Abstract
Purpose
Stereotactic body radiation therapy (SBRT) has emerged as a potential treatment option for local tumor control of primary malignancies of the pancreas. We report on our experience with SBRT in patients with pancreatic adenocarcinoma whom were found not to be candidates for surgical resection.
Methods
The prospective database of the first 20 consecutive patients receiving SBRT for unresectable pancreatic adenocarcinomas and a neuroendocrine tumor under an IRB approved protocol was reviewed. Prior to SBRT, cylindrical solid gold fiducial markers were placed within or around the tumor endoscopically (n=13), surgically (n=4), or percutaneously under CT-guidance (n=3) to allow for tracking of tumor during therapy. Mean radiation dose was 25 Gray (range 22–30Gy) delivered over 1–3 fractions. Chemotherapy was given to 68% of patients in various schedules/timing.
Results
Patients had a mean gross tumor volume of 57.2 cm3 (range 10.1–118 cm3) before SBRT. The mean total gross tumor volume reduction at 3 and 6 months after SBRT were 21 and 38%, respectively (P<0.05). Median follow-up was 14.57 months (range 5–23 months). The overall rate of freedom from local progression at 6 and 12 months were 88 and 65%. The probability of overall survival at 6 and 12 months were 89 and 56%. No patient had a complication related to fiducial markers placement regardless of modality. The rate of radiation induced adverse events was: grade 1–2 (11%) and grade 3 (16%). There were no grade 4/5 adverse events seen.
Conclusion
Our preliminary results showed SBRT as a safe and likely effective local treatment modality for pancreatic primary malignancy with acceptable rate of adverse events.
Keywords: pancreatic adenocarcinoma, radiosurgery, CyberKnife, stereotactic body radiation therapy, fiducial placement
Introduction
More than 40,000 individuals are diagnosed annually with tumors of the pancreas in the United States (1). Of these, less than 20% are amenable to definitive surgical management due to advanced stage of local disease, distant metastasis, or co-morbid medical conditions (2). Unresectable pancreatic cancer carries a poor prognosis with limited non-surgical effective treatment options. At this time, successful systemic therapies for unresectable pancreatic cancer are poorly developed. Gemcitabine and/or 5-FU are routinely offered to patients, but have not had a significant impact on survival with a median overall survival of less than 1 year for non-surgically removed primary tumors of the pancreas (3).
Stereotactic radiosurgery (SRS) is a technique that allows precise delivery of a large ablative radiation dose in 1 to 5 fractions to the target field (neoplasm) while sparing normal surrounding tissue. This technique allows radiation to be delivered with sub-millimeter precision and with a rapid radiation fall-off from the target field thus sparing normal tissue from high dose of radiation. Initially developed for intracranial lesions, SRS resulted in local control rates above 80–90% for the treatment of brain metastases (4). When SRS is used in extracranial tumors, it is also called stereotactic body radiation therapy (SBRT). Nonetheless, its use in extracranial tumors had been limited due to the inherent movement of abdominal organs that occurs during the respiratory cycle. One such device that tracks tumors during respiration and automatically adjusts during patient positioning is the CyberKnife® system, which consists of three key components: i) an advanced, lightweight linear accelerator (LINAC), ii) a robotic arm which can point the LINAC from a wide variety of angles, and iii) a tumor tracking system. This system tracks a patient’s abdominal tumor during respiration via two simultaneous mechanisms: a) internal fluoroscopic monitoring of fiducial markers placed in or around the tumor; and b) external monitoring through a camera system that model the chest wall motion and adjusts the linear accelerator movement simultaneously. Thus, SBRT by the Cyberknife® system uses real-time tracking of implanted fiducial markers combined with real-time respiratory motion modeling to achieve sub-millimeter accuracy by continually detecting and correcting for tumor motion throughout treatment. It was reported that the average treatment delivery precision was 0.3 ± 0.1 mm as measured at three different CyberKnife® facilities (5). We have acquired experienced with SBRT in the treatment of liver malignancies and others have shown preliminary encouraging results in the treatment of pancreatic tumors (6–8). We report our initial experience with 19 patients who underwent SBRT for unresectable pancreatic adenocarcinoma and one additional patient with a pancreatic neuroendocrine tumor (PNET).
Materials and Methods
Patient Population
A prospective database of 20 patients treated with SBRT at University Hospitals-Case Medical Center between November 2007 and November 2010 for non-resectable pancreatic tumors was reviewed under an IRB approved protocol. 19 patients met the inclusion criteria for enrolment including: i) biopsy proven pancreatic adenocarcinoma, ii) unresectable disease and iii) life expectancy of at least 12 weeks. Two of them had localized pancreatic recurrence after pancreaticoduodenectomy and adjuvant chemoradiation. Three of them underwent biliary bypass prior to SBRT and 68% underwent chemotherapy. Another patient received SBRT for unresectable PNET; however, this patient was not included in the statistical analysis.
Stereotactic Body Radiation Therapy (SBRT)
All patients were evaluated by a multidisciplinary team including HPB/oncologic surgeons, medical oncologists and radiation oncologists. They were discussed in a multidisciplinary gastrointestinal tumor board. All patients were staged with contrast enhanced computerized tomography (CT scan), magnetic resonance imaging (MRI), and/or positron emission tomography scan (PET). Subsequent imaging for treatment plan development and contouring was obtained as needed.
Cylindrical solid gold fiducial markers 3 to 5 mm in size (Best Medical International, Springfield, VA) were placed either endoscopically (n=12), surgically (n=4) or under CT guidance (n=3). 3 to 6 fiducial were placed within or around the tumor tissue and at a minimum distance of 2 cm between adjacent markers. One week was provided between markers placement and imaging studies for SBRT treatment planning simulation to allow for fiducial settling. Patients were then brought to the SBRT suite. They were immobilized using an alpha cradle, and fitted with a synchrony vest during simulation and treatment. Patients underwent imaging in the SBRT immobilized position. These scans were imported into the Multiplan™ treatment planning system version 2.05 (Accuray inc., Sunnyvale, CA) and digitally fused. Tumor definition, normal tissue constraints, and final treatment plan were approved by the attending radiation oncologist, the attending surgeon and the medical physicist. Regional lymph nodes were not included in the treatment plan. 100 to 300 6 MV X-ray beams were used for each plan.
Prior to each treatment fraction, patients were pre-medicated with Dexamethasone (Roxane, OH) and Ondansetron (Ethex, MO) and they were continuously monitored during treatment. SBRT was performed under real-time kilovoltage camera fiducial tracking and real-time respiratory motion modeling using a separate Synchrony® Respiratory Tracking System (Accuray inc., Sunnyvale, CA). Average treatment time per fraction was 2 hours. Patients were aimed to be assessed every 3 months after completion of treatment by physical exam and imaging. CT, MRI, and/or PET scans were performed at each follow-up. The maximum tumor diameter and the gross tumor volume (GTV) were measured using the Multiplan™ treatment planning system version 2.05 (Accuray inc., Sunnyvale, CA) and the images were imported into ADAC Pinnacle Radiation Therapy Planning system with 3D volume algorithms.
Local response to SBRT was graded by RECIST 1.1 (Response Evaluation Criteria in Solid Tumors version 1.1) to describe change in treated tumor lesion (9; 10). This grading system has four tumor response grades (Table 1), Complete Response: disappearance of all target lesions; Partial Response: At least a 30% decrease in the sum of the longest diameter (LD) of target lesions, taking as reference the baseline sum LD; Progressive disease: At least a 20% increase in the sum of the LD of target lesions, taking as reference the smallest sum LD recorded since the treatment started or the appearance of one or more new lesions; Stable disease: Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum LD since the treatment started (10). To further evaluate a partial tumor response to SBRT we developed a grading system (Table 2) taking in consideration tumor volume (7). Partial response grade I: At least a 10% decrease in tumor volume but less than 30% from original tumor volume; Partial response grade II: A decrease in volume ≥30% but <50% from original tumor volume; Partial response grade III: A decrease in tumor volume ≥50%. In some instances the tumor volume was similar to the original but the enhancement and PET activity vanished; we considered these particular cases as a grade III partial response.
Table 1.
RECIST criteria
| CR | Disappearance of all target lesions |
| PR | At least a 30% decrease in the sum of the LD of target lesions, taking as reference the baseline sum LD |
| PD | At least a 20% increase in the sum of the LD of target lesions, taking as reference the smallest sum LD recorded since the treatment started or the appearance of one or more new lesions |
| SD | Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum LD since the treatment started |
Complete response (CR); Partial response (PR); Progressive disease (PD); Stable disease (SD); Longest diameter (LD).
Table 2.
Grading of partial response based on tumor volume from original tumor
| Grade I | A decrease in tumor volume ≥10% but <30% from original tumor volume |
| Grade II | A decrease in tumor volume ≥30% but <50% from original tumor volume |
| Grade III | A decrease in tumor volume ≥50% or no enhancement |
Local or distant recurrent disease was graded as well by the following scale (Table 3), Grade 1: local recurrence (tumor progression within or at the periphery of the radiation field) with two subgroups, Grade 1a: 1 local recurrence and Grade 1b: >1 local recurrences; Grade 2: Distant intra-abdominal recurrence (new tumor >3 cm away from the radiation field or in another organ); Grade 3: distant extra-abdominal recurrence; and Grade 4: A combination of local and distant recurrences (7).
Table 3.
Grading of recurrent disease
| Grade 0 | No recurrence |
| Grade 1a | 1 local (same-organ) |
| Grade 1b | >1 local (same organ) |
| Grade 2 | Distant intra-abdominal |
| Grade 3 | Distant extra-abdominal |
| Grade 4 | Local + distant |
Local recurrence: tumor progression within or at the periphery of the radiation field; Distant recurrence: new tumor >3 cm away from the radiation field or in another organ.
Adverse events
Adverse events after SBRT were graded on 1–5 scale according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE v3.0). Causes were attributed to either surgery, placement of fiducial markers, chemotherapy, radiation therapy or related to medical comorbidities.
Statistical Analysis
A database of clinical, imaging and radiation variables was created and maintained in a prospective manner. Subject and tumor characteristics were expressed in mean, standard deviation and percentages. Paired t-tests were used to compare tumor volume before and after treatment. Kaplan-Meier product-limit curves were generated to calculate overall survival (OS) and freedom from local progression (FFLP). Local progression is defined as any progression of the disease adjacent to the treated area. OS was calculated from the date of SBRT to the day of last follow-up or death. Log-Rank test were used to compare estimates of survival between subgroups of patients. Patients who were still alive were censored on March 2011. Statistical routines were performed using JMP Statistical Discovery Software version 9.0 (SAS Institute, Cary, NC). P-values <0.05 were considered statistically significant.
Results
Patient characteristics and demographics are summarized in Table 4. Our cohort of patients with pancreatic adenocarcinoma consisted of 8 males and 11 females (n=19) with a mean age of 74.5 years (range: 54–91 years). The median follow-up among survival patients was 9 months (range: 5.8–23.1 months). Mean tumor volume was 57.2 cm3 (range: 10.1–118 cm3). 14 patients were treated with 20–25 Gy in one fraction and 5 patients were treated with 24–30 Gy in three fractions. The median prescription dose was 25 Gray (20–30 Gy) at a median prescription isodense line of 70%.
Table 4.
Demographics and baseline tumor characteristics of patients with non-resectable pancreatic adenocarcinoma treated with SBRT
| Subjects | 19 |
| Mean age (range) | 74 (54–91) |
| Gender (M:F) | 8:11 (ratio 0.7:1) |
| Initial tumor characteristics | |
| Number of tumors | 1 |
| Mean Max diameter (range) | 4.7 (2.3–9.1) |
| Mean Volume (range) | 57.2 (10.1–118) |
| Prior therapy (# of patients) | |
| Surgery | 6 (32%) |
| Chemotherapy | 13 (68%) |
| Radiation therapy | 1 (5%) |
Age (years); Max diameter (cm); Tumor volume (cm3)
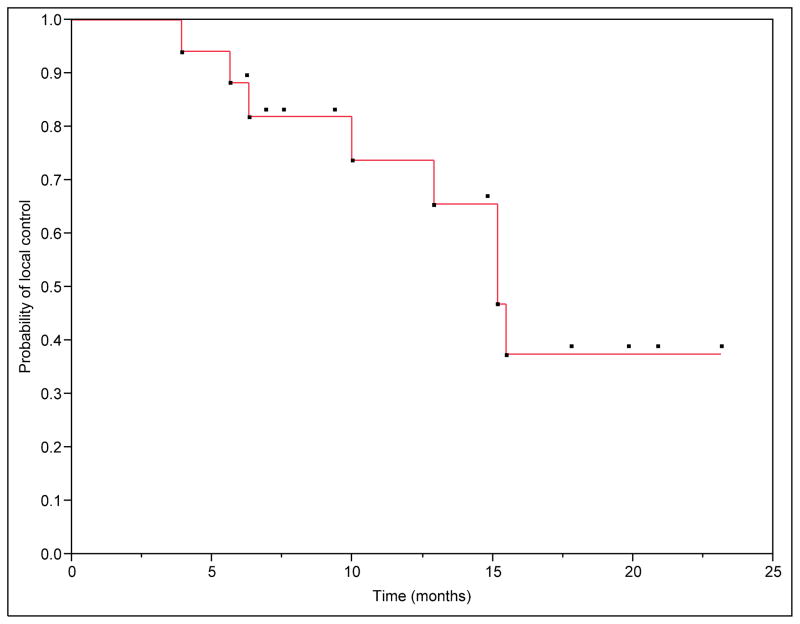
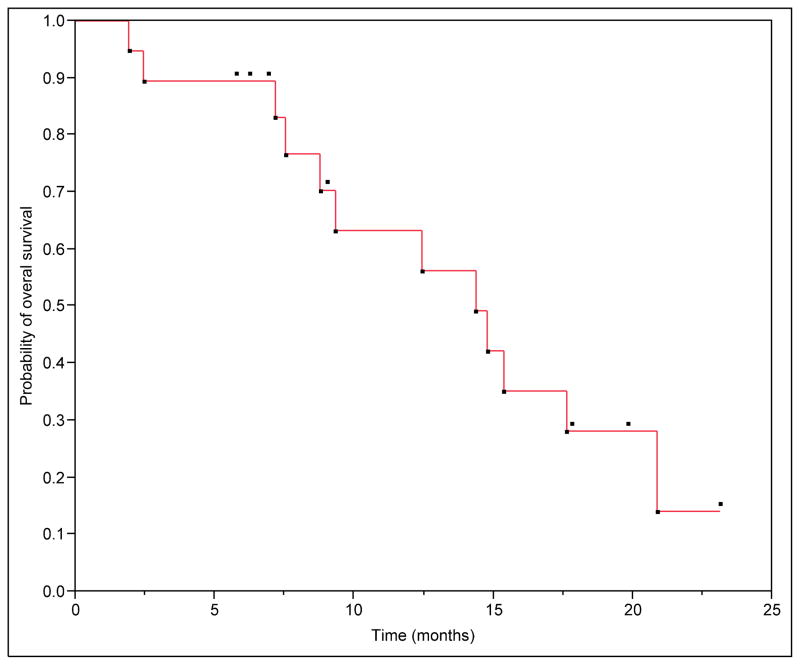
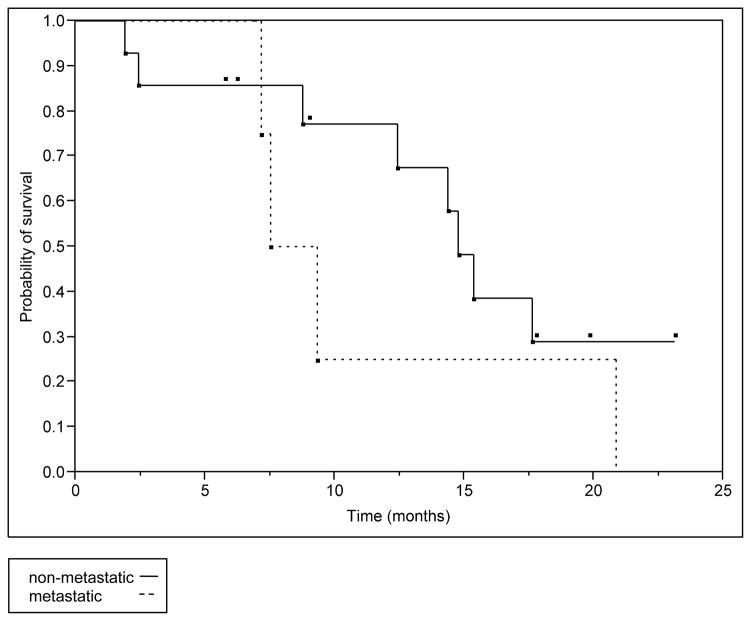
We have initial follow-up imaging after SBRT at a median of 3 months (range: 1–5 months) in 84% of patients and a second follow-up imaging at a median of 9 months (range: 4.7–22.9) in 53% of patients. We were unable to determine initial tumor response in one patient due to medical related death before follow-up imaging at 2 months. Two patients did not have follow-up imaging in our center. Local control was achieved in 81% of the patients. Freedom from local progression (FFLP) rates at 6 months and 1 year were 88 and 65%, respectively (Figure 1). The median time to local progression was 11.43 months (range: 3.9 to 15.47). The median overall survival (OS) for the group was 14.37 months. The estimated 6 months and 1 year survival was 89 and 56% (Figure 2). In the subgroup of patients with metastatic disease at the time of SBRT the estimated 6 months and 1 year survival was 75 and 25%, however, this was not statistically different from the non-metastatic group with a Log-Rank test value of P=0.29 (Figure 3).
Figure 1. Overall freedom from local progression from the time of SBRT.
Median time to local progression was 11.43 months (range: 3.9–15.47 months). Freedom from local progression (FFLP) rates at 6 months and 1 year were 88 and 65%.
Figure 2. Overall survival from the time of SBRT.
Median overall survival for the group was 14.37 months. The estimated 6 months and 1 year survival was 89 and 56%.
Figure 3. Overall survival for metastatic and non-metastatic groups.
In the initially metastatic group the median survival time was 8.43 months versus 14.77 months in the non-metastatic group. However this difference was not statistically significant. (Log-Rank test p=0.29)
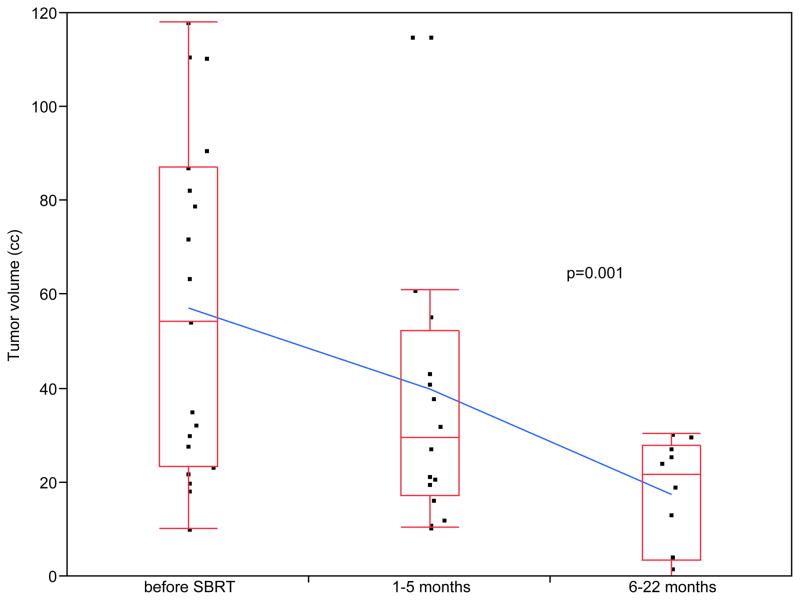
The mean tumor volume reduction among the 16 patients with initial follow-up imaging studies at 3 months was 21% (P=0.005 by single sided paired t-test). The overall mean tumor volume reduction at last follow-up imaging was 38% (P=0.001 by single sided paired t-test, Figure 4). Using RECIST criteria, 2 patients (13%) had complete response, 5 patients (31%) had partial response and 6 patients (38%) had stable disease at the time of last follow-up. Only 3 patients (19%) had progressive disease and 3 patients did not have follow-up imaging. Using our developed partial tumor response grading system, 38% of patients had a grade 3 response, 19% of patients had a grade 2 response and 13% had a grade 1 response at the time of their last follow-up. Six patients (32%) developed distant metastasis to the liver at a median time of 12.45 months after SBRT (range: 3.9–15.47 months, Table 5).
Figure 4. Tumor response in patients with non-resectable pancreatic tumors treated with Stereotactic Body Radiation Therapy (SBRT).
38% of patients (6/16) with adenocarcinoma of the pancreas had a partial tumor response grade 3, 19% of patients (3/16) had a grade 2 response and 13% of patients (2/16) had a partial tumor response grade 1 at the time of their last follow-up. Tumor volume decreased from an initial volume of 57.2±35.7 cc to a final volume of 33.3±34.9 cc (p=0.001 by one-tailed paired t-test). One patient died from medical issues before follow-up imaging 2 months post-therapy. Two patients did not have follow-up imaging in our center.
Table 5.
Tumor response and recurrence of malignancy in patients treated with SBRT for non-resectable pancreatic adenocarcinoma.
| Subjects | 19* |
| Last follow-up imaging (mean, range) | 7.3 months (1–23) |
| Tumor response* (RECIST) | |
| PR | 5 (31%) |
| SD | 6 (38%) |
| CR | 2 (13%) |
| PD | 3 (19%) |
| Partial tumor response grade* | |
| Grade 1 | 2 (13%) |
| Grade 2 | 3 (19%) |
| Grade 3 | 6 (38%) |
| Tumor recurrence* | |
| Grade 0 (no recurrence) | 7 (44%) |
| Grade 1a | 3 (19%) |
| Grade 1b | 0 |
| Grade 2 | 1 (6%) |
| Grade 3 | 2 (13%) |
| Grade 4 | 3 (19%) |
Follow-up imaging was available only in 16 patients.
No complications were observed during placement of fiducial markers. Two patients (11%) presented grade 1–2 adverse events including fatigue, nauseas and one asymptomatic pyloric ulcer that responded to medical treatment. Three patients (16%) presented grade 3 gastrointestinal ulcers which responded to medical therapy. One patient had a sterile fluid collection drained revealing necrotic pancreatic tissue. Two patients with baseline pain had pain management procedures performed after SBRT including celiac plexus block and RFA ablation of posterior spinal rami. One patient expired within 3 months of SBRT prior to obtaining post-treatment imaging. Each death was reviewed and considered not related to SBRT complications.
Additionally, a 55 years old morbidly obese male received SBRT for a pancreatic nonfunctional neuroendocrine tumor of 2.5 cm diameter (15.5 cm3 volume). It was treated to a dose of 30 Gy in 3 fractions to the 80% isodose curve. This patient had a complete tumor response at 31 months after SBRT. Patient is still alive and free of disease 3 years after treatment and 4 years after diagnosis.
Discussion
Analysis of 19 patients treated with SBRT for unresectable adenocarcinomas of the pancreas are presented. 69% of patients responded to SBRT as judged by > 10% decrease in gross tumor volume (GTV) in 11 patients and arrest of tumor growth in 2 patients. No patient had local tumor recurrence on imaging 3 months after SBRT. At nine months follow-up two patients recurred locally and one patient recurred locally and distally to the liver. Six patients (35%) developed distant metastasis to the liver at a median time of 12.45 months after SBRT (range: 3.9–15.47 months).
Two patients (11%) were admitted 6 and 8 months after SBRT with bleeding gastric and duodenal ulcers which responded to medical therapy. On review of their delivered plan, radiation doses to affected mucosal areas were below normal tolerance dose, making their etiology unlikely from SBRT. Because of the reported risk of GI ulceration, fractionated SBRT with smaller doses per fraction may reduce the risk of luminal gastrointestinal toxicity while maintaining local control. We modified our protocol to provide SBRT in 3 fractions along with chemotherapy similar to the regimen reported by the Stanford group (11). The published literature reports a rate of GI ulceration in approximately 10% in patients treated with SBRT for pancreatic adenocarcinoma (2; 3; 6; 11–16).
Two patients treated in our series presented with recurrent pancreatic adenocarcinoma after pancreaticoduodenectomy and chemoradiation. One of them developed distant recurrence to the liver two months after SBRT. Treatment of unresectable tumor recurrence has been established as a method for local control in these lesions. This suggests local control can be achieved with SBRT in locally recurrent pancreatic cancer that is refractory to multimodal treatment. Nevertheless, overall survival ultimately depends on the development of systemic disease, which further supports the use for a multimodal approach with SBRT and chemotherapy. We report a local control rate of 65% at 12 months. This is comparable to the current literature in which the FFLP at 12 months is between 48.5 to 84% (table 6) (6; 8), indicating that only 35% of our patients could potentially died of uncontrolled local disease rather than systemic failure (6). In addition to the increase in overall survival obtained with SBRT in the treatment of pancreatic adenocarcinoma, the importance of achieving local control should not be undermined. Preventing or delaying local recurrence with SBRT not only decreases tumor burden, but may also offer palliative benefit. Untreated local disease can lead to significant pain, gastric outlet obstruction, biliary obstruction, and other morbidities that decrease quality of life (2). Thus, SBRT should also be considered as a palliative option for unresectable pancreatic adenocarcinoma.
Table 6.
Local control and Median Overall Survival rates in patients after SBRT for adenocarcinoma of the pancreas
| Series | # Patients | Prior XRT (%) | Chemo (%) | FFLP (%) | Median OS (months) |
|---|---|---|---|---|---|
| Stanford Phase I (3) | 15 | 2 (13) | 3 (20) | 77 | 11 |
| Stanford Boost Phase II (13) | 16 | 16 (100) | 16 (100) | 94 | 8.3 |
| Stanford Gencitabine (11) | 16 | 0 (0) | 16 (100) | 81 | 11.4 |
| Stanford Composite (16) | 77 | 9 (12) | 74 (96) | 84 | 6.4 |
| Danish Phase II (12) | 22 | 0 (0) | 6 (27) | 57 | 5.7 |
| Deaconess (15) | 32 | 3 (13) | 32 (100) | 79 | NR |
| Georgetown (14) | 28 | 28 (100) | 28 (100) | 86 | 5.3 |
| Pittsburgh (8) | 71 | 15 (21) | 64 (90) | 48.5 | 10.3 |
| Case | 19 | 0 (0) | 13 (68) | 65 | 14.4 |
|
| |||||
| TOTAL | 296 | 73 (25) | 252 (85) | 74.6±14.7 | 9.1±3.2 |
FFLP: Freedom from local progression, OS: Overal survival, Total FFLP and OS (mean±SD)
Gemcitabine or 5-FU is commonly prescribed to patients with pancreatic adenocarcinoma and cholangiocarcinoma. At our institution all candidates for SBRT were evaluated by medical oncologists. 68% of our patients underwent chemotherapy with gemcitabine. We acknowledge that many of these patients with locally advanced cancer have other medical co-morbidities or poor performance status that may preclude chemotherapy. It is our practice that all patients with unresectable pancreatic malignancies are offered multimodal treatment including chemotherapy when medically feasible in addition to the locally ablative treatment of SBRT. Thus, using SBRT earlier in the natural history of cancer progression (before second-line chemotherapy) may lead to improved outcomes (17).
Pancreatic neuroendocrine tumors (PNETs) are relatively rare cancers. They are usually treated with surgical resection as the only potential cure. Contessa et al. in 2009 was the first in reporting a series of 36 patients treated with external beam radiotherapy for palliation of unresectable PNETs. They reported a 90% rate of clinical response rate achieving symptomatic palliation and a median overall survival of 2 years (18). We have successfully treated 1 patient with a nonfunctional PNET obtaining a complete response at 31 months and achieving 3 years survival and local control after SBRT. It is difficult to draw a conclusion from a single case; however, this type of tumor appears to be radiosensitive and suitable for SBRT treatment.
SBRT for unresectable pancreatic tumors has excellent local control rates and low adverse events from radiotherapy or fiducial marker placement (2; 3; 6; 8; 11–15). In previous publications fiducial markers were placed safely by an endoscopic approach near the porta hepatis, gastroesophageal junction, and pancreas in 11/13 (84.6%) patients. Fiducial markers could not be placed endoscopically in two patients because the linear scope was unable to pass to the pancreatic head due to gastric outlet obstruction or retrocrural location of tumor (19). Currently only gold fiducials are available for use with 19 gauge FNA needle that can be manually back-loaded into the tip of the needle. Compatibility with a 22 gauge needle would facilitate easier deployment of fiducial markers within a tumor. The ability to deploy multiple markers in one pass would shorten procedure duration and decrease risk of bleeding or infectious complications (20). In our study multiple approaches were used to place fiducial markers. Twelve patients had endoscopic placement for pancreatic tumors without complication. Three patients had CT-guided placement to the pancreatic tumor. One pancreatic tumor had a hypervascular appearance and duodenal narrowing on endoscopy making it difficult to pass endoscope to second portion of duodenum. Four patients had fiducial markers placed surgically without any complications. In our experience, surgical placement of fiducial markers is enhanced by use of the intra-operative ultrasound to detect the tumor(s).
RECIST is a commonly used method to report tumor response to treatment based on measurement of maximal diameter in the abdominal tumor(s) (9; 10). However, this grading system is not the optimal way to assess the entire tumor’s response to therapy. Measurement of largest diameter is a snapshot into tumor effect that can occur after chemotherapy or loco-regional therapy versus effect on tumor volume. Gross tumor volume (GTV) is precisely measured in three dimensions during treatment planning for SBRT. The same process can be used after loco-regional therapy including SBRT to assess tumor volume. We report the change in tumor volume after SBRT based on measuring GTV using the Multiplan™ system. Amongst the current literature, there are vague and inconsistent definitions of tumor response and recurrence. Without a precise and uniform classification system it is difficult to compare results among studies and centers. We encourage the development of a grading system for abdominal tumor response and recurrence using change in tumor volume after SBRT. We encourage its use in similar cohorts in an attempt to further evaluate different treatment modalities among centers.
It has been suggested that radiographic response analysis should be set no earlier than 4–6 months after SBRT to assess tumor response in clinical trials (21). Future long term data from randomized clinical trials are needed to determine the role of SBRT in the treatment of tumors of the pancreas. It appears that SBRT can play a primary role in the local control of these malignancies. The sample size and follow-up of this study are smaller to other published SBRT studies but this study adds to the existing literature pertaining to SBRT for pancreatic malignancies. Within the limitations of a relatively small sample size we have demonstrated that SBRT is a safe form of therapy for unresectable tumors of the pancreas with a 65% local response rate with 14 months mean follow-up. Future studies should focus on the development of strategies to define the role of SBRT in the treatment of pancreatic tumors.
Conclusions
SBRT is a safe and effective local treatment option for unresectable pancreatic adenocarcinoma. In the multidisciplinary management of these tumors, SBRT adds to our armamentarium of local treatment modalities especially for non-operable patients. Fiducial markers can be placed endoscopically, under CT-guidance, or surgically for planning and treatment of pancreatic tumors with minimal adverse events. Further prospective studies are ongoing to determine long-term response and survival after SBRT for pancreatic malignancies.
Acknowledgments
We are grateful to the staff and residents in the Department of Surgery, Medicine, Radiology, and Radiation Oncology, University Hospital-Case Medical Center, Cleveland, Ohio. Rafael A. Ibarra was supported by a Ruth L. Kirschstein National Research Service Award NIH/NIDDK (T32-DK007319).
List of Abbreviations
- SBRT
Stereotactic Body Radiotherapy
- CT
Computerized Tomography
- MRI
Magnetic Resonance Imaging
- PET
Positron Emission Tomography
- Gy
Gray
- DVT
Deep Venous Thrombosis
- GI
Gastrointestinal
- HPB
Hepatopancreatobiliary
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
K Goyal, Email: kushgoyal@gmail.com.
D Einstein, Email: doug@einsteinfamily.com.
RA Ibarra, Email: doctorrafaelibarra@gmail.com.
M Yao, Email: min.yao@uhhospitals.org.
C Kunos, Email: charles.kunos@uhhospitals.org.
R Ellis, Email: rod.ellis@uhhs.com.
J Brindle, Email: james.brindle@uhhospitals.org.
D Singh, Email: deepjot.singh@va.gov.
J Hardacre, Email: jeffrey.hardacre@uhhospitals.org.
Y Zhang, Email: yuxia.zhang@uhhospitals.org.
J Fabien, Email: jeffrey.fabien@uhospitals.org.
J Brindle, Email: james.brindle@uhhospitals.org.
G Funkhouser, Email: gary.funkhouser@uhhosptals.org.
M Machtay, Email: mitchell.machtay@uhhospitals.org.
JR Sanabria, Email: juan.sanabria@uhhospitals.org.
Reference List
- 1.American Cancer Society. Cancer Facts and Figures 2008. Atlanta: American Cancer Society; 1-1-0008. Ref Type: Report. [Google Scholar]
- 2.Chang BW, Saif MW. Stereotactic body radiation therapy (SBRT) in pancreatic cancer: is it ready for prime time? JOP. 2008;9:676–682. [PubMed] [Google Scholar]
- 3.Koong AC, Le QT, Ho A, Fong B, Fisher G, Cho C, Ford J, Poen J, Gibbs IC, Mehta VK, Kee S, Trueblood W, Yang G, Bastidas JA. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Young RF. The role of the gamma knife in the treatment of malignant primary and metastatic brain tumors. CA Cancer J Clin. 1998;48:177–188. doi: 10.3322/canjclin.48.3.177. [DOI] [PubMed] [Google Scholar]
- 5.Yu C, Main W, Taylor D, Kuduvalli G, Apuzzo ML, Adler JR., Jr An anthropomorphic phantom study of the accuracy of Cyberknife spinal radiosurgery. Neurosurgery. 2004;55:1138–1149. doi: 10.1227/01.neu.0000141080.54647.11. [DOI] [PubMed] [Google Scholar]
- 6.Chang DT, Schellenberg D, Shen J, Kim J, Goodman KA, Fisher GA, Ford JM, Desser T, Quon A, Koong AC. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 7.Goyal K, Einstein D, Yao M, Kunos C, Barton F, Singh D, Siegel C, Stulberg J, Sanabria J. Cyberknife stereotactic body radiation therapy for nonresectable tumors of the liver: preliminary results. HPB Surg. 2010;2010 doi: 10.1155/2010/309780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rwigema JC, Parikh SD, Heron DE, Howell M, Zeh H, Moser AJ, Bahary N, Quinn A, Burton SA. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol. 2011;34:63–69. doi: 10.1097/COC.0b013e3181d270b4. [DOI] [PubMed] [Google Scholar]
- 9.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van GM, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Schellenberg D, Goodman KA, Lee F, Chang S, Kuo T, Ford JM, Fisher GA, Quon A, Desser TS, Norton J, Greco R, Yang GP, Koong AC. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678–686. doi: 10.1016/j.ijrobp.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 12.Hoyer M, Roed H, Sengelov L, Traberg A, Ohlhuis L, Pedersen J, Nellemann H, Kiil BA, Eberholst F, Engelholm SA, von der MH. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol. 2005;76:48–53. doi: 10.1016/j.radonc.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Koong AC, Christofferson E, Le QT, Goodman KA, Ho A, Kuo T, Ford JM, Fisher GA, Greco R, Norton J, Yang GP. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320–323. doi: 10.1016/j.ijrobp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Lominska CE, Nasr NM, Silver NL, Gagnon GJ. Salvage Stereotactic Radiosurgery for Locally Recurrent Previously Irradiated Pancreatic Cancer. International Journal of Radiation Oncology*Biology*Physics. 2008;72:S276–S277. [Google Scholar]
- 15.Mahadevan A, Shanmugam L, Kaplan I, Brennan D, Lu X, Pleskow D, Vollmer C, Callery M. Fractionated Radiosurgery for Pancreas Cancer. International Journal of Radiation Oncology*Biology*Physics. 2007;69:S307. [Google Scholar]
- 16.Chang DT, Schellenberg D, Shen J, Kim J, Goodman KA, Fisher GA, Ford JM, Desser T, Quon A, Koong AC. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 17.Lee MT, Kim JJ, Dinniwell R, Brierley J, Lockwood G, Wong R, Cummings B, Ringash J, Tse RV, Knox JJ, Dawson LA. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27:1585–1591. doi: 10.1200/JCO.2008.20.0600. [DOI] [PubMed] [Google Scholar]
- 18.Contessa JN, Griffith KA, Wolff E, Ensminger W, Zalupski M, Lawrence TS, Ben-Josef E. Radiotherapy for pancreatic neuroendocrine tumors. Int J Radiat Oncol Biol Phys. 2009;75:1196–1200. doi: 10.1016/j.ijrobp.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 19.Pishvaian AC, Collins B, Gagnon G, Ahlawat S, Haddad NG. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest Endosc. 2006;64:412–417. doi: 10.1016/j.gie.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 20.Van DJ, Varadarajulu S, Jin Z. EUS 2008 Working Group document: evaluation of EUS-guided implantation therapy (with video) Gastrointest Endosc. 2009;69:S49–S53. doi: 10.1016/j.gie.2008.10.058. [DOI] [PubMed] [Google Scholar]
- 21.Kavanagh BD, Scheftera TE, Wersall PJ. Liver, renal, and retroperitoneal tumors: stereotactic radiotherapy. Front Radiat Ther Oncol. 2007;40:415–426. doi: 10.1159/000106051. [DOI] [PubMed] [Google Scholar]