Abstract
In this study, changes in growth parameters and nutrient intake were compared in Chinese children (ages 30–60 months) with picky eating (PE) behaviors and weight-for-height ≤25th percentile, who were randomized to receive nutrition counseling alone (NC; n = 76) or with a nutritional milk supplement (NC + NS; n = 77) for 120 days. Increases in weight-for-height z-scores were significantly greater in the NC + NS group at days 30 and 90 and over the entire study period (all P < 0.05), but not at day 120. Increases in weight-for-age z-scores were significantly greater in the NC + NS group at day 90 (P = 0.025) and over the entire study period (P = 0.046). Mean intakes of energy, protein, carbohydrate, docosahexaenoic acid, arachidonic acid, calcium, phosphorous, iron, zinc, and vitamins A, C, D, E, and B6 were significantly higher in the NC + NS group at days 60 and 120 (all P < 0.01). Thus, in young children with PE behaviors, nutritional supplementation given as an adjunct to NC resulted in greater improvements in nutrient intake compared with NC alone. Growth parameters differed between groups at several timepoints during the study, but not at day 120.
Keywords: picky eating, eating behavior, growth, nutrient intake, preschool children
Introduction
Children who eat slowly, consume a limited number of foods, lack interest in food, have no regular eating pattern, are reluctant to try new foods, and/or consume small amounts of foods are often described as having picky eating (PE) behaviors.1–7 Caregiver-reported PE behaviors are common, with a reported prevalence in young children of 19–50%.8–11 Often a physician is consulted because of caregiver anxiety over the child’s PE behaviors,4,12–15 which can become a chronic issue, lasting for more than two years in up to 40% of affected children.4 Although the specific causes of PE behaviors are not well established at this time, contributing factors may include high maternal control of feeding,16 parental pressure to eat,17–19 higher levels of child emotionality,20 greater sensitivity to sensory stimuli,21 and introduction of complementary foods before age six months.22
Children with PE behaviors may have lower intakes of total calories, protein, vitamins, minerals, and specific food groups such as vegetables and fruits.1,3,23 As a consequence, children with PE behaviors often weigh less than children without PE behaviors.8,12,14,24 Indeed, children with a lower body mass index or body weight in the lower weight-for-age or weight-for-height percentiles are more likely to be picky eaters than those with higher values.11,12,24,25 Consuming a variety of foods within and across food groups is suggested to improve micronutrient intakes and provide exposure to a wider range of key dietary components, including fiber and phytochemicals;26,27 thus, the limited dietary variety associated with PE behaviors28 may not be optimal. A child’s PE behaviors can also contribute to attempts by the caregiver to change eating behaviors by force or coercion, which may exacerbate feeding issues.29,30 Therefore, it is important to address PE behaviors at an early age to support growth, adequate nutrient intake, and positive caregiver–child interactions that contribute to healthy development.30
Nutrition counseling (NC), which includes education and behavior modification strategies designed to improve eating habits, is considered a primary component of standard care for the management of children with PE behaviors.31–33 As good eating habits often take time to become established, a nutrient-enriched supplement administered in conjunction with NC may help improve nutrient intake and support adequate growth while PE behaviors are being addressed by NC. Others have reported significantly greater increases in growth parameters among children with PE behaviors who received NC plus a nutritional supplement for 90 days, but the total dietary intake was not analyzed.34 The objective of this study was to compare growth parameters and nutrient adequacy in children with PE behaviors who received either NC alone or NC with a nutritional milk supplement (NS), in order to expand current clinical evidence on outcomes associated with the addition of NS to standard nutrition and behavioral counseling in this population.
Methods
Subjects/Study design
This was a multicenter, open-label, randomized, controlled trial conducted between February and December 2010 in the People’s Republic of China (Xinhua Hospital, affiliated to Shanghai Jiaotong University School of Medicine; Nanjing Maternity and Child Health Care Hospital, affiliated to Nanjing Medical University; Jinan Children’s Hospital; and Wuxi People’s Hospital) and Hong Kong (Prince of Wales Hospital). Children ages 30–60 months with PE behaviors and whose weight-for-height was ≤25th percentile according to WHO Child Growth Standards35 were eligible for inclusion. Presence of PE behaviors was determined by caregiver’s report of common PE behaviors (eg, child consumes a limited number of foods and/or exhibits strong preferences for a limited number of foods; child is unwilling to try new foods; child eats slowly, lacks interest in eating, and/or does not eat enough). Exclusion criteria included current acute or chronic illness, food allergies, lactose intolerance, dietary restrictions precluding dairy foods, any genetic disorder or any other type of metabolic, cardiovascular, gastrointestinal, pancreatic, or hepatic diseases that could compromise growth and/or food intake, cognitive or developmental disorders, or medications that could influence study outcomes.
Enrolled children were randomized in blocks of 4 to receive either NC alone or NC + NS in a 1:1 allocation ratio using a computer-generated randomization list stratified by study site (SAS version 9.1.3, Cary, North Carolina, USA). The randomization list was prepared before study initiation by a statistician at a third-party data management company (Excel PharmaStudies, Inc., Beijing, China) and then stored by this company until completion of data collection. Group assignment information was sealed in opaque envelopes, which were opened by investigators upon enrollment of an eligible subject.
The study comprised a baseline period, when a three-day food record was completed by caregivers at home, followed by a 120-day intervention period and a post-study telephone call 14 days later. Study visits were conducted at days 30, 60, 90, and 120. The study was conducted according to the principles of the International Conference on Harmonization Guideline for Good Clinical Practice36 and the Declaration of Helsinki,37 and approved by each institution’s ethics committee (primary committee: Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine). Written informed consent was obtained from caregivers prior to the start of the study. The study was registered at ClinicalTrials.gov (NCT01823302).
The primary outcome measure was change in weight-for-height z-score (WHZ) from baseline to day 120. Secondary outcome measures were changes in WHZ from baseline to days 30, 60, and 90 and over the entire study period; changes in weight-for-age z-scores (WAZ) and height-for-age z-scores (HAZ) from baseline to days 30, 60, 90, and 120 and over the entire study period; dietary macro- and micronutrient intakes at days 60 and 120; quantitative ultrasound (QUS) measurements of the radius and tibia; and incidence of common illnesses.
Nutrition counseling
NC was provided at each visit by physicians (China) or dietitians (Hong Kong) who were blinded to the treatment assignment. NC providers developed individualized dietary strategies aimed at improving the child’s eating behaviors using the baseline three-day food record in conjunction with the Chinese Nutrition Society Maternal and Child Nutrition Branch dietary guidelines for children.38 These guidelines aim to help children establish healthy eating habits and were reinforced during NC sessions. Examples of guidance included: scheduled times and fixed locations for meals and snacks; age-appropriate portions; distraction-free mealtime environment; and providing a caregiver role model at meals.
Food record/dietary assessment
Caregivers prospectively recorded the child’s food intake on three consecutive days (2 weekdays and 1 weekend day) prior to study visits at baseline and on days 60 and 120. Details of foods and drinks, including brand name and amount consumed, were recorded. A Dietary Nutrition Survey Guide,39 containing pictures of 180 common foods in typical household containers, was provided to caregivers, along with instructions to help them estimate portion sizes while completing the food record. Nutrient intakes were calculated by trained study personnel at one center using food records and the Nutrition Data System for Research dietary analysis program (Nutrition Coordinating Center, University of Minnesota, USA). The NS was included in the dietary analysis using a default nutritional supplement from the program database and adjusting for nutrient differences with the NS. Nutrient intakes were expressed as a percentage of the recommended intake values in China.40 To maintain blinding, NC providers did not have access to the three-day food records from days 60 and 120.
Nutritional supplement
The NS was a milk-based powder (S-26 PE GOLD, Wyeth Nutrition, Singapore) prepared by caregivers according to label instructions. Study personnel at each site instructed the caregiver in the use of the NS and were not involved in providing NC. The NS provided 200 kcal/serving and a balanced combination of macronutrients (14% energy from protein, 54% carbohydrate, and 32% fat) and micronutrients (see Appendix for detailed nutrient composition). Children in the NC + NS group were asked to consume at least two 230 mL servings/day, in accordance with the standard usage instructions for the NS. For children in the NC + NS group, a questionnaire was completed monthly by caregivers to record consumption and willingness to consume the NS.
Anthropometry
Child’s height (SZG-180 Height Meter; Nantong Yuejian Anthropometry Apparatus Co., Ltd, Nantong City, China; scale division: 1 mm) and weight (RGT-120 Weighing Scale, Nantong Weighing Apparatus Factory, Nantong City, China; scale division: 0.05 kg) were assessed at baseline and at monthly study visits. Measurements were expressed as z-scores (2006 WHO Child Growth Standards).35 Study personnel performing anthropometric measurements were blinded to group assignment.
Bone quality
Bone quality was assessed at baseline and day 120 using QUS (Sunlight Omnisense® 7000P; Sunlight Medical, Petah Tikva, Israel), which measured speed of sound at the tibia and radius, and compared data with an age-specific reference database of more than 1500 children and adolescents in urban China.
Common illnesses
Incidences of diarrhea and upper and lower respiratory tract infections, based on specific definitions provided by study personnel, were recorded at each study visit and by telephone contact between visits; incidences of these illnesses occurring in the interim periods were also reported by caregivers.
Adverse events
Adverse events (AEs) were recorded throughout the study.
Statistical analysis
A minimum of 60 children per group was required to provide 90% power to detect a mean (standard deviation [SD]) between-group difference of 0.3 (0.5) for WHZ at the 5% significance level.34 Assuming 10% dropout and 10% non-evaluable rates, enrollment of 150 children (75 per group) was required for adequate power.
The efficacy analyzable population (randomized children with baseline and ≥1 post-baseline endpoint data and, for the NC + NS group, had consumed ≥1 serving of NS) was used for efficacy analyses. The safety population (all randomized children in the NC group and all randomized children who had consumed ≥1 serving of the NS in the NC+NS group) was used for the analysis of AEs.
For growth parameters, a mixed model for repeated measures (MMRM) analysis was done based on the changes from baseline in z-scores, with visit, treatment group, and treatment-by-visit interaction included and baseline z-score as a covariate. P-values for comparison of adjusted mean changes (adjusted for baseline z-score values) were obtained for days 30, 60, 90, and 120 and for the entire 120-day study period (ie, simultaneously taking the changes at all timepoints into account). The MMRM was also used to assess nutrient intakes between the two groups; as these data were non-normally distributed, analysis was based on the ranks of the observed values, except phosphorous (mean values used). The Hochberg step-up procedure41 was used to adjust for multiple comparisons with regard to the dietary intake variables. Between-group comparisons of QUS measurements were performed using ANCOVA, with baseline value as a covariate.
Fisher’s Exact Test was used to compare incidence of common illnesses between groups. The frequency and percentage of children who gave each of the responses to the NS acceptance questionnaire were summarized, and the reported number and percentage of children with AEs were summarized for each group.
Results
Subjects
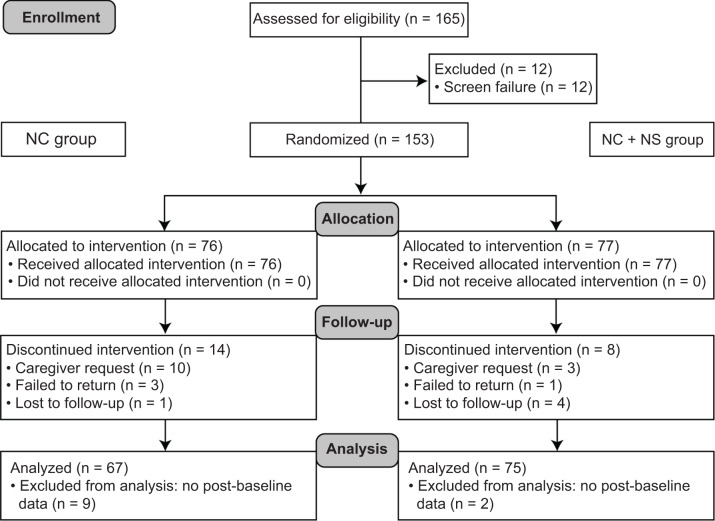
Of the 153 randomized children, 142 were included in the efficacy analyzable population (Fig. 1). The safety population included all randomized subjects (NC + NS group, n = 77; NC group, n = 76).
Figure 1.
Participant flow.
Abbreviations: NC, nutrition counseling; NS, nutritional milk supplement.
Demographic and baseline data were comparable between groups (Table 1). Mean baseline WHZ, WAZ, and HAZ were below zero and comparable between groups. Caregiver and household characteristics (including household size, smokers, education level, occupation, and income) did not differ significantly between groups. Nutritional intake at baseline was also generally similar between groups (Table 2).
Table 1.
Baseline characteristics (efficacy analyzable population).
| NC GROUP (n = 67) |
NC + NS GROUP (n = 75) |
|
|---|---|---|
| Age, years | 3.6 (0.7) | 3.8 (0.7) |
| Sex, male, % | 43.3 | 49.3 |
| Weight, kg | 13.3 (1.7) | 13.5 (1.9) |
| Height, cm | 98.2 (6.4) | 99.3 (7.1) |
| Weight-for-height percentile† | 10.4 (6.3) | 10.7 (7.3) |
| z-scores‡ | ||
| Weight-for-height | −1.4 (0.4) | −1.4 (0.5) |
| Weight-for-age | −1.1 (0.7) | −1.2 (0.7) |
| Height-for-age | −0.4 (1.1) | −0.6 (1.1) |
Table 2.
Dietary intakes and percentage of Chinese RNI at baseline and days 60 and 120 for total energy, macro- and micronutrients.
| NC GROUP | NC + NS GROUP | |||||
|---|---|---|---|---|---|---|
| BASELINE (n = 67) |
DAY 60 (n = 63) |
DAY 120 (n = 61) |
BASELINE (n = 75) |
DAY 60 (n = 71) |
DAY 120 (n = 67) |
|
| Total energy, kcal/day | ||||||
| Median (% RNI) | 1013 (75) | 955 (71) | 1027 (77) | 1018 (76) | 1124** (82) | 1209*** (89) |
| (Q1, Q3) | (869, 1140) | (785, 1180) | (840, 1208) | (814, 1120) | (959, 1306) | (1013, 1398) |
| Carbohydrate, g/day | ||||||
| Median (% RNI) | 126 (ND) | 110 (ND) | 128 (ND) | 120 (ND) | 139*** (ND) | 150*** (ND) |
| (Q1, Q3) | (111, 142) | (92, 135) | (97, 147) | (99, 141) | (114, 170) | (127, 176) |
| Protein, g/day | ||||||
| Median (% RNI) | 43.8 (95) | 42.2 (94) | 45.8 (102) | 42.9 (94) | 52.1** (108) | 50.4* (107) |
| (Q1, Q3) | (35.4, 54.5) | (32.4, 53.1) | (36.6, 57.2) | (36.1, 51.7) | (37.4, 61.8) | (42.1, 61.3) |
| Total fat, g/day | ||||||
| Median (% RNI) | 35.2 (ND) | 35.0 (ND) | 37.2 (ND) | 38.8 (ND) | 40.8 (ND) | 42.3 (ND) |
| (Q1, Q3) | (29.1, 43.9) | (25.9, 47.1) | (28.7, 43.8) | (29.3, 47.8) | (32.2, 48.9) | (36.1, 53.5) |
| DHA, g/day | ||||||
| Median (% RNI) | 0.06 (ND) | 0.05 (ND) | 0.05 (ND) | 0.06 (ND) | 5.45*** (ND) | 6.30*** (ND) |
| (Q1, Q3) | (0.02, 0.11) | (0.03, 0.10) | (0.03, 0.10) | (0.03, 0.09) | (3.78, 6.37) | (4.99, 6.80) |
| AA, g/day | ||||||
| Median (% RNI) | 0.12 (ND) | 0.10 (ND) | 0.13 (ND) | 0.11 (ND) | 7.80*** (ND) | 9.13*** (ND) |
| (Q1, Q3) | (0.08, 0.16) | (0.08, 0.16) | (0.09, 0.15) | (0.08, 0.16) | (5.47, 9.26) | (7.24, 9.88) |
| Calcium, mg/day | ||||||
| Median (% RNI) | 438 (69) | 452 (69) | 451 (71)† | 429 (62) | 667*** (93) | 686*** (100) |
| (Q1, Q3) | (353, 587) | (352, 556) | (339, 548) | (333, 538) | (524, 835) | (550, 828) |
| Iron, mg/day | ||||||
| Median (% RNI) | 7.4 (62) | 7.2 (60) | 8.3 (69) | 7.5 (62) | 10.6*** (88) | 10.8*** (90) |
| (Q1, Q3) | (5.9, 9.1) | (5.5, 10.2) | (5.8, 10.4) | (5.8, 9.9) | (7.7, 12.5) | (9.7, 12.8) |
| Zinc, mg/day | ||||||
| Median (% RNI) | 5.5 (56) | 5.5 (57) | 6.0 (56) | 5.5 (54) | 8.2*** (80) | 8.4*** (82) |
| (Q1, Q3) | (4.6, 6.4) | (4.3, 7.0) | (4.6, 7.1) | (4.2, 6.5) | (6.4, 9.9) | (7.4, 9.7) |
| Vitamin A, mcg/day | ||||||
| Median (% RNI) | 313(59) | 330 (60) | 362 (70) | 351 (65) | 554*** (103) | 579*** (107) |
| (Q1, Q3) | (231, 458) | (221, 467) | (262, 439) | (214, 502) | (416, 757) | (441, 762) |
| Vitamin C, mg/day | ||||||
| Median (% RNI) | 45.4 (67) | 31.2 (52) | 37.3 (57) | 39.3 (62) | 60.4*** (91) | 64.8*** (103) |
| (Q1, Q3) | (23.7, 66.4) | (19.7, 53.7) | (19.7, 54.1) | (25.5, 65.0) | (46.0, 82.4) | (49.0, 77.2) |
| Vitamin D, mcg/day | ||||||
| Median (% RNI) | 3.7 (37) | 3.9 (39) | 3.9 (39) | 3.8 (38) | 7.0*** (70) | 7.9*** (79) |
| (Q1, Q3) | (2.6, 4.9) | (2.4, 5.3) | (2.5, 5.2) | (2.2, 4.7) | (5.4, 9.0) | (5.5, 8.7) |
| Vitamin E, mg/day | ||||||
| Median (% RNI) | 2.9 (70) | 3.3 (74) | 3.6 (87) | 3.4(77) | 6.6*** (151) | 7.6*** (173) |
| (Q1, Q3) | (2.2, 4.0) | (2.1, 4.6) | (2.3, 4.8) | (2.5, 4.7) | (5.2, 8.6) | (6.1, 9.2) |
| Folate, mcg DFE/day | ||||||
| Median (% RNI) | 250 (152) | 243 (147) | 282 (166) | 258 (147) | 294 (155) | 296 (161) |
| (Q1, Q3) | (197, 295) | (190, 333) | (187, 358) | (207, 325) | (212, 358) | (219, 365) |
| Lutein, mcg/day | ||||||
| Median (% RNI) | 631 (ND) | 475 (ND) | 579 (ND) | 551 (ND) | 595 (ND) | 557 (ND) |
| (Q1, Q3) | (335, 1093) | (282, 880) | (354, 1069) | (325, 900) | (334, 912) | (396, 1138) |
| Magnesium, mg/day | ||||||
| Median (% RNI) | 140 (120) | 123 (108) | 135 (115) | 130 (108) | 143** (120) | 142 (115) |
| (Q1, Q3) | (106, 163) | (95, 153) | (100, 163) | (100, 166) | (113, 186) | (118, 168) |
| Phosphorus, mg/day | ||||||
| Mean (% RNI) | 689 (149) | 677 (146) | 694 (150) | 679 (143) | 841*** (177) | 841*** (178) |
| (SD) | (202) | (227) | (212) | (212) | (252) | (230) |
| Vitamin B6, mg/d | ||||||
| Median (% RNI) | 0.83 (151) | 0.74 (143) | 0.82 (147) | 0.81 (148) | 1.00*** (188) | 1.03*** (185) |
| (Q1, Q3) | (0.67, 1.00) | (0.56, 1.11) | (0.61, 1.03) | (0.60, 1.04) | (0.77, 1.23) | (0.86, 1.27) |
| Vitamin B12, mcg/d | ||||||
| Median (% RNI) | 2.5 (256) | 2.5 (249) | 2.6 (240) | 2.3 (236) | 2.7 (259) | 2.6 (242) |
| (Q1, Q3) | (1.9, 3.2) | (1.9, 3.5) | (1.9, 3.3) | (1.7, 3.6) | (2.0, 3.8) | (1.9, 3.6) |
Notes: Asterisks denote significance as demonstrated when the P value from the MMRM analyses between treatment groups was lower than the significance level adjusted using the Hochberg step-up procedure. The following P values, denoting significant differences from the NC group, were reported:
P < 0.01;
P < 0.001;
P < 0.0001. % RNI data were analyzed by descriptive statistics only.
n = 60 (calcium intake data for one subject was invalid at this timepoint).
Abbreviations: Q1, 25th percentile; Q3, 75th percentile; AA, arachidonic acid; DFE, dietary folate equivalents; NC, nutrition counseling; ND, not defined (Chinese RNI not specified for this nutrient); NS, nutritional milk supplement; RNI, recommended nutrient intake; SD, standard deviation.
Growth parameters
Mean WHZ increased in both groups over the course of the study (Fig. 2A). The adjusted mean (standard error [SE]) change from baseline to day 120 in WHZ was 0.23 (0.06) in the NC + NS group and 0.11 (0.06) in the NC group (between-group difference, 0.12 [95% confidence interval (CI), −0.04, 0.29]; P = 0.137). Although the difference between groups at day 120 was not significant, increases in WHZ were significantly higher in the NC + NS group at days 30 (P = 0.047) and 90 (P = 0.021) and also for the entire study period (between-group difference, 0.13 [95% CI, 0.01, 0.25]; P = 0.029). Mean WAZ also increased in both groups during the study (Fig. 2B). Compared with increases in the NC group, adjusted mean (SE) increases in WAZ in the NC + NS group were significantly greater at day 90 (0.03 [0.03] vs 0.14 [0.03]; P = 0.025) and for the entire study period (between-group difference, 0.08 [95% CI, 0.00, 0.16], P = 0.046). No significant between-group differences were observed in mean change in HAZ at any timepoint or for the entire study period (Fig. 2C).
Figure 2.
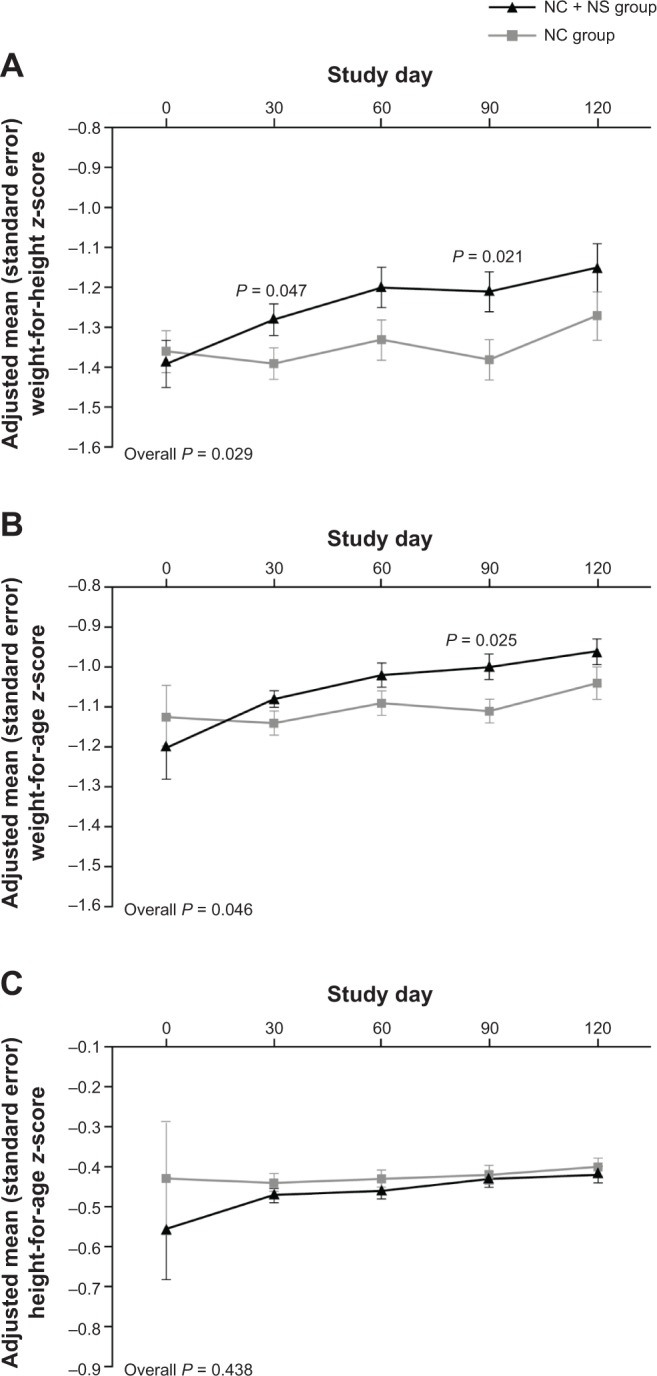
Adjusted mean (A) weight-for-height, (B) weight-for-age, and (C) height-for-age z-scores.
Abbreviations: NC, nutrition counseling; NS, nutritional milk supplement.
Nutrient intake
Dietary intake results are presented in Table 2. Median total energy intake increased from baseline at days 60 and 120 in the NC + NS group and slightly decreased in the NC group at day 60. Median total energy intake was significantly higher in the NC + NS group at both timepoints compared with the NC group (P < 0.001 for each). Significantly greater intakes of carbohydrates and protein were also observed at days 60 and 120 for the NC + NS compared with the NC group (P < 0.01). While intakes of docosahexaenoic acid and arachidonic acid were significantly higher at days 60 and 120 in the NC + NS group compared with the NC group (P < 0.001), between-group differences in total fat intake were not significant at any timepoint. Additionally, there was a significantly greater intake of several micronutrients, including calcium, phosphorous, iron, zinc, and vitamins A, C, D, E, and B6, in the NC + NS group versus the NC group at days 60 and 120 (P < 0.001).
Nutrient adequacy was assessed by expressing nutrient intakes as a percentage of Chinese Recommended Nutrient Intake (% RNI) values40 (Table 2). For calcium, iron, zinc, and vitamins A, C, and D, baseline % RNI values in both of the groups were low (range, 37–69%). Change in % RNI over the study interval was greater in the NC + NS group when compared with the NC group, with values approaching or exceeding 100% for several nutrients, including total energy, calcium, iron, zinc, and vitamins A, C, D, and E.
Additional findings
The z-scores for QUS bone measures were not significantly different between groups at day 120. Additionally, the incidence of common illnesses (diarrhea and upper and lower respiratory tract infections) did not differ between groups. AEs were reported in 81.8 and 71.1% of children in the NC + NS and NC groups, respectively. The most frequent AEs were upper respiratory tract infection, pyrexia, and diarrhea. Two children (2.6%) in the NC + NS group experienced moderate constipation or mild diarrhea considered to be study related. Serious AEs occurred in three children (2.0%), all considered unrelated to the study.
At least one serving of NS was consumed daily by more than 75% of children in the NC + NS group at days 60 and 120. Consumption rate was consistent, with a mean (SD) consumption of 344 (161) and 371 (155) mL/day at days 60 and 120, respectively. At day 120, 57.3% of caregivers reported that their child liked the taste of the NS and 68.0% reported that their child was willing to drink the supplement daily.
Discussion
This study evaluated the effects of an oral NS provided in addition to standard care with NC on growth parameters and nutrient adequacy in children with PE behaviors. The provision of an NS as an adjunct to NC was associated with significantly greater increases in overall protein and energy intake, as well as the intake of key micronutrients including iron, zinc, calcium, and vitamin A. The NS was readily accepted and well tolerated, which is notable given the study population comprised children with PE behaviors.
Adequate nutrient intake is required for healthy growth and development. The dietary intake results from the current study show that nutritional supplementation can contribute to the management of potential risks associated with PE behaviors, by increasing the intake of essential nutrients for growth and development in children whose dietary intake may be inadequate and whose growth may be toward the lower end of the normal range. The mean consumption of NS over the course of the study represents an additional 350 kcal/day, plus micronutrients important for children with PE behaviors, who may be at risk of inadequate intakes of zinc, iron, and vitamins A and D.42 Intakes of these nutrients were below Chinese RNI values at baseline in both of the groups, while intakes at study days 60 and 120 among children receiving NC + NS were significantly greater than those receiving NC only. Changes in % RNI indicate that the micronutrient status of children receiving NC + NS was improved relative to those receiving NC alone.
With respect to growth parameters, the between-group comparison across the entire 120-day study period, taking into account the differences at each of the four post-baseline visits, revealed an overall difference in the changes in WHZ and WAZ between the NC and NC + NS groups. However, there were no significant differences between groups for change in WHZ and WAZ at day 120. The larger incremental gains in WHZ and WAZ observed in the NC group between days 90 and 120 suggest the possibility that NC alone may require several months before improved eating habits are established and changes in growth parameters are observed. This is consistent with findings from prior studies of nutrition counseling and behavior modification interventions, which indicate that a duration of at least 12 months is important to the success of these interventions.43 In addition, there were no significant differences between groups for change in HAZ, possibly due to the relatively short study duration; however, this parameter increased at each visit in both groups.
PE behaviors affect the child, caregivers, family, and healthcare providers, and can create caregiver anxiety about the child’s growth and development.14 Strategies to change eating behaviors include nutrition education and behavioral therapy for the child and caregivers to improve mealtime behavior, acceptance of new foods, and overall nutrient intake.30,32,33 Dietary counseling has been used in a variety of feeding problems in children and is generally considered effective.31,44–46
Few studies have been conducted in children with PE behaviors to document the effects of management strategies such as NC + NS. One other randomized, controlled PE interventional trial has been published;34 this was conducted in 92 children aged 36–60 months from Taiwan and the Philippines with PE behaviors and weight-for-height below the 25th percentile. Although dietary intake was not assessed, the study found greater increases in growth parameters over the course of the study in children receiving nutrition counseling plus an oral nutritional supplement versus those receiving counseling without supplementation.
A relationship between PE behaviors and inadequate growth has been reported in some studies,8,11,12,24,25 but not found by others.2,4 Nonetheless, combined interventions such as NC + NS may be useful to alleviate caregiver anxiety about PE, while also correcting poor eating behaviors and dietary insufficiencies. As demonstrated here, the addition of NS to standard care with NC can have a positive impact on nutrient intake in children with PE behaviors, particularly as an immediate source of nutritional support while long-term effects of NC on dietary behaviors develop. Overall, the long-term aim of interventions for children with PE behaviors is to improve eating patterns and support appropriate growth and weight gain;32,33 the use of supplementation without counseling to improve dietary intake should be discouraged in this population. Further research is needed to determine optimal timing and use of NS as an adjunct to NC, to ensure the achievement of both the behavioral and nutritional objectives of PE interventions.
Strengths of this study include the study design, including a comprehensive dietary assessment, and the specific study population, as children with PE behaviors have rarely been studied in China or other Asian countries. Potential limitations include the relatively short study duration and small sample size for evaluating some of the additional/secondary outcomes. The effects of interventions on bone quality and height occur over time, and a longer study period is likely necessary for differences to be seen in these parameters. Additionally, unless a larger sample is enrolled or a population with nutritional or social disadvantage is studied, it may be difficult to detect significant differences in the incidence of common childhood illnesses and infections. Lastly, this study relied on parent/caregiver reports to identify children with PE behaviors for inclusion in the study. Although this approach has been shown to be valid,47 additional measures to more fully characterize the patterns and degree of PE using new approaches48 would be useful in future studies.
Conclusion
In summary, this study demonstrated that NS added to NC improves nutrient adequacy in young Chinese children with PE behaviors and WHZ ≤25th percentile. This result supports the use of multiple intervention strategies to promote better nutritional outcomes in this population. Nutrition education and behavior modification remain essential tools to help improve eating habits in children with PE behaviors and to establish life-long healthy dietary practices. This study suggests that NS may be helpful as part of a treatment plan for picky eaters, especially during the early phase of NC, when changes in dietary habits may not yet be sufficient to significantly improve nutrient intake. Long-term studies are warranted to assess the enduring health and developmental outcomes associated with PE behavior and the overall effects of nutritional counseling and supplementation in this population.
Supplementary Data
Appendix 1.
Nutrient composition of the milk supplement.
| NUTRIENT | AMOUNT PER SERVING (230 mL) |
|---|---|
| Energy, kcal | 200 |
| Protein, g | 7 |
| Total fat, g | 7 |
| Arachidonic acid, mg | 5.2 |
| Docosahexaenoic acid, mg | 3.6 |
| Available carbohydrates, g | 27 |
| Nucleotides, mg | 5.2 |
| Taurine, mg | 9.4 |
| L-Carnitine, mg | 3.4 |
| Lutein, mcg | 40 |
| Vitamin A (retinol), mcg | 200 |
| Carotenes, mcg | 42 |
| Vitamin D (cholecalciferol), mcg | 3.3 |
| Vitamin E (d-alpha tocopherol), mg | 2.2 |
| Vitamin K, mcg | 13 |
| Vitamin B1 (thiamine), mg | 0.26 |
| Vitamin B2 (riboflavin), mcg | 320 |
| Vitamin B6 (pyridoxine), mcg | 250 |
| Vitamin B12 (cyanocobalamin), mcg | 0.5 |
| Niacin, mcg | 1388 |
| Folic acid (dietary folate equivalents), mcg | 19 |
| Pantothenic acid, mcg | 1000 |
| Biotin, mcg | 4.9 |
| Vitamin C (ascorbic acid), mg | 24 |
| Choline, mg | 60 |
| Inositol, mg | 15 |
| Calcium, mg | 260 |
| Phosphorus, mg | 170 |
| Magnesium, mg | 28 |
| Iron, mg | 3.8 |
| Zinc, mg | 2.4 |
| Manganese, mcg | 225 |
| Copper, mcg | 175 |
| Iodine, mcg | 19 |
| Sodium, mg | 118 |
| Potassium, mg | 475 |
| Chloride, mg | 275 |
Acknowledgments
The authors thank Dr. Chung Mo Chow for recruiting and assessing subjects, and Ms. Flora YY Kwok and Dr. Yvonne YF Ho (Department of Pediatrics, The Chinese University of Hong Kong, Prince of Wales Hospital) for providing nutritional counseling and collecting study data. The authors were fully responsible for content and editorial decisions for this manuscript, with editorial support provided by Jocelyn Woodcock and Carol Cooper of Caudex Medical, funded by Wyeth Nutrition.
Footnotes
ACADEMIC EDITOR: Joseph Zhou
FUNDING: This study was sponsored by Wyeth Nutrition, a Nestlé business. XS, MT, DZ, TFL, and FZ received funding for the conduct of the study from Wyeth Nutrition.
COMPETING INTERESTS: NPH, RN, DLT, and MY are employees of Wyeth Nutrition, King of Prussia, PA, USA. JG is an employee of Wyeth Nutritional (China) Company Ltd., Shanghai, China. WMH is an employee of Wyeth (Hong Kong) Holding Company Ltd., Hong Kong SAR, China. TFL has received research funding, conference sponsorship, speaker’s fees unrelated to the present study from GlaxoSmithKline (Biologicals) Limited, Wyeth Nutritional (Indonesia) Limited, and FrieslandCampina. TFL received a grant from Wyeth (Hong Kong) for this study. Other authors disclose no potential conflicts of interest.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
Author Contributions
XS, MT, DZ, TFL, FZ, JG, WMH, RN and MY participated in the design of the study. XS, MT, DZ, TFL, and FZ were responsible for subject recruitment and data collection. Dietary data processing was conducted by XS. XS, NPH, RN, DLT, and MY made substantial contributions to data interpretation and manuscript preparation. All authors contributed to and approved the manuscript prior to submission.
REFERENCES
- 1.Carruth BR, Skinner J, Houck K, Moran J, Coletta F, Ott D. The phenomenon of “Picky Eater”: a behavioral marker in eating patterns of toddlers. J Am Coll Nutr. 1998;17(2):180–186. doi: 10.1080/07315724.1998.10718744. [DOI] [PubMed] [Google Scholar]
- 2.Carruth BR, Skinner JD. Revisiting the picky eater phenomenon: neophobic behaviors of young children. J Am Coll Nutr. 2000;19(6):771–780. doi: 10.1080/07315724.2000.10718077. [DOI] [PubMed] [Google Scholar]
- 3.Galloway AT, Fiorito L, Lee Y, Birch LL. Parental pressure, dietary patterns, and weight status among girls who are picky eaters. J Am Diet Assoc. 2005;105(4):541–548. doi: 10.1016/j.jada.2005.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mascola AJ, Bryson SW, Agras WS. Picky eating during childhood: a longitudinal study to age 11 years. Eat Behav. 2010;11:253–257. doi: 10.1016/j.eatbeh.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reau NR, Senturia YD, Lebailly SA, Christoffel KK. Infant and toddler feeding patterns and problems: normative data and a new direction. Pediatric Practice Research Group. J Dev Behav Pediatr. 1996;17:149–153. [PubMed] [Google Scholar]
- 6.Dovey TM, Staples PA, Gibson EL, Halford JCG. Food neophobia and “picky/fussy” eating in children: a review. Appetite. 2008;50:181–193. doi: 10.1016/j.appet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Jacobi C, Schmitz G, Agras WS. Is picky eating an eating disorder? Int J Eat Disord. 2008;41:626–634. doi: 10.1002/eat.20545. [DOI] [PubMed] [Google Scholar]
- 8.Carruth BR, Ziegler PJ, Gordon A, Barr SI. Prevalence of picky eaters among infants and toddlers and their caregivers’ decisions about offering a new food. J Am Diet Assoc. 2004;104:S57–S64. doi: 10.1016/j.jada.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Goh DYT, Jacob A. Perception of picky eating among children in Singapore and its impact on caregivers: a questionnaire survey. Asia Pac Fam Med. 2012;11(1):5. doi: 10.1186/1447-056X-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Equit M, Palmke M, Becker N, Moritz AM, Becker S, von Gontard A. Eating problems in young children—a population-based study. Acta Paediatr. 2013;102(2):149–155. doi: 10.1111/apa.12078. [DOI] [PubMed] [Google Scholar]
- 11.Orun E, Erdil Z, Cetinkaya S, Tufan N, Yalcin SS. Problematic eating behaviour in Turkish children aged 12–72 months: characteristics of mothers and children. Cent Eur J Public Health. 2012;20(4):257–261. doi: 10.21101/cejph.a3748. [DOI] [PubMed] [Google Scholar]
- 12.Ekstein S, Laniado D, Glick B. Does picky eating affect weight-for-length measurements in young children? Clin Pediatr (Phila) 2010;49(3):217–220. doi: 10.1177/0009922809337331. [DOI] [PubMed] [Google Scholar]
- 13.Leung AK, Marchand V, Sauve RS. The picky eater: The toddler or preschooler who does not eat. Paediatr Child Health. 2012;17(8):455–460. doi: 10.1093/pch/17.8.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright CM, Parkinson KN, Shipton D, Drewett RF. How do toddler eating problems relate to their eating behavior, food preferences, and growth? Pediatrics. 2007;120(4):e1069–e1075. doi: 10.1542/peds.2006-2961. [DOI] [PubMed] [Google Scholar]
- 15.Japan Ministry of Health, Labour, Welfare The National Nutrition Survey of Infant and Toddlers. 2005. [Accessed January 24, 2014]. Available at http://www.mhlw.go.jp/houdou/2006/06/h0629-1.html.
- 16.Morrison H, Power TG, Nicklas T, Hughes SO. Exploring the effects of maternal eating patterns on maternal feeding and child eating. Appetite. 2013;63:77–83. doi: 10.1016/j.appet.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Powell FC, Farrow CV, Meyer C. Food avoidance in children. The influence of maternal feeding practices and behaviours. Appetite. 2011;57(3):683–692. doi: 10.1016/j.appet.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Gregory JE, Paxton SJ, Brozovic AM. Maternal feeding practices, child eating behaviour and body mass index in preschool-aged children: a prospective analysis. Int J Behav Nutr Phys Act. 2010;7:55. doi: 10.1186/1479-5868-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haycraft E, Blissett J. Predictors of paternal and maternal controlling feeding practices with 2- to 5-year-old children. J Nutr Educ Behav. 2012;44(5):390–397. doi: 10.1016/j.jneb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Hafstad GS, Abebe DS, Torgersen L, von Soest T. Picky eating in preschool children: the predictive role of the child’s temperament and mother’s negative affectivity. Eat Behav. 2013;14(3):274–277. doi: 10.1016/j.eatbeh.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Farrow CV, Coulthard H. Relationships between sensory sensitivity, anxiety and selective eating in children. Appetite. 2012;58(3):842–846. doi: 10.1016/j.appet.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Shim JE, Kim J, Mathai RA, STRONG Kids Research Team Associations of infant feeding practices and picky eating behaviors of preschool children. J Am Diet Assoc. 2011;111:1363–1368. doi: 10.1016/j.jada.2011.06.410. [DOI] [PubMed] [Google Scholar]
- 23.Dubois L, Farmer AP, Girard M, Peterson K. Preschool children’s eating behaviours are related to dietary adequacy and body weight. Eur J Clin Nutr. 2007;61:846–855. doi: 10.1038/sj.ejcn.1602586. [DOI] [PubMed] [Google Scholar]
- 24.Jansen PW, Roza SJ, Jaddoe VW, et al. Children’s eating behavior, feeding practices of parents and weight problems in early childhood: results from the population-based Generation R Study. Int J Behav Nutr Phys Act. 2012;9:130. doi: 10.1186/1479-5868-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubois L, Farmer A, Girard M, Peterson K, Tatone-Tokuda F. Problem eating behaviors related to social factors and body weight in preschool children: a longitudinal study. Int J Behav Nutr Phys Act. 2007;4:9. doi: 10.1186/1479-5868-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steyn NP, Nel JH, Nantel G, Kennedy G, Labadarios D. Food variety and dietary diversity scores in children: are they good indicators of dietary adequacy? Public Health Nutr. 2006;9(5):644–650. doi: 10.1079/phn2005912. [DOI] [PubMed] [Google Scholar]
- 27.Tucker KL. Eat a variety of healthful foods: old advice with new support. Nutr Rev. 2001;59(5):156–158. doi: 10.1111/j.1753-4887.2001.tb07005.x. [DOI] [PubMed] [Google Scholar]
- 28.Falciglia GA, Couch SC, Gribble LS, Pabst SM, Frank R. Food neophobia in childhood affects dietary variety. J Am Diet Assoc. 2000;100:1474–1478. 1481. doi: 10.1016/S0002-8223(00)00412-0. [DOI] [PubMed] [Google Scholar]
- 29.Kerzner B. Clinical investigation of feeding difficulties in young children: a practical approach. Clin Pediatr (Phila) 2009;48(9):960–965. doi: 10.1177/0009922809336074. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell GL, Farrow C, Haycraft E, Meyer C. Parental influences on children’s eating behaviour and characteristics of successful parent-focussed interventions. Appetite. 2013;60(1):85–94. doi: 10.1016/j.appet.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Tseng AG, Biagioli FE. Counseling on early childhood concerns: sleep issues, thumb sucking, picky eating, and school readiness. Am Fam Physician. 2009;80(2):139–142. [PubMed] [Google Scholar]
- 32.McCormick V, Markowitz G. Picky eater or feeding disorder? Strategies for determining the difference. Adv NPs PAs. 2013;4(3):18–22. [PubMed] [Google Scholar]
- 33.Ong C, Phuah KY, Salazar E, How CH. Managing the “picky” eater dilemma. Singapore Med J. 2014;55(4):184–190. doi: 10.11622/smedj.2014049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alarcon PA, Lin LH, Noche M, Jr, et al. Effect of oral supplementation on catchup growth in picky eaters. Clin Pediatr (Phila) 2003;42:209–217. doi: 10.1177/000992280304200304. [DOI] [PubMed] [Google Scholar]
- 35.WHO Multicentre Growth Reference Study Group. de Onis M. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006;95:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 36.ICH Expert Working Group Guideline for good clinical practice E6(R1) 1996. [Accessed January 24, 2014]. Available at http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdf.
- 37.World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. 2008. [Accessed January 24, 2014]. Available at http://www.wma.net/en/30publications/10policies/b3/ [PubMed]
- 38.Chinese Nutrition Society, Maternal and Child Nutrition Branch . Chinese Dietary Guidelines for Pregnant and Lactating Women and 0–6 Year Old Children. Beijing: People’s Medical Press; 2007. [Google Scholar]
- 39.Zhai F, editor. A Dietary Nutrition Survey Guide. Beijing: Science Press; 2006. [Google Scholar]
- 40.Chinese Nutrition Society . The Dietary Guidelines for Chinese Residents. Lhasa: Tibet People Press; 2008. [Google Scholar]
- 41.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- 42.World Health Organization, Food and Agricultural Organization of the United Nations . Guidelines on food fortification with micronutrients. Geneva, Switzerland: World Health Organization; 2006. [Accessed January 24, 2014]. Available from: http://www.who.int/nutrition/publications/micronutrients/9241594012/en/ [Google Scholar]
- 43.Knai C, Pomerleau J, Lock K, McKee M. Getting children to eat more fruit and vegetables: a systematic review. Prev Med. 2006;42:85–95. doi: 10.1016/j.ypmed.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Ashworth A, Ferguson E. Dietary counseling in the management of moderate malnourishment in children. Food Nutr Bull. 2009;30(3 Suppl):S405–433. doi: 10.1177/15648265090303S304. [DOI] [PubMed] [Google Scholar]
- 45.Jeong SJ. Nutritional approach to failure to thrive. Korean J Pediatr. 2011;54(7):277–281. doi: 10.3345/kjp.2011.54.7.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernard-Bonnin AC. Feeding problems of infants and toddlers. Can Fam Physician. 2006;52(10):1247–1251. [PMC free article] [PubMed] [Google Scholar]
- 47.Jacobi C, Agras WS, Bryson S, Hammer LD. Behavioral validation, precursors, and concomitants of picky eating in childhood. J Am Acad Child Adolesc Psychiatry. 2003;42:76–84. doi: 10.1097/00004583-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Tharner A, Jansen PW, Kiefte-de Jong JC, et al. Toward an operative diagnosis of fussy/picky eating: a latent profile approach in a population-based cohort. Int J Behav Nutr Phys Act. 2014;11:14. doi: 10.1186/1479-5868-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1.
Nutrient composition of the milk supplement.
| NUTRIENT | AMOUNT PER SERVING (230 mL) |
|---|---|
| Energy, kcal | 200 |
| Protein, g | 7 |
| Total fat, g | 7 |
| Arachidonic acid, mg | 5.2 |
| Docosahexaenoic acid, mg | 3.6 |
| Available carbohydrates, g | 27 |
| Nucleotides, mg | 5.2 |
| Taurine, mg | 9.4 |
| L-Carnitine, mg | 3.4 |
| Lutein, mcg | 40 |
| Vitamin A (retinol), mcg | 200 |
| Carotenes, mcg | 42 |
| Vitamin D (cholecalciferol), mcg | 3.3 |
| Vitamin E (d-alpha tocopherol), mg | 2.2 |
| Vitamin K, mcg | 13 |
| Vitamin B1 (thiamine), mg | 0.26 |
| Vitamin B2 (riboflavin), mcg | 320 |
| Vitamin B6 (pyridoxine), mcg | 250 |
| Vitamin B12 (cyanocobalamin), mcg | 0.5 |
| Niacin, mcg | 1388 |
| Folic acid (dietary folate equivalents), mcg | 19 |
| Pantothenic acid, mcg | 1000 |
| Biotin, mcg | 4.9 |
| Vitamin C (ascorbic acid), mg | 24 |
| Choline, mg | 60 |
| Inositol, mg | 15 |
| Calcium, mg | 260 |
| Phosphorus, mg | 170 |
| Magnesium, mg | 28 |
| Iron, mg | 3.8 |
| Zinc, mg | 2.4 |
| Manganese, mcg | 225 |
| Copper, mcg | 175 |
| Iodine, mcg | 19 |
| Sodium, mg | 118 |
| Potassium, mg | 475 |
| Chloride, mg | 275 |