of injury. A previous study showed that mGluR agonists protect neurons from oxidative stress (22). We exposed OPCs to culture medium with lowered concentrations of cystine (0 or 3 μM) to cause oxidative stress by depleting intracellular GSH (4). Although DHPG (100 μM) had no significant protection against OPC death induced by total deprivation of cystine (zero cystine) (data not shown), DHPG did significantly prevent OPC death induced by partial deprivation of cystine (3 μM cystine) at 24 h (Fig. 4B). Furthermore, DHPG exhibited a dose-dependent protective effect on OPC death induced by partial cystine deprivation, and the mGluR antagonist MCPG (500 μM) reversed the DHPG effect (Fig. 4C). Cystine deprivation-induced OPC death was prevented by Euk-134 (30 μM) but not by NBQX (50 μM) (Fig. 4B), indicating that this form of oxidative stress is independent of iGluR activation. Taken together, these results indicate that mGluR activation by DHPG can counteract oxidative stress induced by both excitotoxic and nonexcitotoxic conditions.
Fig. 4.
DHPG protects OPCs from nonexcitotoxic and excitotoxic forms of oxidative stress. (A) Effect of Euk-8, Euk-134 (30 μM each), or NBQX (50 μM) on kainate-(50 μM) or OGD-(2 h) induced OPC toxicity at 24 h. (B) Effect of DHPG (100 μM), Euk-134 (30 μM), or NBQX (50 μM) on cystine deprivation-(3μM cystine) induced OPC toxicity at 24 h. (C) Dose response of the effect of DHPG on cystine deprivation-(3 μM cystine) induced OPC toxicity at 24 h in the absence or presence of the mGluR antagonist MCPG (500 μM). *, P < 0.001 vs. none.
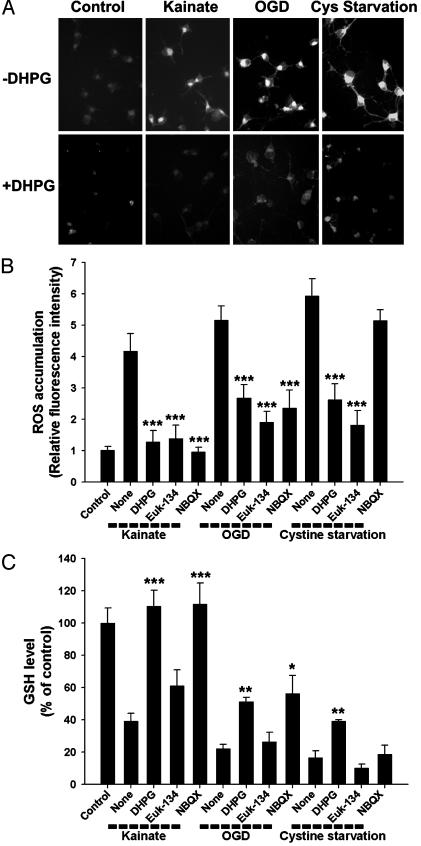
DHPG Attenuates Oxidative Stress to OPCs by Maintaining Intracellular GSH Levels. To further examine the effect of mGluR activation on oxidative stress, we directly measured intracellular ROS accumulation in the excitotoxic and nonexcitotoxic injury models. Exposure of OPCs to kainate (50 μM), OGD (2 h), or cystine deprivation (3 μM cystine) all dramatically induced ROS accumulation at 12 h (when no cell loss was apparent), as assessed by cytoplasmic fluorescence generated from ROS oxidation of dihydrorhodamine 123 (4, 13). In each paradigm, ROS accumulation was markedly prevented by DHPG (100 μM) (Fig. 5 A and B) or Euk-134 (30 μM) (Fig. 5B). NBQX (50 μM) prevented ROS accumulation induced by kainate exposure or OGD but not by cystine deprivation (Fig. 5B), consistent with cell survival data (Fig. 4B).
Fig. 5.
DHPG attenuates intracellular ROS accumulation and GSH loss. (A) Representative cell images of ROS accumulation in the absence or presence of DHPG (100 μM) 12 h after exposure of OPCs to kainate (50 μM), OGD (2 h), or cystine deprivation (3 μM cystine). (B and C) Quantitative ROS accumulation GSH levels and the effect of DHPG (100 μM), Euk-134 (30 μM), or NBQX (50μM). *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. none in the corresponding condition.
Next, we examined whether the DHPG effect on oxidative stress could be linked to intracellular GSH levels. Kainate (50 μM), OGD (2 h), and cystine deprivation (3 μM cystine) all caused GSH loss at 12 h, and the GSH loss induced by each was markedly attenuated by DHPG (100 μM) (Fig. 5C). As expected, the AMPA/kainate receptor antagonist NBQX (50 μM) was effective in preventing intracellular GSH loss induced by kainate exposure or OGD but not by cystine deprivation (Fig. 5C). The antioxidant Euk-134 (30 μM) did not significantly affect GSH levels (Fig. 5C), consistent with the demonstration in several models that antioxidants can prevent ROS accumulation and cell death without affecting GSH levels (4, 13, 23). Taken together, these results indicate that mGluR activation by DHPG attenuates both oxidative stress downstream of excitotoxic iGluR activation and nonexcitotoxic forms of oxidative stress by maintaining intracellular GSH levels.
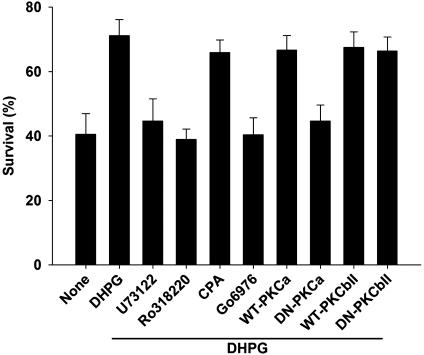
PKCα Activation Is Required for the Modulating Effect of DHPG on OGD-Induced OPC Injury. OGD in culture simulates the hypoxiaischemia involved in vivo in the pathogenesis of PVL and other brain disorders, produces both GluR-dependent and -independent injury, and may cause both excitotoxic and nonexcitotoxic forms of oxidative stress. Thus, we further investigated the possible signaling mechanisms underlying the modulating effect of DHPG on OGD-induced OPC injury. The stimulation of group I mGluRs activates, via G proteins, PLC, which in turn catalyzes inositol triphosphate (IP3) and diacylglycerol (DAG) production from membrane phosphatides. IP3 induces the release of Ca2+ from internal stores, and DAG, the activation of PKC (11). We found that the PLC inhibitor U73122 (1 μM) or the PKC inhibitor Ro318220 (500 nM), but not the inhibitor for intracellular Ca2+ mobilization, cyclopiazonic acid (20 μM), eliminated the protective effect of DHPG (100 μM) on OGD-induced OPC death (Fig. 6). Furthermore, the selective inhibitor for PKCα and -β isoforms, Go6976 (1 μM), had an effect similar to the nonselective PKC inhibitor Ro318220 (500 nM) (Fig. 6). We then examined the effect of genetically altered PKCα and -β on the protective effect of DHPG (100 μM) on OGD-induced OPC death. Infection of OPCs with dominant-negative type PKCα- but not PKCβII-expressing retrovirus abolished the DHPG effect compared to respective wild-type controls (Fig. 6).
Fig. 6.
PKCα activation is required for the modulating effect of DHPG on OGD-induced OPC injury. PLC inhibition with U73122 (1 μM), PKC inhibition with Ro318220 (500 nM) or Go6976 (1 μM), or dominant-negative-type PKCα, but not the inhibition of intracellular Ca2+ mobilization with cyclopiazonic acid (20 μM) or dominant-negative-type PKCβII, abolishes the protective effect of DHPG-(100 μM) on OGD-(2 h) induced OPC death. DN, dominant negative.
The concentrations of the pharmacological inhibitors used in these experiments were chosen as the doses that gave maximal response but minimal cytotoxicity when continuously present. Neither the pharmacological inhibitor nor the infection with retrovirus carrying each wild-type or dominant-negative PKC isoform per se affected OGD-induced OPC death in the absence of DHPG (data not shown). Taken together, the data indicate that the signaling cascade involving group 1 mGluRs/PLC/PKCα is responsible for the modulating effect of DHPG on OGD-induced OPC injury.
The mGluR-Modulating Effects Are Absent in MBP-Expressing Mature OLs. Finally, we examined whether group 1 mGluR activation by DHPG is protective in mature OLs after excitotoxic or nonexcitotoxic forms of injury. We have previously demonstrated that mature, as opposed to immature, OLs are resistant to toxicity induced by kainate (14), OGD (5), and oxidative stress (4). This maturation-dependent vulnerability of OLs critically underlies age-dependent hypoxic-ischemic white-matter injury and PVL (3, 5, 7, 8). To induce significant toxicity in MBP-expressing mature OLs, day 10 cultures were subjected to more severe insults (1 mM kainate, 6-h OGD, or total cystine deprivation). We found that DHPG (0-500 μM) had no effect on cell viability at 24 h in these injury paradigms (data not shown). These results suggest that the mGluR effect may be absent in mature OLs, consistent with our data indicating that mGluR expression is greatly diminished on mature cells (Fig. 1). Importantly, OPCs, rather than mature OLs, are the cellular substrate of PVL (3), and the mGluR-modulating effect on injury to OPCs, which exhibit enhanced expression of these receptors, may represent age-specific therapeutic strategies for PVL.
Discussion
This study reports a previously undescribed developmental regulation of mGluR expression across the OL lineage. Metabotropic GluRs are transiently expressed in OPCs but are down-regulated in mature OLs. Furthermore, we demonstrate that activation of group 1 mGluRs, but not other groups of mGluRs, reduces excitotoxic OPC injury induced by kainate exposure or OGD by controlling downstream oxidative stress and by maintaining intracellular GSH levels. This modulating effect of mGluRs on OPC excitotoxicity is not due to endocytosis of AMPA/kainate receptors from the cell surface, which occurs in neurons in response to mGluR activation, but requires activation of PKCα. In addition, group 1 mGluR activation on OPCs also prevents nonexcitotoxic forms of oxidative stress by maintaining intracellular GSH levels. These results reveal a previously uncharacterized role for mGluR activation in preventing OL excitotoxicity and oxidative stress, both of which contribute importantly to the pathogenesis of PVL and many other cerebral white-matter disorders (1). Although iGluRs mediate OL excitotoxicity (5, 24, 25), drugs that target iGluRs may not be therapeutically useful because of the ubiquitous involvement of these receptors in normal neurotransmission throughout the CNS. On the other hand, pharmacological manipulation of mGluRs may have relatively favorable profiles of adverse effects. This study identifies group 1 mGluRs as potential new therapeutic targets for treating such disorders as PVL, in which OL excitotoxicity and/or oxidative stress play an important role.
Previous neuronal studies showed that mGluRs can modulate neurotransmission and neurotoxicity. Stimulation of different mGluR subtypes has distinct effects on excitotoxic injury to neurons. Generally, group 1 mGluRs facilitate, whereas group 2 and 3 mGluRs limit, neuronal excitotoxicity (26, 27). In contrast, the present study reveals that activation of group 1 mGluRs, but not other groups of mGluRs, mitigates excitotoxic injury to OPCs. Two reasons may account for this discrepancy between neurons and OPCs. First, neuronal excitotoxicity is mediated predominantly by N-methyl-d-aspartate (NMDA) receptors on neurons, whereas OPCs express only AMPA/kainate receptors (28), which mediate excitotoxicity to these cells. The interplay between mGluRs and iGluRs of the NMDA vs. AMPA/kainate types may be different. Second, unlike in OPCs, mGluRs in neurons may modulate synaptic transmission via both pre- and postsynaptic mechanisms and thus influence glutamatergic activity and glutamate excitotoxicity in an anatomically and functionally distinct manner due to the heterogeneous localization of specific mGluR subtypes with distinct functional properties.
Finally, our study demonstrates that activation of group 1 mGluRs regulates intracellular ROS and GSH levels during OL injury. Metabotropic GluRs have long been linked to numerous signaling events such as phosphorylation and dephosphorylation of proteins, protein-protein interactions, and transactivation of genes (11, 29), all of which may contribute to the regulation of apoptotic/antiapoptotic molecules, cellular redox status, and GSH metabolism. Our study demonstrates that the modulating effect of group 1 mGluRs on hypoxic-ischemic OL injury requires activation of PKCα after G protein coupling to PLC. PKCα activation has been shown to reduce oxidative stress and cellular injury in various biological preparations (30-34). Future studies are needed to identify targets of PKCα responsible for the mGluR effect on ROS suppression in OLs. Although the complete mechanistic details of how mGluR activation controls oxidative stress remain to be fully elucidated, this study establishes a previously undescribed biological function for group 1 mGluRs in preventing OL excitotoxicity and oxidative stress, the two major forms of OL injury seen in many cerebral white-matter disorders.
Acknowledgments
We thank Ling Dong and Ben Williams for technical assistance and Rajeev Sivasankaran and Zhigang He (Children's Hospital, Harvard Medical School) for kindly providing wild-type or dominant-negative PKC-expressing retrovirus. This work was supported by National Institutes of Health Grants R01 NS31718 (to F.E.J.), P01 NS38475 (to J.J.V., P.A.R., and F.E.J.), and T32 AG00222 (to W.D.); by the William Randolph Hearst Fund (to W.D. and H.W.); and by Mental Retardation Research Center Grant P30 HD18655.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; GluR, glutamate receptor; GSH, glutathione; iGluR, ionotropic GluR; mGluR, metabotropic GluR; OGD, oxygen-glucose deprivation; OL, oligodendrocyte; OPC, OL precursor cell; PLC, phospholipase C; PVL, periventricular leukomalacia; ROS, reactive oxygen species; DHPG, (R,S)-3,5-dihydroxyphenylglycine; MCPG, (S)-methyl-4-carboxyphenylglycine; NBQX, 6-nitro-7-sulfamoylbenzo(f)quinoxaline-2,3-dione; MBP, myelin basic protein.
References
- 1.Volpe, J. J. (2001) in Neurology of the Newborn (Saunders, Philadelphia), 4th Ed., pp. 217-276.
- 2.Volpe, J. J. (2003) Pediatrics 112, 176-180. [DOI] [PubMed] [Google Scholar]
- 3.Back, S. A., Luo, N. L., Borenstein, N. S., Levine, J. M., Volpe, J. J. & Kinney, H. C. (2001) J. Neurosci. 21, 1302-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Back, S. A., Gan, X., Li, Y., Rosenberg, P. A. & Volpe, J. J. (1998) J. Neurosci. 18, 6241-6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng, W., Rosenberg, P. A., Volpe, J. J. & Jensen, F. E. (2003) Proc. Natl. Acad. Sci. USA 100, 6801-6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng, W. & Poretz, R. D. (2003) Neurotoxicology 24, 161-178. [DOI] [PubMed] [Google Scholar]
- 7.Follett, P. L., Rosenberg, P. A., Volpe, J. J. & Jensen, F. E. (2000) J. Neurosci. 20, 9235-9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fern, R. & Moller, T. (2000) J. Neurosci. 20, 34-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshioka, A., Yamaya, Y., Saiki, S., Kanemoto, M., Hirose, G., Beesley, J. & Pleasure, D. (2000) Brain Res. 854, 207-215. [DOI] [PubMed] [Google Scholar]
- 10.Choi, D. W. (1994) Prog. Brain Res. 100, 47-51. [DOI] [PubMed] [Google Scholar]
- 11.Conn, P. J. & Pin, J.-P. (1997) Annu. Rev. Pharmacol. Toxicol. 37, 205-237. [DOI] [PubMed] [Google Scholar]
- 12.Vincent, A. M. & Maiese, K. (2000) Exp. Neurol. 166, 65-82. [DOI] [PubMed] [Google Scholar]
- 13.Li, J., Lin, J. C., Wang, H., Peterson, J. W., Furie, B. C., Furie, B., Booth, S. L., Volpe, J. J. & Rosenberg, P. A. (2003) J. Neurosci. 23, 5816-5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg, P. A., Dai, W., Gan, X. D., Ali, S., Fu, J., Back, S. A., Sanchez, R. M., Segal, M. M., Follett, P. L., Jensen, F. E., et al. (2003) J. Neurosci. Res. 71, 237-245. [DOI] [PubMed] [Google Scholar]
- 15.Finkbeiner, S., Tavazoie, S. F., Maloratsky, A., Jacobs, K. M., Harris, K. M. & Greenberg, M. E. (1997) Neuron 19, 1031-1047. [DOI] [PubMed] [Google Scholar]
- 16.Wang, H. & Joseph, J. A. (2000) Free Radic. Biol. Med. 28, 1222-1231. [DOI] [PubMed] [Google Scholar]
- 17.Sivasankaran, R., Pei, J., Wang, K. C., Zhang, Y. P., Shields, C. B., Xu, X. M. & He, Z. (2004) Nat. Neurosci. 7, 261-268. [DOI] [PubMed] [Google Scholar]
- 18.Luyt, K., Varadi, A. & Molnar, E. (2003) J. Neurochem. 84, 1452-1464. [DOI] [PubMed] [Google Scholar]
- 19.Kelland, E. E. & Toms, N. J. (2001) Eur. J. Pharmacol. 424, R3-R4. [DOI] [PubMed] [Google Scholar]
- 20.Snyder, E. M., Philpot, B. D., Huber, K. M., Dong, X., Fallon, J. R. & Bear, M. F. (2001) Nat. Neurosci. 4, 1079-1085. [DOI] [PubMed] [Google Scholar]
- 21.Liu, H. N., Giasson, B. I., Mushynski, W. E. & Almazan, G. (2002) J. Neurochem. 82, 398-409. [DOI] [PubMed] [Google Scholar]
- 22.Sagara, Y. & Schubert, D. (1998) J. Neurosci. 18, 6662-6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oka, A., Belliveau, M. J., Rosenberg, P. A. & Volpe, J. J. (1993) J. Neurosci. 13, 790-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matute, C., Sanchez-Gomez, M. V., Martinez-Millan, L. & Miledi, R. (1997) Proc. Natl. Acad. Sci. USA 94, 8830-8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald, J. W., Althomsons, S. P., Krzysztof, L. H., Choi, D. W. & Goldberg, M. P. (1998) Nat. Med. 4, 291-297. [DOI] [PubMed] [Google Scholar]
- 26.Nicoletti, F., Bruno, V., Copani, A., Casabona, G. & Knopfel, T. (1996) Trends Neurosci. 19, 267-271. [DOI] [PubMed] [Google Scholar]
- 27.Bruno, V., Battaglia, G., Copani, A., D'Onofrio, M., Di Iorio, P., De Blasi, A., Melchiorri, D., Flor, P. J. & Nicoletti, F. (2001) J. Cereb. Blood Flow Metab. 21, 1013-1033. [DOI] [PubMed] [Google Scholar]
- 28.Patneau, D. K., Wright, P. W. & Wisden, W. (1994) Neuron 12, 357-371. [DOI] [PubMed] [Google Scholar]
- 29.Michaelis, E. K. (1998) Prog. Neurobiol. 54, 369-415. [DOI] [PubMed] [Google Scholar]
- 30.Itano, Y., Ito, A., Uehara, T. & Nomura, Y. (1996) J. Neurochem. 67, 131-137. [DOI] [PubMed] [Google Scholar]
- 31.Smith, C. A., Williams, G. T., Kingston, R., Jenkinson, E. J. & Owen, J. J. (1989) Nature 337, 181-184. [DOI] [PubMed] [Google Scholar]
- 32.Cuvillier, O., Pirianov, G., Kleuser, B., Vanek, P. G., Coso, O. A., Gutkind, S. & Spiegel, S. (1996) Nature 381, 800-803. [DOI] [PubMed] [Google Scholar]
- 33.May, W. S., Tyler, P. G., Ito, T., Armstrong, D. K., Qatsha, K. A. & Davidson, N. E. (1994) J. Biol. Chem. 269, 26865-26870. [PubMed] [Google Scholar]
- 34.Jarvis, W. D., Fornari, F. A., Traylor, R. S., Martin, H. A., Kramer, L. B., Erukulla, R. K., Bittman, R. & Grant, S. (1996) J. Biol. Chem. 271, 8275-8284. [DOI] [PubMed] [Google Scholar]