Abstract
Background
We investigated whether the anti-atherosclerosis of adiponectin (APN) relates to the reduction of oxidative stress. We observed the overexpression of adiponectin gene with different titers on atherosclerosis (AS) models of high-fat apolipoprotein E-deficient (ApoE−/−) mice.
Material/Methods
We divided 48 male ApoE−/− mice into 4 groups: control group, high-fat diet group, low adiponectin group, and high adiponectin group. The low and high adiponectin group mice were treated with recombinant adenovirus expressing mice adiponectin (Ad-APN) with low-dose adiponectin 1.0×108 p.f.u. and high-dose adiponectin 5.0×108 p.f.u. via the tail every 2 weeks and given a high-fat diet for the last 8 weeks. On the 14th day after injection, blood samples were obtained from the vena cava.
Results
Along with increased serum adiponectin, serum superoxide dismutase (SOD) activity increased (P<0.05) and concentration of malondialdehyde (MDA) was decreased (P<0.05). Levels of total cholesterol (TC), triglyceride (TG), and low-density lipoprotein cholesterol (LDL-C) were decreased, especially TC and LDL-C (P<0.05). A real-time fluorescent quantitative polymerase chain reaction test was used to analyze levels of mRNA expression for endothelial nitric oxide synthase (eNOS) and adiponectin in the aorta. Along with increased adiponectin, the mRNA expression of eNOS in the aorta was increased significantly (P<0.05). The lesion formation in the aortic sinus was inhibited by 25% and 31% in the low-APN group and high-APN group, respectively (P<0.05). Along with the increase of adiponectin doses, the damage of atherosclerosis gradually eased. However, the differences between the low-APN group and high-APN group had no statistical significance.
Conclusions
Adiponectin may protect the aorta from atherosclerosis injury by reducing oxidative stress, reducing lesion formation size in the aortic root and reducing TC, TG, and LDL-C in serum. The molecular mechanism may involve preservation of SOD, reducing MDA in serum, and increasing eNOS and adiponectin mRNA expression in the aorta.
MeSH Keywords: Adiponectin, Atherosclerosis, Nitric Oxide Synthase Type III, Oxidative Stress, Superoxide Dismutase
Background
Cardiovascular disease has the highest worldwide mortality rate, surpassing heart disease and cancer [1]. Atherosclerosis is an important cause of cardiovascular disease. The occurrence and mortality rate of atherosclerosis is increasing. Most of the pathological changes take place in middle-aged persons and elderly people, with the fastest development in those ages 40–49 years [2–4]. Although it has been studied for many years, the cause and mechanism of atherosclerosis remains poorly defined [5]. However, some risk factors e.g., dyslipidemia, hypertension, smoking, diabetes, and dyslipidemia) and hereditary factors have been proposed, of which the formation mechanism is still not fully understood [6].
Adiponectin (APN), also referred to as ACRP30 (adipocyte complement-related 30 kDa protein), is a specific adipokine secreted by adipose tissue. Adiponectin is found in human plasma at concentrations of 3–30 μg/ml [7,8]. Adiponectin contains a collagen-repeat domain and a globular domain with a sequence homology to complement factor C1q. The human adiponectin gene is located on chromosome 3q27, with a length of about 16kb, including 3 explicit factors and 2 introns. Through full genetic group scanning, this region was found to be a sensitive area for metabolic syndrome, type-II diabetes and cardiovascular disease [9]. It is well established that adiponectin levels in plasma are negatively associated with accumulation of body fat, particularly visceral fat [10]. Clinical studies indicate a close association of low adiponectin levels with many obesity-related disorders [11,12]. It has correlations with obesity, insulin resistance, and type-II diabetes, and also has anti-inflammation and anti-atherosclerosis action [13,14]. As an adipose-derived hormone, adiponectin has been reported to have vascular protective effects. Previous studies showed that adiponectin is the only anti-inflammation factor negatively correlated with cardiovascular disease. Low level of adiponectin is a separate type of risk factor for the occurrence of atherosclerosis disease [15,16]. Atherosclerotic endothelial dysfunction, particularly at early stages, is primarily caused by the dysregulation of endothelial nitric oxide synthase (eNOS) enzymatic activity and inactivation of nitric oxide (NO) through oxidative stress [17]. Thus, recent research to improve endothelial function in atherosclerosis has focused on the promotion of NO production and prevention of NO inactivation by oxidative stress. The integral role of the endothelium in vascular health and of endothelial dysfunction in atherosclerosis has generated considerable interest in the potential for reversal of endothelial dysfunction with various therapies [18].
Consistent with these clinical findings, several experimental studies indicated a protective role by reducing oxidative stress in complications of obesity. In adiponectin knockout (KO) mice (adiponectin−/− mice), ACh-induced aortic vasorelaxation was impaired, accompanied by increased superoxide (O2•−) and peroxynitrite (ONOO−) production. eNOS expression was conserved in adiponectin−/− mice, but NO and eNOS phosphorylation were significantly reduced [19]. Moreover, Xiuping Chen [20] previously found that reciprocal regulation between adiponectin and lectin-like oxidized LDL (ox-LDL) receptor (LOX)-1 amplifies oxidative stress and ox-LDL uptake, leading to endothelial dysfunction in atherosclerosis. However, the interaction and association between adiponectin and eNOS, serum superoxide dismutase (SOD) activity, and concentration of malondialdehyde (MDA) in atherosclerosis have not been established. In this study, we examined the effects of adiponectin and SOD, MDA, and eNOS on the aorta in ApoE KO mice. More importantly, we elucidated the reciprocal association between different doses of adiponectin and oxidative stress, laying the theoretical foundation for treatment of atherosclerosis with adiponectin.
Material and Methods
Mouse model of atherosclerosis
[Grade SPF] ApoE is a major apolipoprotein, which acts as a ligand for low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) receptors in the liver. ApoE−/− mice, a well established animal model of atherosclerosis, show remarkable hypercholesterolemia and severe atherosclerosis. ApoE−/− mice, age 12 weeks, male, with weight of 20–22 g, donated by Shanghai Southern Biomodel Research Center, bred in and grown by the Experiment Animal Department of First Affiliated Hospital of Xinjiang Medical University [SYXK(Xin)2010-0003, AAALAC accredited number: 001279], were fed in the feeding room with specific-pathogen free (SPF). All the procedures related to the animal experiments were approved (IACUC-20130709010) by the Animal Ethics Committee of First Affiliated Hospital of Xinjiang Medical University. The ApoE−/− mice were housed in an animal facility equipped with 12:12-h light-dark cycles and allowed free access to chow and water. A high-fat diet (Jiangsu Medicine Co., Ltd.) containing 35% kcal from fat, 45% kcal from carbohydrates, and 1.25% cholesterol was fed for 8 weeks to induce obesity and atherosclerosis. We randomly divided 48 healthy adult male ApoE−/− mice into 4 groups: a control group, a high-fat diet group, a low-dose adiponectin group, and a high-dose adiponectin group, with 12 mice in each group. The control group (CON) was fed normal chow and the high-fat diet group (HFD) was fed a high fat diet for 8 weeks. Adenovirus vector containing the gene for full-length mouse adiponectin (Ad-APN) was obtained from Shenzhen Byrne Biotechnology Co., Ltd. Human embryonic kidney (HEK)-293 AD cells were used for the construction of pDC316-mCMV-EGFP-mAdipoq and pDC316-mCMV-EGFP adenovirus vector, then adenovirus packaging. The low-dose adiponectin (low-APN) group was injected with Ad-APN 1.0×108 p.f.u. via tail veins every 2 weeks and the high-dose adiponectin (high-APN) group was injected with Ad-APN 5.0×108 p.f.u. and fed a high-fat diet during the experiment. After animals were anesthetized with pentobarbital sodium (50 mg/kg i.p.), blood samples were obtained from the vena cava.
Pathological detection of vascular tissue
The lesion site in the blood vessels of the root part of the aortic arch of mice was biopsied, fixed with paraformaldehyde, dehydrated to become transparent, paraffin embedded, and sliced. Hematoxylin and eosin staining was done. Analysis using ImageJ software was performed to determine the atherosclerosis-damaged area of the blood vessels and the lesion rate of the arterial lumen.
Measurement of blood parameters
A whole blood sample was held for 30 min at room temperature to allow clotting. The sample was centrifuged at 3000 g for 10 min at 4°C. The serum was transferred into separate tubes without disturbing blood clots and stored at −80°C. A fully automatic biochemistry analyzer (HITACHI 7020 Automatic Analyzer) was used to detect the total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Double-antibody sandwich ELISA method was used to determine the serum concentration of adiponectin. Colorimetry was conducted to determine the activity of super oxide dismutase (SOD) and concentration of malondialdehyde (MDA). Enzyme-labeling (Bio-Rad Benchmark Plus), blood parameters measurement reagent (America Roche Diagnostics Co., Ltd.), as well as adiponectin-ELISA (America Biomerica Company), MAD, and SOD reagent kits (Nanjing Jiancheng Bioengineering Institute) were also used.
Determination of mRNA levels
A real-time fluorescent quantitative polymerase chain reaction test was used to assess gene expression. Total RNAs were extracted from the arteries of mice by means of Trizol (Invitrogen) according to the manufactures’ instructions. One μg DNase I-treated (Thermo) total RNA was reverse transcribed using Primer Script First Strand cDNA Synthesis Kit (Promega). The reverse transcription and real-time fluorescent quantization were performed according to the instructions. A PCR device (BIO-RAD My Cycler), electrophoresis apparatus electrophoresis (Liuyi brand DYY-6D), and high-speed freezing centrifugation (HC-3018R) were used. APN with amplified with a fragment length of 128 bp (Takara taq). The reaction occurred under reaction conditions of 94°C, 55°C, 30 s, and 72°C 30 s for 35 cycles. The eNOS with amplified fragment length of 75 bp were processed under reaction conditions of 94°C for 30 s, 55°C for 30 s, 72°C for 30 s, and 35 cycles using a DNA purification kit (Tiangen Biotech Co., Ltd.). cDNA was amplified using the QuantiFast SYBR® Green Real-time PCR Master Mix (Qiagen) in 96-well optical reaction plates on a fluorescent quantization PCR device (CFX96) according to the manufactures’ protocol. The cycling parameters were: 95°C for 5 min and then 40 cycles of 95°C for 10 s, 60°C for 30 s, followed by a melting curve analysis. Samples were run in triplicate. The SQ mean values of the target gene primers were compared with those of β-actin-specific primers using the double standard curve method. The primers used in real-time PCR were: (see Table 1).
Table 1.
Sequences of primers for quantitative RT-PCR.
| Genes | Forward | Reverse |
|---|---|---|
| β-actin | 5′-GCTCTTTTCCAGCCTTCCTT-3′ | 5′-CTTCTGCATCCTGTCAGCAA-3′ |
| APN | 5′-AGGTTGGATGGCAGGC-3′ | 5′-GTCTCACCCTTAGGACCAAGAA-3′ |
| eNOS | 5′-TGTCTGCGGCGATGTCACT-3′ | 5′-CATGCCGCCCTCTGTTG-3′ |
Statistical analysis
All parameters determined in this study are presented as mean ± standard error of the mean. Statistical analyses were performed using one-way analysis of variance (ANOVA) or t-test. P<0.05 was considered significant.
Results
Inhibition of the formation of atherosclerosis plaques by adiponectin
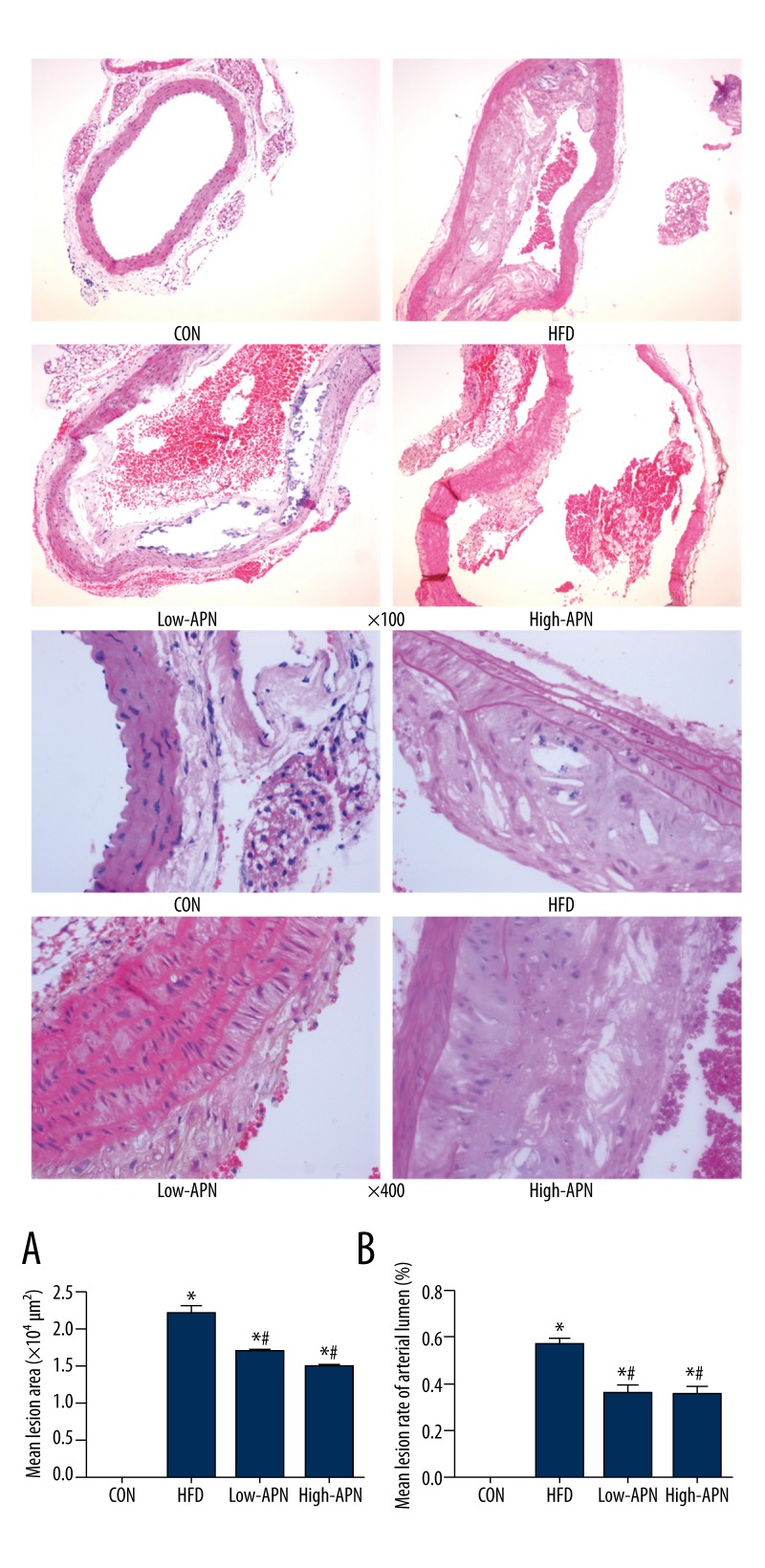
In the control group, there was no atherosclerosis damage in the ApoE−/− mice. Compared with those in the control group, obvious atherosclerosis damage occurred to the aorta in the high-fat diet group and the plaques were clear with cholesterol crystals forming necrotic tissue and foam cell cavities. Compared with those in the high-fat diet group, the atherosclerosis damage area in the low-APN group and high-APN group decreased remarkably, by 25% and 31%, respectively (P<0.05) and the mean arterial lumen lesion rate was reduced significantly (P<0.05) (Figure 1). The pathological cutting imaging showed that, compared with those in the low-APN group, the atherosclerosis damage area in the high-APN group was less, but the difference between the 2 groups was not statistically significant.
Figure 1.
Effect of Ad-APN treatment on atherosclerotic lesions (HE staining) in each ApoE−/− mouse group (n=12 each) with low and high (×100 and ×400) magnification. Mean lesion area and mean lesion rate of arterial lumen in each group ApoE−/− mice 14 days after Ad-APN injection (n=12 each). (A) Average lesion area of each mouse was determined from aortic root. The value is mean ±SEM. (B) Mean lesion rate of arterial lumen. The value are means ±SEM. * P<0.05 vs. CON group; # P<0.05 vs. HFD group.
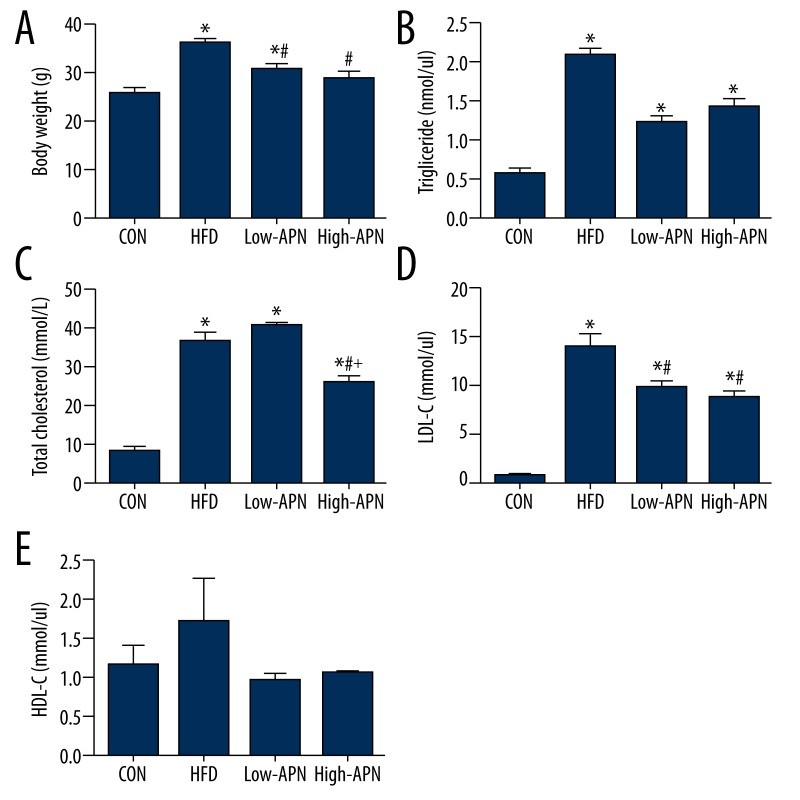
Effects of Ad-APN on body weight and hyperlipidemia
During postnatal development, there was no significant difference in body weight (male, ApoE−/− mice 12 weeks of age); mice in the 4 groups showed almost the same body weight. After 8 weeks of high-fat diet, compared with those in the control group, the body weight of mice in the high-fat diet group increased significantly (P<0.05) and the levels of TC, TG, LDL-C in the serum increased most significantly (P<0.05), and the levels of HDL-C increased but not significantly. Compared with those in the high-fat diet group, the weight of mice in the low-APN group and high-APN group decreased significantly (P<0.05); LDL-C in the low-APN group decreased significantly (P<0.05); and the TC and LDL-C of the high-APN group decreased significantly (P<0.05). Comparing the high-APN group with the low-APN group, TC in the high-APN group decreased significantly (P<0.05), but the differences of TG, LDL-C, and HDL-C had no statistical significance (Figure 2).
Figure 2.
Effects of Ad-APN on body weight and blood parameters in serum after Ad-APN injection (n=12 each). (A) Mean body weight. (B) Triglyceride. (C) Total cholesterol. (D) Low-density lipoprotein cholesterol. (E) High-density lipoprotein cholesterol. The value are means ± SEM. * P<0.05 vs. CON group; # P<0.05 vs. HFD group; + P<0.05 vs. low-APN group.
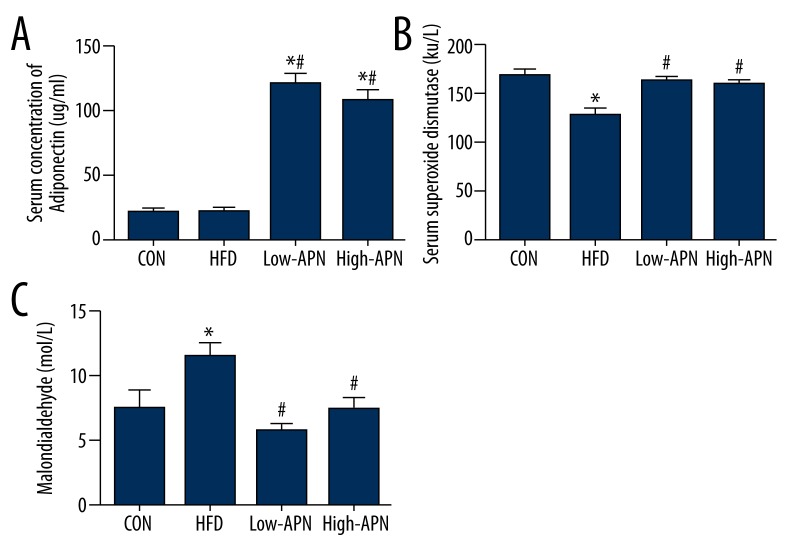
Effects of adiponectin on molecules in serum related to oxidative stress
Compared with those in the control group, the level of adiponectin and activity of SOD in the serum of the high fat diet group decreased significantly (P<0.05) and MDA increased significantly (P<0.05). Compared with those in the high-fat diet group, the level of adiponectin in the high-APN group increased significantly (P<0.05) and the activity of SOD increased significantly (P<0.05), MDA decreased significantly (P<0.05); the level of adiponectin in the low-APN group increased significantly (P<0.05), the activity of SOD increased significantly (P<0.05), and MDA decreased significantly (P<0.05). Comparing the high-APN group with the low-APN group, the difference between them had no statistical significance (Figure 3).
Figure 3.
Comparison of the level of serum adiponectin, SOD, and MDA in different groups (n=12 each). (A) Serum concentration of adiponectin. (B) Activity of superoxide dismutase. (C) Serum concentration of malondialdehyde. The values are means ± SEM. * P<0.05 vs. CON group; # P<0.05 vs. HFD group.
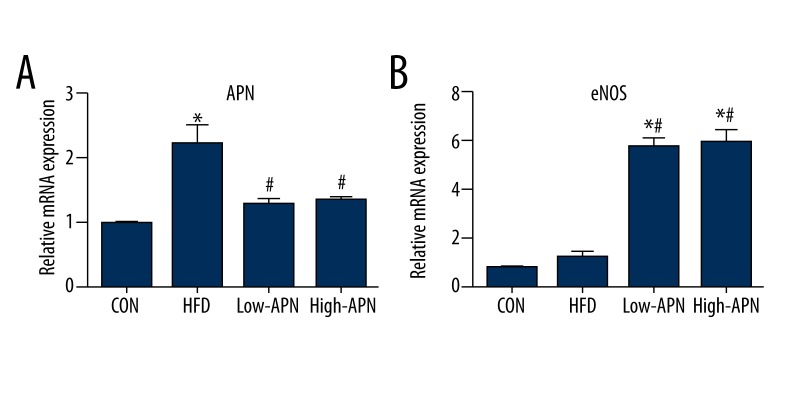
Effects of adiponectin on mRNA expression of molecules related to oxidative stress
To test the anti-oxidative stress effects of adiponectin, we examined mRNA expression of adiponectin and eNOS, respectively. In contrast to mice in the CON group, expression of adiponectin was higher in the high-fat diet group and in the Ad-APN group. In contrast to CON mice, expression of eNOS was significantly higher in the Ad-APN group and was decreased in the high-fat diet group. The incremental increase of adiponectin in ApoE−/− mice treated with Ad-APN resulted in an increase in mRNA expression compared with control group mice. In addition, levels of mRNA expression for eNOS were comparable among groups (P<0.05). Comparing the high-APN group with the low-APN group, the difference had no statistical significance (Figure 4).
Figure 4.
Exogenous adiponectin reduces atherosclerosis-induced oxidative stress. (A) mRNA expression of adiponectin in the aortic vasculature of mice in different groups (n=12, each). (B) mRNA expression of eNOS in the aortic vasculature of mice in different groups (n=12, each). The value are means ±SEM. * P<0.05 vs. CON group; # P<0.05 vs. HFD group.
Discussion
Reduced NO bioavailability by decreased production and inactivation of NO is associated with the initiation, progression, and complications of atherosclerosis [21,22]. Adiponectin deficiency leads to increased oxidative stress and inflammation under high-fat diet conditions in animal models [23,24]. However, the functional significance of suppression of oxidative stress processes by adiponectin in obesity/atherosclerosis has not been addressed in the ApoE−/− atherosclerosis mouse model. Our major findings are: 1) exogenous supplementation of serum protein adiponectin using adenovirus improves obesity-induced atherosclerosis. Importantly, our study showed that the area of atherosclerosis plaques of the ApoE−/− mice shrank gradually along with the rising level of exogenous adiponectin; 2) this improvement is due, at least in part, to downregulation of oxidative stress by increasing eNOS mRNA expression in the aorta, increasing the activity of SOD, and reducing levels of MDA; and 3) exogenous supplementation of serum protein adiponectin normalizes dyslipidemia by reducing serum TC and TG and LDL-C, which suggests that adiponectin reduces cholesterol and TG, and the weight decreased significantly.
Adiponectin reduces atherosclerosis plaque formation of ApoE−/− mice
Atherosclerosis has been regarded as an inflammatory disease. The initial atherosclerotic lesion consists of monocytes/macrophages and T lymphocytes [25].The first change that precedes the formation of lesions of atherosclerosis is endothelial injury, which is mediated by various inflammatory stimuli, including TNF-α [26]. Experimental studies have indicated that adiponectin has potential anti-atherogenic and anti-inflammatory properties [27,28]. When the vascular endothelium is injured, adiponectin accumulates in the subintimal space of the arterial wall through its interaction with collagens in the vascular intima [29]. Adiponectin dose-dependently inhibited TNF-α-induced monocytes adhesion and expression of endothelial-leukocyte adhesion molecule-1, E-selectin, vascular cell adhesion molecule-1 (VCAM-1), and intracellular adhesion molecule-1 (ICAM-1) on the endothelium [30]. It has been suggested that the intracellular signal by which adiponectin suppressed adhesion molecule expression is inhibition of endothelial nuclear transcription factor-κB (NF-κB) signaling through the activation of cAMP protein kinase A [31]. A recent report demonstrated that adiponectin directly stimulates production of NO in endothelial cells. This direct stimulation depends on the pathway of phospatidylinositol-3-kinase (PI3K) involving phosphorylation of endothelial NO synthase (eNOS) at ser 1179 by AMPK [32]. Previous studies showed that elevated plasma adiponectin suppresses the development of atherosclerosis in vivo. Fourteen days after Ad-APN treatment, the lesion formation in aortic sinus was inhibited in Ad-APN-treated ApoE−/− mice by 30% compared with control mice. The lipid droplets became smaller compared with control mice [24]. Another experiment indicated that adiponectin deficiency does not accelerate the formation of atherosclerotic lesions in lean tissue in LDL receptor-deficient mice [33]. These discrepancies may derive from differences in strains, age, and disease status of the animals, as well as the dose and effective concentration of adiponectin. In our study, compared with those in the high-fat diet group, the damage area of atherosclerosis and the mean lesion rate of arterial lumen were both reduced significantly in the low-APN group and high-APN group. The area of atherosclerosis damage in the high-APN group lessened, but the difference between the 2 groups had no statistical significance, perhaps because the dose difference did not reach the threshold.
Adiponectin downregulates oxidative stress by increasing eNOS mRNA expression in the aorta, increases the activity of SOD, and reduces levels of MDA in serum
It was demonstrated that average adiponectin levels decreased significantly in the plasma of patients with obesity and obesity-related diseases, such as diabetes, coronary artery diseases, and hypertension, but excessive oxidative stress increased in these diseases. The multiple mechanisms involved, such as NAD(P)H oxidase, xanthine oxidase, mitochondrial respiratory chain, arachidonic acid metabolites, and uncoupled eNOS, can be inhibited by interventions such as exercise and medication [34,35]. Therefore, some scholars believed that adiponectin is correlated with oxidative stress, which can inhibit the expression of adiponectin on the transcription level, while adiponectin inhibits the oxidative stress [36]. Globular adiponectin (gAd) is a protein hydrolysis product of adiponectin existing in the plasma, which has much stronger pharmacological action on myocytes than does full-length adiponectin. The gAd can inhibit the generation of superoxidation of the endothelial cells. Motoshima et al. cultured bovine aortic endothelial cells (BAECs) to investigate the influence of gAd on oxidized LDL inducing ROS in the use of reconstructed gAd. When the gAd and oxidized LDL were used jointly to make the culturing, the release level of reactive oxygen species (ROS) of the BAECs has no similarity to those observed without any cultivation of reagents in the control group. These results showed that gAd can inhibit the release of ROS induced by BAECS, and that adiponectin can regulate oxidative stress [37].
Some experiments found that in the adiponectin gene-deficient mouse model, the vascular reproduction was damaged, but the revascularization could be realized through the expression of adenovirus-mediation adiponectin. In addition, supplementary exogenous adiponectin has been proven to be able to stimulate vascular reproduction and block vessels of the basement membrane in corneal transplantation animals [38]. Adiponectin is able to stimulate the migration and differentiation of endothelial cells to the capillary vascular-like structure to prevent the death of endothelial cells, as well as protect the endothelial cells inside the vasculature through the promotion of phosphorylation of eNOS and increase of NO synthesis and biological activity. On the one hand, adiponectin decreases in obesity and obesity-related diseases; the mechanism can be explained through the low-APN mass formed by blood stasis induced by increased oxidative stress, but the detailed regulation mechanism is still not completely understood [39,40]. On the other hand, adiponectin adjusts the oxidative stress to prevent the development of chronic diseases, such as coronary diseases, hypertension, and type 2 diabetes, and thus it may be a useful biomarker of oxidative stress on the adiponectin levels in vivo in plasma [41]. Our study showed that, along with the increase of exogenous adiponectin supplement, the adiponectin levels increased in the serum, the activity of SOD increased, and the MDA levels decreased; thus, adiponectin inhibited the oxidative stress in atherosclerosis mice.
At present, the mechanism by which adiponectin regulates oxidative stress is still unclear but may be interpreted with the following several mechanisms. First, the mechanism may be through inhibition of phosphorylation of mitogen-activated protein kinase (MAPK), that is, gAd suppresses the activity of NAD(P)H oxidase, to reduce the phosphorylation level of p42/p44 MAPK to inhibit the release of hyperoxide. The second is to affect the activation of peroxisome proliferator-activated receptor α(PPAR-α). The interaction between the adiponectin and adiponectin receptors (AdipoR1 and AdipoR2) activates the signaling molecule PPAR-α, the activation of which is related to the oxidation of fatty acid stimulated by adiponectin. The activation of PPAR-α can regulate the redox state in the cells to inhibit the signal transduction of nuclear transcription factor-κB (NF-κB), which is sensitive to oxidation-reduction. The adipokines activate NF-κB and then increase the production of NO and ROS, which participates in the formation of oxidized LDL, while the latter can activate NF-κB to induce the generation of extra ROS, and then to mediate the oxidative stress. The third mechanism may be by inhibition of signaling transduction ways of NF-κB. Adiponectin activates the cyclic adenosine monophosphate protein kinase A(cAMP-PKA) signaling pathways and simultaneously the inhibition of NF-κB signals mediates part of the anti-inflammation action of adiponectin [42–44]. We hypothesized that adiponectin might modulate atherosclerosis through inhibition of the NF-κB pathway. Further studies need to investigate adiponectin mediation of the action of NF-κB in the oxidative stress mechanism.
Adiponectin normalizes obesity and dyslipidemia
Atherosclerosis is an abnormality in lipid metabolism that is characterized by high TG, oxidized LDL, and low LDL-cholesterol levels [45]. The atherogenic process is initiated by the formation of oxidized LDL-cholesterol in the arterial wall, resulting in the recruitment of circulating monocytes and differentiation into macrophages [46]. Lipid accumulation causes dysregulation of adipokine production, which strongly contributes to the onset of inflammation and atherosclerosis [47]. Adiponectin – exhibiting antihyperglycemic, antiatherogenic, and anti-inflammatory properties – could have important clinical benefits in development of therapies for prevention of and/or for the treatment of obesity and obesity-related disease. A recent study showed that adiponectin, when overexpressed in macrophages of mice, resulted in decreased plasma cholesterol and triglyceride levels compared with control mice [48]. Adiponectin, when overexpressed in diabetes mice, resulted in decreased plasma triglyceride levels without changing total cholesterol levels [23]. Pair-feeding revealed that overexpression of globular adiponectin indeed markedly reduced body weight gain of ob/ob mice. These data suggest that overexpression of globular adiponectin primarily increased energy expenditure, and consequently increased food intake [49]. However, in our study, exogenous adiponectin supplementation significantly reduced total cholesterol and LDL-C body weight without HDL-C levels. The possible reason is that ApoE is a major apolipoprotein that acts as a ligand for low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) receptors in the liver. Atherogenic metabolic disturbances may not always be adequately reflected by the level of HDL-C, because VLDL contains more cholesterol and the relative cholesterol content of VLDL increased across TG quintiles [50–52].
Conclusions
The present study demonstrates that adiponectin mediated suppression of oxidative stress and lipid production in mice. Furthermore, exogenous adiponectin supplementation reduces atherosclerosis lesion formation size and rate in the aorta and reduces TC, TG, and LDL-C levels. The molecular mechanism may involve preservation of APN, SOD, reducing MDA in serum, increasing eNOS, and adiponectin mRNA expression in the aorta. Therefore, besides the treatment of basic diseases, it is quite significant to restore the level of adiponectin and completeness of the vasculature through reducing oxidative stress. This could promote pathological and physiological studies on obesity-related diseases and studies on the mechanism by which adiponectin regulates oxidative stress. Collectively, these findings give new insights into the linkage between adiponectin and oxidative stress in the course of atherosclerosis. However, the most effective dose of adiponectin was not been determined in this study, also the mechanism by which adiponectin regulates oxidative stress is still unclear. Therefore, further studied are needed to evaluate the therapeutic potential of these observations.
Footnotes
Source of support: The study was supported by the National Science Foundation of China (NSFC), China (No. 81160042), and by the Xinjiang Key Laboratory of Medical animal Model Research (No. XJDX1103-2012-02)
References
- 1.Lehrke M, Lebherz C. AAV-Mediated Gene Therapy for Atherosclerosis. Curr Atheroscler Rep. 2014;16(9):434. doi: 10.1007/s11883-014-0434-0. [DOI] [PubMed] [Google Scholar]
- 2.Blum A. HMG-CoA reductase inhibitors (statins), inflammation, and endothelial progenitor cells-New mechanistic insights of atherosclerosis. Biofactors. 2014;40(3):295–302. doi: 10.1002/biof.1157. [DOI] [PubMed] [Google Scholar]
- 3.Montagnana M, Danese E, Lippi G. Genetic risk factors of atherothrombosis. Pol Arch Med Wewn. 2014 doi: 10.20452/pamw.2409. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Jamkhande PG, Chandak PG, Dhawale SC, et al. Therapeutic approaches to drug targets in atherosclerosis. Saudi Pharm J. 2014;22(3):179–90. doi: 10.1016/j.jsps.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki M, Minami A, Nakanishi A, et al. Atherosclerosis and tumor suppressor molecules. Int J Mol Med. 2014;34(4):934–40. doi: 10.3892/ijmm.2014.1866. [DOI] [PubMed] [Google Scholar]
- 6.Sessa R, Pietro MD, Filardo S, et al. Infectious burden and atherosclerosis: A clinical issue. World J Clin Cases. 2014;16;2(7):240–9. doi: 10.12998/wjcc.v2.i7.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagli N, Ozturk U, Karaca I, et al. Adiponectin levels in coronary artery ectasia. Heart Vessels. 2009;24(2):84–89. doi: 10.1007/s00380-008-1087-0. [DOI] [PubMed] [Google Scholar]
- 8.Lehrke M, Greif M, Broedl UC, et al. MMP-1 serum levels predict coronary atherosclerosis in humans. Cardiovasc Diabetol. 2009;8:50. doi: 10.1186/1475-2840-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekmekci H, Ekmekci OB. The role of adiponectin in atherosclerosis and thrombosis. Clin Appl Thromb Hemost. 2006;12(2):163–68. doi: 10.1177/107602960601200203. [DOI] [PubMed] [Google Scholar]
- 10.Ohashi K, Shibata R, Murohara T, et al. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol Metab. 2014;25(7):348–55. doi: 10.1016/j.tem.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Nakatsuji H, Kishida K, Sekimoto R, et al. Accumulation of adiponectin in inflamed adipose tissues of obese mice. Metabolism. 2014;63(4):542–53. doi: 10.1016/j.metabol.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Van Stijn CM, Kim J, Barish GD, et al. Adiponectin expression protects against angiotensin II-mediated inflammation and accelerated atherosclerosis. PLoS One. 2014;9(1):e86404. doi: 10.1371/journal.pone.0086404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashino Y, Jackson JL, Hirata T, et al. Effects of exercise on C-reactive protein, inflammatory cytokine and adipokine in patients with type 2 diabetes: a meta-analysis of randomizedcontrolled trials. Metabolism. 2014;63(3):431–40. doi: 10.1016/j.metabol.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Komura N, Maeda N, Mori T, et al. Adiponectin protein exists in aortic endothelial cells. PLoS One. 2013;8(8):e71271. doi: 10.1371/journal.pone.0071271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeuchi S, Wada K, Uozumi Y, et al. Adiponectin receptor 1 expression is associated with carotid plaque stability. Neurol India. 2013;61(3):249–53. doi: 10.4103/0028-3886.115063. [DOI] [PubMed] [Google Scholar]
- 16.Adya R, Tan BK, Chen J, et al. Protective actions of globular and full-length adiponectin on human endothelial cells: novel insights into adiponectin-induced angiogenesis. J Vasc Res. 2012;49(6):534–43. doi: 10.1159/000338279. [DOI] [PubMed] [Google Scholar]
- 17.Cheraghi N, Dai H, Raghuveer G. Vitamin D deficiency is associated with atherosclerosis-promoting risk factor clustering but not vascular damage in children. Med Sci Monit. 2012;18(12):CR687–92. doi: 10.12659/MSM.883593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motawi T, Shaker O, Taha N, et al. Genetic variations in E-selectin and ICAM-1: relation to atherosclerosis. Med Sci Monit. 2012;18(6):CR381–89. doi: 10.12659/MSM.882908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Cui Y, Peng DQ. The role of monocyte phenotype switching in peri-procedural myocardial injury and its involvement in statin therapy. Med Sci Monit. 2013;19:1006–12. doi: 10.12659/MSM.889661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Zhang H, McAfee S, et al. The reciprocal relationship between adiponectin and LOX-1 in the regulation of endothelial dysfunction in ApoE knockout mice. Am J Physiol Heart Circ Physiol. 2010;299:H605–12. doi: 10.1152/ajpheart.01096.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drummond GR, Sobey CG. Endothelial NADPH oxidizes: which NOX to target in vascular disease? Trends Endocrinol Metab. 2014 doi: 10.1016/j.tem.2014.06.012. pii: S1043-2760(14)00123-4. [DOI] [PubMed] [Google Scholar]
- 22.Shaw A, Doherty MK, Mutch NJ, et al. Endothelial cell oxidative stress in diabetes: a key driver of cardiovascular complications? Biochem Soc Trans. 2014;42(4):928–33. doi: 10.1042/BST20140113. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Zhang H, Chen J, et al. Adiponectin abates diabetes-induced endothelial dysfunction by suppressing oxidative stress, adhesion molecules, and inflammation in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2012;303(1):H106–15. doi: 10.1152/ajpheart.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okamoto Y, Kihara S, Ouchi N, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106(22):2767–70. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 25.Sasaguri Y, Tanimoto A. Role of macrophage-derived histamine in atherosclerosis – chronic participation in the inflammatory response. J Atheroscler Thromb. 2004;11(3):122–30. doi: 10.5551/jat.11.122. [DOI] [PubMed] [Google Scholar]
- 26.Dessein PH, Tsang L, Solomon A, et al. Adiponectin and atherosclerosis in rheumatoid arthritis. Mediators Inflamm. 2014;2014:358949. doi: 10.1155/2014/358949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fayad R, Pini M, Sennello JA, et al. Adiponectin deficiency protects mice from chemically induced colonic inflammation. Gastroenterology. 2007;132(2):601–14. doi: 10.1053/j.gastro.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 28.Ohman MK, Shen Y, Obimba CI, et al. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2008;117(6):798–805. doi: 10.1161/CIRCULATIONAHA.107.717595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishida M, Moriyama T, Ishii K, et al. Effects of IL-6, adiponectin, CRP and metabolic syndrome on subclinical atherosclerosis. Clin Chim Acta. 2007;384(1–2):99–104. doi: 10.1016/j.cca.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Wu LL. Adiponectin and interleukin-6 in inflammation-associated disease. Vitam Horm. 2012;90:375–95. doi: 10.1016/B978-0-12-398313-8.00014-2. [DOI] [PubMed] [Google Scholar]
- 31.Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102(11):1296–301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Montagnani M, Funahashi T, et al. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278(45):45021–26. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 33.Nawrocki AR, Hofmann SM, Teupser D, et al. Lack of association between adiponectin levels and atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2010;30(6):1159–65. doi: 10.1161/ATVBAHA.109.195826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S, Park Y, Dellsperger KC, et al. Exercise training improves endothelial function via adiponectin-dependent and independent pathways in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2011;301(2):H306–14. doi: 10.1152/ajpheart.01306.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee S, Park Y, Zuidema MY, et al. Effects of interventions on oxidative stress and inflammation of cardiovascular diseases. World J Cardiol. 2011;3(1):18–24. doi: 10.4330/wjc.v3.i1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai XJ, Li CJ, Chen L, et al. A hypothesis: adiponectin mediates anti-atherosclerosis via adventitia-AMPK-iNOS pathway. Med Hypotheses. 2008;70(5):1044–47. doi: 10.1016/j.mehy.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Motoshima H, Wu X, Mahadev K, et al. Adiponectin suppresses proliferation and superoxide generation and enhances eNOS activity in endothelial cells treated with oxidized LDL. Biochem Biophys Res Commun. 2004;315(2):264–71. doi: 10.1016/j.bbrc.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 38.Parker-Duffen JL, Nakamura K, Silver M, et al. T-cadherin is essential for adiponectin-mediated revascularization. J Biol Chem. 2013;288(34):24886–97. doi: 10.1074/jbc.M113.454835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owens CD, Kim JM, Hevelone ND, et al. Novel adipokines, high molecular weight adiponectin and resistin, are associated with outcomes following lower extremity revascularization with autogenous vein. J Vasc Surg. 2010;51(5):1152–59. doi: 10.1016/j.jvs.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang SS, Huang PH, Chen YH, et al. Association of adiponectin with future cardiovascular events in patients after acute myocardial infarction. J Atheroscler Thromb. 2010;17(3):295–303. doi: 10.5551/jat.3533. [DOI] [PubMed] [Google Scholar]
- 41.Kondo M, Shibata R, Miura R, et al. Caloric restriction stimulates revascularization in response to ischemia via adiponectin-mediated activation of endothelial nitric-oxide synthase. J Biol Chem. 2009;284(3):1718–24. doi: 10.1074/jbc.M805301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishii M, Shibata R, Kondo K, et al. Vildagliptin stimulates endothelial cell network formation and ischemia-induced revascularization via an endothelial nitric oxide synthase-dependent mechanism. J Biol Chem. 2014 doi: 10.1074/jbc.M114.557835. pii: jbc.M114.557835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bharrhan S, Chopra K, Arora SK, et al. Down-regulation of NF-κB signalling by polyphenolic compounds prevents endotoxin-induced liver injury in a rat model. Innate Immun. 2012;18(1):70–79. doi: 10.1177/1753425910393369. [DOI] [PubMed] [Google Scholar]
- 44.Hulsmans M, Geeraert B, Arnould T, et al. PPAR agonist-induced reduction of Mcp1 in atherosclerotic plaques of obese,insulin-resistant mice depends on adiponectin-induced Irak3 expression. PLoS One. 2013;8(4):e62253. doi: 10.1371/journal.pone.0062253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mierzecki A, Kłoda K, Bukowska H, et al. Association between low-dose folic acid supplementation and blood lipids concentrations in male and female subjects with atherosclerosis risk factors. Med Sci Monit. 2013;19:733–39. doi: 10.12659/MSM.889087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamauchi T, Kamon J, Waki H, et al. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–68. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 47.Tian L, Luo N, Klein RL, et al. Adiponectin reduces lipid accumulation in macrophage foam cells. Atherosclerosis. 2009;202(1):152–61. doi: 10.1016/j.atherosclerosis.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo N, Liu J, Chung BH, et al. Macrophage adiponectin expression improves insulin sensitivity and protects against inflammation and atherosclerosis. Diabetes. 2010;59(4):791–99. doi: 10.2337/db09-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida S, Fuster JJ, Walsh K. Adiponectin attenuates abdominal aortic aneurysm formation in hyperlipidemic mice. Atherosclerosis. 2014;235:339–46. doi: 10.1016/j.atherosclerosis.2014.05.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nigro E, Scudiero O, Monaco ML, et al. New Insight into Adiponectin Role in Obesity and Obesity-Related Diseases. Biomed Res Int. 2014;2014:658913. doi: 10.1155/2014/658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo R, Li W, Liu B, et al. Resveratrol protects vascular smooth muscle cells against high glucose-induced oxidative stress and cell proliferation in vitro. Med Sci Monit Basic Res. 2014;20:82–92. doi: 10.12659/MSMBR.890858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karwowski W, Naumnik B, Szczepański M, et al. The mechanism of vascular calcification – a systematic review. Med Sci Monit. 2012;18(1):RA1–11. doi: 10.12659/MSM.882181. [DOI] [PMC free article] [PubMed] [Google Scholar]