The activation of Dictyostelium STATc by hyperosmotic stress is essential for a complete transcriptional response. Two TKL enzymes function semiredundantly to phosphorylate STATc, and the two proteins differ in domain organization and move to different parts of the stressed cell.
Abstract
When Dictyostelium cells are hyperosmotically stressed, STATc is activated by tyrosine phosphorylation. Unusually, activation is regulated by serine phosphorylation and consequent inhibition of a tyrosine phosphatase: PTP3. The identity of the cognate tyrosine kinase is unknown, and we show that two tyrosine kinase–like (TKL) enzymes, Pyk2 and Pyk3, share this function; thus, for stress-induced STATc activation, single null mutants are only marginally impaired, but the double mutant is nonactivatable. When cells are stressed, Pyk2 and Pyk3 undergo increased autocatalytic tyrosine phosphorylation. The site(s) that are generated bind the SH2 domain of STATc, and then STATc becomes the target of further kinase action. The signaling pathways that activate Pyk2 and Pyk3 are only partially overlapping, and there may be a structural basis for this difference because Pyk3 contains both a TKL domain and a pseudokinase domain. The latter functions, like the JH2 domain of metazoan JAKs, as a negative regulator of the kinase domain. The fact that two differently regulated kinases catalyze the same phosphorylation event may facilitate specific targeting because under stress, Pyk3 and Pyk2 accumulate in different parts of the cell; Pyk3 moves from the cytosol to the cortex, whereas Pyk2 accumulates in cytosolic granules that colocalize with PTP3.
INTRODUCTION
The metazoan STATs are “fast-track,” plasma membrane–to-nucleus signal transducers that are, most often, tyrosine phosphorylated by Janus kinases (JAKs; Bromberg and Darnell, 2000). Activation is initiated when a cytokine binds to and directs clustering of its cell surface receptor. This results in activation of specific JAKs, which are associated with the receptor chains. The JAKs undergo autophosphorylation and also phosphorylate a specific tyrosine residue on the receptor. This site is bound by the SH2 domain of the STAT. The JAK then tyrosine phosphorylates the STAT at a site proximal to its C-terminus, and this directs SH2 domain–mediated binding to a partner STAT to form an active, dimeric transcription factor.
In addition to their specific ligands, metazoan STATs 1 and 3 can be activated by hyperosmotic stress (Gatsios et al., 1998). The mechanistic basis for this response is unknown, but substitution of sorbitol by urea, a membrane-permeant osmolyte, leaves the response unaffected. This suggests that activation results from a cellular shape change rather than a change in intracellular osmolarity. Dictyostelium STATc can also be activated either by ligand stimulation or hyperosmotic stress, and we recently presented evidence that both act by inducing changes in cytoskeletal organization that alter the shape of the cell (Araki, Tsujioka, et al., 2003; Araki and Williams, 2012). This pathway is important in eliciting a full response because 20% of stress-regulated genes show an altered reaction to hyperosmolar sorbitol in a STATc-null strain (Na et al., 2007).
The activating ligand for STATc is DIF-1, a chlorinated polyketide (Fukuzawa, Abe, et al., 2003). STATc tyrosine phosphorylation is regulated, by both DIF-1 and hyperosmotic stress, via controlled dephosphorylation of transiently phosphorylated STATc molecules (Araki, Langenick, et al., 2008). The cognate protein tyrosine phosphatase, PTP3, was identified by substrate trapping using a mutant, active-site C-to-S, form of PTP3. In response to DIF-1 or stress, PTP3 becomes phosphorylated on two serine residues, S448 and S747, and these modifications correlate with a reduction in its activity.
In marked contrast to other amoebozoans, such as Acanthamoeba (Clarke et al., 2013), Dictyostelium has no orthodox tyrosine kinases, that is, enzymes that contain the diagnostic amino acid sequence tracts that distinguish tyrosine kinases from serine–threonine kinases. However, there are 66 predicted tyrosine kinase–like (TKL) enzymes in the Dictyostelium kinome (Goldberg, Manning, et al., 2006). Five of these, first identified by expressing cDNA libraries in Escherichia coli and immunologically detecting phosphotyrosine in individual bacterial colonies, selectively phosphorylate tyrosine residues (Adler et al., 1996; Nuckolls et al., 1996). Recently we characterized one of them, Pyk2, and showed it to be a direct, constitutive activator of STATc (Araki et al., 2012); in both the presence and the absence of DIF, Pyk2 autophosphorylates on tyrosine residues (Figure 1). Then, via a STATc SH2-domain-Pyk2 phosphotyrosine–mediated interaction, Pyk2 binds to and phosphorylates STATc.
FIGURE 1:
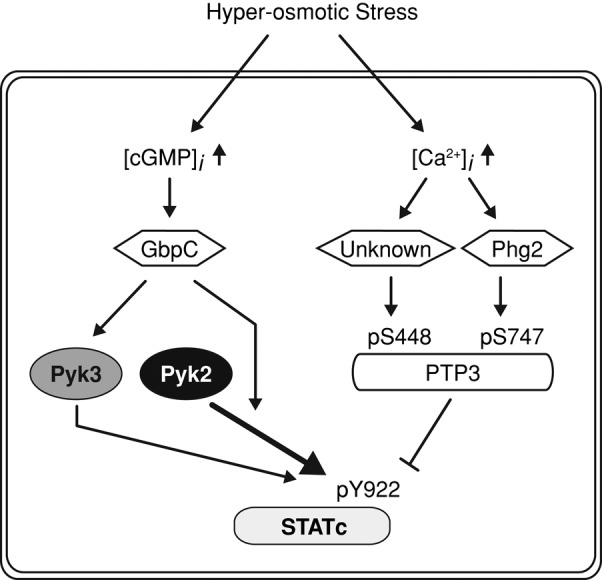
Scheme for the STATc activation pathways. STATc activation scheme that involves coordinated stress regulation over the phosphatase (PTP3) and the kinase arms (two TKLs; Pyk2 and Pyk3). The calcium/second messenger pathway and the serine/threonine group TKL kinase, Phg2, are involved in PTP3 regulation. The hyperosmotic stress-induced intracellular cGMP increase and the cGMP-binding protein, GbpC, are suggested to affect Pyk2 and Pyk3 activities in the STATc stress response.
The most closely related kinase to Pyk2 in the Dictyostelium kinome is Pyk3, another of the five TKLs identified by its expression in E. coli. The pyk3-null mutant, in an Ax3 genetic background, displays prolonged activation of STATc after exposure to DIF-1 (Lee et al., 2008). However, this may be strain dependent because we are not able to reproduce the observation in Ax2 cells; pyk3− cells treated with DIF show similar kinetics to the parent strain, but the level of activation is ∼30% lower (Supplemental Figure S1). There has been no side-by-side analysis of the pyk2− and pyk3− mutants in response to stress, which is a significantly stronger and more enduring activator than DIF-1. Therefore we analyze, in parallel, the respective roles of Pyk2 and Pyk3 in the STATc stress activation pathway in mutants generated in Ax2 cells using the several levels of analysis that have already been applied to the DIF-activation of Pyk2, that is, tyrosine autophosphorylation, pull-down assay with STATc, and STATc activation in an immunoprecipitation (IP) kinase assay.
The signaling pathway upstream of PTP3, on the phosphatase arm of the STATc stress activation pathway, is in part understood. Pharmacological evidence suggests that calcium signaling stimulates the two serine kinase activities that down-regulate PTP3 (Araki et al., 2010). Thus drugs that elevate intracellular calcium levels activate STATc, and at least one of them, 2,5,-di(tert-butyl)-1-4-benzohydroquinone (BHQ), leads to increased serine phosphorylation at one of the regulatory sites: S747 (Figure 1). Analysis of a gene disruptant suggests that Phg2, a serine/threonine-specific member of the TKL group, lies in the pathway that represses PTP3 activity by modifying it on S747 (Figure 1; Vu et al., 2014). We presented a pull-down analysis showing that Phg2 binds directly to STATc. The same study showed, in agreement with the data we present here, that deletion of Pyk3 leads to partial reduction in the plateau value of stress-induced STATc activation.
What of the kinase arm of the postulated scheme? Hyperosmotic stress induces an elevation of the intracellular cGMP concentration, and cGMP acts as the second messenger that mediates several responses (Kuwayama et al., 1996; Oyama, 1996; van Haastert and Kuwayama, 1997; Bosgraaf et al., 2002). Biochemical evidence suggests that cGMP also functions as a second messenger on the hyperosmotic stress signaling pathways (Figure 1). Thus exposure to a membrane-permeant derivative, 8Br-cGMP, activates STATc and directs transcription of stress-dependent genes (Araki, Tsujioka, et al., 2003; Na et al., 2007). By analysis of cells expressing myc-tagged Pyk2 and Pyk3 in an in vitro kinase assay, we show that both enzymes directly increase tyrosine phosphorylation of STATc in response to cGMP. These results are supported by genetic analyses of STATc activation, showing that both kinases are required for a full stress response and that the double null is entirely unresponsive.
Although there are these significant similarities between signaling through Pyk2 and Pyk3, there is a major structural difference between them. Like metazoan JAKs but unlike Pyk2, Pyk3 contains a TKL domain that is located C-terminal to a pseudokinase domain. Pseudokinase domains contain substitutions of what were believed to be residues essential for catalysis (reviewed by Eyers and Murphy, 2013). They comprise 10% of the human kinome (Manning et al., 2002). In the case of JAKs, they are a universally conserved feature, and they function as negative regulators that act intermolecularly to repress tyrosine kinase activity (Saharinen et al., 2000; Staerk et al., 2005). We present data showing that the pseudokinase domain of Pyk3 functions in a similar manner. We then provide a potential explanation for the functional redundancy of Pyk2 and Pyk3 by showing that each moves to a different part of the cell under stress conditions.
RESULTS
Pyk2− and pyk3− mutants display retarded stress activation of STATc, and the double mutant is entirely unresponsive
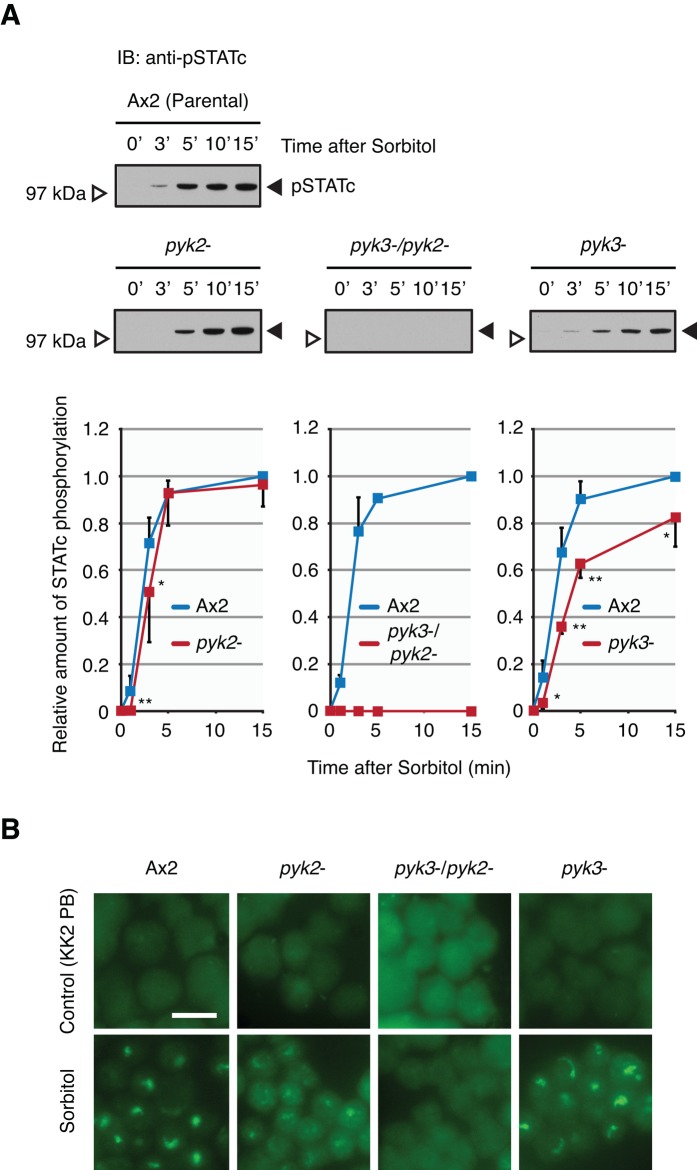
In marked contrast to the response to DIF-1, for which the pyk2− mutant is totally unresponsive (Araki et al., 2012), the STATc response of pyk2− cells to hyperosmotic stress is only minimally diminished; relative to the parental Ax2 strain, there is just a 1-min delay in STATc activation, and a very similar plateau level is achieved (Figure 2A). This suggests that, in the case of stress but not DIF-1, another kinase is almost fully competent to subsume the function of Pyk2 as an activator of STATc. The logical candidate for this role is Pyk3; it has the most closely related kinase domain in the kinome (51% identity in the kinase domain, Supplemental Figure S4A), it is one of the five TKLs that autophosphorylate on tyrosine when expressed in E. coli, and two entirely independently constructed and analyzed null strains for pyk3 (pyk3− strains, Supplemental Figure S2) show a 20–50% reduction in their sorbitol-induced activation of STATc (Figure 2A; Vu et al., 2014). This suggests that Pyk2 is partially able to substitute the function of Pyk3. However, the most striking result is observed with the double mutant, pyk2−/pyk3−. This strain is completely unresponsive to hyperosmotic stress–induced activation of STATc (Figure 2A).
FIGURE 2:
Characterization of the pyk2−, pyk3−, and double-null strains. (A) Hyperosmotic stress–induced activation of STATc in parental Ax2 and pyk2−, pyk3−, and double-null strains. Parental Ax2 cells and null cells were developed in shaken suspension for 4 h and then treated with sorbitol at 200 mM for the indicated times. Tyrosine phosphorylation of STATc was assayed by Western transfer, using phospho-STATc antibody CP22. The same blot was reprobed with total-STATc antibody 7H3 (unpublished data). The combined data from several such experiments are shown as fold relative to sorbitol-induced Ax2 (at 15 min) ± SD. Student's t test: *p < 0.05; **p < 0.01. (B) Nuclear enrichment of STATc in parental Ax2 and pyk2−, pyk3−, and double-null strains. Cells developed as in A were treated with sorbitol at 200 mM for 5 min. The nuclear accumulation of STATc was assayed immunohistochemically, using total STATc antibody. KK2 PB–treated cells were used as control, as it is the vehicle for sorbitol. Bar, 10 μm.
Analysis of the nuclear accumulation of STATc in the three mutant strains leads to the same conclusions. Even though the accumulation data are by their nature less easily quantified, it is clear that neither of the single mutants is greatly reduced in sorbitol-induced STATc accumulation, whereas the double mutant is unresponsive (Figure 2B). Again, these data suggest that the two kinases are virtually interchangeable in their ability to confer stress responsiveness, but that no other kinase is able to subsume their function.
Development of the pyk2−/pyk3− double mutant is hypersensitive to stress
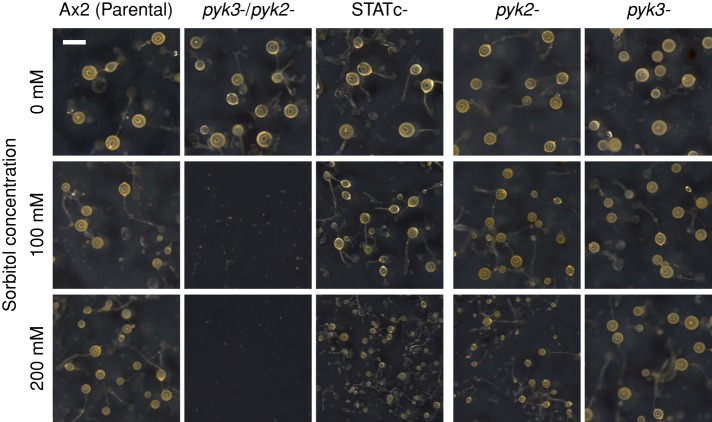
The developmental phenotype of the three null mutants was assayed using cells starved on filters, placed on water pads, and containing different amounts of sorbitol. Development was compared with that of parental Ax2- and STATc-null cells (Figure 3). Without stress, there were no major changes in the sizes of the fruiting body in any of the strains. However, in the presence of 100 mM sorbitol—a concentration that hardly affects development of the other two strains—development of the double-null strain is almost entirely arrested at an early stage: just a few tiny fruiting bodies are formed. The pattern for the double-null strain is similar with 200 mM sorbitol to that at 100 mM, but now STATc-null (Thewes et al., 2012) and pyk2− strains form slightly smaller fruiting bodies. These results indicate that Pyk2 and Pyk3 have functions that are additional to the activation of STATc. What might these targets be? There is a major effect of hyperosmotic stress on one specific tyrosine phosphorylation; actin is heavily tyrosine phosphorylated within a few minutes after stress is applied. However, this response is retained in the three null mutants (unpublished data).
FIGURE 3:
Developmental phenotype of the pyk2−/pyk3− double-null strain. Single- and double-null strains were developed on black nitrocellulose filters supported by water pads with various concentrations of sorbitol. Parental Ax2, STATc-null, and single-null strains are also shown. Photos were taken at 48 h after starvation. Bar, 500 μm.
Specific gene expression in the pyk2−/pyk3− double mutant is hypersensitive to stress
To determine the effects of the various null mutations on specific gene expression, we analyzed, by quantitative real-time PCR (qPCR), the sorbitol induction of three genes known to require STATc for their optimal inducibility: rtoA, sigG, and an uncharacterized gene (Dictybase ID DDB_G0277667). The three genes are maximally induced in the parental (Ax2) strain and somewhat less well induced in the pyk2− or pyk3− strain (Figure 4A). In the double-null strain, and as expected in the STATc-null strain, sorbitol inducibility is abolished and there is even, perhaps, slight repression of gene expression by sorbitol.
FIGURE 4:
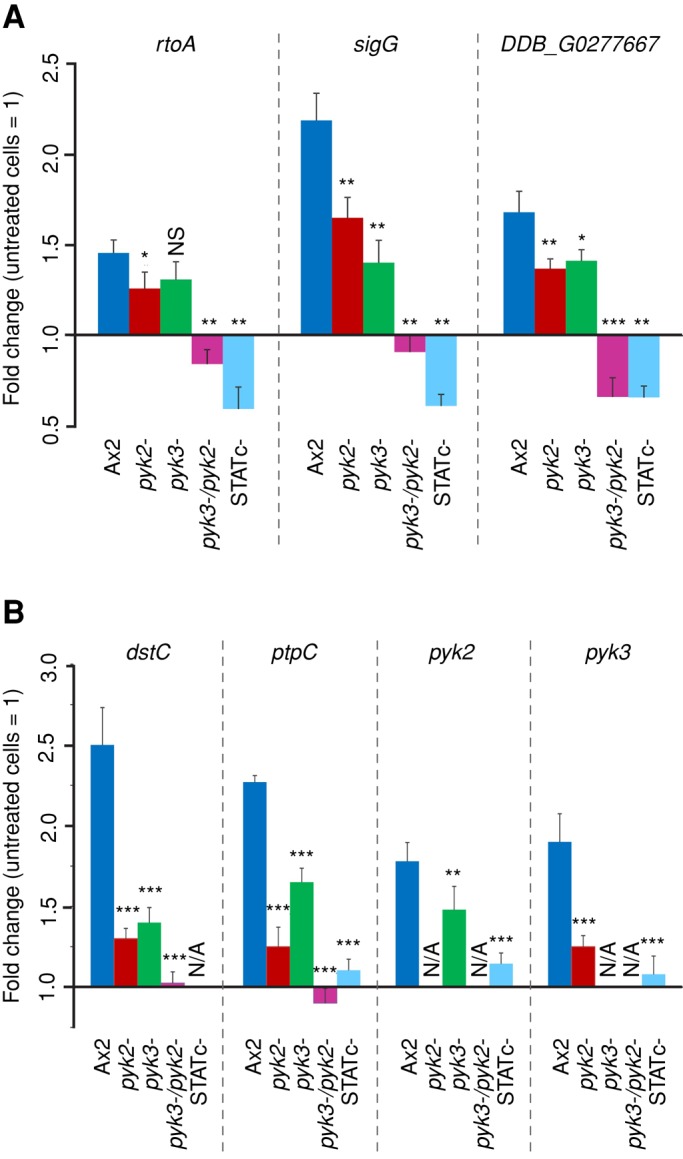
Transcriptional regulation of STATc target genes and STATc pathway genes. Real-time PCR analysis of selected STATc-dependent genes was carried out with cDNAs from Ax2 wild-type, pyk2−, pyk3−, pyk2−/pyk3−, and STATc− cells either treated for 15 min with 200 mM sorbitol or left untreated. (A) Differential expression of STATc target genes rtoA, sigG , and DDB_G0277667. (B) Differential expression of STATc pathway genes dstc, ptpC, pyk2, and pyk3. The data are expressed as means of fold change in comparison to untreated cells. Fold changes and SEM of three independent experiments with three measurements each. N/A, not applicable. Unpaired t test: *p < 0.05; **p < 0.01; ***p < 0.001; NS, not significant. Statistical significance as compared with expression values in Ax2 cells is indicated.
Previous work showed that there is a positive feedback between STATc and other members of the stress signaling pathway; STATc itself (dstC) and the gene encoding PTP3 (ptpC), the protein tyrosine-phosphatase that deactivates STATc, both require STATc for optimal inducibility. We therefore analyzed the inducibility of four of the pathway component genes—dstC, ptpC, pyk2, and pyk3—in the different mutant backgrounds. The data mirror those seen with the three, nonpathway STATc-responsive genes (Figure 4A); the single-null, pyk2− and pyk3−, strains show reduced expression of the other, pathway-specific genes, whereas the double-null mutant shows no significant inducibility for them (Figure 4B).
Pyk3 is autophosphorylated on tyrosine
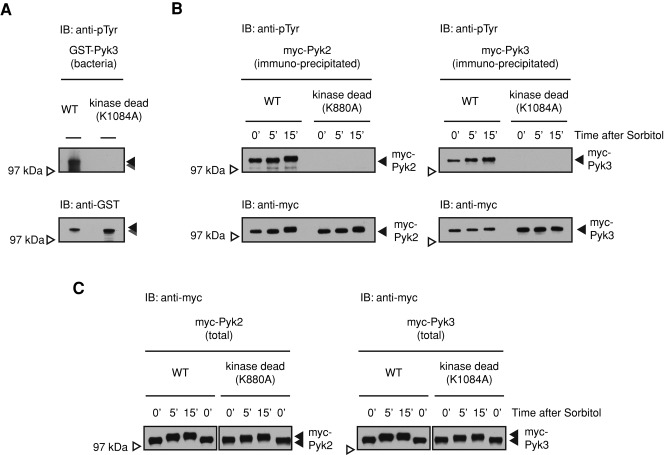
The initiating step in the interaction of Pyk2 and STATc is the autocatalytic tyrosine phosphorylation of Pyk2 (Araki et al., 2012). The data in Figure 5, A–C, show that Pyk3 is similarly modified. First, we analyzed GST-Pyk3 protein purified from E. coli, for which tyrosine is not normally phosphorylated, using a general phosphotyrosine-specific antibody (Figure 5A). There is a smear of protein, with the largest species at the predicted size of GST-Pyk3; the smear below presumably comprises breakdown products. Tyrosine phosphorylation of Pyk3 was ablated when K1084, in the predicted site of ATP binding (equivalent to K72 in PKA; Supplemental Figure S4A), of the TKL domain (KI) was mutated to alanine to create a “kinase-dead” form. We next showed that myc-Pyk3 isolated from Dictyostelium cells displays the same behavior.
FIGURE 5:
Characterization of the Pyk3 protein. (A) Bacterially expressed Pyk3 is autophosphorylated on tyrosine. Glutathione S-transferase (GST)–fused Pyk3 protein and its kinase-dead form were expressed in and purified from E. coli. The kinase-dead form was generated by introducing a point mutation in the ATP-binding site of the KI kinase domain (K1084 to alanine; Supplemental Figures S3 and S4A). The tyrosine phosphorylation level of purified proteins was assayed by Western transfer with the general phosphotyrosine antibody 4G10. (B) Tyrosine phosphorylation in vivo of Pyk2 and Pyk3. Myc-Pyk2 proteins or myc-Pyk3 proteins were overexpressed (OE) in Dictyostelium Ax2 cells using an actin15 promoter to yield OE cells. The myc-tagged OE proteins were immunopurified with the anti-myc antibody and analyzed with the general phosphotyrosine antibody 4G10. (C) Hyperosmotic stress–induced mobility shift of Pyk2 and Pyk3. Total lysates of myc-Pyk2 OE or myc-Pyk3 OE cells were separated on nongradient gel (8% Tris-Glycine gel) to analyze mobility shifts after sorbitol treatment for the indicated times.
Ax2 cells were transformed with myc-Pyk3 or its kinase-dead form. These were then either left unstressed or stressed for the indicated times, and myc-Pyk3 was purified by immunoprecipitation (IP) of the myc tag. Myc-Pyk3 and its kinase-dead form were analyzed by Western transfer using a general phosphotyrosine-specific antibody (Figure 5B). This analysis shows that myc-Pyk3 is, like myc-Pyk2 (included as a positive control), tyrosine phosphorylated and that the level of modification increases with time of sorbitol treatment. That the modification is due to tyrosine autophosphorylation is demonstrated by the complete lack of signal generated by the kinase-dead form of the fusion proteins (Figure 5B). This is based on quantitative evidence in Figure 5B, but there is a slight displacement over time that is consistent with phosphorylation; the phosphorylated band migrates slightly more slowly in samples isolated from stressed cells. This was confirmed using nongradient gels instead of gradient gels (Figure 5C), in which the separation is higher. Because the tyrosine autophosphorylation activity is absent from the kinase-dead enzyme, we assume that the shift is due to some other modification, such as serine/threonine phosphorylation.
Pyk3 and STATc interact in a manner similar to Pyk2 and STATc
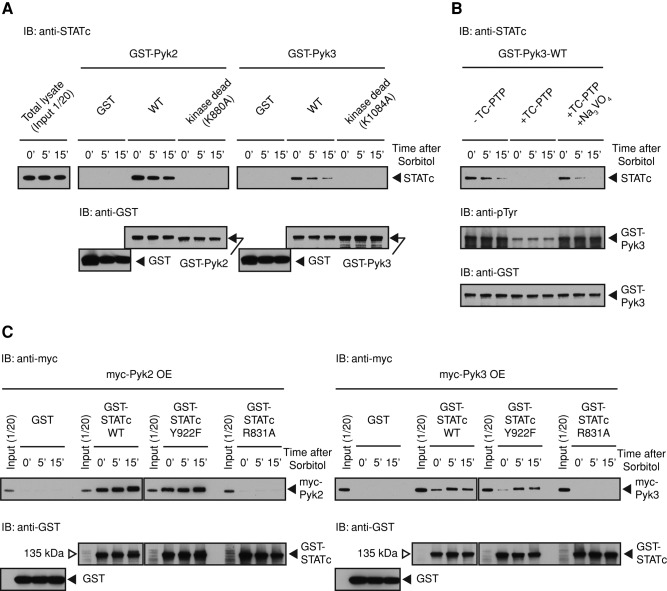
The interaction between Pyk2 and STATc can readily be demonstrated using Pyk2-GST fusion protein, produced in E. coli, as a binding substrate for STATc in a “pull-down” assay (Araki et al., 2012). The same holds true for Pyk3. In both cases there is a basal level of STATc binding to Pyk2 and Pyk3 that decreases somewhat after exposure to sorbitol (Figure 6A). Thus binding of STATc to the two kinases is semiconstitutive. The reason for a decrease is unclear, but one possibility is that some change in STATc, such as serine/threonine phosphorylation, is induced by high osmolarity, and this inhibits stress-induced binding to STATc in some unknown way. Interaction between Pyk2 and STATc occurs when STATc binds to a site or sites of tyrosine autophosphorylation on Pyk2 (Araki et al., 2012). When a similar experiment is performed using GST-Pyk3 and its kinase-dead form rather than GST-Pyk2, it yields identical results (Figure 6A).
FIGURE 6:
Pyk3 and STATc physically interact. (A) Analysis of the interaction of GST-Pyk2 and GST-Pyk3 with STATc. Ax2 cells were developed in suspension for 4 h and then left untreated or exposed to sorbitol as in Figure 2A. Cells were lysed, and the extracts were subjected to pull-down assay using GST-Pyk2-WT or GST-Pyk2-K880A, its kinase-dead form, and GST-Pyk3-WT or GST-Pyk3-K1084A. Bound STATc was detected using total STATc antibody 7H3. The blot was reprobed with a GST antibody as a loading control. (B) Tyrosine phosphorylation–dependent GST-Pyk3 binding to STATc. GST-Pyk3 was bound to glutathione beads, and the beads were either left untreated or digested with TC-PTP. A parallel reaction was performed on GST-Pyk3 beads in the presence of sodium orthovanadate, an inhibitor of the enzyme, left untreated or induced with sorbitol, and assayed as in A. The blot was reprobed with a GST antibody as a loading control, and the same samples were reanalyzed with a general phosphotyrosine antibody 4G10. (C) Pull-down analysis of Pyk3 by parental and mutant forms of STATc. Myc-Pyk2 OE or myc-Pyk3 OE cell lysate from control cells, or cells induced with sorbitol, were subjected to pull-down assay using GST-STATc or its R831A and Y922F mutant forms as in A. The blot was reprobed with a GST antibody as a loading control.
Again, just as with Pyk2 (Araki et al., 2012), biochemical analysis using human recombinant T-cell protein tyrosine phosphatase (TC-PTP) shows that a decrease in tyrosine phosphorylation of Pyk3 causes a massive reduction in binding to STATc (Figure 6B). This reduction in binding is prevented by inclusion of vanadate, an inhibitor of TC-PTP, in the reaction mix. In addition, as with Pyk2, point mutation of the ultraconserved R residue (R831 equivalent to R175 in v-Src) within the STATc SH2 domain ablates binding to GST-Pyk3 (Figure 6C). In contrast, but as expected, mutation of the C-terminus-proximal Y922 residue, which is essential for dimerization of STATc, has no effect on the binding of STATc to Pyk3.
Exposure to a hyperosmolar concentrations of sorbitol increases the activity of Pyk2 and Pyk3 as assayed in vitro in an IP kinase reaction
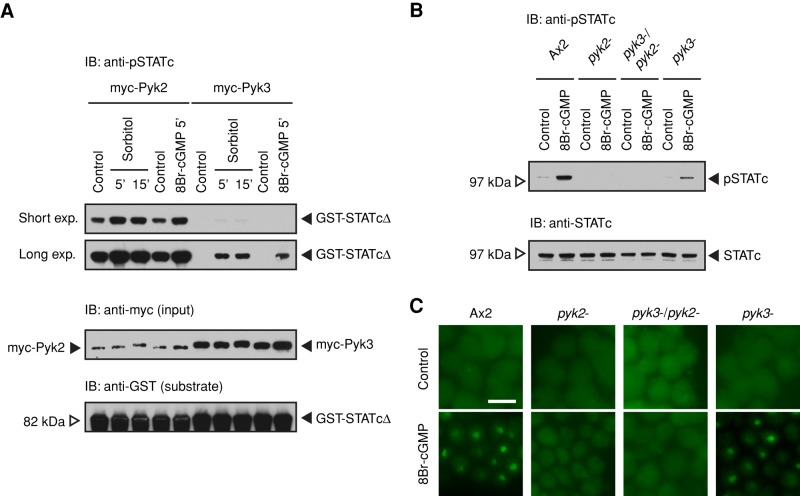
We previously used an IP kinase assay to provide a measure of the activity level of myc-tagged, immunopurified Pyk2 kinase before, and at times after, exposure to DIF-1. In this assay, cells expressing the myc-tagged fusion protein are preexposed to inducer. Then the fusion protein is purified by immunoprecipitation and used in an in vitro kinase assay with GST-STATcΔ, which contains the approximate C-terminal half (amino acids [aa] 463–932) of STATc as the substrate. The strength of signal obtained in a Western blot, using phosphospecific antibody to Y922 of STATc, should be in some way proportional to the amount of active kinase present at the time of induction.
In the foregoing assay, there is, as expected (Araki et al., 2012), a high basal level of phosphorylation with immunoprecipitates from cells expressing myc-Pyk2 (Figure 7A). The uninduced myc-Pyk3 level is very much lower. When cells are exposed to 200 mM sorbitol and analyzed after 5 and 15 min, there is a rise in STATc phosphorylation on Y922 (Figure 7A). Because Pyk2 displays such a high basal level, the activation ratio is higher for myc-Pyk3, but the induced signal is much lower. These data suggest that stress acts by stimulating Pyk2 and Pyk3 activity.
FIGURE 7:
In vitro tyrosine phosphorylation of STATc by Pyk2 and Pyk3 and the role of intracellular cGMP. (A) Tyrosine phosphorylation of STATc using immunopurified kinases. Ax2 cells transformed with myc-Pyk2 or myc-Pyk3, at 4 h of suspension development, were treated with sorbitol for 5 and 15 min or 8Br-cGMP (a membrane-permeable cGMP analogue at 20 mM) for 5 min, then lysed, and the enzyme immunopurified from them was assayed for STATc tyrosine kinase activity with GST-STATcΔ as substrate in a phospho-STATc Western assay. (B) 8Br-cGMP induced activation of STATc in single- and double-null cells Tyrosine phosphorylation of STATc was assayed after 8Br-cGMP treatment at 20 mM for 5 min. The blot was reprobed with total STATc antibody 7H3. (C) 8Br-cGMP induced nuclear enrichment of STATc in single- and double-null cells. Nuclear localization of STATc after 8Br-cGMP treatment for 5 min was analyzed by immunofluorescence staining with total STATc antibody. Bar, 10 μm.
A membrane-permeant form of cGMP activates STATc selectively in pyk3− cells
Although Pyk2 and Pyk3 are both stress inducible, as determined in the IP kinase assay, further analysis suggests that the two kinases use partially different upstream signaling pathways. First, there is the major difference in relative activity level between the two kinases. However, copy number differences between different kinase fusion proteins could, in principle, render results not directly comparable. We circumvent this problem by using the myc tag to monitor amount loaded and to correct for the loading differences. The Pyk2 activity always greatly exceeds the Pyk3 activity. Second, there is a difference in responsiveness to the second messenger that is used.
Cyclic GMP is the second messenger responsible for several aspects of the osmotic stress response, and 8Br-cGMP, a membrane-permeant derivative of cGMP, induces tyrosine phosphorylation of STATc (Araki, Tsujioka, et al., 2003). As measured using the IP kinase assay, both Pyk2 and Pyk3 are activated as STATc kinases when cells are treated with 8Br-cGMP (Figure 7A). Again, Pyk2 shows a higher basal level of activity and only an approximate twofold to threefold increase in the presence of 8Br-cGMP. Pyk3 has a very low basal level and a comparatively high induction ratio. As will be discussed, these results are supported by the genetic analyses of the three null strains (Figure 7, B and C); full activation by 8Br-cGMP depends on the presence of an intact pyk2 gene, and this is true in the single, pyk2−, or the double null with pyk3.
Pyk3 is regulated by a pseudokinase domain–mediated mechanism similar to that of metazoan JAKs
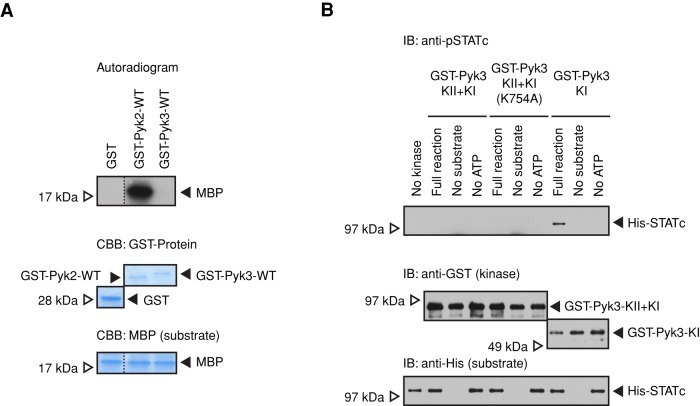
Another major difference between Pyk2 and Pyk3 is the presence in Pyk3 of both a pseudokinase domain and an adjacent, C-terminus-proximal TKL domain. The metazoan JAKs have a similar structure, and there the pseudokinase domain of JAK2 serves to negatively regulate the kinase activity of the C-terminal tyrosine kinase domain. To determine whether Pyk3 uses a similar mechanism, we first established a system in which the kinase activity of recombinant GST-tagged enzyme could be assayed in-trans using isotopically γ-labeled ATP. The substrate chosen was myelin basic protein (MBP). Whereas Pyk2 directed efficient 32P incorporation into MBP, the Pyk3 activity was in comparison negligible (Figure 8A). We then concentrated on the TKL (KI) and pseudokinase (KII) domains of Pyk3 (Supplemental Figure S3), using histidine (His)-STATc as the substrate in an in vitro kinase reaction and monitoring the reaction using the phospho-STATc antibody CP22 (Figure 8B). In the construct GST-Pyk3-KI, where KI was expressed in isolation from KII, the TKL directed tyrosine phosphorylation of His-STATc on Y922. In contrast, in the construct GST-Pyk3-KII+KI, where KI was expressed in the presence of KII, the TKL domain did not direct tyrosine phosphorylation of His-STATc on Y922. Thus the presence of the KII domain represses the kinase activity of the KI domain.
FIGURE 8:
In vitro kinase activity of Pyk3. (A) Kinase activities of recombinant GST-Pyk2 and GST-Pyk3 proteins. Bacterially expressed fusion proteins were used in a kinase reaction with MBP as general kinase substrate and by isotopic labeling. The reaction products were separated by SDS–PAGE, and the activities were detected by autoradiography. The loadings of the GST-fusion protein and MBP substrate were verified by gel staining with Coomassie blue. (B) In vitro tyrosine phosphorylation of STATc using foreshortened segments of Pyk3. Bacterially expressed GST-Pyk3 kinase domain KII+KI, KI only, and KII+KI with a point mutation of a putative ATP-binding site in the pseudokinase domain (K754A; shown in Supplemental Figures S3 and S4B) were used in a kinase reaction with His-STATc as substrate. The reaction products were assayed for STATc tyrosine phosphorylation by Western transfer with the phospho-STATc antibody CP22. Same samples were probed with anti-GST antibody and anti-polyhistidine (anti-His) antibody as loading controls.
Recently it was shown that the JH2 (pseudokinase) domain of JAK2 functions as an active protein kinase, but its activity is only 10% that of JH1 and it binds Mg-ATP in an unorthodox manner. Phosphorylation of S523 is the primary event in JAK2 activation. It enhances the subsequent autophosphorylation of T570 (Ungureanu et al., 2011; Bandaranayake, Ungureanu, et al., 2012). Although the homology is relatively poor around this region, we provisionally identified an ATP-binding site (K754) in the Pyk3 KII domain (equivalent to K581 in the JAK2 JH2 domain; Supplemental Figure S4B). However, in an in vitro kinase assay, bacterially expressed GST-Pyk3-KII+KI domain with a K754A mutation does not show any STATc kinase activity (Figure 8B). This is difficult to interpret, however, because it may be a genuine negative result or may be due to an incorrect alignment in the region of K754.
On induction with sorbitol, Pyk2 and Pyk3 localize to different parts of the cell
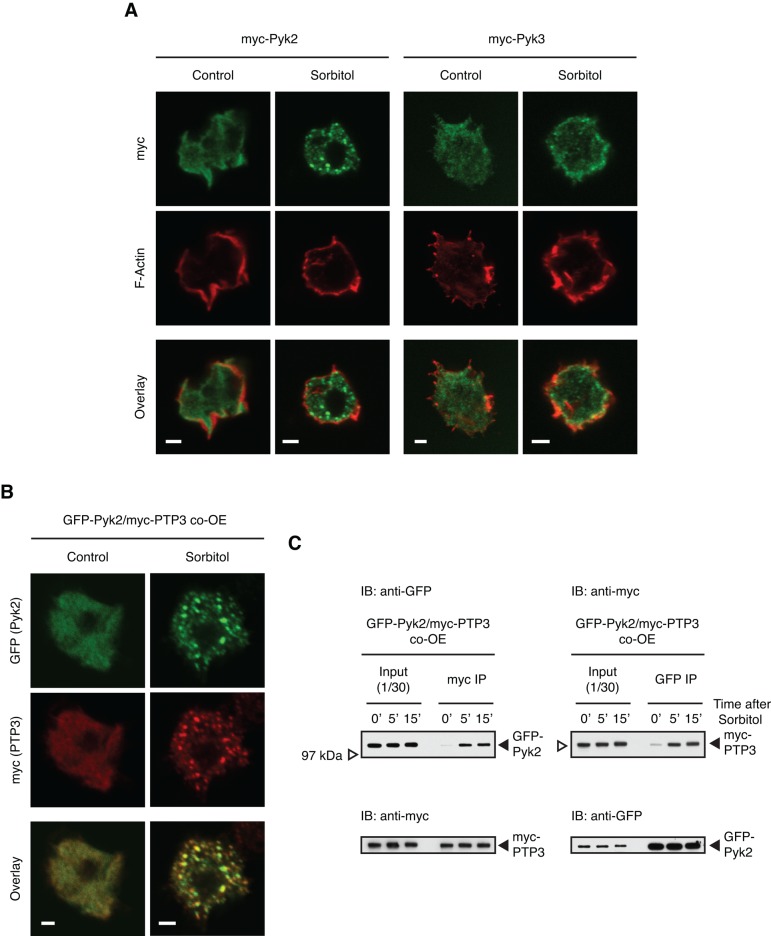
A further difference between Pyk2 and Pyk3 became apparent when the intracellular compartmentalization of the myc-tagged proteins was analyzed. Before sorbitol treatment, Pyk2 is localized predominantly in the cytosol, but after treatment, it becomes concentrated in cytoplasmic granules. PTP3 also becomes localized to granules after osmotic shock (Figure 9A), and immunostaining of double transformants shows that most of the granules contain both proteins (Figure 9B). Before sorbitol treatment, Pyk3 is also enriched in the cytosol, but, after exposure to sorbitol, the amount at the cortex increases, where it often colocalizes with F-actin (Figure 9A). Biochemical coimmunoprecipitation analysis confirms that there is a Pyk2 and PTP3 interaction and that it increases after sorbitol treatment (Figure 9C).
FIGURE 9:
Subcellular localization of Pyk2 and Pyk3 after hyperosmotic stress. (A) Different localization of Pyk2 and Pyk3. Myc-Pyk2– or myc-Pyk3–overexpressing cells plated on glass cover slides were treated with sorbitol for 5 min, then fixed for indirect immunofluorescence staining with anti-myc antibody, followed by treatment with fluorescent dye–conjugated phalloidin for F-actin staining. Single confocal Z- images of myc signal (green) and F-actin (red) and overlay images. Bar, 2 μm. (B) Colocalization of Pyk2 and PTP3 at stress-induced granules. After 5 min of sorbitol treatment, GFP-Pyk2 and myc-PTP3 co-overexpressed (co-OE) cells were fixed for indirect immunofluorescence staining with anti-GFP and anti-myc antibodies. Single confocal Z-images of GFP (green), myc (red), and overlay images. Bar, 2 μm. (C) Physical interaction of Pyk2 and PTP3 after hyperosmotic stress. GFP-Pyk2 and mycPTP3 co-OE cells were subjected to the coimmunoprecipitation assay with anti-myc or anti-GFP antibody. The interactions with immunopurified proteins were analyzed by Western transfer with anti-GFP or anti-myc antibody. The blot was reprobed with the antibody used for immunoprecipitation.
DISCUSSION
Hyperosmotic stress directs activation of two redundant STATc TKLs
Dictyostelium does not encode orthodox, receptor tyrosine kinases. Instead, it encodes a very high number of TKL proteins, and five of these were isolated, some repeatedly, in screens for kinase domains that phosphorylate on tyrosine when expressed in E. coli. They play diverse roles. Pyk1 (SplA) is necessary for maintaining spore integrity (Nuckolls et al., 1996), Pyk2 is necessary for the activation of STATc by DIF-1 (Araki et al., 2012), Pyk3 is (in Ax3 cells) necessary for the decrease in STATc tyrosine phosphorylation that occurs subsequent to DIF-1 activation (Lee et al., 2008), Pyk4 (ZakA) is the kinase that activates GSK3 (Kim et al., 1999), and Pyk5 is of unknown function. Here we showed that the two most closely related of the five kinases, Pyk2 and Pyk3, are also functionally homologous, because they direct net phosphorylation of STATc when cells are subjected to hyperosmotic stress. The fact that these two kinases activate STATc in response to stress, whereas only one kinase, Pyk2, transiently, activates STATc in response to DIF-1, presumably explains why activation by DIF-1 is much more short lived and diminished relative to stress activation. This difference is amplified because stress directs a sorbitol-regulated increase in STATc phosphorylation, both by decreasing the activity of PTP3 and by increasing the activity of Pyk2 and Pyk3, whereas DIF-1 affects only the phosphatase arm of the pathway (Araki et al., 2012).
We show that, as does Pyk2, Pyk3 binds to and directly phosphorylates STATc when cells are stressed. When assayed as a single-null mutant, neither kinase is necessary for a response, although the pyk3 null shows a slower reaction than the pyk2 null, which is barely delayed in its response. The strongest evidence for a functional interaction between Pyk2 and Pyk3 derives from the analysis of the stress reaction in the double null; it is entirely unresponsive. Thus one or other of the two kinases must be present if there is to be stress activation of STATc. A similar situation applies when we analyze expression of genes that require STATc for their stress inducibility. These include two genes, ptpC and STATc (dstC) itself, that form part of a positive feedback loop that regulates the stress response. Inactivation of the STATc gene has only a minor effect on fruiting body size, manifest under stressful conditions. However, the fact that simultaneous disruption of pyk2 and pyk3 renders development highly stress sensitive suggests that Pyk2 and Pyk3 have essential targets additional to STATc.
The pyk2-null strain has a higher dependence on intracellular cGMP than the pyk3-null strain
The kinase activation pathway, as originally defined and then assumed to be a single pathway, uses cGMP as its second messenger. The kinases define two apparently parallel pathways of stress-regulated STATc activation. Both activation pathways can use cGMP as second messenger but with different extents of dependence. This conclusion derives from analysis of STATc activation in the three possible mutant strains: pyk2−, pyk2−/pyk3−, and pyk3−. The pyk2− strain and pyk2−/pyk3− strains are essentially inactivatable, but the pyk3− strain is only 40% reduced in 8Br-cGMP–induced activity. These results indicate that Pyk2 can partially replace Pyk3 but not vice versa. This can be explained if the Pyk2 pathway overlaps to some degree with the Pyk3 pathway. Hence the results may correlate with the in vitro kinase assay data, which indicate a much higher level of (semiconstitutive) Pyk2 activity than (regulated) Pyk3 activity. Cross-talk between signaling pathways might then be biased toward the much more active, Pyk2 pathway.
Pyk3 contains a pseudokinase domain that functions similarly to those of metazoan JAKs
Additional evidence for partially discrete activation pathways is indirect and derives from consideration of the structures of the two proteins. Pyk2 contains a single TKL domain, whereas Pyk3 has a closely related TKL domain and a pseudokinase domain. The “pseudokinase” domain of JAK2 is now known to function as a low-activity kinase that exerts a repressive effect on the active TKL domain. It will be of interest to determine whether an analogous mechanism is at play with Pyk3. What we have been able to show is that the pseudokinase domain of Pyk3 exerts a repressive effect on the activity of the kinase (KI) domain. What we have not yet proven is that stress induces autophosphorylation at a specific regulatory site or that weak kinase activity of the pseudokinase domain is involved in the negative regulation.
What distinguishes Pyk2 and Pyk3: why two kinases for the same job?
We showed that when cells are stressed, the two kinases accumulate in different subcellular compartments; Pyk3 accumulates in the cortex and Pyk2 accumulates in granules. We suggest therefore that STATc requires activation in both the cortex and in intracellular granules and that it achieves this segregation by interacting with two differentially targeted TKLs. PTP3 also accumulates in granules when cells are stressed (Gamper et al., 1999), and we showed by immunostaining that PTP3 in the granules is most often colocalized with Pyk2. Moreover Pyk2 and PTP3 coimmunoprecipitate, and the amount of complex increases with time of stress. There is therefore the possibility of a preformed signaling complex in which the kinase and its cognate phosphatase interact in a transitory way with STATc. What of Pyk3? Of interest, GbpC, the cGMP-binding protein that partially mediates the stress response (Araki et al., 2010), also translocates to cortex after stress (Kortholt, van Egmond, et al., 2012), but we do not yet know whether GbpC colocalizes with Pyk3.
MATERIALS AND METHODS
Cell culture, development, and induction
Dictyostelium strain Ax2 (G. Gerisch isolate) cells were grown axenically at 21°C in HL-5 medium (Ashworth and Watts, 1970), starved, and sorbitol induced as described previously (Araki, Tsujioka, et al., 2003). For development in stress conditions, starved cells were plated at a density of 1.5 × 107 cells/cm2 on a black nitrocellulose membrane (HABP filter; Millipore, Billerica, MA) on a water pad. Cells were allowed to develop in a humid box for 48 h.
Real-time PCR
RNA isolation and real-time PCR were essentially done as described (Farbrother et al., 2006). The primers (Supplemental Table S1) were purchased from Metabion (Munich, Germany).
Immunological analysis
The phospho-STATc monoclonal antibody CP22 was used to detect STATc Y922 phosphorylation by Western transfer. STATc immunostaining and nonphosphospecific Western transfer analyses were performed using total STATc antibody 7H3 (Araki, Tsujioka, et al., 2003). Antiphosphotyrosine antibody 4G10 (Millipore) was used to monitor general phosphotyrosine modification, anti-myc antibody 9E10 was used for myc-tagged proteins, anti–green fluorescent protein (GFP) antibody 4E10 (Roche Diagnostics, Mannheim, Germany) was used for GFP-tagged proteins, and anti–polyhistidine peroxidase–conjugated antibody (Sigma-Aldrich, St. Louis MO) was used for His-tagged proteins. For immunoprecipitation of myc-tagged proteins or GFP-tagged proteins, 1–6 ml of cell suspension at 107 cells/ml was harvested and lysed in 1 ml of mNP40 lysis buffer (50 mM Tris HCl, pH 8.0, 150 ml NaCl, 1.0% NP40, 50 mM NaF, 2 mM EDTA, pH 8.0, 2 mM Na-pyrophosphate, 2 mM benzamidine, 1 mM phenylmethylsulfonyl fluoride, and Complete EDTA-free protease inhibitor cocktail [Roche Diagnostics]) for 10 min on ice. After preclearing by centrifugation, the supernatant was incubated with anti-myc antibody or anti-GFP antibody for 30 min at 4ºC with gentle rocking, followed by another 1-h incubation with Dynabeads Protein-G (Life Technologies, Paisley, United Kingdom) or Protein G-agarose (Roche Diagnostics). Beads were washed four times in mNP40 buffer and proteins eluted by boiling in SDS gel sample buffer.
STATc nuclear localization assay were performed with total STATc antibody 7H3 as described previously (Araki and Williams, 2012). For subcellular localization analysis of myc-tagged proteins, cells with myc-tagged protein were developed for 3.5 h in suspension culture and then plated on glass cover slides for 30 min. Cells were treated with sorbitol for 5 min and then fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min. After quenching (with 150 mM glycine and 0.1% Triton X-100 in PBS) for 10 min, samples were blocked with antibody dilution solution (AbDil; 2% bovine serum albumin, 0.1% Triton X-100 and 0.1% NaN3 in PBS). Cells were stained with anti-myc antibody and then with fluorescent dye (Alexa 488 or 594)–conjugated secondary antibody (Life Technologies). The samples were treated with fluorescent dye (Alexa5 94 or 488)–conjugated phalloidin (Life Technologies) for F-actin staining before mounting. For double staining of GFP-tagged Pyk2 proteins and myc-tagged PTP3 proteins, co-overexpressed cells were treated and fixed as described, then stained with mouse anti-GFP antibody (Roche Diagnostics) and rabbit anti-myc antibody (Santa Cruz Biotechnology, Santa Cruz, CA), followed by secondary antibodies (Alexa 488–conjugated anti-mouse antibody and Alexa 594–conjugated anti-rabbit antibody [Life technologies]). Single Z-images were acquired with an inverted confocal microscope (Leica DMRBE model SP2; Leica Microsystems, Wetzlar, Germany).
Construction of the Pyk3 disruption vector and of tagged proteins
In the disruption construct, Pyk3 sequences encompassing the TKL domain and the pseudokinase domain were replaced with a blasticidin resistance cassette (Supplemental Figure S2). For the myc-Pyk3 construct, Pyk3 was tagged at its N-terminus by addition of the 11-aa myc epitope, EQKLISEEDLN. The myc-Pyk3 fusion was cloned under the transcriptional control of the semiconstitutive actin 15 promoter and inserted into a G418 resistance vector. For the GST-Pyk3 constructs, pyk3 cDNA was prepared from 4-h-developed cells with the RNeasy Mini Kit (Qiagen, Hilden, Germany) and Superscript III One-Step RT-PCR system with Platinum Taq DNA Polymerase (Life Technologies), and Pyk3 (WT [full length], aa 1–1339; KII+KI, aa 677–1339; KI, aa 1007–1339) was tagged at its N-terminus by insertion into the E. coli expression vector pGEX-5X-1 (GE-Healthcare Bio-Sciences, Uppsala, Sweden). Pyk3 recombinant proteins are summarized in Supplemental Figure S3. GST-STATc (full length, aa 1–932; or Δ, aa 463–932) in pGEX-5X-1 and His-STATc (full length) in pET-28a were constructed as described previously (Araki et al., 2012). Point mutant forms of Pyk3 were generated using a PCR-based method. Bacterially expressed tagged proteins were purified with glutathione-Sepharose 4B (GE-Healthcare Bio-Sciences) or TALON metal affinity resin (Clontech Laboratories, Mountain View, CA).
GST pull-down assays and T-cell protein tyrosine phosphatase digestion
GST pull-down assays with bacterially expressed GST-Pyk2, GST-Pyk3, and GST-STATc proteins and dephosphorylation of GST tagged proteins with T-cell protein tyrosine phosphatase were performed as described previously (Araki et al., 2012).
In vitro kinase assay with bacterially expressed proteins
Bacterially expressed GST-Pyk2 WT, GST-Pyk3 (WT [full length], KII+KI, KII+KI with K754A mutation and KI), MBP (Sigma-Aldrich), and His-STATc proteins were used for this assay. For isotopic labeling, GST-Pyk2, GST-Pyk3, and GST proteins (typically 1 μg/reaction) were incubated with MBP (3 μg/reaction) as the substrate and [32P]γ-ATP (0.185 MBq/reaction; PerkinElmer, Boston, MA) mixed with unlabeled ATP (final concentration, 0.2 mM) in HEPES kinase buffer (20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES]–NaOH, pH 7.5, 30 mM NaCl, 10 mM MgCl2, 0.02% NP40, and 1 mM dithiothreitol) at 21°C for 30 min. The kinase reaction was terminated by boiling in SDS gel sample buffer. After separation by SDS gel electrophoresis, kinase activity was detected by autoradiography. For immunological detection, GST-Pyk3 protein was incubated with His-STATc (0.5 μg/reaction, purified from bacteria) as the substrate and 0.2 mM ATP in HEPES kinase buffer at 21°C for 30 min. The kinase reaction was terminated by boiling in SDS gel sample buffer, and activities were detected by Western analysis with phospho-STATc antibody CP22.
Immunoprecipitation kinase assay using myc-Pyk2 and myc-Pyk3
Myc-Pyk2 or myc-Pyk3 was immunopurified as described, but, instead of four washes in mNP40 buffer, the myc-Pyk3/Dynabeads complex was washed twice in mNP40 high-salt washing buffer, containing 1 M instead of 150 mM NaCl, with a further three washes in mNP40 buffer without EDTA. The complex was incubated in HEPES kinase buffer with GST-STATcΔ, which contains the approximate C-terminal half (aa 463–932) of STATc as the substrate (typically 0.5 μg/reaction, purified from bacteria) and 0.2 mM ATP at 4°C for 2 h. The reaction was terminated by boiling in SDS gel sample buffer. GST-STATcΔ protein was subjected to SDS–PAGE and detected with the phospho-STATc antibody CP22 after Western transfer.
Supplementary Material
Acknowledgments
The work was funded by a Wellcome Trust Program Grant (082579/Z) to J.G.W., the German Research Foundation (EI 399/4-1) and Köln Fortune to L.E., and a Grant-in-Aid from the Japan Society for the Promotion of Science (24510307) to T.K.
Abbreviations used:
- 8Br-cGMP
8 bromo-cyclic GMP
- DIF-1
differentiation-inducing factor-1
- JAK
Janus kinase
- MBP
myelin basic protein
- STAT
signal transducer and activator of transcription
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-07-1182) on August 20, 2014.
REFERENCES
Boldface names denote co–first authors.
- Adler K, Gerisch G, von Hugo U, Lupas A, Schweiger A. Classification of tyrosine kinases from Dictyostelium discoideum with two distinct, complete or incomplete catalytic domains. FEBS Lett. 1996;395:286–292. doi: 10.1016/0014-5793(96)01053-8. [DOI] [PubMed] [Google Scholar]
- Araki T, Kawata T, Williams JG. Identification of the kinase that activates a nonmetazoan STAT gives insights into the evolution of phosphotyrosine-SH2 domain signaling. Proc Natl Acad Sci USA. 2012;109:E1931–E1937. doi: 10.1073/pnas.1202715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Langenick J, Gamper M, Firtel RA, Williams JG. Evidence that DIF-1 and hyper-osmotic stress activate a Dictyostelium STAT by inhibiting a specific protein tyrosine phosphatase. Development. 2008;135:1347–1353. doi: 10.1242/dev.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Tsujioka M, Abe T, Fukuzawa M, Meima M, Schaap P, Morio T, Urushihara H, Katoh M, Maeda M, et al. A STAT-regulated, stress-induced signalling pathway in Dictyostelium. J Cell Sci. 2003;116:2907–2915. doi: 10.1242/jcs.00501. [DOI] [PubMed] [Google Scholar]
- Araki T, van Egmond WM, van Haastert PJ, Williams JG. Dual regulation of a Dictyostelium STAT by cGMP and Ca2+ signalling. J Cell Sci. 2010;123:837–841. doi: 10.1242/jcs.064436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Williams JG. Perturbations of the actin cytoskeleton activate a Dictyostelium STAT signalling pathway. Eur J Cell Biol. 2012;91:420–425. doi: 10.1016/j.ejcb.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth JM, Watts DJ. Metabolism of the cellular slime mould Dictyostelium discoideum grown in axenic culture. Biochem J. 1970;119:175–182. doi: 10.1042/bj1190175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaranayake RM, Ungureanu D, Shan Y, Shaw DE, Silvennoinen O, Hubbard SR. Crystal structures of the JAK2 pseudokinase domain and the pathogenic mutant V617F. Nat Struct Mol Biol. 2012;19:754–759. doi: 10.1038/nsmb.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosgraaf L, Russcher H, Smith JL, Wessels D, Soll DR, van Haastert PJ. A novel cGMP signalling pathway mediating myosin phosphorylation and chemotaxis in Dictyostelium. EMBO J. 2002;21:4560–70. doi: 10.1093/emboj/cdf438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg J, Darnell JE., Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- Clarke M, Lohan AJ, Liu B, Lagkouvardos I, Roy S, Zafar N, Bertelli C, Schilde C, Kianianmomeni A, Bürglin TR, et al. Genome of Acanthamoeba castellanii highlights extensive lateral gene transfer and early evolution of tyrosine kinase signaling. Genome Biol. 2013;14:R11. doi: 10.1186/gb-2013-14-2-r11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyers PA, Murphy JM. Dawn of the dead: protein pseudokinases signal new adventures in cell biology. Biochem Soc Trans. 2013;41:969–974. doi: 10.1042/BST20130115. [DOI] [PubMed] [Google Scholar]
- Farbrother P, Wagner C, Na J, Tunggal B, Morio T, Urushihara H, Tanaka Y, Schleicher M, Steinert M, Eichinger L. Dictyostelium transcriptional host cell response upon infection with Legionella. Cell Microbiol. 2006;8:438–456. doi: 10.1111/j.1462-5822.2005.00633.x. [DOI] [PubMed] [Google Scholar]
- Fukuzawa M, Abe T, Williams JG. The Dictyostelium prestalk cell inducer DIF regulates nuclear accumulation of a STAT protein by controlling its rate of export from the nucleus. Development. 2003;130:797–804. doi: 10.1242/dev.00303. [DOI] [PubMed] [Google Scholar]
- Gamper M, Kim E, Howard PK, Ma H, Hunter T, Firtel R. Regulation of Dictyostelium protein-tyrosine phosphatase-3 (PTP3) through osmotic shock and stress stimulation and identification of pp130 as a PTP3 substrate. J Biol Chem. 1999;274:12129–12138. doi: 10.1074/jbc.274.17.12129. [DOI] [PubMed] [Google Scholar]
- Gatsios P, Terstegen L, Schliess F, Häussinger D, Kerr IM, Heinrich PC, Graeve L. Activation of the Janus kinase/signal transducer and activator of transcription pathway by osmotic shock. J Biol Chem. 1998;273:22962–22968. doi: 10.1074/jbc.273.36.22962. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Manning G, Liu A, Fey P, Pilcher KE, Xu Y, Smith JL. The Dictyostelium kinome—analysis of the protein kinases from a simple model organism. PLoS Genet. 2006;2:e38. doi: 10.1371/journal.pgen.0020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim L, Liu J, Kimmel AR. The novel tyrosine kinase ZAK1 activates GSK3 to direct cell fate specification. Cell. 1999;99:399–408. doi: 10.1016/s0092-8674(00)81526-3. [DOI] [PubMed] [Google Scholar]
- Kortholt A, van Egmond WN, Plak K, Bosgraaf L, Keizer-Gunnink I, van Haastert PJ. Multiple regulatory mechanisms for the Dictyostelium Roco protein GbpC. J Biol Chem. 2012;287:2749–2758. doi: 10.1074/jbc.M111.315739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwayama H, Ecke M, Gerisch G, van Haastert PJ. Protection against osmotic stress by cGMP-mediated myosin phosphorylation. Science. 1996;271:207–209. doi: 10.1126/science.271.5246.207. [DOI] [PubMed] [Google Scholar]
- Lee NS, Rodriguez M, Kim B, Kim L. Dictyostelium kinase DPYK3 negatively regulates STATc signaling in cell fate decision. Dev Growth Differ. 2008;50:607–613. doi: 10.1111/j.1440-169X.2008.01058.x. [DOI] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Na J, Tunggal B, Eichinger L. STATc is a key regulator of the transcriptional response to hyperosmotic shock. BMC Genomics. 2007;8:123. doi: 10.1186/1471-2164-8-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuckolls GH, Osherov N, Loomis WF, Spudich JA. The Dictyostelium dual-specificity kinase splA is essential for spore differentiation. Development. 1996;122:3295–3305. doi: 10.1242/dev.122.10.3295. [DOI] [PubMed] [Google Scholar]
- Oyama M. cGMP accumulation induced by hypertonic stress in Dictyostelium discoideum. J Biol Chem. 1996;271:5574–5579. [PubMed] [Google Scholar]
- Saharinen P, Takaluoma K, Silvennoinen O. Regulation of the Jak2 tyrosine kinase by its pseudokinase domain. Mol Cell Biol. 2000;20:3387–95. doi: 10.1128/mcb.20.10.3387-3395.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staerk J, Kallin A, Demoulin JB, Vainchenker W, Constantinescu SN. JAK1 and Tyk2 activation by the homologous polycythemia vera JAK2 V617F mutation: cross-talk with IGF1 receptor. J Biol Chem. 2005;280:41893–41899. doi: 10.1074/jbc.C500358200. [DOI] [PubMed] [Google Scholar]
- Thewes S, Krohn S, Schmith A, Herzog S, Stach T, Weissenmayer B, Mutzel R. The calcineurin dependent transcription factor TacA is involved in development and the stress response of Dictyostelium discoideum. Eur J Cell Biol. 2012;91:789–799. doi: 10.1016/j.ejcb.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Ungureanu D, Wu J, Pekkala T, Niranjan Y, Young C, Jensen ON, Xu CF, Neubert TA, Skoda RC, Hubbard SR, et al. The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling. Nat Struct Mol Biol. 2011;18:971–976. doi: 10.1038/nsmb.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haastert PJ, Kuwayama H. cGMP as second messenger during Dictyostelium chemotaxis. FEBS Lett. 1997;410:25–28. doi: 10.1016/s0014-5793(97)00416-x. [DOI] [PubMed] [Google Scholar]
- Vu LH, Araki T, Na J, Clemen CS, Williams JG, Eichinger L. Identification of the protein kinases Pyk3 and Phg2 as regulators of the STATc-mediated response to hyperosmolarity. PLoS One. 2014;9:e90025. doi: 10.1371/journal.pone.0090025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.