Abstract
Background:
Invasive fungal infections cause excessive morbidity and mortality in premature neonates and severely ill infants.
Methods:
Safety and efficacy outcomes of micafungin were compared between prematurely and non-prematurely born infants <2 years of age. Data were obtained from all completed phase I–III clinical trials with micafungin that had enrolled infants (<2 years of age) that were listed in the Astellas Clinical Study Database. Demographics, adverse events, hepatic function tests and treatment success data were extracted and validated by the Astellas biostatistical group for all micafungin-treated patients, <2 years of age, using the unique patient identifier.
Results:
One-hundred and sixteen patients included in 9 clinical trials, 48% premature [birth weight (BW) <2500 g and/or gestational age <37 weeks], 52% non-premature, received ≥1 dose of micafungin. Among premature patients, 14.5% were low BW (1500–2499 g), 36.4% very low BW (1000–1499 g) and 49.1% extremely low BW (<1000 g). Ninety patients (78%) completed the studies; 13 [11% (4 premature)] died. Significantly more non-premature than premature patients discontinued treatment (P = 0.003). Treatment-related adverse events were recorded in 23% of patients with no difference between groups. More extremely low BW (n = 4, 15%) and very low BW (n = 8, 40%) infants experienced treatment-related adverse events than low BW (n = 0) and there was no relation to micafungin dose or duration. For a subgroup of 30 patients with invasive candidiasis, treatment success was achieved in 73% in both premature and non-premature groups. Prophylaxis was successful in 4/5 non-premature hematopoietic stem cell transplant patients.
Conclusion:
Micafungin has a safe profile in premature and non-premature infants with substantial efficacy.
Keywords: pediatric, neonatal, micafungin, candidiasis, antimicrobial
Prevalence of invasive fungal infection (IFI) has increased in very low birth weight (VLBW) preterm neonates (<1500 g), as more neonates born at the youngest gestational ages survive past the immediate postnatal period.1,2 These neonates are not yet fully immunocompetent and often require invasive procedures, such as the use of central venous catheters and endotracheal tubes. Additionally, they are commonly exposed to broad-spectrum antibiotics, parenteral nutrition, H2 blockers and corticosteroids. All these factors place neonates at high risk of IFI, particularly from Candida species. Invasive Candida infection (ICI) causes severe morbidity and mortality in VLBW preterm neonates in neonatal intensive care units.3–5 In multicenter studies, ICI has an associated mortality rate of 21–32%, increasing to 40–50% in the most immature infants [ie, <28 weeks gestational age and extremely low birth weight (ELBW), ie, <1000 g].6–9 Late neurodevelopmental impairment is common in ELBW infants who develop ICI.4 The challenge is to establish adequate prevention and treatment strategies in such vulnerable populations.
Research has focused on identifying efficacious and well-tolerated antifungal drugs that can prevent and treat neonatal IFI. Historically, first-line options for treating neonatal IFI have been amphotericin B deoxycholate (AMB-D) and fluconazole.10 However, nephrotoxicity limits AMB-D and uncertainties remain about its appropriate dosing and safety in premature infants.10 An alternative AMB formulation, liposomal amphotericin B (L-AMB), is effective in VLBW infants with few reports of nephrotoxicity,11–13 although adequate trial data do not exist. Fluconazole is only active against yeasts14–16 and some species such as Candida glabrata and Candida krusei may be fluconazole resistant.17,18 The echinocandins (anidulafungin, caspofungin and micafungin) are the most recently developed antifungal drug class, with broad-spectrum efficacy against Candida species including fluconazole-resistant strains and some activity against Aspergillus species. Among the echinocandins, the efficacy and safety of micafungin have been studied in clinical trials in neonates19 along with pharmacokinetic studies.20–22 The European Medicines Agency has licensed micafungin as the only echinocandin for neonatal use based on these data.
The inclusion of pediatric patients (<2 years of age), including full-term and premature neonates, in Phase I–III clinical trials of micafungin provided the opportunity to analyze patient-level data in this population. The aim of this study was to review the data regarding the safety and efficacy of micafungin in neonates and infants <2 years of age. We focused on the outcomes of premature compared with non-premature infants, with further stratification of premature infants based on degree of prematurity.
METHODS
Design and Data Sources
This retrospective study was conducted through database analysis of the demographic, safety and efficacy data obtained during all completed Phase I–III clinical trials in the Astellas Clinical Study Database. The trials were conducted from 1999 to 2012. Inclusion criteria were all infants <2 years of age treated with micafungin, prophylactically or for confirmed/suspected ICI or other IFI, regardless of dosages and duration of micafungin treatment.
Patient Population
All patients <2 years of age, regardless of gestational age, birth weight (BW) and indication, who had received at least 1 micafungin dose, were included in the analysis.
For the purpose of comparison, patients were categorized as non-premature if born at ≥37 weeks or premature if <37 weeks + 0 days gestational age and/or <2500 g at birth, including those readmitted to hospital and whose underlying condition was no longer prematurity but associated sequelae, such as bronchopulmonary dysplasia, intracranial hemorrhage or gastrointestinal disease of surgical interest.
Patients categorized as premature were further subcategorized by degree of prematurity, according to BW as infants with low birth weight (LBW): 1500−2499 g at birth; VLBW: 1000−1499 g at birth or ELBW: <1000 g at birth. Whenever BW was not available, we used an approximate correlation of gestational age with BW: LBW: 32 weeks + 0 days to 36 weeks + 6 days; VLBW: 28 weeks + 0 days to 31 weeks + 6 days or ELBW: ≤27 weeks + 6 days. For patients whose gestational age and BW were not available, an estimate of prematurity status and grading was made based on the weight and age of the patients at study baseline. Medical history notes from case report forms were reviewed to further clarify prematurity where necessary as certain conditions, such as those listed above, indicate a premature birth.
Outcomes and Statistical Analysis
Patient Demographics, Medical History and Disposition
Demographics, medical history, disposition and micafungin exposure were summarized for all patients, separately for non-premature and premature patients and for premature patients further subcategorized as LBW, VLBW or ELBW.
Safety
Safety data were recorded for all patients, separately for non-premature and premature patients and for premature patients further subcategorized as LBW, VLBW or ELBW.
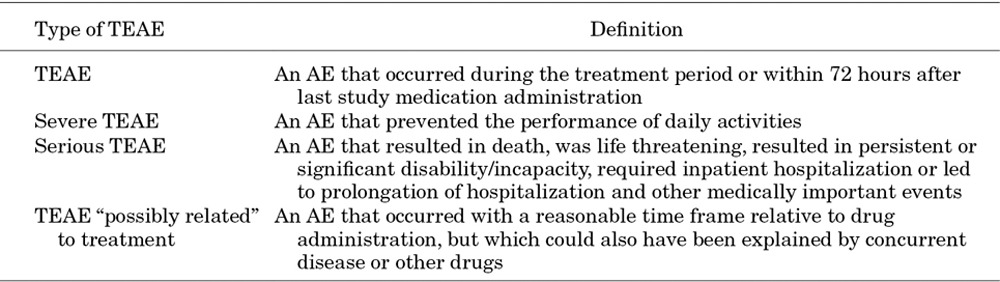
Safety was assessed by identifying treatment-emergent adverse events (TEAEs), severe TEAEs, serious TEAEs, treatment-related TEAEs (TR-TEAEs) and TEAEs leading to discontinuation of study treatment. TEAEs were judged to be related, including “possibly related” or “not assessable” TEAEs or not related to study medication by the investigator. Relevant definitions for TEAEs can be found in Table 1. Adverse events (AEs) were classified according to the Medical Dictionary for Regulatory Activities version 12 (http://www.meddra.org/).
TABLE 1.
Definitions of TEAE
Hepatic function was assessed by laboratory tests for alanine aminotransferase (ALT) and aspartate aminotransferase (AST), alkaline phosphatase (ALP) and total bilirubin. Serum creatinine was measured to assess renal function. The number and percent of patients who had either ALT or AST elevations >3, 5 or 10 times the upper limit of normal (ULN) value of 35 U/L and bilirubin elevations >2, 4 or 6 times ULN value of 1.0526 mg/dL (18 μmol) at least once during the treatment period were evaluated. This also applied to patients who had conjoint elevations of ALT or AST >3 times ULN and total bilirubin of either 1.5 or 2 times ULN. “Conjoint” was defined as 2 laboratory measures either from within the same sample or from 2 separate samples taken within 3 days. For patients from the Phase III randomized, active-controlled study of micafungin19 shifts between baseline (before therapy) and during the study or at end of treatment (EOT) in ALT, AST, ALP (relevant change >3 times ULN), total bilirubin and serum creatinine (relevant change >2 times ULN) were evaluated.
Efficacy
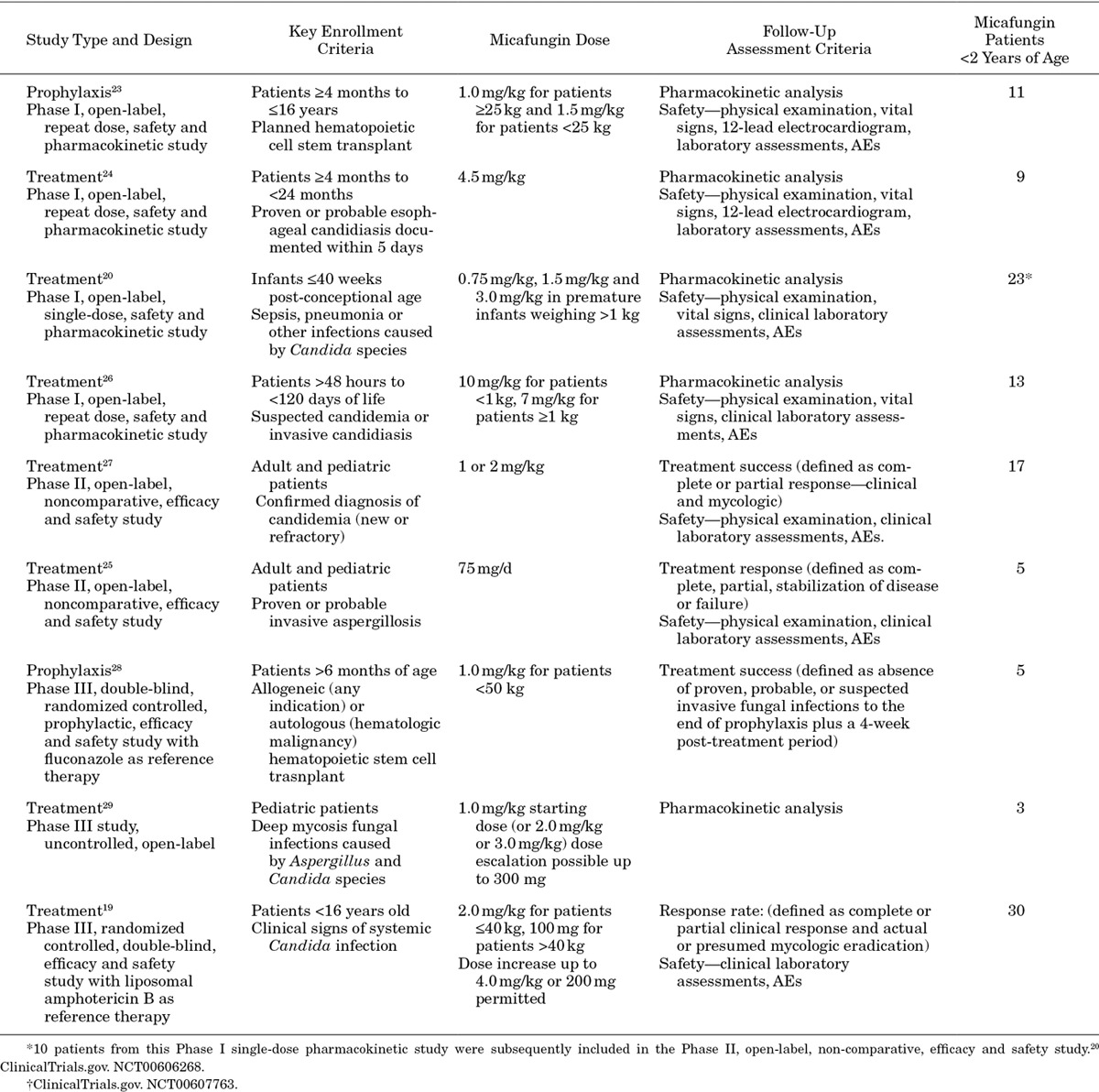
Of the 9 trials analyzed,19,20,23–29 4 incorporated efficacy outcome measures (Table 2).19,25,27,28 Of these 4, data from the 2 Phase III studies—the only double-blind studies with active comparators—are provided here. For the prophylactic study,28 efficacy was measured as treatment success defined as the absence of a proven or probable IFI up to EOT plus a 4-week, post-treatment period. For the ICI study,19 clinical response was defined as a complete or partial resolution of signs and symptoms, and mycologic response was defined as eradication or presumed eradication of the ICI. Treatment success rate was calculated as the ratio of the total number of treatment successes to the total number of patients and described where applicable by prematurity status.
TABLE 2.
Clinical Trials Included in the Systematic Review
Statistics
All eligible study data were pooled on a patient level and analyzed. Descriptive statistics for continuous variables included the number of subjects (n), mean and standard deviation. For categorical variables, percentages were calculated using the number of known values as denominator. Fisher’s exact test was used to compare underlying conditions, treatment discontinuations, TEAEs, liver enzymes and bilirubin between non-premature and premature patients and also to evaluate the relationship between TEAE occurrence and micafungin dose (mg/kg) and duration (days). Wilcoxon rank sum test was used to compare mean daily micafungin dose and mean duration of micafungin treatment. Differences were considered significant if P < 0.05. Data analysis was generated using SAS/STAT software, Version 9.1 (2000–2004), SAS Institute Inc, Cary, NC.
RESULTS
Studies Retrieved
Nine clinical trials were identified, including 4 Phase I pharmacokinetic,20,23,24,26 2 Phase II noncomparative25,27 and 3 Phase III studies19,28,29 (Table 2).
Patient Demographics, Medical History and Disposition
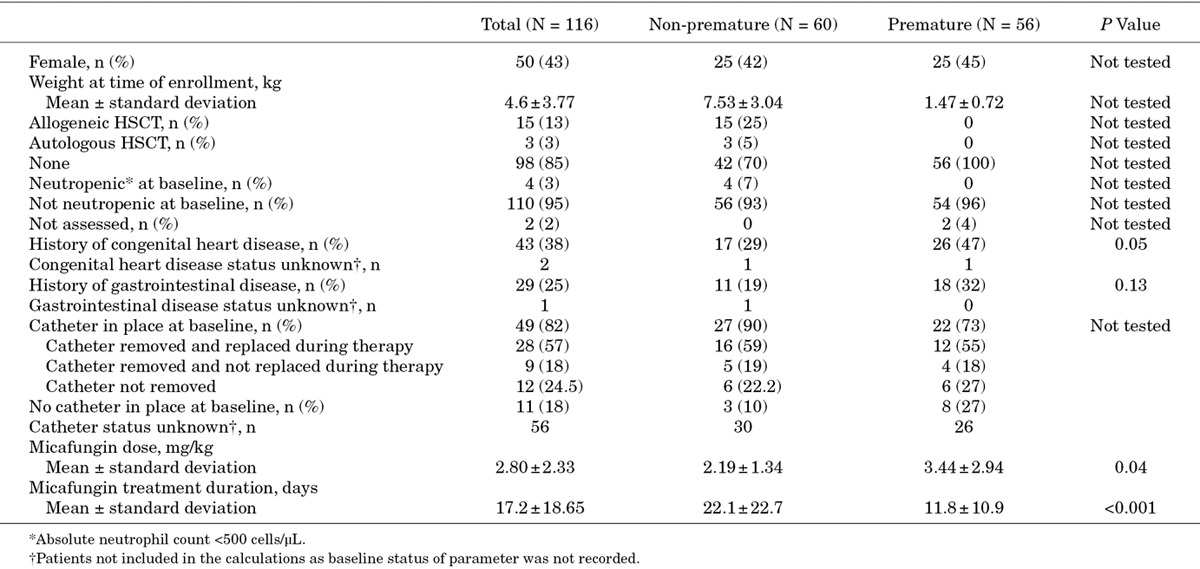
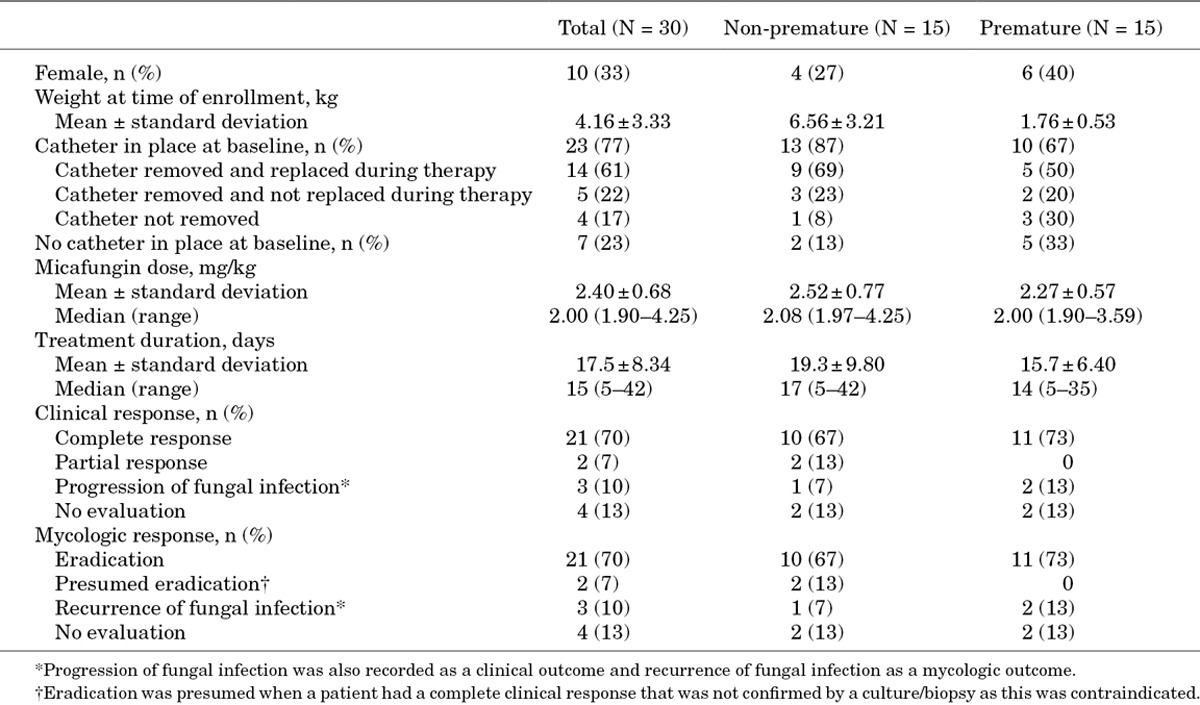
From the 9 eligible studies, 116 patients <2 years of age were identified as having received at least 1 micafungin dose and were included in the pooled analysis. Of these, 60 (52%) were non-premature and 56 (48%) were premature; Table 3 shows demographics and medical history. For the premature group, where gestational age was recorded (n = 25), the mean age was 26.5 ± 2.7 weeks.
TABLE 3.
Demographics and Medical History for All Patients and by Prematurity Status
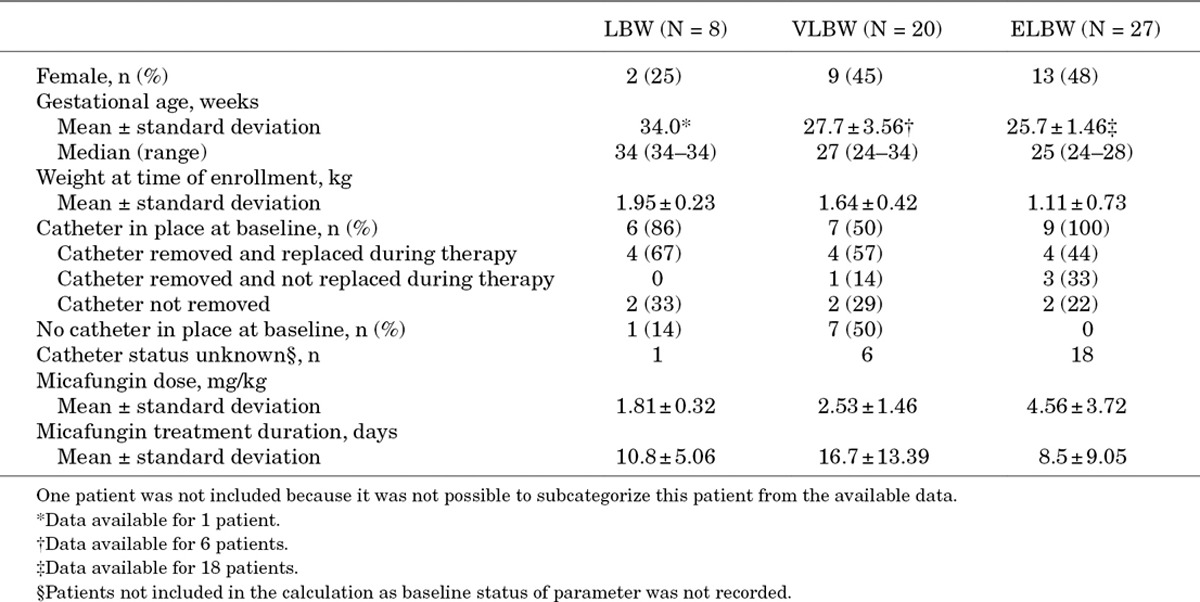
Of the 56 premature patients, 55 were further categorized by degree of prematurity as LBW (n = 8), VLBW (n = 20) or ELBW (n = 27). BW was recorded for 12 patients and BW was extrapolated from recorded gestational age for 13 patients. An estimate of prematurity status and grading was made based on the weight and age of the patients at study baseline for 30 patients. It was not possible to subcategorize 1 patient from the available data. Table 4 summarizes patient demographics and medical history.
TABLE 4.
Demographics and Medical History for Premature Patients Categorized by Degree of Prematurity
Ninety (78%) patients completed the studies, 13 (11%) died, 6 (5%) were lost to follow up, 4 (3%) did not complete studies for non-specified reasons and data were missing for 3 patients. Twenty-four (21%) patients discontinued treatment, with significantly more non-premature than premature infants discontinuing (32% vs. 9%, respectively; P = 0.003).
Of the 13 patients who died, 9 were non-premature. Death was deemed unrelated to micafungin in the majority of cases. Death in 2 non-premature patients was considered a result of lack of efficacy. The first patient suffered from Aspergillus endocarditis and received combination therapy with L-AMB, micafungin and flucytosine for 134 days. The second patient died after 8 days’ L-AMB and micafungin treatment for Candida peritonitis and sepsis because of Candida albicans.
For the 55 patients categorized by degree of prematurity, 45 (82%) completed the study: 5 (63%) LBW, 16 (80%) VLBW and 24 (89%) ELBW. Two patients in both the LBW and VLBW groups were lost to follow up and 1 was lost in the ELBW group. Mortality was low with 4 deaths: 1 LBW patient, 2 VLBW and 1 ELBW. One LBW (13%), 2 (10%) VLBW and 2 (7%) ELBW patients discontinued treatment. Tables 3 and 4 show the mean daily dose and duration of micafungin for premature and non-premature patients.
Safety
TEAEs occurred in 105 (91%) patients. There was no significant difference in the incidence of severe, serious or TR-TEAEs between non-premature and premature infants (Fig. 1). Incidence of overall TEAEs was similar across patients categorized by degree of prematurity. Although more ELBW and VLBW infants seemed to experience severe or serious TEAEs and TR-TEAEs than LBW infants, differences were not statistically significant (Fig. 2). No relationship was apparent between the occurrence of TEAEs, severe TEAEs, serious TEAEs and TR-TEAEs with micafungin dose or treatment duration in non-premature and premature groups or for premature patients categorized by degree of prematurity.
FIGURE 1.
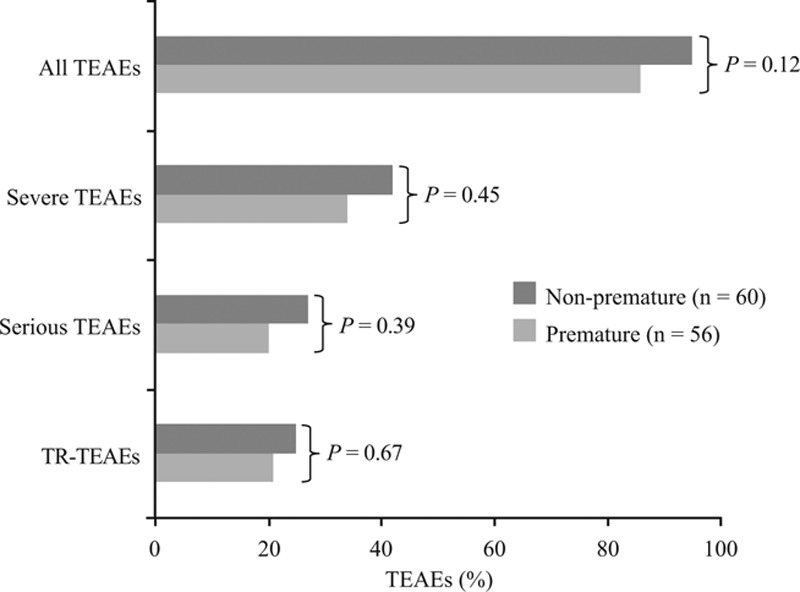
TEAEs for patients by prematurity status. Statistical comparison of non-premature versus premature groups was by Fisher’s exact test.
FIGURE 2.
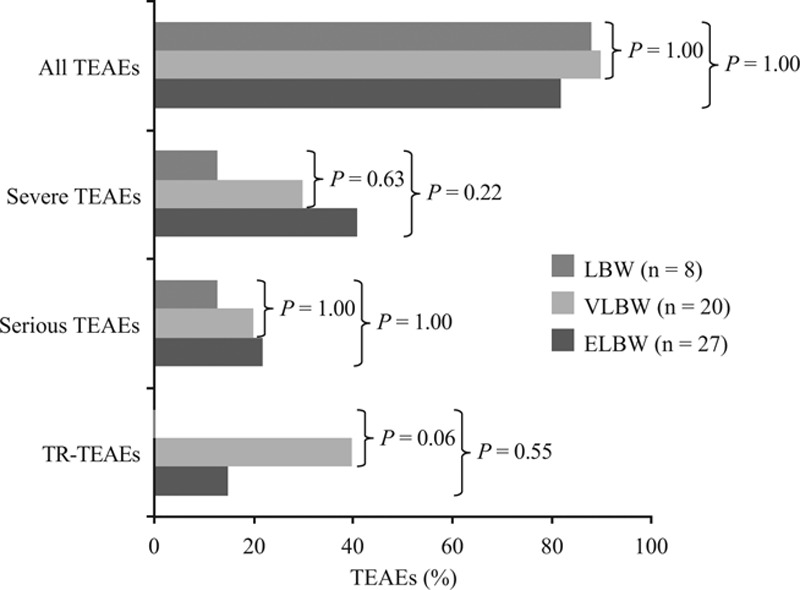
TEAEs for premature patients categorized by degree of prematurity. Statistical comparison against LBW group as the comparator was by Fisher’s exact test.
Figure 3 shows the most common TEAEs occurring in 10% or more of non-premature or premature patients. Eleven TEAEs led to 9 (8%) patients discontinuing treatment: 2 (4%) premature and 7 (12%) non-premature patients (P = 0.17). TEAEs leading to discontinuation in non-premature infants were increased liver enzymes (n = 3), cardiac failure (n = 2), decreased oxygen saturation, neutropenia, intracranial hemorrhage and hypotension (all n = 1). Cardio-respiratory arrest led to treatment discontinuation in an LBW patient and an abnormal liver test led to treatment discontinuation in an ELBW patient.
FIGURE 3.
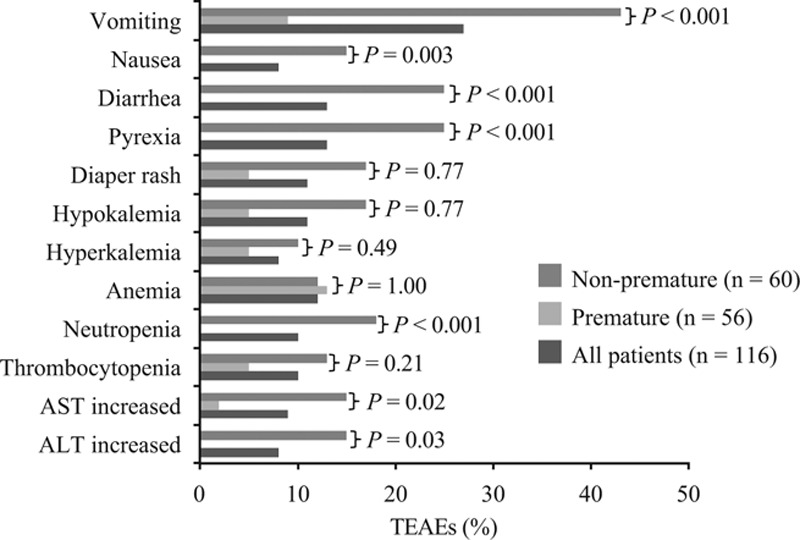
Incidence of TEAEs (>10% in either group) for all patients and for patients by prematurity status. Statistical comparison of non-premature versus premature groups was by Fisher’s exact test.
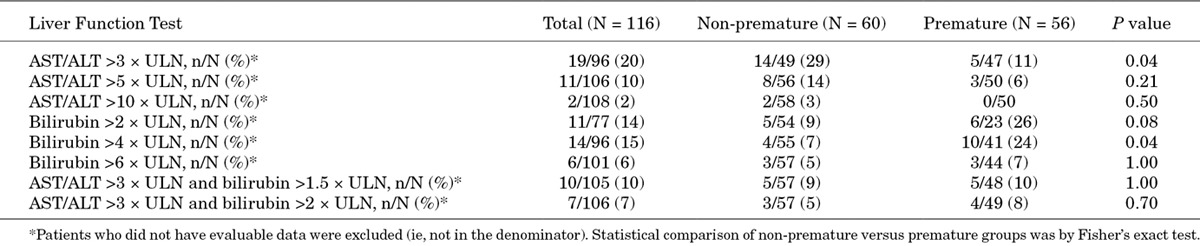
Regarding liver function, there were more non-premature infants with elevated ALT or AST levels than premature infants. In contrast, the incidence of bilirubin levels above ULN during the study was higher in premature patients than non-premature patients. Conjoint elevations of ALT or AST and bilirubin were similar between groups (Table 5).
TABLE 5.
Incidence of Clinically Significant Elevations in Liver Function Test at Least Once During Therapy for All Patients and for Patients by Prematurity Status
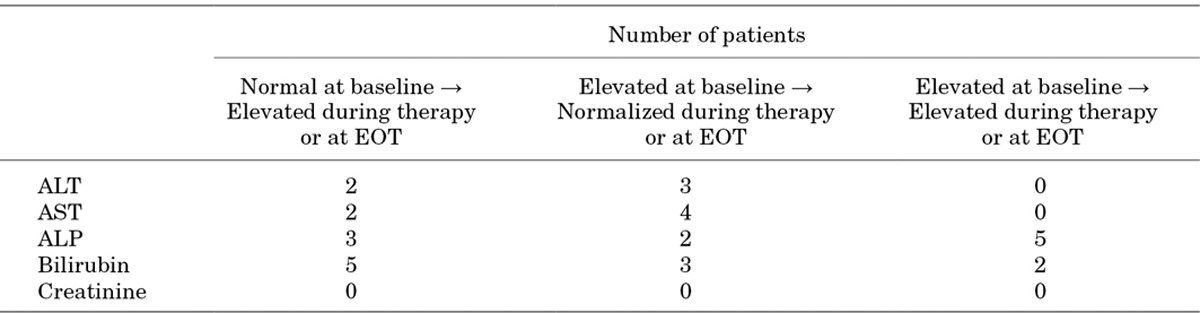
For the majority of the 30 neonates from the ICI study,19 whose ALT, AST, ALP, bilirubin and creatinine levels were calculated before, during and at EOT, there were no elevations in these parameters at any time. There was a lack of consistency in the shift patterns of these clinical laboratory evaluations in patients who did experience elevated levels. Although a few patients had elevated ALT, AST, ALP and bilirubin levels during therapy, or at EOT, from normal baseline levels, others had elevated levels at baseline which then either normalized or remained elevated during therapy or at EOT (Table 6). Serum creatinine elevations were not observed at any time.
TABLE 6.
Shifts in Liver Enzymes, Bilirubin and Creatinine From Baseline That Occurred During Therapy or at End of Treatment in the 30 Neonate Patients From the Phase III Randomized, Active-Controlled Study of Micafungin19
Efficacy
The ICI study19 included 30 patients <2 years of age who had received at least 1 micafungin dose. Of these, 15 were premature. None had undergone hematopoietic stem cell transplantation (HSCT) or were neutropenic. C. albicans, Candida parapsilosis and Candida tropicalis were the most common pathogens in both groups, accounting for 43% (n = 13), 20% (n = 6), and 17% (n = 5) of Candida species, respectively, whereas, C. krusei, Candida guilliermondii, Candida utilis and Candida lipolytica accounted for single cases. The most common infection site was the blood in 93% (n = 28) of patients. Mean micafungin daily dose and treatment duration were similar between non-premature and premature patients (Table 7). Overall, treatment success was achieved in 73% of both non-premature and premature patients. The majority of patients had a complete clinical response and complete mycologic eradication (Table 7). For the 3 patients in whom ICI recurred, the species was C. albicans.
TABLE 7.
Demographics, Medical History, and Treatment Success of Patients <2 Years Old From the Phase III Randomized, Active-Controlled Study in Invasive Candidiasis Infection19
Five non-premature patients <2 years of age from the prophylaxis study28 were included in this analysis (mean weight: 11.28 ± 3.17 kg). All 5 had undergone allogeneic HSCT, none had neutropenia and catheter status was unknown. Mean daily micafungin dose was 0.99 ± 0.02 mg/kg and treatment duration was 33.6 ± 10.9 days. Micafungin prophylaxis was successful in 4 patients after allogeneic HSCT and was not evaluable in 1.
DISCUSSION
This is the first study to date to comprehensively review prospectively collected data from randomized controlled trials on the safety and efficacy of an echinocandin, that is, micafungin, in infants <2 years of age, and compare non-premature and premature patients and those with various grades of prematurity. Our analysis found no unexpected safety concerns with micafungin in either premature or non-premature infants, even with the use of high daily doses and long treatment courses. Our results confirm findings that AEs because of micafungin in this population tend to be mild and seldom lead to treatment discontinuation.30,31
This safety analysis in premature neonates and infants <2 years of age supports and expands results found in a review of 6 clinical trials of micafungin in pediatric patients aged ≤16 years.32 Together, the 2 analyses show that micafungin is well-tolerated by children of all ages, including premature neonates. In our analysis, the incidence of severe, serious or TR-TEAEs was similar in premature and non-premature infants. Although there appeared to be more severe, serious and TR-TEAEs in ELBW and VLBW infants than in those with LBW, an analysis of these TEAEs showed that there was no relationship with micafungin dose or treatment duration. This is consistent with studies in other patient groups, including older children, where no trends were observed between AEs and micafungin dose or treatment duration.32–34
AMB-D, a mainstay of IFI therapy, has a poor safety profile mainly associated with nephrotoxicity, hypokalemia and infusion-related local reactions.35 AMB-D is generally better tolerated by neonates than adults;36 however, severe nephrotoxicity has been reported in VLBW infants.37 L-AMB was developed as a safer alternative to AMB-D, but a high incidence (16.7%) of AEs leading to treatment discontinuation has been reported in pediatric patients treated for ICI with L-AMB.19 In contrast to L-AMB, the incidence of AEs leading to treatment discontinuation with micafungin in this study was lower (3.8%)19 and similar to the current analysis (3.4%). These results are consistent with the pooled risk of treatment discontinuation of >10% for AMB preparations and 2.5−3.8% for fluconazole, caspofungin and micafungin reported by Wang et al,38 and confirm that micafungin is a well-tolerated alternative to AMB preparations in pediatric populations.
In the pooled analysis of micafungin-treated pediatric patients,32 nearly one-half had elevated baseline AST or ALT levels and one-third had elevated baseline bilirubin. The proportions of patients with elevated liver enzymes or bilirubin during treatment were far less, at 7.7% for AST, 11.7% for ALT and 4.9% for bilirubin. It is likely that in our analysis the incidence of elevated AST/ALT and bilirubin levels included patients who had raised levels at baseline or even during treatment as a result of their underlying condition, rather than being associated with drug treatment. Shifts in AST and ALT levels were examined in the current analysis at baseline and during treatment in the 30 patients <2 years of age treated with micafungin for invasive candidiasis;19 this showed a number of patients with high AST, ALT or bilirubin levels at baseline who normalized their values during therapy.
Neonate deaths that occurred in the studies analyzed were deemed by the participating investigator to be unrelated to micafungin in the majority of cases, although 2 deaths were attributed to a lack of efficacy. In our opinion, 1 patient, who was treated with L-AMB and micafungin for Candida peritonitis and sepsis because of C. albicans, did die because of failure of the combination therapy, as this Candida species is often susceptible to echinocandins and L-AMB. The other patient’s death was, in our opinion, not related to micafungin; this patient suffered from Aspergillus endocarditis and was on combination therapy with L-AMB, micafungin and flucytosine. As echinocandins may not be fungicidal for Aspergillus, especially in a patient with cardiac vegetations who is on combination therapy, we consider this death cannot be attributed to micafungin failure.
Micafungin pharmacokinetics demonstrate a shorter half-life and faster clearance relative to younger age in children39 and lower weight in neonates,20 indicating pediatric dosing should perhaps be modified based on age or weight. The pharmacokinetics of elevated micafungin doses have been examined in a small number of young infants.22,26 These data have prompted proposals to use high (up to 10 mg/kg) micafungin doses to treat ICI in neonates in case of potential central nervous system infection.40–42 Clinical trial data do demonstrate a micafungin dose of 2 mg/kg effectively treats pediatric patients with ICI with success rates similar to L-AMB.19 Treatment outcomes in pediatric ICI have been reported to be similar for the different antifungal classes (67% efficacy rate for triazoles, 73% for polyenes, 73% for echinocandins).43 The 73% success rate observed with micafungin in current analysis of neonates from the ICI study19 is similar to these results. Micafungin has also been shown to be efficacious against Candida in VLBW premature infants in a separate study.44 Concerns regarding the efficacy of echinochandins, including micafungin, against C. parapsilosis, the most common non-C. albicans fungal pathogen in neonates,43,45–48 have been raised because of higher in vitro minimum inhibitory concentrations. However, in clinical trials, the high treatment success rates of micafungin against this strain are similar to C. albicans and C. glabrata.27,49
In addition to treating diagnosed infections, micafungin might also be seen as an attractive alternative to fluconazole as antifungal prophylaxis as it is active against Candida including fluconazole-resistant Candida species such as C. glabrata and C. krusei and against Aspergillus species. In the single study of micafungin versus fluconazole as antifungal prophylaxis in adult and pediatric HSCT patients, micafungin was superior to fluconazole (P = 0.03) during the neutropenic phase after transplantation.28 Among 5 patients <2 years of age evaluated in this current analysis, 4 had no breakthrough IFI during neutropenia and the fifth was lost to follow up. Results of this prophylaxis efficacy analysis are limited by the small number of patients <2 years of age included (n = 5).
Our study has some limitations. As the data analyzed came from the Astellas Clinical Study Database, this study is not a systematic review of all micafungin studies in infants <2 years of age but rather a review of data obtained during the drug’s clinical development. The retrospective design of the study may also be considered a limitation. However, the strength of the data analyzed here derives from the prospective and interventional design of the 9 Phase I–III clinical trials, and from the inclusion in the analysis of the largest number of patients <2 years of age who were prospectively treated with a single antifungal (n = 116). An additional strength of this systematic analysis is the subcategorization of premature patients and comparison of micafungin efficacy and safety in patients with different degrees of prematurity. Neither this analysis, nor the 9 studies from which the data were derived, were designed to test for significant differences between subgroups. The statistical comparisons our study provides confirm the hypothesis of no difference in the safety profile of micafungin in premature infants versus those born at full term, to a greater extent than can be ascertained by reviewing descriptive statistics of the individual studies alone. Caveats including lack of statistical powering and multiplicity of testing should be considered when interpreting, in particular, the significant differences between subgroups with regards to TEAEs.
In conclusion, in the 9 multicenter trials analyzed, micafungin was well-tolerated in premature and non-premature neonates <2 years of age and few TR-TEAEs leading to treatment discontinuation were reported.
These data suggest that micafungin can be a valuable and well-tolerated, first-line option for the treatment of ICI in premature and non-premature infants <2 years of age.
ACKNOWLEDGMENTS
Statistical assistance was provided by Dorothea Wessiepe (Metronomia Clinical Research GmbH, Munich, Germany) and medical writing assistance was provided by Lisa Thomas (Connect2CME, Westerham, Kent, United Kingdom) funded by Astellas Pharma Europe Ltd.
Footnotes
This analysis and publication were funded by Astellas Pharma Europe Ltd. Paolo Manzoni has served as a scientific advisor and been a member on speaker bureaux for Astellas Pharma and Pfizer. Chunzhang Wu is an employee Astellas Pharma Global Development. Lorraine Tweddle is an employee Astellas Pharma Europe. Emmanuel Roilides has received research grants of significant value from Enzon, Gilead, Merck, Pfizer and Schering, has served as a scientific advisor for Astellas, Gilead, Merck, Pfizer, and Schering and been a member on speaker bureaux for Astellas, Aventis, Cephalon, Gilead, GlaxoSmithKline, Merck, Pfizer, Sanofi-Pasteur, Schering and Wyeth.
The authors have no other funding or conflicts of interest to disclose.
REFERENCES
- 1.Brecht M, Clerihew L, McGuire W. Prevention and treatment of invasive fungal infection in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2009;94:F65–F69. doi: 10.1136/adc.2007.133769. [DOI] [PubMed] [Google Scholar]
- 2.Roilides E. Invasive candidiasis in neonates and children. Early Hum Dev. 2011;87(Suppl 1):S75–S76. doi: 10.1016/j.earlhumdev.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 3.Roilides E, Farmaki E, Evdoridou J, et al. Neonatal candidiasis: analysis of epidemiology, drug susceptibility, and molecular typing of causative isolates. Eur J Clin Microbiol Infect Dis. 2004;23:745–750. doi: 10.1007/s10096-004-1210-9. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, et al. National Institute of Child Health and Human Development Neonatal Research Network. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. 2006;117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 5.Zaoutis TE, Heydon K, Localio R, et al. Outcomes attributable to neonatal candidiasis. Clin Infect Dis. 2007;44:1187–1193. doi: 10.1086/513196. [DOI] [PubMed] [Google Scholar]
- 6.Makhoul IR, Sujov P, Smolkin T, et al. Epidemiological, clinical, and microbiological characteristics of late-onset sepsis among very low birth weight infants in Israel: a national survey. Pediatrics. 2002;109:34–39. doi: 10.1542/peds.109.1.34. [DOI] [PubMed] [Google Scholar]
- 7.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2 pt 1):285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 8.López Sastre JB, Coto Cotallo GD, Fernández Colomer B Grupo de Hospitales Castrillo. Neonatal invasive candidiasis: a prospective multicenter study of 118 cases. Am J Perinatol. 2003;20:153–163. doi: 10.1055/s-2003-40008. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin DK, DeLong E, Cotten CM, et al. Mortality following blood culture in premature infants: increased with Gram-negative bacteremia and candidemia, but not Gram-positive bacteremia. J Perinatol. 2004;24:175–180. doi: 10.1038/sj.jp.7211068. [DOI] [PubMed] [Google Scholar]
- 10.Hope WW, Castagnola E, Groll AH, et al. ESCMID Fungal Infection Study Group. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. 2012;18(suppl 7):38–52. doi: 10.1111/1469-0691.12040. [DOI] [PubMed] [Google Scholar]
- 11.Juster-Reicher A, Leibovitz E, Linder N, et al. Liposomal amphotericin B (AmBisome) in the treatment of neonatal candidiasis in very low birth weight infants. Infection. 2000;28:223–226. doi: 10.1007/s150100070040. [DOI] [PubMed] [Google Scholar]
- 12.Weitkamp JH, Poets CF, Sievers R, et al. Candida infection in very low birth-weight infants: outcome and nephrotoxicity of treatment with liposomal amphotericin B (AmBisome). Infection. 1998;26:11–15. doi: 10.1007/BF02768745. [DOI] [PubMed] [Google Scholar]
- 13.Manzoni P, Galletto P, Rizzollo S, et al. Liposomal amphotericin B does not induce nephrotoxicity or renal function impairment in premature neonates. Early Hum Dev. 2012;88(suppl 2):S86–S91. doi: 10.1016/S0378-3782(12)70024-5. [DOI] [PubMed] [Google Scholar]
- 14.Castagnola E, Jacqz-Aigrain E, Kaguelidou F, et al. Fluconazole use and safety in the nursery. Early Hum Dev. 2012;88(suppl 2):S11–S15. doi: 10.1016/S0378-3782(12)70005-1. [DOI] [PubMed] [Google Scholar]
- 15.Frattarelli DA, Reed MD, Giacoia GP, et al. Antifungals in systemic neonatal candidiasis. Drugs. 2004;64:949–968. doi: 10.2165/00003495-200464090-00003. [DOI] [PubMed] [Google Scholar]
- 16.Manzoni P, Benjamin DK, Jr, Franco C, et al. Echinocandins for the nursery: an update. Curr Drug Metab. 2013;14:203–207. [PubMed] [Google Scholar]
- 17.Zaoutis TE, Foraker E, McGowan KL, et al. Antifungal susceptibility of Candida spp. isolated from pediatric patients: a survey of 4 children’s hospitals. Diagn Microbiol Infect Dis. 2005;52:295–298. doi: 10.1016/j.diagmicrobio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Pfaller MA, Messer SA, Hollis RJ, et al. Variation in susceptibility of bloodstream isolates of Candida glabrata to fluconazole according to patient age and geographic location in the United States in 2001 to 2007. J Clin Microbiol. 2009;47:3185–3190. doi: 10.1128/JCM.00946-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Queiroz-Telles F, Berezin E, Leverger G, et al. Micafungin Invasive Candidiasis Study Group. Micafungin versus liposomal amphotericin B for pediatric patients with invasive candidiasis: substudy of a randomized double-blind trial. Pediatr Infect Dis J. 2008;27:820–826. doi: 10.1097/INF.0b013e31817275e6. [DOI] [PubMed] [Google Scholar]
- 20.Heresi GP, Gerstmann DR, Reed MD, et al. The pharmacokinetics and safety of micafungin, a novel echinocandin, in premature infants. Pediatr Infect Dis J. 2006;25:1110–1115. doi: 10.1097/01.inf.0000245103.07614.e1. [DOI] [PubMed] [Google Scholar]
- 21.Hope WW, Smith PB, Arrieta A, et al. Population pharmacokinetics of micafungin in neonates and young infants. Antimicrob Agents Chemother. 2010;54:2633–2637. doi: 10.1128/AAC.01679-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith PB, Walsh TJ, Hope W, et al. Pharmacokinetics of an elevated dosage of micafungin in premature neonates. Pediatr Infect Dis J. 2009;28:412–415. doi: 10.1097/INF.0b013e3181910e2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ClinicalTrials.gov. NCT00606268. A study of safety and pharmacokinetics of repeated dose of micafungin as antifungal prophylaxis in children and adolescents who undergo hematopoietic stem cell tansplantation. 2014. Available at: http://clinicaltrialgov/ct2/show/NCT00606268?term=NCT00606268&rank=1.
- 24.ClinicalTrials.gov. NCT00607763. Study of mycamine in infants and toddlers with fungal nfections to evaluate safety and blood levels of the drug. 2014. Available at: http://clinicaltrialgov/ct2/show/NCT00607763?term=NCT00607763&rank=1. Accessed September 9, 2013.
- 25.Denning DW, Marr KA, Lau WM, et al. Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis. J Infect. 2006;53:337–349. doi: 10.1016/j.jinf.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamin DK, Jr, Smith PB, Arrieta A, et al. Safety and pharmacokinetics of repeat-dose micafungin in young infants. Clin Pharmacol Ther. 2010;87:93–99. doi: 10.1038/clpt.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostrosky-Zeichner L, Kontoyiannis D, Raffalli J, et al. International, open-label, noncomparative, clinical trial of micafungin alone and in combination for treatment of newly diagnosed and refractory candidemia. Eur J Clin Microbiol Infect Dis. 2005;24:654–661. doi: 10.1007/s10096-005-0024-8. [DOI] [PubMed] [Google Scholar]
- 28.van Burik JA, Ratanatharathorn V, Stepan DE, et al. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004;39:1407–1416. doi: 10.1086/422312. [DOI] [PubMed] [Google Scholar]
- 29.Tabata K, Katashima M, Kawamura A, et al. Linear pharmacokinetics of micafungin and its active metabolites in Japanese pediatric patients with fungal infections. Biol Pharm Bull. 2006;29:1706–1711. doi: 10.1248/bpb.29.1706. [DOI] [PubMed] [Google Scholar]
- 30.Wiederhold NP, Cota JM, Frei CR. Micafungin in the treatment of invasive candidiasis and invasive aspergillosis. Infect Drug Resist. 2008;1:63–77. [PMC free article] [PubMed] [Google Scholar]
- 31.Kawada M, Fukuoka N, Kondo M, et al. Pharmacokinetics of prophylactic micafungin in very-low-birth-weight infants. Pediatr Infect Dis J. 2009;28:840–842. doi: 10.1097/INF.0b013e3181a0cfd1. [DOI] [PubMed] [Google Scholar]
- 32.Arrieta AC, Maddison P, Groll AH. Safety of micafungin in pediatric clinical trials. Pediatr Infect Dis J. 2011;30:e97–e102. doi: 10.1097/INF.0b013e3182127eaf. [DOI] [PubMed] [Google Scholar]
- 33.Jarvis B, Figgitt DP, Scott LJ. Micafungin. Drugs. 2004;64:969–982; discussion 983. doi: 10.2165/00003495-200464090-00004. [DOI] [PubMed] [Google Scholar]
- 34.Kohno S, Masaoka T, Yamaguchi H, et al. A multicenter, open-label clinical study of micafungin (FK463) in the treatment of deep-seated mycosis in Japan. Scand J Infect Dis. 2004;36:372–379. doi: 10.1080/00365540410020406. [DOI] [PubMed] [Google Scholar]
- 35.Horwitz E, Shavit O, Shouval R, et al. Evaluating real-life clinical and economical burden of amphotericin-B deoxycholate adverse reactions. Int J Clin Pharm. 2012;34:611–617. doi: 10.1007/s11096-012-9654-y. [DOI] [PubMed] [Google Scholar]
- 36.Turkova A, Roilides E, Sharland M. Amphotericin B in neonates: deoxycholate or lipid formulation as first-line therapy - is there a ‘right’ choice? Curr Opin Infect Dis. 2011;24:163–171. doi: 10.1097/QCO.0b013e328343614e. [DOI] [PubMed] [Google Scholar]
- 37.Baley JE, Kliegman RM, Fanaroff AA. Disseminated fungal infections in very low-birth-weight infants: therapeutic toxicity. Pediatrics. 1984;73:153–157. [PubMed] [Google Scholar]
- 38.Wang JL, Chang CH, Young-Xu Y, et al. Systematic review and meta-analysis of the tolerability and hepatotoxicity of antifungals in empirical and definitive therapy for invasive fungal infection. Antimicrob Agents Chemother. 2010;54:2409–2419. doi: 10.1128/AAC.01657-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seibel NL, Schwartz C, Arrieta A, et al. Safety, tolerability, and pharmacokinetics of Micafungin (FK463) in febrile neutropenic pediatric patients. Antimicrob Agents Chemother. 2005;49:3317–3324. doi: 10.1128/AAC.49.8.3317-3324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ascher S, Smith PB, Benjamin DK., Jr Safety of micafungin in infants: insights into optimal dosing. Expert Opin Drug Saf. 2011;10:281–286. doi: 10.1517/14740338.2011.545345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caudle KE, Inger AG, Butler DR, et al. Echinocandin use in the neonatal intensive care unit. Ann Pharmacother. 2012;46:108–116. doi: 10.1345/aph.1Q346. [DOI] [PubMed] [Google Scholar]
- 42.Watt K, Benjamin DK, Jr, Cohen-Wolkowiez M. Pharmacokinetics of antifungal agents in children. Early Hum Dev. 2011;87(Suppl 1):S61–S65. doi: 10.1016/j.earlhumdev.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinbach WJ, Roilides E, Berman D, et al. International Pediatric Fungal Network. Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. Pediatr Infect Dis J. 2012;31:1252–1257. doi: 10.1097/INF.0b013e3182737427. [DOI] [PubMed] [Google Scholar]
- 44.Kawaguchi C, Arai I, Yasuhara H, et al. Efficacy of micafungin in treating four premature infants with candidiasis. Pediatr Int. 2009;51:220–224. doi: 10.1111/j.1442-200X.2008.02726.x. [DOI] [PubMed] [Google Scholar]
- 45.Oeser C, Vergnano S, Naidoo R, et al. Neonatal invasive fungal infection in England 2004–2010. Clin Microbiol Infect. doi: 10.1111/1469-0691.12578. [DOI] [PubMed] [Google Scholar]
- 46.Vogiatzi L, Ilia S, Sideri G, et al. Invasive candidiasis in pediatric intensive care in Greece: a nationwide study. Intensive Care Med. 2013;39:2188–2195. doi: 10.1007/s00134-013-3057-y. [DOI] [PubMed] [Google Scholar]
- 47.Chitnis AS, Magill SS, Edwards JR, et al. Trends in Candida central line-associated bloodstream infections among NICUs, 1999–2009. Pediatrics. 2012;130:e46–e52. doi: 10.1542/peds.2011-3620. [DOI] [PubMed] [Google Scholar]
- 48.Pammi M, Holland L, Butler G, et al. Candida parapsilosis is a significant neonatal pathogen: a systematic review and meta-analysis. Pediatr Infect Dis J. 2013;32:e206–e216. doi: 10.1097/INF.0b013e3182863a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pappas PG, Rotstein CM, Betts RF, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2007;45:883–893. doi: 10.1086/520980. [DOI] [PubMed] [Google Scholar]


